Abstract
Separating acetylene from light hydrocarbon mixtures like ethylene is a very important process for downstream industrial applications. Herein, we report a new MOF [CuL2(SiF6)] (UTSA-220, L = (1E,2E)-1,2-bis(pyridin-4-ylmethylene)hydrazine) with dual functionalities featuring optimal pore size with strong binding sites for acetylene. UTSA-220 exhibits apparently higher uptake capacity for C2H2 than those for other light hydrocarbons. The potential of this material for trace C2H2 removal from C2H4 has also been demonstrated by a dynamic breakthrough experiment performed with C2H2/C2H4 (1/99 v/v) under simulated industrial conditions. According to the dispersion-corrected density functional theory (DFT-D) simulation, SiF62− and azine moieties serve as preferential binding sites for C2H2, indicating the feasibility of the dual functionalities incorporated in UTSA-220 for adsorbent-based C2H2 separations.
Keywords: Metal–organic frameworks, Light hydrocarbons, Gas separation, Acetylene
Graphical Abstract

INTRODUCTION
The separation of light hydrocarbons is widely regarded as important processes in petrochemistry.1,2 Among them, acetylene (C2H2) is the source of many organic chemicals in industry, such as α-ethynyl alcohols, vinyl compounds, and acrylic acid derivatives.3 C2H2 usually coexists with carbon dioxide (CO2), ethylene (C2H4) or other light hydrocarbons, because C2H2 is mainly manufactured by cracking of hydrocarbons or partial combustion of methane (CH4). Apart from this, C2H2 is a common impurity in the production of olefins like C2H4. The presence of C2H2 in C2H4 largely affects the downstream C2H4 polymerization reaction by poisoning the catalyst.2 Thus, the significance of C2H2/C2H4 separation is comparable to that of some important separations such as C2H4/C2H6, C3H4/C3H6 and C3H6/C3H8.4–8 Considerable difficulty has been encountered in the removal of C2H2 from the coexisting species for industrial requirements, because these small molecules are similar in physical properties such as molecular sizes, electronic structures, and volatilities.9 Traditional cryogenic distillation method suffers from the extraordinarily high energy and capital input, which propels us to develop alternative approaches to addressing the issue more efficiently.1 Separation by means of adsorption with porous materials is perceived as a promising way to replace the traditional distillation method and has experienced significant development in recent years.4–8,10–13
As a new generation of porous materials, metal–organic frameworks (MOFs) have attracted vast attention in exploring their application potential in various fields,14–21 including but not limited to gas storage,22–25 separation,6,26–34 catalysis,35–38 sensing,24,39,40 and drug delivery.41,42 The diverse properties and functions of MOFs stem from their highly modifiable pore structure and surface.43,44 With rational choice of the building blocks (metal ions/clusters and organic ligands), the topology, pore size, surface functionality of MOFs can be tailored specifically to fit into application scenarios like gas separation.45,46 For C2H2 removal and enrichment, the control over pore size and pore functionalities are found effective in developing MOFs with desired performance.8,47,48
Recently, a series of MOFs containing SiF62− moieties have been reported to have excellent abilities to separate C2H2 from C2H4 as a combined outcome of the molecular sieving effect and strong hydrogen bonding interactions between SiF62− and C2H2 molecules.8,49 Herein, we report a new MOF [CuL2(SiF6)] (termed UTSA-220) based on an azine based ligand L (L = (1E,2E)-1,2-bis(pyridin-4-ylmethylene)-hydrazine), featuring a bifunctionalized environment for C2H2 accommodation in the framework. This MOF shows a three-dimensional (3D) network with one-dimensional (1D) channels that provide optimal pore size and strong binding sites for C2H2. Gas sorption studies reveal that UTSA-220 selectively adsorbs C2H2 over other light hydrocarbons at ambient temperature. A dynamic breakthrough experiment performed with a C2H2/C2H4 (1/99 v/v) mixture further demonstrates UTSA-220 is feasible to separate C2H2 from C2H4 under practical conditions.
EXPERIMENTAL SECTION
Synthesis of UTSA-220.
Attempts to obtain single crystals were unsuccessful. Thus, bulk powder of this MOF was synthesized as follows. A methanolic solution of copper hexafluorosilicate was made by dissolving 22.4 mg (0.1 mmol) CuSiF6·xH2O into 10 mL methanol. The CuSiF6 solution was added dropwise with constant stirring into a methanolic ligand solution prepared by dissolving 42 mg (0.2 mmol) L into 10 mL methanol. The suspension was stirred at room temperature for 30 min before the powder product was separated from the solution and washed with methanol for 3 times with a centrifuge. The product was transferred to a gas sorption tube and activated under vacuum at room temperature for 24 h before gas sorption measurements.
Breakthrough Experiment.
The breakthrough experiments were conducted on a self-built instrument (see Supporting Information) with a gas mixture of C2H2/C2H4 (1/99 v/v) at room temperature (298 K) and 1 bar. The MOF solid was packed into a ϕ2 × 70 mm stainless-steel column under the protection of N2 gas in a glovebox. The packed column was flushed with helium gas at a rate of 40 mL/min for 2 h at room temperature to further activate the sample prior to measurements. The flow rate of the C2H2/C2H4 mixture was set at 2 mL/min and was first directed to a blank column to stabilize the gas flow before being switched to the adsorbent column to initiate the breakthrough experiment. The effluent composition was evaluated by a gas chromatography (GC) with a thermal conductivity detector (detection limit 0.1 ppm). Between two tests, the adsorbent was regenerated by helium flow (40 mL/min) for 12 h to guarantee a complete removal of the adsorbed gases. The desorption test on breakthrough instrument was performed at room temperature by switching the feed gas to He gas. The He flow was maintained at 20 mL/min. The effluent composition was measured by GC until no detectable C2H2 and C2H4 was found from the effluent.
RESULTS AND DISCUSSION
Structure and Synthesis.
The dropwise addition of a methanolic solution of L ligand into a CuSiF6·xH2O methanolic solution at room temperature with constant stirring affords the light purple crystalline powder of UTSA-220. Based on its powder X-ray diffraction pattern, the rough structure of UTSA-220 was determined to crystallize in the space group of C2/m and has one Cu atom, in which two L ligands and one SiF6 group were found in the asymmetric unit (Figure S1 and Table S1). In this MOF, Cu atom is coordinated by four N atoms equatorially and two F atoms axially (Figure 1a–c). The four N donors come from four bidentate L ligands while the two F atoms are from two bridging SiF62− anions. The obtained structure is a 2-fold interpenetrated 3D network (Figure S2). Since the organic linker L is not parallel but tilted with respect to the axial direction of coordinated Cu2+ ion, UTSA-220 features two kinds of 1D channels along the a-axis (Figure 1b,d). Owing to interpenetration, the sizes of two channel windows are narrowed to 3.0 × 3.2 Å2 and 4.0 × 6.5 Å2 (Figure 1d), with the width of the larger channel between 4.5 and 5.5 Å and that of smaller one 3.1–4.8 Å (Figure 1e,f). The total guest-accessible volume for UTSA-220 is estimated to be 40%. The thermogravimetric analysis of the as-synthesized sample shows that the solvent molecules can be easily removed around ambient temperature, and framework decomposition is at around 250 °C (Figure S3).
Figure 1.
Building units (a) and structure (b) of UTSA-220. The channels of UTSA-220 are viewed along the a-axis. (c) The coordination sphere of Cu atom in UTSA-220. (d) Illustration of the channel dimensions of UTSA-220 viewed along the a-axis. Cross section of the larger (e) and smaller (f) channels viewed along b-axis. Color code: Cu, turquoise; F, bright green; Si, orange; C, gray; N, blue; H, white.
Gas Sorption Properties.
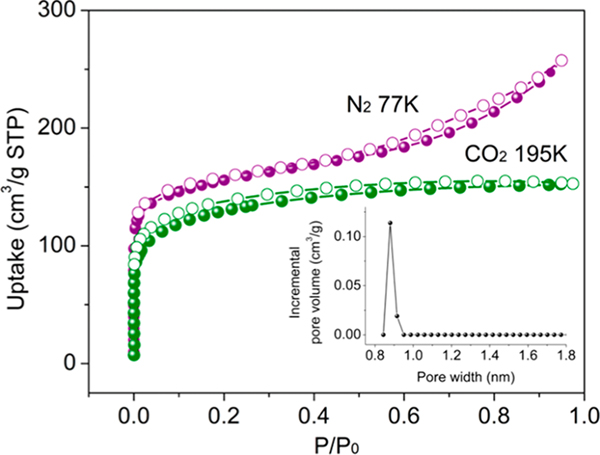
To investigate the porosity and channels in UTSA-220, the N2 sorption isotherms at 77 K and CO2 sorption at 195 K were collected. The obtained N2 sorption curve is a typical type I isotherm with the Brunauer–Emmett–Teller (BET) and Langmuir surface areas as 577 and 825 m2/g, respectively. With the aid of nonlocal density functional theory (NLDFT), the pore size distribution diagram was also obtained from the N2 adsorption data, showing a narrow distribution of approximately 8.8 Å (Figure 2, inset). Notably, the measured pore volume of UTSA-220 is 0.35 cm3/g based on 77 K N2 isotherm (at P/P0 = 0.85), which is consistent with the theoretical value of 0.33 cm3/g from crystal structure, implying the well retention of its porosity. In contrast, the zinc analogue of UTSA-220 shows far lower measured pore volume,50 which might be attributed to its high humidity sensitivity.
Figure 2.
Gas sorption isotherms of UTSA-220. N2 at 77 K (violet) and CO2 at 195 K (olive). The inset graph shows its incremental pore size distribution obtained from N2 isotherms at 77 K.
Because of the suitable pore size and favorably functionalized channels, UTSA-220 is suitable for selective adsorption of C2H2 over other light hydrocarbons. Gas sorption tests were performed on UTSA-220 at 298 K (Figure 3a) and 273 K (Figure S4) with C2H2, CO2, C2H4, C2H6, CH4 and N2. UTSA-220 show distinct adsorption amount of these gases. At 298 K and 100 kPa (1 bar), C2H2 has the highest uptake (3.40 mmol/g) among the series, followed by CO2 (3.38 mmol/g), C2H4 (2.53 mmol/g), C2H6 (2.14 mmol/g), CH4 (0.59 mmol/g) and N2 (0.18 mmol/g) (Figure 3a). At 273 K, the same sequence is also observed in corresponding gas uptakes (Figure S4). Dual-site Langmuir isotherm model was employed to fit the two sets of data at different temperatures to calculate the isosteric heat of adsorption (denoted as −Qst) of these gases (Table S2). At zero-coverage, the −Qst for C2H2, CO2, C2H4 and C2H6 are calculated to be 29, 27, 24 and 28 kJ/mol, respectively (Figure 3b). It should be noted that UTSA-220 adsorbs C2H2 rapidly at low pressure, with C2H2 uptake at 15 kPa reaching 2.40 mmol/g at 298 K. By contrast, the uptakes of CO2 (1.56 mmol/g), C2H4 (1.22 mmol/g), C2H6 (1.10 mmol/g) and CH4 (0.12 mmol/g) at the same condition are considerably lower. Such pronounced difference in the uptakes between C2H2 and other gas species reveals that UTSA-220 is very promising for C2H2 related separation. To assess the separation ability of UTSA-220, the ideal adsorbed solution theory (IAST) selectivity was calculated on the C2H2/C2H4 (1/99 v/v) and C2H2/CH4 (1/99 v/v) mixtures (Figure 3c, Figure S5). The C2H2/C2H4 (1/99 v/v) adsorption selectivity at 100 kPa is 10, suggesting that UTSA-220 has the potential to effectively remove trace C2H2 from C2H4 to yield high quality monomer for polyethylene production. More impressively, the C2H2/CH4 (1/99 v/v) selectivity is as high as 358 at 100 kPa (Table 1). We also calculate the equimolar IAST selectivity for C2H2/C2H4, C2H2/CO2 and C2H2/C2H6 to explore the potential to perform C2H2 separation from these gas mixtures. Based on the results, UTSA-220 has moderately high selectivities in these separation scenarios, with the selectivity values as 8.8 (C2H2/C2H4), 4.4 (C2H2/CO2) and 14 (C2H2/C2H6), respectively (Figure 3d, Table 1).
Figure 3.
(a) C2H2, CO2, C2H4, C2H6, CH4 and N2 adsorption isotherms of UTSA-220 at 298 K. (b) Isosteric heat of adsorption of C2H2, CO2, C2H4, C2H6 in UTSA-220. (c) IAST selectivity of C2H2/CH4 (1/99 v/v) and C2H2/C2H4 (1/99 v/v) at 298 K in UTSA-220. (d) IAST selectivity of C2H2/C2H6 (50/50 v/v), C2H2/C2H4 (50/50 v/v) and C2H2/CO2 (50/50 v/v) at 298 K in UTSA-220.
Table 1.
Summary of the IAST Selectivities of C2H2/C2H4 (1/99 v/v), C2H2/CH4 (1/99 v/v), C2H2/C2H4 (50/50 v/v), C2H2/CO2 (50/50 v/v) and C2H2/C2H6 (50/50 v/v) in UTSA-220 at 100 kPa
| mixture | component proportion | IAST selectivity |
|---|---|---|
| C2H2/C2H4 | 1/99 | 10 |
| C2H2/CH4 | 1/99 | 358 |
| C2H2/C2H4 | 50/50 | 8.8 |
| C2H2/CO2 | 50/50 | 4.4 |
| C2H2/C2H6 | 50/50 | 14 |
To delve into the adsorption mechanism of C2H2 and preferential C2H2 adsorption sites in UTSA-220, we carried out computational investigations using dispersion-corrected density functional theory (DFT-D). Two C2H2 adsorption sites are found within the framework, which are located at two different channels, respectively (Figure 4a). In Site I, C2H2 molecule is oriented almost parallel to the c-axis, with both hydrogen atoms pointing at two F atoms from two SiF62− units. On both ends of the C2H2 molecule, the H···F distances are 1.994 Å (Figure 4b), suggesting C2H2 forms hydrogen bonds with the surrounding F atoms. In Site II, C2H2 molecule is almost parallel with the ab plane, and is in an orientation with both H in proximity to two F atoms. Since H···F distances are 2.420 Å (Figure 4c), the C2H2 molecule in Site II also has hydrogen bonds formed. Each C2H2 molecule interacts with two F atoms from SiF62− units located on opposite sides of the channel simultaneously. The calculated static binding energies for two sites are −48.5 and −45.8 kJ/mol, respectively, which accounts for the highly selective adsorption of C2H2 over other light hydrocarbons. The adsorption of C2H2 follows the similar mechanism as those in previously reported SIFSIX MOFs,8,49,51 which highly relies on the optimal pore structure that matches well with the molecular shape of C2H2. Moreover, the simulation at ambient pressure reveals that there exists an extra interaction between C2H2 and azine groups with modest strength in UTSA-220 (Figure S6). These simulation results serve as a good evidence for our rational design of the framework to achieve desired performance.
Figure 4.
(a) DFT-D simulated adsorption sites for C2H2 in UTSA-220 and (b, c) their close contacts with the framework. Atom color code: F, bright green; Si, orange; Cu, turquoise; C in framework, gray; C in Site I, violet; C in Site II, golden; H, white; and N, blue.
Breakthrough Experiment.
To evaluate whether the material is capable to separate C2H2/C2H4 mixture in simulated industrial conditions, dynamic breakthrough experiments were conducted on UTSA-220 with a mixed gas of C2H2/C2H4 (1/99 v/v) using a homemade apparatus (Figure S7) at a flow rate of 2 mL/min at 298 K and 1 bar. C2H4 was first monitored at the column outlet after a short period of time since the start of the experiment, while C2H2 was still fully captured by the adsorbent and retained in the column (Figure 5). At this stage, a polymer-grade pure C2H4 was generated from the column with no measurable C2H2 (<0.1 ppm), indicating UTSA-220 is effective to remove trace amount of C2H2 from C2H4. As the adsorbent was close to saturation, C2H2 emerged from the column and gradually increased in concentration until all C2H2 in the gas mixture can be probed. The retention time for C2H2 and C2H4 are 48 min/g and 10 min/g, respectively. In order to test whether UTSA-220 can be utilized as a reusable adsorbent, the column was successively regenerated using a He flow at a rate of 20 mL/min at room temperature and put to breakthrough experiments for four times (Figures S8 and S9). Each time, breakthrough time is around 48 min/g, suggesting the recyclable feature of UTSA-220.
Figure 5.
Experimental column breakthrough curves of UTSA-220 material for C2H2/C2H4 (1/99 v/v) separation at 298 K and 1 bar.
CONCLUSION
To sum up, a new MOF with dual functionalities was successfully synthesized based on an azine ligand (1E,2E)-1,2-bis(pyridin-4-ylmethylene)hydrazine for efficient C2H2 removal and purification. Thanks to the dual functionalities, this MOF exhibits selective adsorption of C2H2 over other light hydrocarbons like C2H4. Breakthrough experiments further demonstrate the feasibility to remove trace amount of C2H2 from a mixture of C2H2 and C2H4 with this MOF as the column adsorbent. This work affords a functionalized approach of novel porous materials to advancing important gas separations in relevant energy-intensive petrochemical processes.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the financial support from National Natural Science Foundation of China (21673039, 21573042, 21606163), Fujian Science and Technology Department (2018J07001), Foundation of State Key Laboratory of Coal Conversion (J18-19-610) and the Welch Foundation (AX-1730). We also thank Dr. Shengfei Jin for his help in packing UTSA-220 into the breakthrough column in a glovebox.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssuschemeng.8b05480.
Sample preparation, data collection and analysis (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Yang S; Ramirez-Cuesta AJ; Newby R; Garcia-Sakai V; Manuel P; Callear SK; Campbell SI; Tang CC; Schröder M.Supramolecular binding and separation of hydrocarbons within a functionalized porous metal–organic framework. Nat. Chem 2015, 7, 121–129. [DOI] [PubMed] [Google Scholar]
- (2).Bao Z; Chang G; Xing H; Krishna R; Ren Q; Chen B.Potential of microporous metal–organic frameworks for separation of hydrocarbon mixtures. Energy Environ. Sci 2016, 9 (12), 3612–3641. [Google Scholar]
- (3).Pässler P; Hefner W; Buckl K; Meinass H; Meiswinkel A; Wernicke H-J; Ebersberg G; Müller R; Bässler J; Behringer H; Mayer D.Ullmann’s encyclopedia of industrial chemistry; Wiley-VCH: Weinheim, Germany, 2000; DOI: 10.1002/14356007. [DOI] [Google Scholar]
- (4).Lin R-B; Li L; Zhou H-L; Wu H; He C; Li S; Krishna R; Li J; Zhou W; Chen B.Molecular sieving of ethylene from ethane using a rigid metal–organic framework. Nat. Mater 2018, 17 (12), 1128–1133. [DOI] [PubMed] [Google Scholar]
- (5).Li L; Lin R-B; Krishna R; Li H; Xiang S; Wu H; Li J; Zhou W; Chen B.Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites. Science 2018, 362 (6413), 443–446. [DOI] [PubMed] [Google Scholar]
- (6).Cadiau A; Adil K; Bhatt PM; Belmabkhout Y; Eddaoudi M.A metal-organic framework–based splitter for separating propylene from propane. Science 2016, 353 (6295), 137–140. [DOI] [PubMed] [Google Scholar]
- (7).Li L; Lin R-B; Krishna R; Wang X; Li B; Wu H; Li J; Zhou W; Chen B.Flexible–robust metal–organic framework for efficient removal of propyne from propylene. J. Am. Chem. Soc 2017, 139 (23), 7733–7736. [DOI] [PubMed] [Google Scholar]
- (8).Cui X; Chen K; Xing H; Yang Q; Krishna R; Bao Z; Wu H; Zhou W; Dong X; Han Y; Li B; Ren Q; Zaworotko MJ; Chen B.Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 2016, 353 (6295), 141–144. [DOI] [PubMed] [Google Scholar]
- (9).Zhao X; Wang Y; Li D-S; Bu X; Feng P.Metal–organic frameworks for separation. Adv. Mater 2018, 30 (37), 1705189. [DOI] [PubMed] [Google Scholar]
- (10).Adil K; Belmabkhout Y; Pillai RS; Cadiau A; Bhatt PM; Assen AH; Maurin G; Eddaoudi M.Gas/vapour separation using ultra-microporous metal-organic frameworks: insights into the structure/separation relationship. Chem. Soc. Rev 2017, 46 (11), 3402–3430. [DOI] [PubMed] [Google Scholar]
- (11).Lin R-B; Wu H; Li L; Tang X-L; Li Z; Gao J; Cui H; Zhou W; Chen B.Boosting ethane/ethylene separation within isoreticular ultramicroporous metal–organic frameworks. J. Am. Chem. Soc 2018, 140 (40), 12940–12946. [DOI] [PubMed] [Google Scholar]
- (12).Lin R-B; Xiang S; Xing H; Zhou W; Chen B.Exploration of porous metal–organic frameworks for gas separation and purification. Coord. Chem. Rev 2019, 378, 87–103. [Google Scholar]
- (13).Poliakoff M; Licence P.Green chemistry. Nature 2007, 450, 810–812. [DOI] [PubMed] [Google Scholar]
- (14).Furukawa H; Cordova KE; O’Keeffe M; Yaghi OM The chemistry and applications of metal-organic frameworks. Science 2013, 341 (6149), 1230444. [DOI] [PubMed] [Google Scholar]
- (15).Zhou H-CJ; Kitagawa S.Metal–organic frameworks (MOFs). Chem. Soc. Rev 2014, 43 (16), 5415–5418. [DOI] [PubMed] [Google Scholar]
- (16).Kitagawa S; Kitaura R; Noro S -i. Functional porous coordination polymers. Angew. Chem., Int. Ed 2004, 43 (18), 2334–2375. [DOI] [PubMed] [Google Scholar]
- (17).Wang C; Liu D; Lin W.Metal–organic frameworks as a tunable platform for designing functional molecular materials. J. Am. Chem. Soc 2013, 135 (36), 13222–13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Nugent P; Belmabkhout Y; Burd SD; Cairns AJ; Luebke R; Forrest K; Pham T; Ma S; Space B; Wojtas L; Eddaoudi M; Zaworotko MJ Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. [DOI] [PubMed] [Google Scholar]
- (19).Zhu Q-L; Xu Q.Metal–organic framework composites. Chem. Soc. Rev 2014, 43 (16), 5468–5512. [DOI] [PubMed] [Google Scholar]
- (20).Zhou H-C; Long JR; Yaghi OM Introduction to metal–organic frameworks. Chem. Rev 2012, 112 (2), 673–674. [DOI] [PubMed] [Google Scholar]
- (21).Petit C; Bandosz TJ MOF–graphite oxide composites: combining the uniqueness of graphene layers and metal–organic frameworks. Adv. Mater 2009, 21 (46), 4753–4757. [Google Scholar]
- (22).Cui Y; Li B; He H; Zhou W; Chen B; Qian G.Metal–organic frameworks as platforms for functional materials. Acc. Chem. Res 2016, 49 (3), 483–493. [DOI] [PubMed] [Google Scholar]
- (23).He Y; Zhou W; Qian G; Chen B.Methane storage in metal–organic frameworks. Chem. Soc. Rev 2014, 43 (16), 5657–5678. [DOI] [PubMed] [Google Scholar]
- (24).Chen B; Xiang S; Qian G.Metal–organic frameworks with functional pores for recognition of small molecules. Acc. Chem. Res 2010, 43 (8), 1115–1124. [DOI] [PubMed] [Google Scholar]
- (25).Li H; Wang K; Sun Y; Lollar CT; Li J; Zhou H-C Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21 (2), 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Bloch ED; Queen WL; Krishna R; Zadrozny JM; Brown CM; Long JR Hydrocarbon separations in a metal-organic framework with open iron(II) coordination sites. Science 2012, 335 (6076), 1606–1610. [DOI] [PubMed] [Google Scholar]
- (27).Foo ML; Matsuda R; Hijikata Y; Krishna R; Sato H; Horike S; Hori A; Duan J; Sato Y; Kubota Y; Takata M; Kitagawa S.An adsorbate discriminatory gate effect in a flexible porous coordination polymer for selective adsorption of CO2 over C2H2. J. Am. Chem. Soc 2016, 138 (9), 3022–3030. [DOI] [PubMed] [Google Scholar]
- (28).Hamon L; Llewellyn PL; Devic T; Ghoufi A; Clet G; Guillerm V; Pirngruber GD; Maurin G; Serre C; Driver G; van Beek W; Jolimaître E; Vimont A; Daturi M; Férey G.Co-adsorption and separation of CO2–CH4 mixtures in the highly flexible MIL-53(Cr) MOF. J. Am. Chem. Soc 2009, 131 (47), 17490–17499. [DOI] [PubMed] [Google Scholar]
- (29).Howarth AJ; Katz MJ; Wang TC; Platero-Prats AE; Chapman KW; Hupp JT; Farha OK High efficiency adsorption and removal of selenate and selenite from water using metal–organic frameworks. J. Am. Chem. Soc 2015, 137 (23), 7488–7494. [DOI] [PubMed] [Google Scholar]
- (30).Luo F; Yan C; Dang L; Krishna R; Zhou W; Wu H; Dong X; Han Y; Hu T-L; O’Keeffe M; Wang L; Luo M; Lin R-B; Chen B.UTSA-74: a MOF-74 isomer with two accessible binding sites per metal center for highly selective gas separation. J. Am. Chem. Soc 2016, 138 (17), 5678–5684. [DOI] [PubMed] [Google Scholar]
- (31).Liao P-Q; Huang N-Y; Zhang W-X; Zhang J-P; Chen X-M Controlling guest conformation for efficient purification of butadiene. Science 2017, 356 (6343), 1193–1196. [DOI] [PubMed] [Google Scholar]
- (32).Gelfand BS; Huynh RPS; Mah RK; Shimizu GKH Mediating order and modulating porosity by controlled hydrolysis in a phosphonate monoester metal–organic framework. Angew. Chem., Int. Ed 2016, 55 (47), 14614–14617. [DOI] [PubMed] [Google Scholar]
- (33).Thallapally PK; Tian J; Radha Kishan M; Fernandez CA; Dalgarno SJ; McGrail PB; Warren JE; Atwood JL Flexible (breathing) interpenetrated metal–organic frameworks for CO2 separation applications. J. Am. Chem. Soc 2008, 130 (50), 16842–16843. [DOI] [PubMed] [Google Scholar]
- (34).Rodenas T; Luz I; Prieto G; Seoane B; Miro H; Corma A; Kapteijn F; Llabrés i Xamena FX; Gascon J.Metal–organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater 2015, 14, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Lu G; Li S; Guo Z; Farha OK; Hauser BG; Qi X; Wang Y; Wang X; Han S; Liu X; DuChene JS; Zhang H; Zhang Q; Chen X; Ma J; Loo SCJ; Wei WD; Yang Y; Hupp JT; Huo F.Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation. Nat. Chem 2012, 4, 310–316. [DOI] [PubMed] [Google Scholar]
- (36).Li B; Chrzanowski M; Zhang Y; Ma S.Applications of metal-organic frameworks featuring multi-functional sites. Coord. Chem. Rev 2016, 307, 106–129. [Google Scholar]
- (37).Li B; Leng K; Zhang Y; Dynes JJ; Wang J; Hu Y; Ma D; Shi Z; Zhu L; Zhang D; Sun Y; Chrzanowski M; Ma S.Metal–organic framework based upon the synergy of a Brønsted acid framework and Lewis acid centers as a highly efficient heterogeneous catalyst for fixed-bed reactions. J. Am. Chem. Soc 2015, 137 (12), 4243–4248. [DOI] [PubMed] [Google Scholar]
- (38).Gascon J; Corma A; Kapteijn F; Llabres i Xamena FX Metal organic framework catalysis: quo vadis? ACS Catal. 2014, 4 (2), 361–378. [Google Scholar]
- (39).Hu Z; Deibert BJ; Li J.Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev 2014, 43 (16), 5815–5840. [DOI] [PubMed] [Google Scholar]
- (40).Zhang M; Feng G; Song Z; Zhou Y-P; Chao H-Y; Yuan D; Tan TTY; Guo Z; Hu Z; Tang BZ; Liu B; Zhao D.Two-dimensional metal–organic framework with wide channels and responsive turn-on fluorescence for the chemical sensing of volatile organic compounds. J. Am. Chem. Soc 2014, 136 (20), 7241–7244. [DOI] [PubMed] [Google Scholar]
- (41).Horcajada P; Gref R; Baati T; Allan PK; Maurin G; Couvreur P; Férey G; Morris RE; Serre C.Metal–organic frameworks in biomedicine. Chem. Rev 2012, 112 (2), 1232–1268. [DOI] [PubMed] [Google Scholar]
- (42).Zhao D; Tan S; Yuan D; Lu W; Rezenom YH; Jiang H; Wang L-Q; Zhou H-C Surface functionalization of porous coordination nanocages via click chemistry and their application in drug delivery. Adv. Mater 2011, 23 (1), 90–93. [DOI] [PubMed] [Google Scholar]
- (43).Bai Y; Dou Y; Xie L-H; Rutledge W; Li J-R; Zhou H-C Zr-based metal–organic frameworks: design, synthesis, structure, and applications. Chem. Soc. Rev 2016, 45 (8), 2327–2367. [DOI] [PubMed] [Google Scholar]
- (44).Zhang Y-B; Furukawa H; Ko N; Nie W; Park HJ; Okajima S; Cordova KE; Deng H; Kim J; Yaghi OM Introduction of functionality, selection of topology, and enhancement of gas adsorption in multivariate metal–organic framework-177. J. Am. Chem. Soc 2015, 137 (7), 2641–2650. [DOI] [PubMed] [Google Scholar]
- (45).Wang H; Dong X; Lin J; Teat SJ; Jensen S; Cure J; Alexandrov EV; Xia Q; Tan K; Wang Q; Olson DH; Proserpio DM; Chabal YJ; Thonhauser T; Sun J; Han Y; Li J.Topologically guided tuning of Zr-MOF pore structures for highly selective separation of C6 alkane isomers. Nat. Commun 2018, 9 (1), 1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Horike S; Inubushi Y; Hori T; Fukushima T; Kitagawa S.A solid solution approach to 2D coordination polymers for CH4/CO2 and CH4/C2H6 gas separation: equilibrium and kinetic studies. Chem. Sci 2012, 3 (1), 116–120. [Google Scholar]
- (47).Hu T-L; Wang H; Li B; Krishna R; Wu H; Zhou W; Zhao Y; Han Y; Wang X; Zhu W; Yao Z; Xiang S; Chen B.Microporous metal–organic framework with dual functionalities for highly efficient removal of acetylene from ethylene/acetylene mixtures. Nat. Commun 2015, 6, 7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Li L; Lin R-B; Krishna R; Wang X; Li B; Wu H; Li J; Zhou W; Chen B.Efficient separation of ethylene from acetylene/ethylene mixtures by a flexible-robust metal–organic framework. J. Mater. Chem A 2017, 5 (36), 18984–18988. [Google Scholar]
- (49).Li B; Cui X; O’Nolan D; Wen H-M; Jiang M; Krishna R; Wu H; Lin R-B; Chen Y-S; Yuan D; Xing H; Zhou W; Ren Q; Qian G; Zaworotko MJ; Chen B.An ideal molecular sieve for acetylene removal from ethylene with record selectivity and productivity. Adv. Mater 2017, 29 (47), 1704210. [DOI] [PubMed] [Google Scholar]
- (50).Manna B; Sharma S; Ghosh S.Synthesis and crystal structure of a Zn(II)-based MOF bearing neutral n-donor linker and SiF62– anion. Crystals 2018, 8 (1), 37. [Google Scholar]
- (51).Lin R-B; Li L; Wu H; Arman H; Li B; Lin R-G; Zhou W; Chen B.Optimized separation of acetylene from carbon dioxide and ethylene in a microporous material. J. Am. Chem. Soc 2017, 139 (23), 8022–8028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.