Abstract
There is widespread human exposure to low concentrations of deoxynivalenol (DON), a fungal mycotoxin found in grain-based foods and animal feed, throughout the lifetime. Given that toxicity associated with low-level DON exposure is understudied, this study identified doses that could be used to evaluate long-term toxicity following perinatal exposure. Time-mated Harlan Sprague Dawley (Hsd:Sprague Dawley® SD®) rats were administered 0, 0.03, 0.1, 0.3, 1, or 3 mg/kg/day of DON once daily via gavage from gestational day (GD) 6 through postnatal day (PND) 27. F1 animals were administered the same dose of DON as their respective dams from PND 12–27. DON had no effect on maternal body weight or feed consumption at any dose. In only the 3 mg/kg/day group, F0 females had smaller live litter sizes than controls and pups had significantly lower body weight compared to controls. At study end, F1 body weight was significantly lower than controls and blood samples showed no increases in frequencies of micronucleated immature erythrocytes in either F0 or F1 animals. In summary, doses of DON ≤3 mg/kg/day did not affect maternal survival or body weight. 3 mg/kg/day resulted in decreased body weight in the offspring. The no-observed effect level was 1 mg/kg/day.
Keywords: deoxynivalenol, perinatal, mycotoxin, rats, genotoxicity
Introduction
Deoxynivalenol (DON) is the most commonly detected trichothecene mycotoxin in grain-based foods and animal feed. DON is produced by certain species of the Fusarium fungi and is extremely heat stable, allowing it to resist degradation through processing and cooking of foods (Sobrova et al., 2010). DON has been detected in a variety of wheat products such as cereals, oats, barley, corn, breads, and beers (Panel, 2017). Exposure to DON is widespread as it has been detected in food sources from around the world (Yazar and Omurtag, 2008) and in human urine from various populations (Chen et al., 2017). Estimated human exposure to DON based on dietary surveys ranged from 0.2 to 2.9 μg/kg body weight/day (Panel, 2017) and varies due to regional diet differences.
Acute exposure to DON in humans has been associated with abdominal pain, diarrhea, and vomiting, symptoms which engender an alternative name for DON, vomitoxin. In animals, similar clinical signs have been observed. However, the chronic effects of low-level DON exposure are unknown. Studies in animals commonly report reduced body weight gain and decreased feed consumption, with body weight often being the most sensitive toxicological effect of DON. Other effects that have been observed experimentally include immune dysregulation (Pestka, 2008), hematological disturbances (Wu et al., 2009; Iverson et al., 1995), and steroid hormone disruption (Urbanek et al., 2018). Mechanisms underlying DON toxicity (reviewed in Pestka, 2010 and Sobrova et al., 2010) include inhibition of protein synthesis, inhibition of ribosomal translation, increased expression of cytokines and chemokines, increased oxidative stress, and modulations in mitogen-activated protein kinase signaling pathways that control cell proliferation, differentiation, and apoptosis (Pestka, 2010; Wang et al., 2014).
Human exposure to DON occurs at low levels and throughout life, but the consequences of perinatal DON exposure are not well understood. Not only has DON been detected in urine of pregnant women (Chen et al., 2017), but DON is also predicted to cross the human placenta (Nielsen et al., 2011). Thus, both women and fetuses can be exposed. In mice, continuous exposure of the F0 generation to DON in the diet did not impair fertility but did result in lower F0 body weight, reduced live litter size and lower pup survival and body weight in the top dose group (2 mg/kg) (Khera et al., 1984). Cross-fostering of pups from control and DON-treated dams showed that the reduced survival and body weight may be related to both prenatal and postnatal exposure (Khera et al., 1984). Other studies in mice found prenatal DON exposure (≥2.5 mg/kg) associated with bone malformations of the fetus (Zhao et al., 2012; Khera et al., 1982). In rats, DON exposure (2.5–5 mg/kg) during gestation elicited skeletal malformations in one study (Collins et al., 2006) but not others (Khera et al., 1984; Morrissey, 1984). However, many of the developmental effects are observed in conjunction with significant maternal toxicity, e.g., decreased maternal body weight. Thus, it is unclear how much of the effect of DON is due to maternal toxicity rather than direct effects on the fetus.
The Joint Food and Agriculture and World Health Organization Expert Committee on Food Additives (JECFA) has articulated critical research needs with regard to DON and other trichothecenes, including clarification of species differences in sensitivity, the effects of combined trichothecene exposure, additional genotoxicity studies, and a carcinogenicity study in a second species (rat) (JECFA, 2002). The goal of this study was to provide data that can be used in the design of future studies to address these research needs. Specifically, this study identified doses to evaluate toxicity of DON under environmentally relevant exposure conditions (i.e. exposure to low levels throughout life, including the perinatal period) and investigated in vivo genotoxicity associated with perinatal exposures. Low doses of DON (0.03, 0.1, 0.3, 1, and 3 mg/kg/day) were administered to F0 females during gestation and to F1 offspring early in postnatal life. While administration via feed is considered the most relevant route of exposure, reduced feed consumption is often observed in DON-exposed animals (Morrissey and Vesonder, 1985; Khera et al., 1984; Khera et al., 1986). To avoid this potential confounder, F0 and F1 rats were administered DON via oral gavage.
Materials and Methods
Animals
Studies were conducted at Southern Research (Birmingham, AL). Ten to 12-week-old time-mated (presumed pregnant) female Sprague Dawley rats (Hsd:Sprague Dawley® SD®) were obtained from the vendor (Envigo, Haslett, MI) and housed in solid-bottom, polycarbonate cages lined with irradiated hardwood-chip bedding (Sani-Chips® cage litter, P.J. Murphy Forest Products, Montville, NJ). Females were bred with non-siblings. Irradiated crinkled kraft paper (Crink-l’Nest, The Andersons, Maumee, OH) was provided to F0 females during the study. Presumed pregnant F0 females were singly housed except when with their respective litters during lactation. On PND 4, litters were randomly standardized to eight offspring (as close to four males and four females as possible using the random culling features of the Provantis data collection system). Irradiated NIH-07 certified rodent diet (Zeigler Bros., Inc., Gardners, PA) was available ad libitum throughout the study. Tap water (City of Birmingham, AL) was available ad libitum via an automatic water delivery system. 10 non-mated females were not dosed and included as sentinels for the animal disease screening program.
Animal rooms were maintained at 69–75 °F, humidity 35–65%, with 16 filtered room air changes per hour, and a 12-hour light/dark cycle per day. Room conditions were monitored using the Edstrom Watchdog system. Animal care conformed to the guidelines of the Guide for the Care and Use of Laboratory Animals, the U.S. Department of Agriculture through the Animal Welfare Act (Public Law 99–198), the Public Health Policy on Humane Care and Use of Laboratory Animals (Public Law 99–158) and to the applicable Standard Operating Procedures of Southern Research. This study was conducted in an animal facility accredited by AAALAC International.
Chemical procurement and formulation
Deoxynivalenol (DON; CAS number: 51481–10-8) was obtained from LKT Laboratories, Inc. (Saint Paul, MN) in three lots and were combined (Lot numbers 2597750, 2597589, 2597595). The combined lots were characterized by infrared spectroscopy, 1H and 13C nuclear magnetic resonance spectroscopy, and high-resolution mass spectrometry with exact mass determination. Two microbiology screening assays (enzyme and visual endpoint) also confirmed the presence of DON. The purity was estimated using liquid chromatograph analyses and determined to be ~97%, with three impurity peaks of 2.1%, 0.1%, and 0.1% of the total integrated area. Mass spectrometry identified the major impurity peak to be 7-deoxy-deoxynivalenol and one of the minor impurities to be acetyl-deoxynivalenol.
DON was formulated at 0 (control), 0.006, 0.02, 0.06, 0.2, and 0.6 mg/mL in deionized water. Formulations were analyzed using an ultra-performance liquid chromatography method coupled with ultraviolet absorbance detection (correlation coefficient > 0.99; precision measured as relative standard deviation ≤ 1.2%; accuracy measured as relative error ≤ ±5%). All formulations were within 5% of the target concentration both pre- and post-dose administration. Prior to study start, the stability of DON in the formulation was confirmed for up to 42 days at ambient temperature using the formulation concentration of 0.003 mg/mL.
Exposure
Time-mated females were randomly assigned to control and experimental groups on GD 3 using a computerized randomization procedure to ensure comparable mean body weights across groups. Animals with different mating dates (GD 0) were equally distributed across groups to the extent possible. Each dose group consisted of 10 time-mated females, with the control group and the 1 mg/kg/day group having an extra 6 females for sampling for internal test article concentration analysis. On the first day of dosing, F0 females were 11–13 weeks of age. F0 females were administered 0.03, 0.1, 0.3, 1, or 3 mg/kg/day of DON once daily via gavage starting on GD 6 until PND 27. The dose volume administered was 5 mL/kg using the most recent body weight. Offspring (F1) were given the same dose as their respective dam from PND 12 through PND 27 using a syringe and an appropriately sized needle. Animals were not dosed on the day of scheduled euthanasia, PND 28.
In-life endpoints measured
Body weights (daily), clinical observations (twice daily before 10 am and after 2 pm, at least 6 hours apart), and feed consumption of F0 females (every three days) were recorded throughout the study. Litters born after the second daily observation were recorded as having occurred the following day. A pup was recorded as “cannibalized” if an observation of a partially cannibalized pup was made. Otherwise, the pup was accounted for as “missing, presumed dead.” Body weights of F1 animals were recorded on PND 1, 4, 7, and daily from PND 12–28.
Biological sampling analysis
On GD 18 approximately 2 hours after dosing, three randomly selected, pregnant dams from the control and the 1 mg/kg/day dose group were anesthetized with CO2/O2 and blood was collected via cardiac puncture. Amniotic fluid was collected at laparohysterectomy via needle and syringe and fetuses from these litters were euthanized, pooled by litter, and frozen in liquid nitrogen. On PND 4 approximately 2 hours after dosing, three randomly selected dams were anesthetized with CO2/O2 and blood was collected via cardiac puncture. Blood from the litters of these same dams (4 pups/sex/litter) was collected via decapitation. All samples were flash frozen and stored at − 70°C until chemical analysis. These data are reported in a separate publication (Silinski et al., 2020).
Micronuclei evaluation
The ability of DON to induce chromosomal damage in the form of structural or numerical changes was evaluated using the peripheral blood erythrocyte micronucleus test (OECD Test Guideline 474). On the day of euthanasia for F0 and F1 animals (PND 28), randomly selected animals were anesthetized with CO2/O2 and a blood sample (via the retro-orbital plexus) was obtained from F0 females and from one male and one female offspring of each selected litter from each dose group (N=6). Blood samples were kept refrigerated (4°C), and then packed on cold packs and shipped via overnight courier to the analytical laboratory (Integrated Laboratory Systems, LLC, Research Triangle Park, NC) where they were fixed in ultra-cold methanol (−80°C), as described in the MicroFlowPLUS Kit instruction manual (Litron Laboratories, Rochester, NY). Fixed blood samples were stored in a −80°C freezer for at least 3 days before scoring.
Blood samples were analyzed using automated flow cytometric techniques and MicroFlowPLUS Kit reagents with a Becton-Dickinson FACSCalibur™ dual-laser bench top system. For each blood sample, 20,000 immature polychromatic erythrocytes (PCE; reticulocytes) and approximately 1 × 106 mature normochromatic erythrocytes (NCE) were analyzed to determine the frequency of normal and micronucleated cells, as well as the percentage of PCE among total erythrocytes (PCE + NCE), which provides a measure of bone marrow toxicity. In rats, only the youngest fraction of the PCE population (identified by a highly fluorescing transferrin receptor, CD71+) can accurately be interrogated for presence of micronuclei, as the rat spleen rapidly and efficiently removes damaged erythrocytes soon after they emerge from the bone marrow.
Necropsy
F0 females were not examined at necropsy. A gross macroscopic examination of the thoracic and abdominal viscera was performed for five randomly selected F1 animals/sex/dose group and liver, lung, spleen, thymus, and kidney weights were recorded. Brain weight was collected for three randomly selected F1 animals per sex in the control and the 3 mg/kg/day dose group.
Statistical analyses
Data was collected using the ProvantisTM-hosted system provided by the NTP (Instem Life Sciences Systems, Ltd., Staffordshire, UK). For continuous endpoints, extreme values were identified by the outlier test of Dixon and Massey (1957). To identify outliers for continuous endpoints with litter effects in the F1 generation, all observations across dose groups were fit to a linear mixed effects model with a random litter effect, and the residuals were tested by dose group for outliers using Tukey’s outer fences method (Tukey, 1977). All flagged outliers were examined and implausible values were eliminated from the final analyses. Discrete count endpoints were manually identified for unusual values.
Pairwise comparisons between control and dosed groups for normally distributed endpoints without litter effects (organ weights and gestational/lactational body weights) were analyzed with parametric multiple comparison procedures (Dunnett, 1955, Williams, 1971, Williams, 1972). Comparisons between control and dosed groups for endpoints with skewed distributions (gestational length, feed consumption, litter sizes, survival ratios, dead and percent male F1 pups per litter) were analyzed using the nonparametric multiple comparison methods of Shirley (1977) (as modified by Williams, 1986) and Dunn (1964). For these endpoints, the Jonckheere’s test (1954) was used to assess the significance of dose-related trends, and to determine, at the 0.01 level of significance, whether a trend-sensitive test (the William’s or Shirley test) was more appropriate for pairwise comparisons than a test that does not assume a monotonic dose-related trend (the Dunnett or Dunn test). The Cochran-Armitage test for trend and the Fisher Exact test for pairwise testing were used to analyze binary endpoints (gross pathology) (Gart et al., 1979). Non-pregnant F0 females were excluded from statistical analyses for gestational endpoints. Normally distributed endpoints with litter effects (pre-weaning pup body weights) were analyzed with mixed effects linear models with a random litter effect, and the Dunnett-Hsu procedure (Hsu, 1992) was used to adjust for multiple comparisons. Pup weights were adjusted for litter size by covariate analysis (see below).
Because litter size may have an effect on pup body weight, accounting for litter size in the analysis of pup weights can provide additional insight into the effects of test articles (Hothorn, 2014), by increasing power to detect treatment effects in cases where the litter effect is relatively constant across doses. Preweaning pup body weights were adjusted for live litter size as follows: a linear model was fit to body weights as a function of dose and litter size. The estimated coefficient of litter size was then used to adjust each pup body weight based on the difference between its litter size and the mean litter size. Prestandardization PND 4 F1 body weights were adjusted for PND 1 litter size, and F1 body weights measured between PND 4 poststandardization and PND 21 were adjusted for PND 4 poststandardization litter size. Following adjustment, F1 body weights were analyzed with a linear mixed model with a random litter effect, and a Dunnett-Hsu adjustment for multiple pairwise comparisons.
Genetic toxicity data are summarized as mean ± standard error of the mean for each treatment group. Levene’s test was used to determine if variances among exposed groups were equal at p=0.05 (Levene 1960). When variances were equal, linear regression analysis was used to test for linear trend and Williams’ test was used to evaluate pairwise differences of each exposed group with the vehicle control group (Williams 1971; Williams 1972). When variances were unequal, nonparametric methods were used to analyze the data; Jonckheere’s test was used to evaluate linear trend and Dunn’s test was used to assess the significance of pairwise differences of each exposed group with the vehicle control group. The trend as well as the pairwise differences from the vehicle control group were declared statistically significant if p<0.025. A result was considered positive if the trend test was significant and if at least one exposed group was significantly elevated over the control group, or if two or more exposed groups were significantly increased over the corresponding control group. A response was considered equivocal if only the trend test was significant or if only a single exposed group was significantly increased over the control group.
Results
All study data are available in the NTP Chemical Effects in Biological Systems (CEBS) database: https://doi.org/10.22427/NTP-DATA-002-03342-0004-0000-8.
F0 females
There were no treatment-related clinical observations or mortality during gestation or lactation (CEBS I01, I05). Body weight and body weight gain in F0 females given DON were not significantly different compared to controls throughout gestation and lactation (Figure 1, CEBS I04G). There were sporadic differences in feed consumption during gestation and lactation, but the changes were not consistent and thus were not attributed to DON exposure (Figure 2, CEBS I06).
Figure 1.
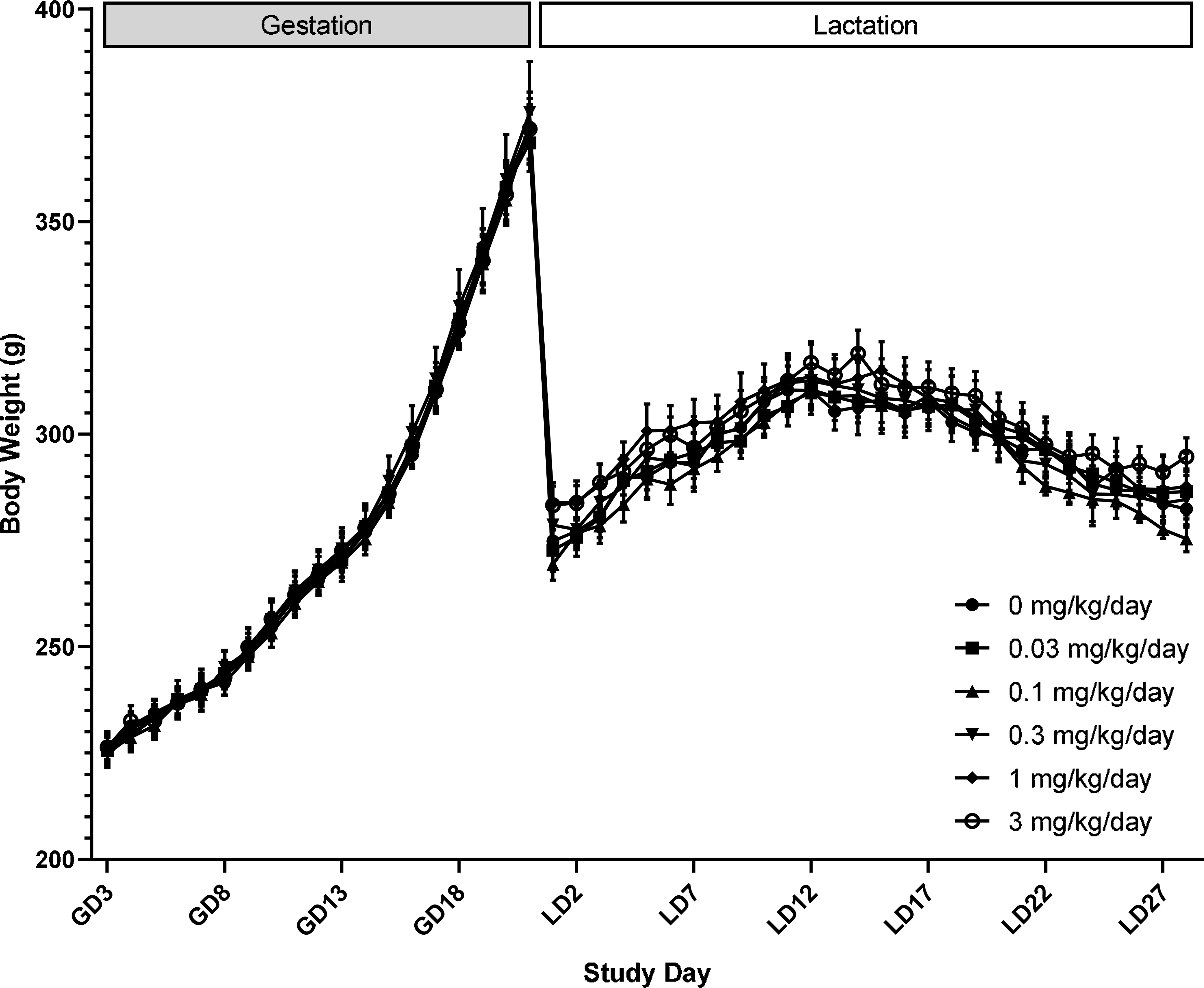
Body weight of female F0 Sprague Dawley rats administered deoxynivalenol via oral gavage starting on gestation (GD) 6 and throughout lactation (LD). Shown are mean ± SEM for each exposure group. N=10 dams, except in the control and 1 mg/kg/day groups which had N=16 during gestation for biological sampling on GD 18 and LD 4. During lactation, N=9 in the control group due to an early death on LD 4 and N=9 in the 1 mg/kg/day group due to a dosing accident on LD 24.
Figure 2.
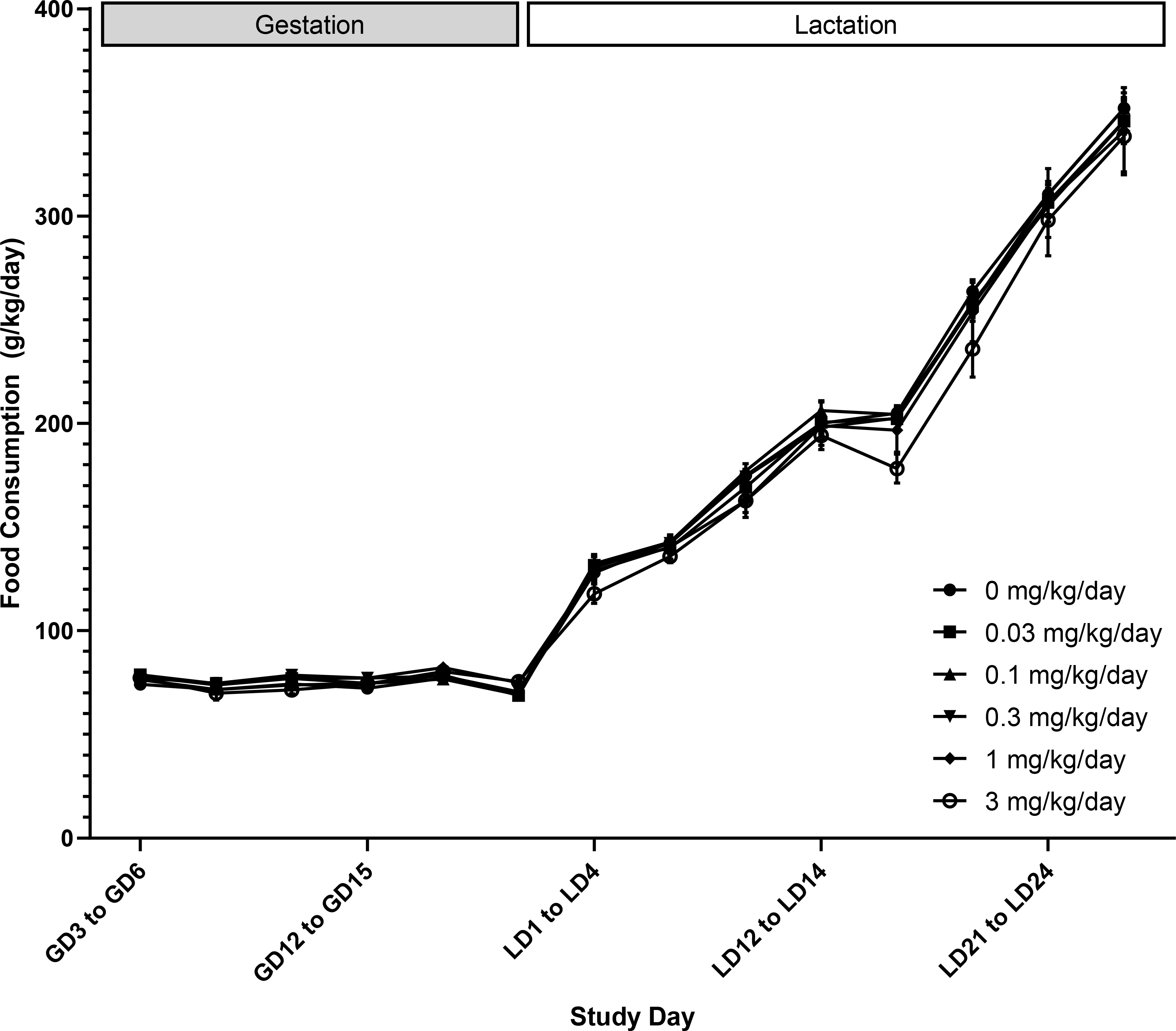
Food consumption (g/kg body weight/day) of female F0 Sprague Dawley rats administered deoxynivalenol via oral gavage starting on gestation (GD) 6 and throughout lactation (LD). Shown are mean ± SEM for each exposure group on each study day. N=10 dams, except in the control and 1 mg/kg/day groups which had N=16 during gestation for biological sampling on GD 18 and LD 4. During lactation, N=9 in the control group due to an early death on LD 4 and N=9 in the 1 mg/kg/day group due to a dosing accident on LD 24.
Live litter size was lower in the 3 mg/kg/day dose group on PND 0. It was not statistically significant, but it was accompanied by a significant decrease in survival on PND 0 (Table 1, CEBS R03) that was primarily driven by pup losses in one litter (4 of 12 died). There were no treatment-related differences in pup survival from PND 1 onwards. Gestation length and sex ratio on PND 0 in DON-treated groups were not different from control (CEBS R02).
Table 1.
Gestation length, litter size, and pup survival in litters from female F0 Sprague Dawley rats administered deoxynivalenol for 6 weeks starting on gestational day 6 via gavage
| 0 mg/kg/day | 0.03 mg/kg/day | 0.1 mg/kg/day | 0.3 mg/kg/day | 1 mg/kg/day | 3 mg/kg/day | |
|---|---|---|---|---|---|---|
| N1 | 12 | 10 | 10 | 10 | 13 | 10 |
| Gestation Length (days) | 21.9 ± 0.1 | 21.9 ± 0.1 | 21.9 ± 0.1 | 22.0 ± 0.0 | 22.0 ± 0.0 | 22.2 ± 0.1 |
| Total Litter Size (PND 0) | 13.1± 0.5 | 13.1 ± 0.4 | 13.9 ± 0.4 | 13.3 ± 1.2 | 12.1 ± 1.0 | 12.1 ± 0.6 |
| Live litter size (PND 0) | 13.0 ± 0.5 | 12.6 ± 0.6 | 13.7 ± 0.4 | 13.1 ± 1.2 | 11.9 ± 1.0 | 11.2 ± 0.7 |
| PND 0 Sex ratio (% Male) | 47.60 ± 4.83 | 44.80 ± 5.59 | 45.91 ± 4.03 | 56.33± 5.42 | 42.92 ± 5.60 | 44.59 ± 6.54 |
| % Survival PND 0–1 | 99 ± 1 | 96 ± 3 | 99 ± 1 | 99 ± 1 | 99 ± 1 | 93 ± 3* |
| % Survival PND 1–4 | 97 ± 1 | 99 ± 1 | 96 ± 2 | 99 ± 1 | 100 ± 0 | 93 ± 5 |
| % Survival postcull2 PND 5–28 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
Statistically significant at p<0.05
Number of dams/litters. Control and 1 mg/kg/day groups had more dams to account for biological sample analyses on PND 4
Litters were culled to 4/sex/litter and N=10 litters per treatment group on PND 4. N=9 litters for the control group.
F1 offspring
To account for potential litter effects, F1 body weights were adjusted and adjusted body weights were used for statistical analyses. There were effects of DON on body weight primarily in the highest dose group, 3 mg/kg/day. In lower dose groups, changes in body weight did not exceed 5% of the control group throughout the study period, and thus changes were not considered related to DON exposure. Before direct dosing of pups on PND 12, the body weight of pups in the 3 mg/kg/day dose group tended to be lower than controls and could be attributed to less body weight gain (Figure 3, CEBS R19, R19G).
Figure 3.
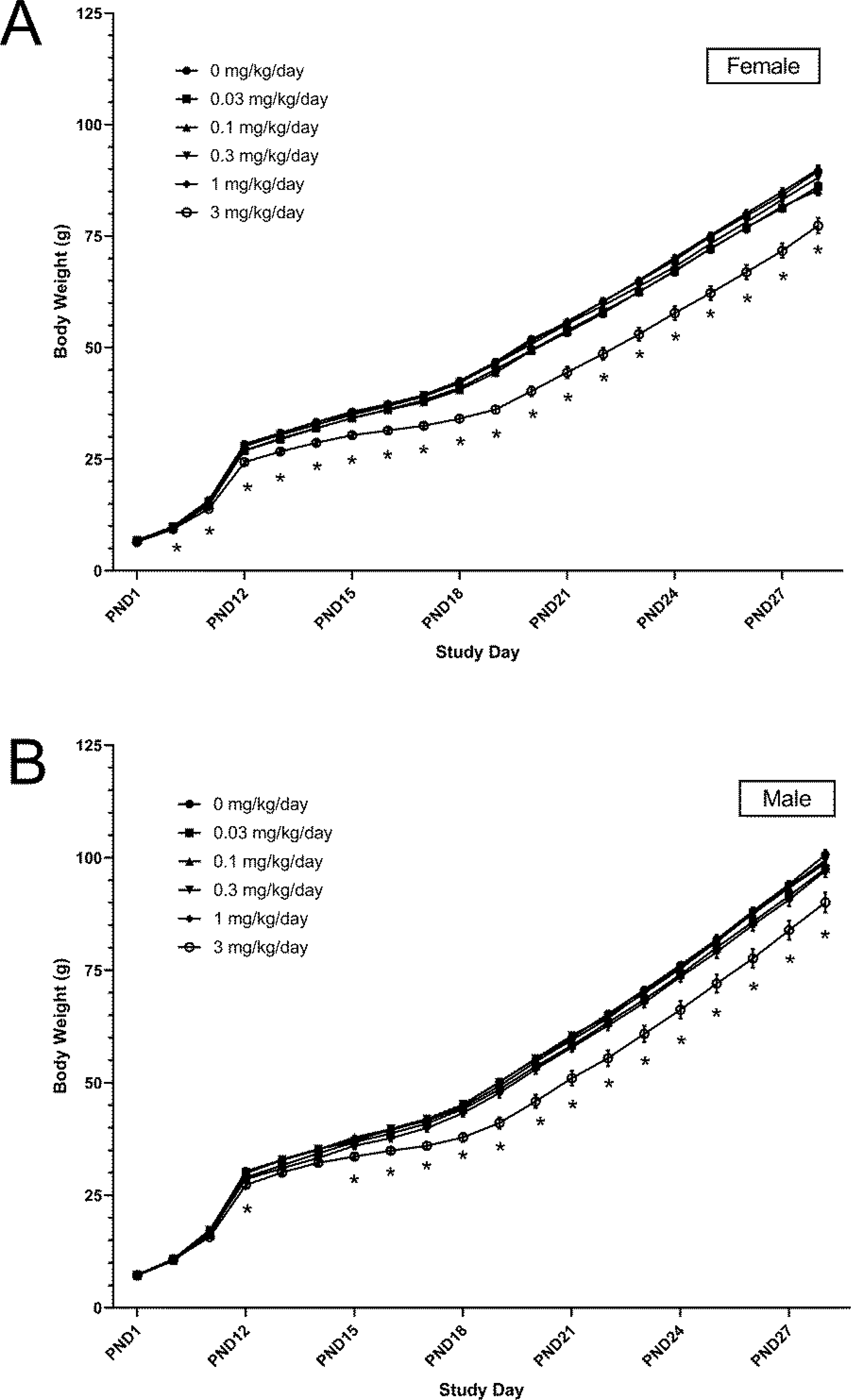
Adjusted body weight of female (A) and male (B) F1 Sprague-Dawley rats from postnatal day (PND) 1 to 28 after exposure to deoxynivalenol in utero beginning on gestation day 6 and continuing through lactation. Shown are mean ± SEM for each exposure group on each study day. N=10 litters, except in the control and 1 mg/kg/day group which had N=12–13 until PND 4 (when pups were taken for biological sampling). From PND 4 onwards, N=9 in the control group due to an early death. *p<0.05 compared to control group.
Body weight was 4–13% lower and body weight gain was 8–23% lower than controls. Effects of 3 mg/kg/day of DON on body weight and body weight gain were more apparent in female pups than male pups. After PND 12, further reductions in body weight were observed in the 3 mg/kg/day dose group, ranging from 8 to 23% lower than controls in both males and females. Starting on PND 15 and until study termination on PND 28, body weight was significantly lower in the 3 mg/kg/day dose group compared to controls; 10% lower in males and 13% lower in females.
At necropsy, there were no treatment-related macroscopic observations in the five F1 animals/sex/dose/group randomly selected for evaluation (CEBS PA46). In female offspring, absolute and relative lung weights were lower in the top two doses (Table 2). Additionally, there was a significant increase in relative spleen weight only at the highest dose group. Absolute, but not relative, kidney and brain weights in the top dose group were significantly lower than controls. These organ weight changes did not appear to be associated with any gross macroscopic observations. No treatment-related differences in organ weight were observed in male offspring (CEBS PA06).
Table 2.
Absolute (mg) and relative organ weights (mg/g body weight) from female F1 Sprague Dawley rats exposed to deoxynivalenol in utero beginning on gestation day 6 and continuing through lactation1
| Unit | 0 mg/kg/day | 0.03 mg/kg/day | 0.1 mg/kg/day | 0.3 mg/kg/day | 1 mg/kg/day | 3 mg/kg/day | |
|---|---|---|---|---|---|---|---|
| Terminal Body Weight | g | 87.58 ± 3.01 (8) | 85.46 ± 1.55 (5) | 87.96 ± 2.27 (5) | 85.00 ± 1.38 (5) | 92.38 ± 2.25 (5) | 73.36 ± 5.14* (8) |
| Brain | g | 1.63 ± 0.03 (3) | NA | NA | NA | NA | 1.53 ± 0.01* (3) |
| mg/g | 20.27 ± 0.98 (3) | NA | NA | NA | NA | 19.53 ± 0.12 (3) | |
| Thymus | g | 0.42 ± 0.04 (5) | 0.39 ± 0.01 (5) | 0.39 ± 0.02 (5) | 0.42 ± 0.02 (5) | 0.45 ± 0.04 (5) | 0.31 ± 0.05 (5) |
| mg/g | 4.16 ± 0.27 (5) | 4.53 ± 0.09 (5) | 4.47 ± 0.17 (5) | 4.99 ±0.21 (5) | 4.82 ± 0.29 (5) | 4.38 ± 0.52 (5) | |
| Lung | g | 1.14 ± 0.10 (5) | 0.97 ± 0.08 (5) | 1.04 ± 0.08 (5) | 0.93 ± 0.04 (5) | 0.90 ± 0.05 (5)* | 0.65 ± 0.07** (5) |
| mg/g | 12.40 ± 0.91 (5) | 11.26 ± 0.81 (5) | 11.95 ± 1.11 (5) | 10.92 ± 0.52 (5) | 9.70 ± 0.40 (5)* | 9.34 ± 0.32** (5) | |
| Liver | g | 4.44 ± 0.29 (5) | 4.16 ± 0.08 (5) | 4.49 ± 0.18 (5) | 4.22 ± 0.11 (5) | 4.74 ± 0.06 (5) | 3.81 ± 0.48 (5) |
| mg/g | 48.39± 2.22 (5) | 48.71 ± 1.22 (5) | 51.11 ± 1.76 (5) | 49.61 ± 0.68 (5) | 51.41 ± 1.20 (5) | 53.98 ± 1.64 (5) | |
| Spleen | g | 0.31 ± 0.01 (5) | 0.31 ± 0.02 (5) | 0.35 ± 0.01 (5) | 0.31 ± 0.01 (5) | 0.32 ± 0.01 (5) | 0.34 ± 0.03 (5) |
| mg/g | 3.45 ± 0.10 (5) | 3.59 ± 0.23 (5) | 3.98 ± 0.11 (5) | 3.68 ± 0.09 (5) | 3.48 ± 0.13 (5) | 5.10 ± 0.76** (5) | |
| Kidney-left | g | 0.47 ± 0.01 (5) | 0.45 ± 0.01 (5) | 0.47 ± 0.02 (5) | 0.45 ± 0.01 (5) | 0.49 ± 0.01 (5) | 0.39 ± 0.04* (5) |
| mg/g | 5.17 ± 0.13 (5) | 5.25 ± 0.13 (5) | 5.32 ± 0.18 (5) | 5.24 ± 0.07 (5) | 5.28 ± 0.19 (5) | 5.54 ± 0.19 (5) | |
| Kidney-right | g | 0.48 ± 0.01 (5) | 0.45 ± 0.01 (5) | 0.48 ± 0.02 (5) | 0.46 ± 0.01 (5) | 0.49 ± 0.01 (5) | 0.40 ± 0.04* (5) |
| mg/g | 5.21 ± 0.10 (5) | 5.32 ± 0.17 (5) | 5.46 ± 0.18 (5) | 5.37 ± 0.07 (5) | 5.36 ± 0.17 (5) | 5.69 ± 0.20 (5) |
NA- not evaluated
Statistically significant at p≤0.05
Statistically significant at p≤0.01
N numbers indicated in parentheses
Treatments to the dams began on gestation day 6; pups continued to be exposed via direct dosing along with dosing of the dam beginning on PND 12 and continuing until PND 27.
Micronucleus assay
No increase in the frequencies of micronucleated immature erythrocytes (PCE) was observed in any treatment group in either the F0 females or F1 males and females, and no significant alterations in the percentage of PCE among total erythrocytes was observed (Table 3 and 4).
Table 3.
Frequencies of micronucleated immature erythrocytes (MN-PCE) in female F0 Sprague Dawley rats administered deoxynivalenol for 6 weeks starting on gestation day 6 by oral gavage1
| Dose (mg/kg/day) | MN-PCE/1000 | %PCE |
|---|---|---|
| 02 | 1.13 ± 0.14 | 0.59 ± 0.09 |
| 0.03 | 1.00 ± 0.15 | 0.88 ± 0.08 |
| 0.1 | 1.21 ± 0.11 | 0.58 ± 0.08 |
| 0.3 | 0.96 ± 0.09 | 0.70 ± 0.13 |
| 1.0 | 1.13 ± 0.37 | 0.73 ± 0.16 |
| 3.0 | 0.92 ± 0.12 | 0.83 ± 0.05 |
| Trend test P3 = | 0.776 | 0.422 |
Six animals per treatment group; 20,000 PCE scored per animal using flow cytometry
Deionized water
Trend test; significance set at P < 0.025
Table 4.
Frequencies of micronucleated immature erythrocytes (MN-PCE) in F1 Sprague Dawley rats exposed to deoxynivalenol in utero beginning on gestation day 6 and continuing through lactation1,2
| Dose (mg/kg/day to dam) | MN-PCE/1000 | %PCE |
|---|---|---|
| Male pups | ||
| 03 | 0.733 ± 0.101 | 5.465 ± 0.247 |
| 0.03 | 0.764 ± 0.079 | 5.898 ± 0.520 |
| 0.1 | 0.817 ± 0.071 | 5.913 ± 0.375 |
| 0.3 | 0.905 ± 0.083 | 5.556 ± 0.250 |
| 1.0 | 0.840 ± 0.040 | 6.564 ± 0.340 |
| 3.0 | 0.825 ± 0.064 | 5.705 ± 0.489 |
| Trend test P4 = | 0.348 | 0.940 |
| Female pups | ||
| 03 | 0.600 ± 0.107 | 5.824 ± 0.243 |
| 0.03 | 0.733 ± 0.094 | 6.204 ± 0.614 |
| 0.1 | 0.833 ± 0.161 | 5.933 ± 0.113 |
| 0.3 | 0.750 ± 0.070 | 5.389 ± 0.142 |
| 1.0 | 0.758 ± 0.121 | 5.848 ± 0.328 |
| 3.0 | 0.642 ± 0.085 | 6.650 ± 0.326 |
| Trend test P4 = | 0.729 | 0.333 |
Treatments to the dams began on gestation day 6; pups continued to be exposed via direct dosing along with dosing of the dam beginning on PND 12 and continuing until PND 27.
Six animals per treatment group; 20,000 PCE scored per animal using flow cytometry
Deionized water
Trend test; significance set at P < 0.025
Discussion
Human exposure to DON and other trichothecene mycotoxins is characterized by lifetime exposures to low doses. As such, this study investigated the effects of perinatal DON exposure (F0: GD 6 through PND 28 and F1: PND 12 through PND 28) in Harlan Sprague Dawley rats. Furthermore, DON was administered via gavage to reduce potential for confounding due to decreased feed consumption. Findings from this study will provide valuable information to begin to address the research needs for DON highlighted by JECFA, such as additional genotoxicity and carcinogenicity assessments.
There were no treatment-related changes in maternal body weight or feed consumption at any of the doses administered (up to 3 mg/kg/day) during gestation or lactation. There were also no treatment-related clinical observations in the F0 females. Due to the lack of body weight changes and clinical observations in F0 females, these doses may be useful for determining if there are developmental toxicities associated with DON apart from maternal toxicity. Live litter size was lower (though not statistically significant) in the highest dose group of 3 mg/kg/day relative to controls. This finding is consistent with Collins et al. where there was no change in viable rat fetuses in the 2.5 mg/kg dose group but significantly fewer fetuses, by over half, in the 5 mg/kg group (Collins et al., 2006). Khera et al. showed no change in live fetuses per litter in mice at doses 0.375 to 1.5 mg/kg/day and slightly fewer live fetuses per litter in rats at 1 mg/kg/day (Khera et al., 1984).
After adjustment for litter effects, lower pup body weight (10–13% less than control) was only observed at the highest dose, 3 mg/kg/day, both prior to and after initiation of direct dosing of F1 animals on PND 12. Lower absolute organ weights observed in F1 females in the top dose group was likely related to lower body weight as relative organ weight was generally unchanged. While the lack of statistically significant organ weight changes in males may suggest differential sensitivity between sexes, it is unclear if the statistically significant differences in females are biologically relevant, especially given the lack of histopathological findings in both males and females. Studies with larger animal numbers and longer F1 exposures are warranted to clarify if there are indeed sex-specific differences. Since there were minimal signs of maternal toxicity during gestation and lactation, the decrease in body weight in F1 animals may be due to direct effects of DON. There is some evidence that DON may affect growth hormone receptor signaling pathways. Exposure of young mice to DON (20 ppm in feed) for 2 to 8 weeks was reported to result in lower levels of two key growth-hormone-induced proteins, insulin-like growth factor 1 and insulin-like growth factor acid-labile subunit (Amuzie and Pestka, 2010). These proteins are essential for postnatal growth and development (Baker et al., 1993). Given the mild decreases in F1 body weight and minimal maternal toxicity, the current study demonstrates that doses of up to 3 mg/kg/day perinatally could be used to probe long-term toxicity of DON and further examine the mechanisms of DON-related growth suppression in rats.
Both the Food and Agriculture Organization of the United Nations and the World Health Organization have proposed a provisional maximum tolerable daily intake (PMTDI) value of 1 μg/kg/day, which is protective of human health in regard to reported toxicities of DON based on the current available literature. The PMTDI value was based on a chronic mouse study showing decreased body weight at doses greater than 0.1 mg/kg/day (Iverson et al., 1995). Results from this present study show a no-observed effect level (NOEL) of 1 mg/kg/day based on changes in body weight in the F1 offspring, which is higher than the 0.1 mg/kg/day NOEL used for establishing the current PMTDI value. Thus, this study supports the use of 1 μg/kg body weight as the PMTDI for DON.
The JECFA report highlighted that “in view of the widespread human exposure to DON, further studies on the genotoxicity of this mycotoxin should be conducted” (JECFA, 2002). Bacterial mutation studies with DON yielded negative results (Knasmüller et al., 1997; Takakura et al., 2014) whereas positive results were reported in some studies that assessed chromosomal and DNA damage. Few in vivo studies, and none involving perinatal exposure, have been conducted with DON. Increases in MN-PCEs (Abdel-Wahhab et al., 2015) and chromosomal aberrations (Abdel-Wahhab et al., 2018) were reported in the bone marrow of adult Sprague Dawley rats exposed to 5 mg/kg/day DON by gavage for 3 weeks These results contrast with those in the present study, where we found that exposures up to 3 mg/kg/day for 28 days did not increase the incidence of MN-PCEs in peripheral blood of either dams or offspring. In vitro, induction of chromosomal aberrations has been reported in a few studies (Hsia et al., 1988; Knasmüller et al., 1997). Overall, the limited data available suggest that DON is not likely to be mutagenic but may have clastogenic potential.
While the concentrations of DON in maternal rat plasma collected in this study exceeded measured or predicted concentrations in humans, they are less than concentrations of DON used for in vitro mechanistic studies. In this study, DON was detected in maternal plasma, amniotic fluid, and in whole fetuses of F0 females exposed to 1 mg/kg/day on GD 18, indicating gestational transfer; concentrations of DON in fetuses (22.9 ng/g) were slightly higher than that in the dams (19.1 ng/mL) (Silinski et al., 2020). On PND 4, DON was detected in dam plasma (17.4 ng/mL) but not detectable in pup plasma, indicating no evidence of lactational DON transfer (Silinski et al., 2020). The average concentration of DON in maternal plasma in this study is lower than what has been observed in mice (Amuzie and Pestka, 2010), but higher than measured or predicted concentrations of DON in humans (Fan et al., 2019; Faeste et al., 2018). For example, the maternal plasma concentrations of DON in this study were ~200-fold higher than the predicted human Cmax in Faeste et al. using an intake of 0.2 μg/kg (2018). On the other hand, studies evaluating the molecular mechanisms of DON in vitro report effects at concentrations 0.02 – 3 μM (Yu et al., 2017), which are higher than the internal levels of DON found in this study (19.1 ng/mL, ~0.064 μM) and in humans (mean: 0.009 μM in plasma; Fan et al., 2019). An understanding of the mechanism of DON at lower doses will clarify which molecular events are relevant to in vivo toxicity.
In conclusion, based on the results of this study, female Harlan Sprague Dawley rats tolerated a dose of up to 3 mg/kg/day of DON during gestation and lactation. Effects on maternal body weight or feed consumption during gestation and lactation were not observed and small, but significant, decreases in body weight were observed in F1 offspring at the top dose of 3 mg/kg/day. There was no evidence of in vivo genotoxicity in either F0 or F1 animals as evaluated by the peripheral blood micronucleus test. The NOEL in this study was 1 mg/kg/day and supports the use of current PMTDI values for DON. Plasma concentrations of DON from animals in this study, other animal studies, and in humans are lower than concentrations that have been used in vitro for evaluating mechanisms. Thus, the use of lower concentrations in vitro will allow for a better understanding of pertinent molecular mechanisms of DON in vivo. Overall, findings from this study set the stage for future studies that can address the knowledge gaps in DON toxicity, such as evaluation of the carcinogenicity of lifetime exposure to DON in rats or the development of comparative studies to enable assessment of aggregate risk to trichothecene mycotoxins.
Highlights.
Perinatal exposure to 3 mg/kg/day deoxynivalenol (DON) reduced body weight in rats
Dam body weight or feed consumption was not affected by ≤3 mg/kg/day oral DON exposure
Micronucleus assay in dam or offspring peripheral blood was negative
Acknowledgements
The authors would like to thank Dr. Kembra Howdeshell and Dr. Sheba Churchill for their review of the manuscript. This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, Intramural Research project ZIA ES103316-04, and performed for the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services, under contract numbers HHSN273201300010C (Southern Research, Birmingham, AL), HHSN273201300009C (Integrated Laboratory Systems, LLC, Research Triangle Park, NC), HHSN273201600011C (Social and Scientific Services, Durham, NC), and HHSN273201400022C (RTI International, Research Triangle Park, NC).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Wahhab MA, El-Kady AA, Hassan AM, Abd El-Moneim OM, and Abdel-Aziem SH (2015). Effectiveness of activated carbon and Egyptian montmorillonite in the protection against deoxynivalenol-induced cytotoxicity and genotoxicity in rats. Food Chem Toxicol 83, 174–182. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahhab MA, El-Nekeety AA, Salman AS, Abdel-Aziem SH, Mehaya FM, and Hassan NS (2018). Protective capabilities of silymarin and inulin nanoparticles against hepatic oxidative stress, genotoxicity and cytotoxicity of Deoxynivalenol in rats. Toxicon 142, 1–13. [DOI] [PubMed] [Google Scholar]
- Amuzie CJ, and Pestka JJ (2010). Suppression of Insulin-Like Growth Factor Acid-Labile Subunit Expression—A Novel Mechanism for Deoxynivalenol-Induced Growth Retardation. Toxicol Sci 113, 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, and Efstratiadis A (1993). Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75, 73–82. [PubMed] [Google Scholar]
- Chen L, Yu M, Wu Q, Peng Z, Wang D, Kuca K, Yao P, Yan H, Nussler AK, Liu L, et al. (2017). Gender and geographical variability in the exposure pattern and metabolism of deoxynivalenol in humans: a review. J Appl Toxicol 37, 60–70. [DOI] [PubMed] [Google Scholar]
- Collins TF, Sprando RL, Black TN, Olejnik N, Eppley RM, Hines FA, Rorie J, and Ruggles DI (2006). Effects of deoxynivalenol (DON, vomitoxin) on in utero development in rats. Food Chem Toxicol 44, 747–757. [DOI] [PubMed] [Google Scholar]
- Dixon WJ, and Massey FJ (1957). Introduction to Statistical Analysis, 2nd edn (New York: McGrawHill Book Company, Inc; ). [Google Scholar]
- Dunn OJ (1964). Multiple comparisons using rank sums. Technometrics 6, 241–252. [Google Scholar]
- Dunnett CW (1955). A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc 50, 1096–1122. [Google Scholar]
- European Food Safety Authority Panel on Contaminents in the Food Chain. (2017). Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA Journal 15, 4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faeste CK, Ivanova L, Sayyari A, Hansen U, Sivertsen T, and Uhlig S (2018). Prediction of deoxynivalenol toxicokinetics in humans by in vitro-to-in vivo extrapolation and allometric scaling of in vivo animal data. Arch Toxicol 92, 2195–2216. [DOI] [PubMed] [Google Scholar]
- Fan K, Xu J, Jiang K, Liu X, Meng J, Di Mavungu JD, Guo W, Zhang Z, Jing J, Li H, et al. (2019). Determination of multiple mycotoxins in paired plasma and urine samples to assess human exposure in Nanjing, China. Environ Pollut 248, 865–873. [DOI] [PubMed] [Google Scholar]
- Gart JJ, Chu KC, and Tarone RE (1979). Statistical issues in interpretation of chronic bioassay tests for carcinogenicity. JNCI 62, 957–974. [PubMed] [Google Scholar]
- Hothorn. (2014) Statistical evaluation of toxicological bioassays- a review. Toxicol. Res. 3, 418–432. [Google Scholar]
- Hsia CC, Wu JL, Lu XQ, and Li YS (1988). Natural occurrence and clastogenic effects of nivalenol, deoxynivalenol, 3-acetyl-deoxynivalenol, 15-acetyl-deoxynivalenol, and zearalenone in corn from a high-risk area of esophageal cancer. Cancer Detect Prev 13, 79–86. [PubMed] [Google Scholar]
- Hsu JC (1992). The factor analytic approach to simultaneous inference in the general linear models. Journal of Computational and Graphical Statistics 1, 151–168. [Google Scholar]
- Iverson F, Armstrong C, Nera E, Truelove J, Fernie S, Scott P, Stapley R, Hayward S, and Gunner S (1995). Chronic feeding study of deoxynivalenol in B6C3F1 male and female mice. Teratog Carcinog Mutagen 15, 283–306. [DOI] [PubMed] [Google Scholar]
- JECFA (2002). Evaluation of certain mycotoxins in food: fifty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives (Geneva, Switzerland: WHO Library Cataloguing-in-Publication Data; ). [Google Scholar]
- Jonckheere AR (1954). A test of significance for the relation between m rankings and k ranked categories. Br J Math Stat Psychol 7, 93–100. [Google Scholar]
- Khera KS, Arnold DL, Whalen C, Angers G, and Scott PM (1984). Vomitoxin (4-deoxynivalenol): effects on reproduction of mice and rats. Toxicol Appl Pharmacol 74, 345–356. [DOI] [PubMed] [Google Scholar]
- Khera KS, Whalen C, and Angers G (1986). A teratology study on vomitoxin (4-deoxynivalenol) in rabbits. Food Chem Toxicol 24, 421–424. [DOI] [PubMed] [Google Scholar]
- Khera KS, Whalen C, Angers G, Vesonder RF, and Kuiper-Goodman T (1982). Embryotoxicity of 4-deoxynivalenol (vomitoxin) in mice. Bull Environ Contam Toxicol 29, 487–491. [DOI] [PubMed] [Google Scholar]
- Knasmüller S, Bresgen N, Kassie F, Mersch-Sundermann V, Gelderblom W, Zöhrer E, and Eckl PM (1997). Genotoxic effects of three Fusarium mycotoxins, fumonisin B1, moniliformin and vomitoxin in bacteria and in primary cultures of rat hepatocytes. Mutat Res 391, 39–48. [DOI] [PubMed] [Google Scholar]
- Levene Howard (1960). “Robust tests for equality of variances”. In Ingram Olkin; Harold Hotelling; et al. (eds.). Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling. Stanford University Press. pp. 278–292. [Google Scholar]
- Morrissey RE (1984). Teratological study of Fischer rats fed diet containing added vomitoxin. Food Chem Toxicol 22, 453–457. [DOI] [PubMed] [Google Scholar]
- Morrissey RE, and Vesonder RF (1985). Effect of deoxynivalenol (vomitoxin) on fertility, pregnancy, and postnatal development of Sprague-Dawley rats. Appl Environ Microbiol 49, 1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JKS, Vikström AC, Turner P, and Knudsen LE (2011). Deoxynivalenol transport across the human placental barrier. Food Chem Toxicol 49, 2046–2052. [DOI] [PubMed] [Google Scholar]
- Pestka JJ (2008). Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25, 1128–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka JJ (2010). Deoxynivalenol: mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol 84, 663–679. [DOI] [PubMed] [Google Scholar]
- Shirley E (1977). A non-parametric equivalent of Williams’ test for contrasting increasing dose levels of a treatment. Biometrics 33, 386–389. [PubMed] [Google Scholar]
- Silinski M, Gilliam J, Fernando R, Robinson V, Germolec D, Cunny H, Huang M, Furr J, and Waidyanatha S (2020). Development of an Analytical Method for Quantitation of Deoxynivalenol by UPLC-MS/MS; a Preliminary Assessment of Gestational and Lactational Transfer in Rats. JAT. doi: 10.1093/jat/bkaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrova P, Adam V, Vasatkova A, Beklova M, Zeman L, and Kizek R (2010). Deoxynivalenol and its toxicity. Interdiscip Toxicol 3, 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura N, Nesslany F, Fessard V, and Le Hegarat L (2014). Absence of in vitro genotoxicity potential of the mycotoxin deoxynivalenol in bacteria and in human TK6 and HepaRG cell lines. Food Chem Toxicol 66, 113–121. [DOI] [PubMed] [Google Scholar]
- Tukey JW (1977). Exploratory Data Analysis (Reading, Massachusetts: Addison-Wesley; ). [Google Scholar]
- Urbanek KA, Habrowska-Górczyńska DE, Kowalska K, Stańczyk A, Domińska K, and Piastowska-Ciesielska AW (2018). Deoxynivalenol as potential modulator of human steroidogenesis. JAT 38, 1450–1459. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture. “Food Security Act of 1985, Subtitle F” (PL 99–198, 23 December 1985) [Google Scholar]
- U.S. Public Health Service. “Health Research Extension Act of 1985” (PL 99–158, 20 November 1985) [Google Scholar]
- Wang Z, Wu Q, Kuča K, Dohnal V, and Tian Z (2014). Deoxynivalenol: signaling pathways and human exposure risk assessment--an update. Arch Toxicol 88, 1915–1928. [DOI] [PubMed] [Google Scholar]
- Williams DA (1971). A test for differences between treatment means when several dose levels are compared with a zero dose control. Biometrics 27, 103–117. [PubMed] [Google Scholar]
- Williams DA (1972). The comparison of several dose levels with a zero dose control. Biometrics 28, 519–531. [PubMed] [Google Scholar]
- Williams DA (1986). A note on Shirley’s nonparametric test for comparing several dose levels with a zero-dose control. Biometrics 42, 183–186. [PubMed] [Google Scholar]
- Wu X, Kohut M, Cunnick J, Bailey T, and Hendrich S (2009). Deoxynivalenol suppresses circulating and splenic leukocyte subpopulations in BALB/c mice: dose response, time course and sex differences. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 26, 1070–1080. [DOI] [PubMed] [Google Scholar]
- Yazar S, and Omurtag GZ (2008). Fumonisins, trichothecenes and zearalenone in cereals. Int J Mol Sci 9, 2062–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Chen L, Peng Z, Nussler AK, Wu Q, Liu L, and Yang W (2017). Mechanism of deoxynivalenol effects on the reproductive system and fetus malformation: Current status and future challenges. Toxicol In Vitro 41, 150–158. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhu X, Wu H, Zhuang D, Yu G, Li X, Li F, and Yu A (2012). Evaluation of fetal skeletal malformations in deoxynivalenol-treated mice using microarray analysis. Arch Environ Contam Toxicol 63, 445–452. [DOI] [PubMed] [Google Scholar]


