Abstract
Oliguric acute kidney injury due to traumatic rhabdomyolysis can be potentially lethal if the proper medical therapy combined with extracorporeal detoxification is not performed. Different extracorporeal techniques are available to overcome this syndrome. Here, we report the first case of removal of myoglobin and successful recovery from acute kidney injury in an elderly septic patient using supra-hemodiafiltration with endogenous reinfusion technique (HFR-Supra) combined with the medical therapy.
Keywords: Acute kidney injury, Extracorporeal detoxification, Hemodiafiltration, HFR-Supra technique, Myoglobin, Rhabdomyolysis
Introduction
Acute kidney injury (AKI) associated with myoglobinuria is the most serious complication of both traumatic and nontraumatic rhabdomyolysis, and it may be potentially lethal [1].
A useful approach to prevent of rhabdomyolysis-induced AKI is counteracting the toxic effects of myoglobin by increasing extracellular volume through aggressive volume expansion [2]. Once overt renal failure develops, the only reliable therapeutic modality is extracorporeal blood detoxification. Some continuous renal replacement therapy (CRRT) modalities like hemodialysis (diffuse, small solute transport), hemofiltration (convective small and medium solute transport), or the combination of both represent helpful options in patients with rhabdomyolysis because of their ability to allow continuous removal of solutes and gradual correction of fluid overload [3].
Unfortunately, in a normal dialysis ward, it is not possible to arrange a CRRT: consequently, when patients with AKI and rhabdomyolysis are admitted, an intermittent dialysis technique is scheduled using dialyzers or procedures able to remove toxins in the range of the middle molecules. Recently, the supra-hemodiafiltration with endogenous reinfusion technique (HFR-Supra, Bellco-Medtronic, Mirandola, Modena, Italy) was applied with the purpose of increasing the access to the middle uremic molecules but avoiding albumin loss during dialysis. This approach has been successfully used in AKI due to myeloma cast nephropathy [4].
Moreover, it is worth to note that HFR is the cheapest technique for AKI patients with rhabdomyolysis in comparison to the other techniques available as shown in Table 1.
Table 1.
Costs of techniques currently available for rhabdomyolysis and AKI in Italy
| Technique | Cost, EUR |
|---|---|
| HFR | 50 |
| CRRT/24 h | 200 |
| HCO (albumin replacement not included) | 850 |
| CPFA | 1,250 |
| Cytosorb | 1,600 |
CPFA, coupled plasma filtration adsorption; CRRT, continuous renal replacement therapy; HCO, high cut off; HFR, hemodiafiltration with on-line endogenous ultrafiltrate reinfusion.
Nonetheless, the HFR-Supra system is upgraded with a hydrophobic resin able to adsorb a wide range of pro- and anti-inflammatory mediators, thus allowing the removal of such molecules. However, there is no report on the use of HFR-Supra to remove myoglobin (molecular weight 17.8 kDa). We present here a case of an 83-year-old man with AKI and rhabdomyolysis successfully treated with the HFR-Supra technique.
Case Report
An 83-year-old man was emergently brought to our Nephrology, Dialysis and Transplantation Unit after falling to the ground and lying on the floor throughout the night because of syncope. At hospital admission, physical examination revealed leg ecchymosis (Fig. 1). The patient was anuric, and laboratory tests suggested the presence of rhabdomyolysis-induced AKI during a septic state.
Fig. 1.
Ecchymosis of the legs at hospital admission.
Sepsis due to acute prostatitis appeared to be the cause of the syncope. Blood determinations revealed increased levels of urea (140 mg/dL), creatinine (7.96 mg/dL), myoglobin (15,560 ng/mL), creatine phosphokinase (CPK: 124,841 U/L), lactate dehydrogenase (LDH: 1,199 U/L), C-reactive protein (CRP: 53.6 mg/dL), procalcitonin (PCT: 505.3 ng/mL), total bilirubin (7.53 mg/dL), direct bilirubin (4.24 mg/dL), aspartate aminotransferase (AST: 749 U/L), alanine aminotransferase (ALT: 410 U/L), prostate-specific antigen (PSA: 662 ng/mL), and white blood cell count (26,460 cells/µL) with 93% neutrophils. Blood levels of sodium were 137 mEq/L, potassium 4.9 mEq/L, calcium 8.7 mg/dL, albumin 2.6 g/dL, and phosphate 4.1 mg/dL. Patient's blood pressure, heart rate, and body temperature were 105/75 mm Hg, 110 bpm, and 37.4°C, respectively. Abdomen ultrasound indicated no hydronephrosis, empty bladder, traces of blood in bladder catheter without indications to urologic surgery.
The review of his medical history did not show previous renal impairment (serum creatinine prior to admission: 0.8 mg/dL) in the presence of some morbidities, namely prostatic hypertrophy (prostate volume: 190 mL; PSA: 9.9 ng/mL), overactive bladder, and recurrent prostatitis. The use of statins was not recorded.
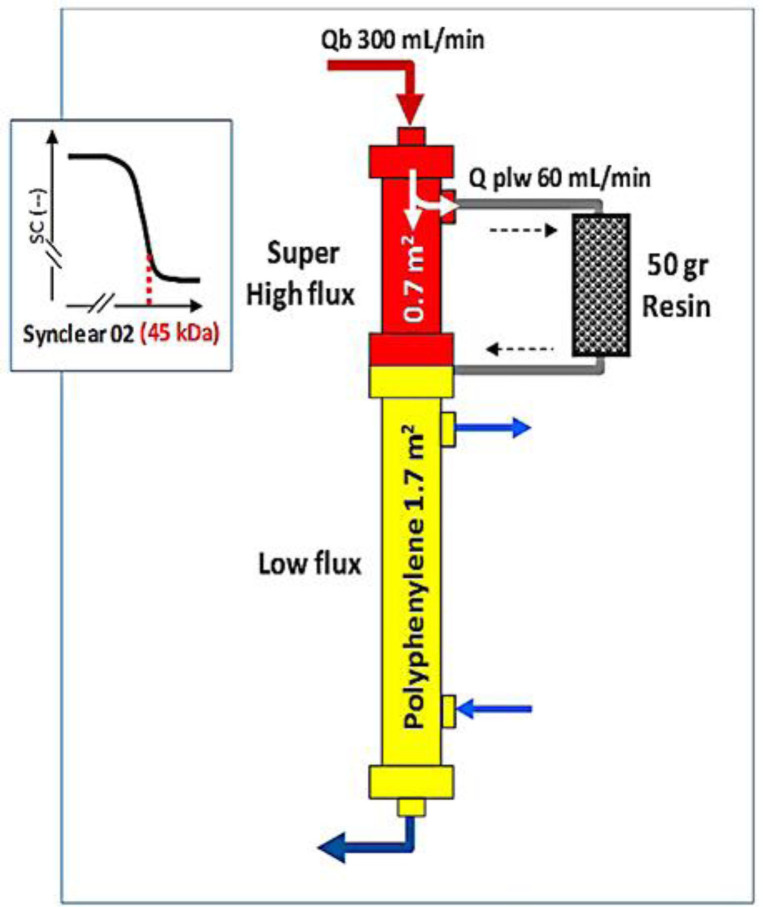
During his hospital stay, medical therapy consisted of volume expansion, diuretic and alpha-agonist, antibiotic treatment. The patient was administered daily 125 mg furosemide by intravenous infusion as diuretic treatment from the first day of admission. Although urea, creatinine, potassium were not high enough to require dialysis, on the first day of admission the patient was anuric. Thus, the same day of admission, a femoral central venous catheter (Mahurkar; Medtronic, Minneapolis, 20 cm in length, diameter 12 Fr) was placed, and extracorporeal treatment through HFR-Supra (Bellco-Medtronic) with endogenous reinfusion was started to remove myoglobin, to reduce the inflammatory status, and to maintain the fluid balance. The system is based on three components: a first filter for ultrafiltration consisting of a super high-flux dialyzer (Synclear 02, 0.7 m2, Bellco-Medtronic) with molecular weight cut off of 45 kDa; an adsorbent cartridge containing 50 g of hydrophobic styrene resin with a surface of 700 m2 for each gram for resin (Suprasorb, Bellco-Medtronic) to regenerate ultrafiltrate; and a second filter consisting of a low flux polyphenylene membrane (1.7 m2, KUF 13 mL/h/mm Hg) where solute and volume removal are performed (Fig. 2).
Fig. 2.
HFR-Supra: schematic diagram. Qb, blood flow; Q plw, plasmatic water flow; kDa, kilodaltons; SC, sieving coefficient.
After 96 h of high-dose furosemide combined with intravenous fluids and HFR-Supra, 24-h urine output was 300 mL but it rose up to 2,400 mL after 8 days. Furosemide was then tapered to 50 mg per day with oral administration. Antibiotic therapy consisted of intravenous infusion of piperacillin/tazobactam switched to meropenem when blood cultures showed growth of E. coliresistant to piperacillin/tazobactam.
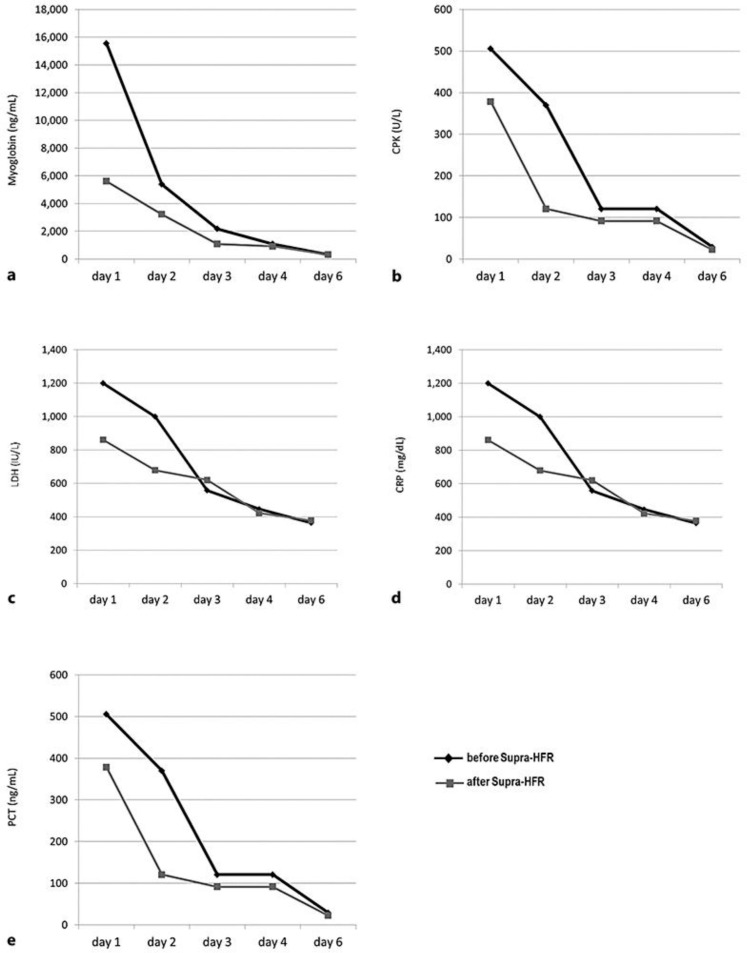
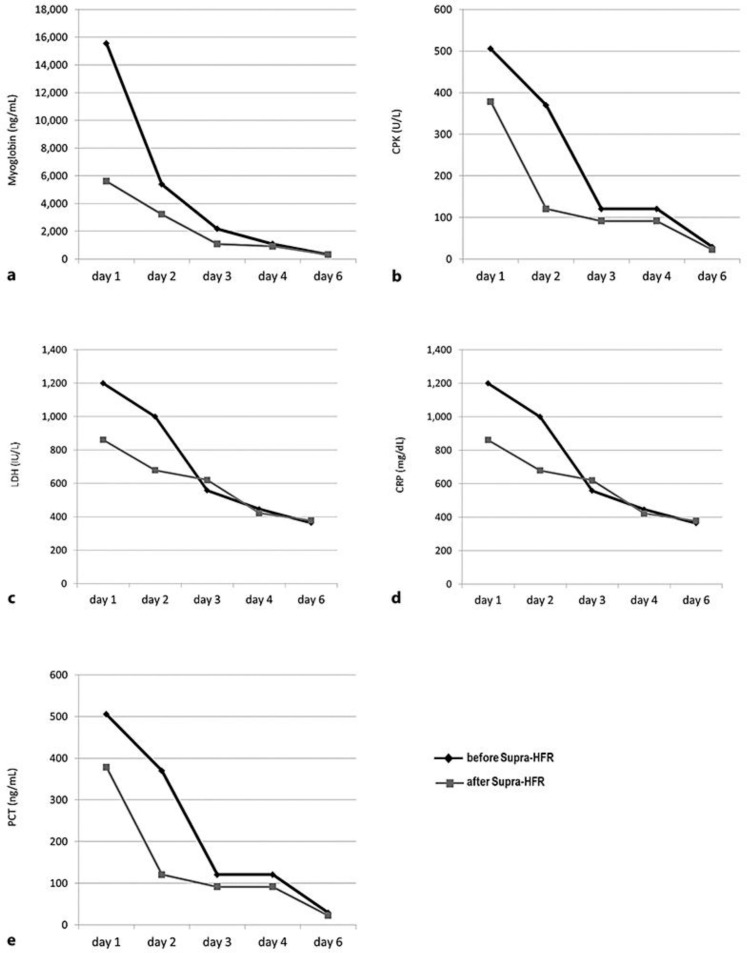
At day 6 from admission, after 5 HFR-Supra sessions, a significant reduction in the levels of myoglobin (98.4%), CPK (99.8%), LDH (72%), CRP (81%), and PCT (98%) was achieved(Fig. 3).
Fig. 3.
Trends in the reduction of circulating levels of myoglobin (a), CPK (b), LDH (c), CRP (d), and PTC (e) over the 5 days of HFR-Supra treatment. The black line with diamonds represents the analyte levels before the dialysis session and the grey line with squares the ones after the dialysis session. CPK, creatine phosphokinase; CRP, C-reactive protein; LDH, lactate dehydrogenase; PCT, procalcitonin.
The HFR-Supra was carried out with 250 mL/min of blood flow. The endogenous ultrafiltrate flow obtained per session was 14 L (Fig. 4).
Fig. 4.

Sorbent cartridge of HFR-Supra during the detoxification process.
The ultrafiltration rate was settled according to the clinical status. Low molecular weight heparin (enoxaparin) 4,000 IU in single bolus on starting dialysis was administered. However, in spite of the normalization of myoglobin and improved signs of systemic inflammation, the renal failure was not recovered. As a consequence, the femoral hemodialysis catheter was removed, a right jugular central venous catheter was placed, and the dialysis therapy was prolonged with 3 sessions of on-line hemodiafiltration (HDF) and 2 sessions of high-flux hemodialysis using the Phylter HF 17G dialyzer (Bellco-Medtronic, polyphenylene 1.7 m2, KUF 57 mL/h/mm Hg, gamma ray sterilization). Three weeks after admission, dialysis was no longer required. At discharge, urine output was 2,300 mL per day, serum levels of urea dropped to 96 mg/dL, creatinine to 3.06 mg/dL, CRP to 3.2 mg/dL, total bilirubin to 0.81 mg/dL, ASL to 27 IU/L, ALT to 25 IU/L, PSA to 61 ng/mL, and white blood cells count to 6,300 cells/µL. The total hospitalization length was 21 days. After 6 months from the last dialysis session, urea was 89 mg/dL, creatinine 2.41 mg/dL, and GFR (CKD-EPI) 24 mL/min.
A written informed consent was obtained from the patient to publish this case report and any accompanying images. The study was conducted in accordance with the Declaration of Helsinki.
Discussion
The main benefit of using HFR-Supra lies in its ability to remove a wide spectrum of large-middle molecules in patients affected by end-stage renal disease on chronic dialysis. To the best of our knowledge, there is no experience on the use of HFR-Supra to treat severe hypermyoglobinemia during AKI. This is the first report of an episode of rhabdomyolysis that was successfully managed with HFR-Supra. This is one of the few reports of acute prostatitis as a cause of sepsis as well: no other source of sepsis was found but acute prostatitis [5, 6, 7]. Compared to standard hemodialysis filters, the advantage of HFR-Supra lies on one hand in the ability to effectively remove myoglobin, on the other, in the possibility to manage AKI in the setting of the normal dialysis ward where CRRT is not feasible. The single case described here is not enough to support strong evidence; our experience suggests that HFR-Supra can be safely provided to AKI patients, when indicated. Nonetheless, it is worth noting that the patient remained dialysis dependent for weeks despite quick, safe, and effective myoglobin by the novel device. The patient did not recover his basal renal function, and chronic kidney disease occurred because 6 months after the last dialysis creatinine was 2.41 mg/dL and the glomerular filtration rate (CKD-EPI) was 24 mL/min. This confirms that renal repair can be maladaptive. The severity, type, and duration of injury as well the age of the patient seem to be risk factors for maladaptive repair [8].
Two authors have previously described cases of rhabdomyolysis treated with a “sister system” called coupled plasma filtration adsorption (CPFA), which was improved for patients admitted to the intensive care unit. Lai et al. [9] reported their experience in using CPFA in one kidney transplant recipient with severe unexplained rhabdomyolysis that partly resembles our patient. In line with our data, the authors achieved a successful decline in myoglobin and PCT by means of 5 consecutive CPFA treatments.
Similarly, Pezzi et al. [10] reported four cases of traumatic rhabdomyolysis patients successfully treated with CPFA to remove myoglobin followed by 14 h of CVVH. Again, consistent with our findings, a clinical improvement in the indices of muscle damage (CK and myoglobin) and AKI (creatinine and potassium) were observed. More recently, the same group has also suggested the early use CPFA along with the infusion therapy, diuretic, and correction of metabolic acidosis to prevent kidney damage in post-traumatic rhabdomyolysis [11].
However, although CPFA is efficient in myoglobin and cytokine removal, its utilization on the dialysis ward is limited to intermittent treatment formats. This kind of application can reduce dialysis adequacy, thus CPFA must be combined with a full-fledged dialysis treatment for the correction of metabolic imbalance, always present in patients with AKI requiring dialysis [12].
The application of an adequate intermittent hemodialysis and an efficient myoglobin removal was also tested with the high cut off (HCO) dialyzer during a case series of rhabdomyolysis-associated AKI. Heyne et al. [13] described patients treated with HCO dialyzers during 4-h intermittent hemodialysis. They obtained a significantly higher myoglobin clearance in comparison to those patients who had undergone sustained low efficiency daily dialysis with high flux filters. Unfortunately, HCO dialyzers require an amount of albumin replacement up to 25 g for 4-h dialysis sessions [14]. Conversely, an important point related to the use of HFR-Supra is represented by the albumin sparing effect that can itself improve the outcome of patients affected by AKI [15].
In our case, a significant reduction in PCT during the HFR-Supra sessions was also observed: the molecular weight of PCT is 13 kDa, and the adsorbing resin can remove it. However, it is difficult to evaluate whether PCT decline is also caused by the concomitant resolution of the underlying inflammatory process. It is worth underlining, however, the ability of the adsorbing resin to remove proinflammatory cytokines, as well as biomarkers of kidney injuries, uremic toxins, fragments of antibodies [16, 17].
Previous studies have shown that the HFR-Supra cartridge retains IL-6 in the ultrafiltrate in chronic patients on hemodialysis confirming the anti-inflammatory effect of HFR-Supra [18]. Esquivas-Motta et al. [19] carried out a crossover study comparing high-flux hemodialysis with Supra cartridge and on-line HDF: the authors observed a significant reduction in activated monocytes after one week of HFR-Supra treatment and a subsequent rise in monocytes when on-line HDF was used.
In summary, although the unicity of the case described here prevents us from drawing any firm conclusion at this moment, HFR-Supra appears to be a safe and helpful option for patients with rhabdomyolysis-induced AKI, combined with the standard medical therapy also in a normal dialysis ward.
Statement of Ethics
The authors have no ethical conflicts to disclose. Written informed consent was obtained from the patient to publish this case report and any accompanying images.
Conflict of Interest Statement
The authors declare no conflict of interest.
Funding Sources
This case report has received no external funding.
Author Contributions
G.D. conception and drafting of the manuscript; M.C. drafting, editing and revision of the manuscript; F.D.F., S.N., M.R., A.S., and A.A. management of the case, collection of clinical data, support in manuscript drafting; G.L.M., main investigator, supervision and revision of the manuscript.
References
- 1.Long B, Koyfman A, Gottlieb M. An evidence-based narrative review of the emergency department evaluation and management of rhabdomyolysis. Am J Emerg Med. 2019 Mar;37((3)):518–23. doi: 10.1016/j.ajem.2018.12.061. [DOI] [PubMed] [Google Scholar]
- 2.Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009 Jul;361((1)):62–72. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- 3.Ronco C. Extracorporeal therapies in acute rhabdomyolysis and myoglobin clearance. Crit Care. 2005 Apr;9((2)):141–2. doi: 10.1186/cc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasquali S, Iannuzzella F, Corradini M, Mattei S, Bovino A, Stefani A, et al. A novel option for reducing free light chains in myeloma kidney: supra-hemodiafiltration with endogenous reinfusion (HFR) J Nephrol. 2015 Apr;28((2)):251–4. doi: 10.1007/s40620-014-0130-8. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Chen J. Septic shock induced by bacterial prostatitis with Morganella morganii subsp. morganii in a postransplantation patient. Case Rep Transplant. 2015;2015:850532. doi: 10.1155/2015/850532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeWitt A, Patel C, Kuapati R, Reddy K. A clear case of MRSA sepsis, of an unexpected origin. J La State Med Soc. 2015 May-Jun;167((3)):156. [PubMed] [Google Scholar]
- 7.Taylor GM, Paratore DM. Septic shock secondary to acute bacterial prostatitis in an HIV-positive male: a novel presentation. Oxf Med Case Rep. 2018 Oct;2018((11)):omy086. doi: 10.1093/omcr/omy086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forni LG, Darmon M, Ostermann M, Oudemans-van Straaten HM, Pettilä V, Prowle JR, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017 Jun;43((6)):855–66. doi: 10.1007/s00134-017-4809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai Q, Di Pietro V, Iesari S, Amabili S, De Luca L, Clemente K, et al. Coupled plasma filtration adsorption in patients with a history of kidney transplantation: report of two cases. Blood Purif. 2015;40((3)):218–22. doi: 10.1159/000437042. [DOI] [PubMed] [Google Scholar]
- 10.Pezzi M, Renda S, Giglio AM, Scozzafava AM, Tiburzi SP, Casella P, et al. The use of coupled plasma filtration adsorption in traumatic rhabdomyolysis. Case Rep Crit Care. 2017;2017:5764961. doi: 10.1155/2017/5764961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pezzi M, Giglio AM, Scozzafava A, Serafino G, Maglio P, Verre M. Early intensive treatment to prevent kidney failure in post-traumatic rhabdomyolysis: Case report. SAGE Open Med Case Rep. 2019;7:2050313X19839529. doi: 10.1177/2050313X19839529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Manna G, Donati G. Coupled plasma filtration adsorption: a multipurpose extracorporeal detoxification therapy. Blood Purif. 2018;46((3)):228–38. doi: 10.1159/000490234. [DOI] [PubMed] [Google Scholar]
- 13.Heyne N, Guthoff M, Krieger J, Haap M, Häring HU. High cut-off renal replacement therapy for removal of myoglobin in severe rhabdomyolysis and acute kidney injury: a case series. Nephron Clin Pract. 2012;121((3-4)):c159–64. doi: 10.1159/000343564. [DOI] [PubMed] [Google Scholar]
- 14.Hutchison CA, Harding S, Mead G, Goehl H, Storr M, Bradwell A, et al. Serum free-light chain removal by high cutoff hemodialysis: optimizing removal and supportive care. Artif Organs. 2008 Dec;32((12)):910–7. doi: 10.1111/j.1525-1594.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- 15.Kritmetapak K, Peerapornratana S, Srisawat N, Somlaw N, Lakananurak N, Dissayabutra T, et al. The impact of macro-and micronutrients on predicting outcomes of critically ill patients requiring continuous renal replacement therapy. PLoS One. 2016 Jun;11((6)):e0156634. doi: 10.1371/journal.pone.0156634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marinez de Francisco AL, Ghezzi PM, Brendolan A, Fiorini F, La Greca G, Ronco C, et al. Hemodiafiltration with online regeneration of the ultrafiltrate. Kidney Int Suppl. 2000 Aug;76:S66–71. doi: 10.1046/j.1523-1755.2000.07608.x. [DOI] [PubMed] [Google Scholar]
- 17.Solano FG, Bellei E, Cuoghi A, Caiazzo M, Bruni F. Radical improvement of signs and symptoms in systemic lupus erythematosus when treated with hemodiafiltration with endogenous reinfusion dialysis. Case Rep Nephrol Dial. 2015 Apr;5((1)):106–12. doi: 10.1159/000381395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riccio E, Cataldi M, Minco M, Argentino G, Russo R, Brancaccio S, et al. Evidence that p-cresol and IL-6 are adsorbed by the HFR cartridge: towards a new strategy to decrease systemic inflammation in dialyzed patients? PLoS One. 2014 Apr;9((4)):e95811. doi: 10.1371/journal.pone.0095811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esquivias-Motta E, Martín-Malo A, Buendia P, Álvarez-Lara MA, Soriano S, Crespo R, et al. Hemodiafiltration with endogenous reinfusion improved microinflammation and endothelial damage compared with online-hemodiafiltration: a hypothesis generating study. Artif Organs. 2017 Jan;41((1)):88–98. doi: 10.1111/aor.12704. [DOI] [PubMed] [Google Scholar]