Abstract
The first case of human babesiosis was reported in the literature in 1957. The clinical disease has sporadically occurred as rare case reports in North America and Europe in the subsequent decades. Since the new millennium, especially in the last decade, many more cases have apparently appeared not only in these regions but also in Asia, South America, and Africa. More than 20,000 cases of human babesiosis have been reported in North America alone. In several cross-sectional surveys, exposure to Babesia spp. has been demonstrated within urban and rural human populations with clinical babesiosis reported in both immunocompromised and immunocompetent humans. This review serves to highlight the widespread distribution of these tick-borne pathogens in humans, their tick vectors in readily accessible environments such as parks and recreational areas, and their phylogenetic relationships.
Keywords: human babesiosis, Babesia spp., Babesia microti, Babesia divergens, Babesia venatorum, Babesia duncani, Babesia crassa
1. Introduction
Babesia spp. are piroplasm parasites of various vertebrate animals with host specificity. Their infections may cause clinical manifestations such as fever, anemia, or even death although asymptomatic infections are not unusual. Some Babesia spp. are zoonotic, causing human infections that result in babesiosis. The very first case of human babesiosis appeared in the literature in 1957. This fatal disease was diagnosed in a 33-year-old asplenic man from Zagreb [1]. The disease was so rare back then that by 1968 only three human cases were reported [2]. Nevertheless, human babesiosis has been emerging in recent years in many geographical regions around the world, particularly in the United States of America (USA), Canada, and China. In the last decade, reviews have been published on the Babesia spp. life cycle, pathogenesis, immunity, diagnosis, and treatment as well as human babesiosis in Europe and China [3,4,5,6]. We reviewed in this manuscript; (a) human babesiosis cases that had been diagnosed as Babesia species by molecular confirmation with attention to cross-sectional surveys, (b) vectors for these Babesia spp., (c) Babesia spp. in tick vectors collected in the recreational areas readily accessible to humans and possible roles by the domestic dog in human babesiosis. We further performed a phylogenetic analysis of the Babesia spp. in human cases and those harbored by ticks recovered in the areas easily accessible to humans. We aimed to initiate a debate on this emerging disease and call the attention of both the medical and veterinary professionals.
2. Human Babesiosis
2.1. Challenge in Diagnosis of Babesia spp. in Humans
Individual Babesia spp. usually have a rather narrow spectrum of hosts, i.e., each with strict host specificity. For instance, Babesia canis causes canine babesiosis in domestic dog (Canis lupus familiaris). So far, no Babesia spp. are found exclusively using only humans as a host although several species have been found infecting humans. Due to technical challenges in the diagnosis of Babesia spp. in humans that have similar morphology and cross-reaction of antibody and antigen, molecular techniques such as PCR and DNA sequencing are often required for species identification. So far, Babesia spp. that have been confirmed infecting humans by molecular methods include B. microti, B. divergens, B. venatorum (Babesia sp., EU1), B. duncani (Babesia sp., WA1), B. crassa, and two yet to be named species Babesia sp. KO1 and Babesia sp. CN1 (Babesia sp. XXB/HangZhou).
2.2. Geographical Distribution
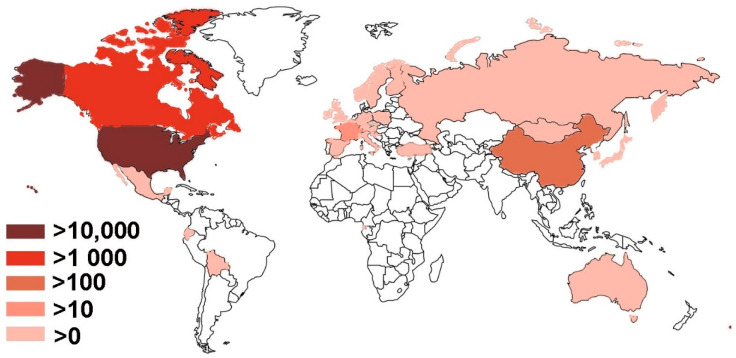
Human babesiosis has been reported in 28 countries on all continents except Antarctica (Table 1 and Figure 1). The data were collected from MEDLINE by searching PubMed using Babesia, OR babesiosis, OR babesiasis AND human case. The resultant titles and abstracts were browsed to identity papers with human cases. The references of those papers were also monitored for additional relevant publications. All countries except the USA, Canada, China, and France, have fewer than ten cases reported and will be discussed in less detail. On the other hand, the USA and Canada, two countries in North America, account for more than 95% of worldwide reported cases and therefore are “the ground zero” of this current emergence of human babesiosis that is mainly attributed to B. microti. Human babesiosis has been a USA notifiable disease nationally since January 2011 (https://www.cdc.gov/parasites/babesiosis/index.html (accessed on 17 February 2021)). The total annual cases reported to CDC are 14,042, i.e., 1126 cases for 2011, 911 for 2012, 1761 for 2013, 1742 for 2014, 2074 for 2015, 1909 for 2016, 2358 for 2017 and 2161 for 2018 (https://www.cdc.gov/parasites/babesiosis/data-statistics/index.html (accessed on 17 February 2021)). No data are available for 2019 yet. Furthermore, a retrospective population-based study was carried out among elderly, over 65 years old, Medicare beneficiaries between 2006 and 2013 using the Centers for Medicare & Medicaid Services (CMS) databases. A total of 10,305 cases had a recorded diagnosis of babesiosis. The top five states with the highest average rate per 100,000 residents annually included Connecticut 46 (total case 1307), Massachusetts 45 (2161), Rhode Island 42 (247), New York 27 (3193), and New Jersey 14 (980). A seasonal peak occurred in the summer between May and October accounting for approximately 75% of all reported cases [7]. It is likely some cases covered in CMS for the years 2011–2013 of 1366, 1219, and 1848 cases, respectively, may be also reported to CDC. The maximum of babesiosis cases in the USA between 2006 and 2018 can be calculated provided that no cases overlap between CDC and CMS data, which were 24,347. The minimal numbers were 20,549 if all CDC cases were included in CMS for the years 2011–2013.
Table 1.
Number of cases and prevalence of human babesiosis worldwide.
| Countries | Number of Case * | Cross-Sectional Survey † | References |
|---|---|---|---|
| Africa | |||
| Equatorial Guinea | 1 (Bm) | [8] | |
| Asia | |||
| China | 16 (Bm); 1 (Bd); 49 (Bv); 1 (CN1); 58 (Bc) | [5,9,10,11,12,13,14,15,16] | |
| Korea | 1 (KO1) | [17] | |
| Japan | 1 (Bm) | [18] | |
| Mongolia | 7.0% (7/100) 1; 3.0% (3/100) 2 | [19] | |
| Australia | |||
| Australia | 1 (Bm); 1 (Bdu) | [20,21] | |
| Europe | |||
| Austria | 1 (Bm); 2 (Bv) | [22,23,24] | |
| Belgium | 1 (Bm) | [25] | |
| British Isles | 6 (Bd) | [25] | |
| Czech | 1 (Bm) | [26] | |
| Croatia | 1 (others) | [25] | |
| Finland | 1 (Bd) | [27] | |
| France | 11 (Bd), 2 (others) | [25,28] | |
| Germany | 1 (Bm); 1 (Bv) | [29,30] | |
| Italy | 1 (Bv) | [23] | |
| Norway | 1 (Bd) | [31] | |
| Poland | 1 (Bm) | [25] | |
| Russia | 1 (Bd) | [32] | |
| Slovenia | 1 (Bc) | [33] | |
| Spain | 2 (Bd); 1 (Bm); 2 (others) | [25,34,35,36] | |
| Sweden | 1 (Bd) | 2.0% (4/197) 1 | [25,37] |
| Switzerland | 1 (Bd) | 1.3% (5/396) 1 | [20,25] |
| Turkey | 2 (Bd) | [38] | |
| North America | |||
| Canada | 1,120 (Bdu); 1 (Bm) | [39,40,41] | |
| Mexico | 4 (Bm) | [42] | |
| USA | 24,363 (Bm); 14 (Bdu); 4 (Bd) | 0.5% (4/879) 1; 2.0% (18/879) 1; 25.0% (48/192) 2 | [7,43,44,45,46,47,48,49] |
| South America | |||
| Bolivia | 3.3% (9/271) 2 | [50] | |
| Ecuador | 1 (Bm) | [51] |
*: Abbreviations of Babesia spp. are B. microti—Bm; B. divergens—Bd; B. venatorum—Bv; B. duncani—Bdu; Babesia sp. KO1-KO1; Babesia sp. CN1-CN1; Bc—B. crassa. †: 1: serology; 2: PCR.
Figure 1.
Geographical distribution of human babesiosis. The darker the color, the more numerous cases there are. There are no reported cases in unfilled countries or regions.
Babesia duncani, the former Babesia sp., WA1, was a new species found in humans in the Western USA, predominantly in Washington and California. So far, a total of 14 human babesiosis cases caused by B. duncani have been reported in the USA, all of them in western states [43]. In addition to B. microti and B. duncani, B. divergens has been reported in Kentucky, Missouri, Michigan, and Washington (Table 1).
The country with the 2nd most numerous reported cases of human babesiosis is Canada where B. microti and B. duncani have been confirmed [39,40]. From 2011 to 2017 data on B. duncani infections in Canadians were collected by tick-borne disease specialists (10 physicians and 10 naturopathic physicians) in the USA and Canada. Cases were found in all ten Canadian provinces ranging from as few as four within Newfoundland and Labrador, seven within Prince Edward Island to as many as 365 within Ontario, and 377 within British Columbia. There was a steady increase in the number of infections annually during the collection period of seven years, ranging from 119 in 2011 to 198 in 2017. In total 1,119 cases were identified [41]. Nevertheless, it is worthy of pointing out that testing in this paper was done at five US laboratories that used in-house assays of B. duncani WA1 IgG by the immunofluorescence assay (IFA) and the DNA probe of the internal transcribed spacer of rRNA. There was no breakdown of which tests were positive and how many samples at each lab. Therefore, more evidence from Public Health laboratories or other researchers is warranted to corroborate these numbers.
China has the 3rd largest number of reported cases of human babesiosis. China is the world’s most populous country, approximately 2.5 times the population of North America, which adds credence to the significance of the reported prevalence in North America. Sixteen cases of human babesiosis were diagnosed with B. microti infections (Table 1). The first Chinese case of B. venatorum infection was from an eight-year-old boy with the diagnosis made based on microscopy, PCR, and DNA sequencing [9]. Additional 48 cases were confirmed. All cases were from immunocompetent individuals. Thirty-two patients had clinical manifestations including fever, headache, fatigue, and dizziness [10]. A survey was carried out from May to July in the Heilongjiang Province of China in 2015 and 2016 among the patients who presented to a local hospital with flu-like symptoms and a tick-bite history. Fifty-eight of the 1125 patients (5.2%) were confirmed to have B. crassa DNA in their whole blood through species-specific PCR and DNA sequencing [11]. Further, a Chinese patient suffering from multiple episodes of mild fever and fatigue of unknown origin over a period of 10 years was diagnosed with babesiosis through microscopy, PCR, and DNA sequencing. The parasite was given a new species name as Babesia sp. XXB/HangZhou since its 18S rRNA sequence was different from, although being clustered with B. microti and B. duncani by phylogenetic analysis [12]. Nevertheless, the new name is inconsistent with the International Code of Zoological Nomenclature (http://iczn.org/iczn/index.jsp (accessed on 17 February 2021)) or in the tradition of Babesia naming. Thus, the name of Babesia sp. CN1 would probably fit better until a valid name is acquired.
2.3. Cross-Sectional Survey of Babesia spp. Infections in Humans
Although human babesiosis has emerged as an important disease and has been notifiable in the USA since 2011, its prevalence among humans has been scarcely addressed. The following cross-sectional data may provide a glimpse.
In a serological survey to detect IgG antibodies against B. microti using IFA, four were positive out of 879 blood donors (0.5%) from Oregon, California, Arizona, North Dakota, Texas, Kansas, Alabama, Maryland and Massachusetts during 2009 [44]. A New Jersey medical center performed multiplex quantitative polymerase chain reaction (qPCR) to detect both B. microti protozoan and Borrelia burgdorferi bacterium in blood samples collected from the residents of three New Jersey counties, an endemic area for both pathogens. Among 192 samples 12 (6.3%) were positive for B. microti alone and 36 (18.7%) had co-infection of both B. microti and B. burgdorferi [45]. In a cross-sectional study in Switzerland from December 1997 to May 1998, blood samples were collected and tested for IgG antibodies to B. microti. Five of 396 (1.3%) were found positive [20]. In a serological survey carried out in Sweden from 2014 to 2015 using IFA, 10.5% (9/86) and 1.2% (1/86) of B. burgdorferi s.l. antibody positive individuals had IgG and IgM antibodies to B. microti, respectively. In contrast in the healthy controls, the rate for the corresponding antibodies was 1.5% (3/197) and 0.5% (1/197), respectively [37]. In 2013 a cross-sectional survey of 271 healthy individuals in two rural communities in Bolivia using microscopy, PCR and DNA sequencing confirmed nine (3.3%) cases having a B. microti infection [50]. A similar cross-sectional study in Mongolia surveyed 100 farmers in 2011, 7% (7/100) and 3.0% (3/100) were found to be positive using IFA and molecular methods including PCR and DNA sequencing, respectively [19]. In a serological survey carried out in Sweden as having been described earlier, 7.0% (6/86) of B. burgdorferi s.l. antibody positive individuals were also IgG positive to B. divergens. In contrast in the healthy controls, the rate for IgG and IgM antibodies were 1.0% (3/197) and 0.5% (1/197), respectively [37]. In a survey of clinical sera collected from the USA pacific regions (Oregon, California) and other states (Arizona, North Dakota, Texas, Kansas, Alabama, Maryland, Massachusetts) during 2008 and 2009 by IFA, 27.0% sera were IgG positive for B. duncani. Furthermore, 18 out of 879 blood donors (2.0%) from these states were IgG positive for the same parasite [44] (Table 1). Collectively, humans appear to be more frequently exposed to Babesia spp. infection than clinically diagnosed cases have indicated in many geographical locations. Therefore, babesiosis should be included in differentials of peoples with fever, especially those with a tick-bite history.
3. Vectors
All Babesia spp. require a hard tick vector in their life cycle as a definitive host. Various hard tick vectors of Babesia spp. infest humans (Table 2). A prospective study performed in the Netherlands between January 2004 and December 2008 examined ticks that had fed on human beings, and individuals were followed for a minimum of 6 months with their clinical outcomes documented. All ticks were Ixodes ricinus and they were tested for tick-borne pathogens including Babesia spp. using PCR and reverse line blotting techniques. 9.1% and 0.3% out of 297 ticks were positive for B. microti and an undetermined Babesia sp., respectively. Clinical symptoms were associated with a tick attachment duration lasting greater than 24 h [52]. It was also found that 14 of the 64 pools of 408 I. ricinus ticks collected from Switzerland tested positive for B. microti by PCR and DNA sequencing [20].
Table 2.
Babesia species causing human babesiosis, their vectors and geographical distribution.
| Babesia spp. | Distribution | Confirmed Tick Vector | Possible Tick Vector |
|---|---|---|---|
| B. microti | North America | I. scapularis | |
| Europe | I. ricinus | H. concinna, R. turanicus | |
| China | I. persulcatus | ||
| Equatorial Guinea | u | ||
| Bolivia | u | ||
| Ecuador | u | ||
| Japan | u | ||
| Mongolia | u | ||
| B. divergens | Europe | I. ricinus | |
| China | u | ||
| USA | u | ||
| B. venatorum (Babesia sp., EU1) | Europe | I. ricinus | I. canisuga |
| China and Mongolia | I. persulcatus; I. ovatus | ||
| Japan | u | ||
| B. duncani (WA1) | North America | Dermacentor albipictus | I. scapularis |
| Australia | u | ||
| Babesia crassa | China | I. persulcatus, H. concinna | |
| Babesia sp. CN1 | China | u | |
| Babesia sp. KO1 | Korea | u |
u: unknown.
High throughout real-time PCR was carried out to screen 7050 I. ricinus nymphs collected within France in 2011, Denmark in 2012 and the Netherlands between 2008 and 2012. Primers and probes were designed for real-time TaqMan PCRs to target various genes of several Babesia spp. including B. microti, B. divergens, B. caballi, B. canis, B. vogeli, B. venatorum, B. bovis, B. bigemina, B. major, and B. ovis. Confirmation was performed by conventional PCR in the amplification of 18S rRNA gene followed by DNA sequencing. Bebesia divergens was found only in ticks collected in Denmark and the Netherlands while B. venatorum was found in all three countries [53]. Ioxdes ricinus ticks collected from Norway between 2006 and 2008 were individually tested for Babesia spp. by PCR and DNA sequencing. A total of 17 out of 1908 (0.9%) ticks were found to be positive for Babesia spp. with B. divergens accounting for less than 30% of the Babesia spp. identified and B. venatorum accounting for 71% of the positive samples [54].
A number of studies have examined B. venatorum transmission in potential vectors. In an experiment of in vitro transmission of the parasite by I. ricinus, nymphs and adult females of field-collected ticks that fed on a glass feeder until fully engorged. The feeder contained 3 mL of sheep red-blood-cells (RBC) with approximately 8% parasitemia. Parasite DNA was detected from eggs and larvae derived from the infected adult females. Furthermore, infected adult females transmitted the Babesia spp. during a new blood meal with parasite DNA being detected in their salivary gland [55]. Ixodes ricinus ticks in Switzerland were found to be 0.8% positive for B. venatorum by next generation sequencing [56]. Ticks were collected from humans in Italy between 1995 and 2011. Among the 334 I. ricinus, 0.3% (one) was positive for B. venatorum [57]. Ixodes ricinus adults were collected from cattle farms (223 ticks) and wild fauna reserve (31 ticks) after full engorgement, 1.3% (three) and 6.5% (two) adult ticks were positive for sporozoites in their salivary gland, respectively. These sporozoites further infected only sheep RBCs [58]. In the Czech Republic, I. ricinus ticks were collected and tested for Babesia spp. by amplifying a fragment of the 18S rRNA gene. Babesia venatorum was confirmed from two positive amplicons [59]. Ixodes persulcatus (819) and Haemaphysalis concinna (260) ticks in China were tested by PCR and DNA sequencing for Babesia spp. Only I. persulcatus was found to be positive (1.0%, 8/819) and DNA sequencing confirmed it as B. venatorum [9,10]. Ixodes persulcatus ticks in Mongolia were also found to be positive for B. venatorum in two studies, one found a prevalence of 3.2% (2/63) [60] and the other found a prevalence of 3.3% (9/275) [61].
The vector and reservoir of B. duncani had been elusive for over two decades since the parasite was first discovered in a patient in Washington State, USA. Lately, DNA of B. duncani was found in the larval and adult stages of the winter tick, Dermacentor albipictus. Overall, a minimum infection prevalence in larvae was 7.2% with the highest rate of 20.7%, whereas rate in adults was 2.1% [43]. The same study also confirmed a primary reservoir was mule deer (Odocoileus henionus) [43].
Ticks were collected from May to July in 2014 in the Heilongjiang Province of China and were analyzed through species-specific PCR and DNA sequencing for B. crassa DNA. Eight of the 1296 (0.6%) I. persulcatus ticks and one of 252 (0.4%) H. concinna ticks were found to be positive [11].
Collectively, I. ricinus has been confirmed a vector for B. microti, B. venatorum and B. divergens in Europe. Ixodes persulcatus is a vector for B. venatorum and B. crassa in Asia. Haemaphysalis concinna is a vector for B. crassa in Asia. Dermacenter albipictus is the vector for B. duncani in North America.
4. Tick Vectors Found in the Recreational Areas Readily Accessible to Humans
Ticks were collected all-year-round in 2011 in the Insugherata Natural Reserve located in northwestern Rome. The ticks that were PCR positive for the 18S rRNA gene of B. microti were Rhipicephalus turanicus, 1.2% (1/85, 29 males and 56 females) and I. ricinus, 12.1% (4/33, 11 males and 22 females) while D. marginatus (1 male and 6 females) and H. punctate, (1 male and 3 females) were found to be negative [62]. Three parks in the Emilia-Romagna Region of Northern Italy were surveyed for ticks from April to October 2010 every 15 days. Picnic areas and footpaths that were frequented by people were selected. DNA was extracted from individual adult ticks or pools of 5 nymphs or 10 larvae from the same area at the same time point followed by PCR and DNA sequencing of piroplasm’s 18S rRNA. In total, 6.4% of the male ticks (2/31), 4.8% of the female ticks (1/21), 9.9% of the nymph tick pools (25/232), and 0.0% of the larvae (0/32) were PCR positive. Eleven including nine pools of nymphs and two adults were positive with B. venatorum. Two pools of nymphs were positive with B. divergens and B. capreoli [63]. Ticks were collected in the Tri-City Landscape Park in northern Poland during 2009–2010. The park was a destination for tourism and leisure among residents of the cities of Gdańsk, Sopot, and Gdynia. 4.5% (34/757) of the I. ricinus ticks were found to be Babesia sp. positive through PCR with B. venatorum being the predominant species found [64]. In a survey in Bratislava, Slovakia, a total of 2799 I. ricinus ticks were collected and this represented an urban/suburban habitat and was characterized by significant human development between 2011 and 2013. Thirty-three ticks (1.2%) were positive for Babesia spp. which included B. microti, B. venatorum, and B. divergens [65]. Ticks were also collected between April and June 2008 from a suburban forest, Sénart Forest, in the southern Paris metropolitan area. This forest has three million visitors annually. Five hundred and fifty-eight out of the 574 ticks identified were I. ricinus ticks. Babesia sp. was tested by PCR and DNA sequencing and among these I. ricinus ticks, an estimated overall prevalence of 1.6% was established. All parasites were identified by DNA sequencing as B. venatorum [66].
In short, ticks collected in areas with high human activities such as parks and picnic areas include confirmed vectors for human babesiosis such as I. ricinus. Further, PCR and DNA sequencing have detected the DNA of many Babesia spp. including B. microti, B. venatorum, and B. divergens in these ticks.
5. Phylogenetic Analysis of Babesia spp. Harbored by Humans, and Ticks on These Hosts
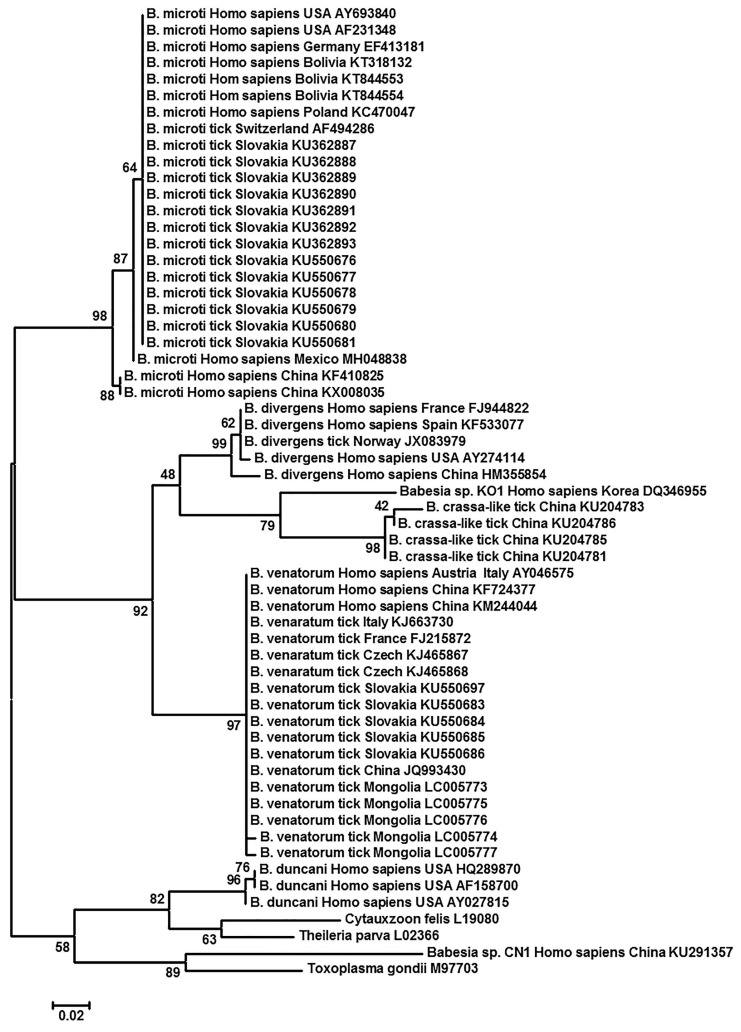
To understand the relationship between Babesia spp. that had been identified from humans, and tick vectors on humans in different geographic regions a phylogenetic analysis was carried out using 18S rRNA sequences of Babesia spp. The trees were rooted with Toxoplasma gondii. Two piroplasm species, one each in the closely related genera of Cytauxzoon (C. felis) and Theileria (T. parva) were also included. The three piroplasm genera are distinguishable from one another by morphological, ultrastructural, and parasite life-cycle characteristics like host preference and host cells of infection [67]. Both Maximum Likelihood (ML) and Neighbor-Joining (NJ) were performed with 1000 bootstrap replications using the free software MEGA (Version 5.2.2) [68]. Both ML and NJ yielded almost identical results. Only the ML result is shown in Figure 2. In general, each of B. venatorum, B. crassa, B. divergens, B. microti, and B. duncani forms its own clade with multiple isolates. It seemed that both Babesia sp. KO1 and Babesia sp. CN1 differed from one another and from other Babesia spp. They may each represent a new Babesia species (Figure 2). Babesia sp. KO1 was most closely related to B. crassa. Interestingly, Babesia sp. CN1 appeared to be closer to T. gondii than to two piroplasms, i.e., C. felis and T. parva (Figure 2). Likely, this may not be a Babesia sp. at all.
Figure 2.
Phylogenetic analysis of 18S rRNA sequences of Babesia spp. found in human and tick vectors. The Maximum Likelihood method was performed in a default setting of the Jukes–Cantor model, uniform rates, and complete depletion with 1000 bootstrap replications. The trees were rooted with Toxoplasma gondii. Two species of piroplasms, one each in the closely related genera of Cytauxzoon (C. felis) and Theileria (T. parva) were also included. Scale bar indicates nucleotide substitutions per site. Numbers at the horizontal lines represent the percentage of replicates of 1000 repeats. The Neighbor-jointing method in the default setting yielded almost identical results as Maximum Likelihood did (Not shown). Each entry was identified in the order of Babesia sp., host, geographical region, and Accession number.
6. Possible Roles of the Domestic Dog in Human Babesiosis
May it even be possible that pet dogs play a role in human babesiosis? Firstly, dogs in China and Russia were found to be infected with B. microti by PCR and DNA sequencing [69,70]. Secondly, ticks collected from pet dogs carry B. microti, B. venatorum, and B. divergens [71,72,73,74,75,76,77,78,79]. These ticks might have obtained Babesia spp. pathogens from other hosts prior to their attachment to the pet dogs. Nevertheless, these pet dogs bring these Babesia infected ticks to the human households, making tick infestation and Babesia spp. transmission to humans a much higher possibility. Lastly, pet dogs living within the same household were incriminated for at least two cases of human babesiosis in the literature [80,81]. One needs to be cautious in interpreting this finding as no evidence presented linked those dogs directly to human infections. Collectively, there is no unequivocal evidence so far in the literature suggesting dogs contribute to human babesiosis. They may play a marginal role. Further experimental data are required to ascertain their roles.
7. Conclusions
This review aimed to address the geographic distribution of the human-infecting Babesia spp., their phylogenetic relationship, and their tick vector worldwide. Human babesiosis is caused by several Babesia spp. that includes but are not limited to, B. microti, B. divergens, B. venatorum, B. duncani, B. crassa, and two Babesia spp. strains i.e., Babesia sp. KO1 and Babesia sp. CN1. The latter two may represent a new Babesia species, which as of yet needs to be further defined and properly named.
The number of human cases that appeared in the literature has been exponentially increased in the last decade. Two countries in North America, USA, and Canada have over twenty thousand and one thousand cases, respectively. China is in distant third place with over one hundred cases. Cases have been reported from all continents with human residence. Several factors may contribute to this rapid increase in confirmed cases of human babesiosis. First is the awareness of the disease in medical professionals, resulting in the correct diagnosis of the disease which would have been mistakenly diagnosed as other infections. The second is through active monitoring and survey studies. Babesiosis is a notifiable disease in the USA. Cross-sectional surveys have been carried out in many regions including Canada and China. Third is global warming, which has expanded tick vector habitats to areas that can be readily accessed by humans such as parks and recreational areas. The fourth factor is transfusion transmission. From 2009 to 2016 in Massachusetts alone 45 of 2578 (1.7%) were transmitted by transfusion [82]. The last, but not least, factor is vertical transmission from an infected mother to her offspring [83].
Author Contributions
A.D. and C.Y. conceived the review. C.Y. did the literature research, performed the phylogenetic analysis, and drafted the manuscript. Y.Y., J.C., and L.K. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the National Key Basic Research Program (973 Program) of China (2015CB150300), National Key Research and Development Program of China (2017YFD0501200), and Ross University School of Veterinary Medicine (41002-2021). APC was paid by the Center one of Ross University School of Verterinary Medicine. The funding source played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Skrabalo Z., Deanovic Z. Piroplasmosis in man; report of a case. Doc. Med. Geogr. Trop. 1957;9:11–16. [PubMed] [Google Scholar]
- 2.Fitzpatrick J.E.P., Kennedy C.C., McGeown M.G., Oreopoulos D.G., Robertson J.H., Soyannwo M.A.O. Human Case of Piroplasmosis (Babesiosis) Nature. 1968;217:861. doi: 10.1038/217861a0. [DOI] [PubMed] [Google Scholar]
- 3.Hildebrandt A., Gray J.S., Hunfeld K.-P. Human Babesiosis in Europe: What clinicians need to know. Infection. 2013;41:1057–1072. doi: 10.1007/s15010-013-0526-8. [DOI] [PubMed] [Google Scholar]
- 4.Ord R.L., Lobo C.A. Human Babesiosis: Pathogens, Prevalence, Diagnosis and Treatment. Curr. Clin. Microbiol. Rep. 2015;2:173–181. doi: 10.1007/s40588-015-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou X., Xia S., Huang J.-L., Tambo E., Zhuge H.-X., Zhou X.-N. Human babesiosis, an emerging tick-borne disease in the People’s Republic of China. Parasit. Vctors. 2014;7:509. doi: 10.1186/s13071-014-0509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krause P.J. Human babesiosis. Int. J. Parasitol. 2019 doi: 10.1016/j.ijpara.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Menis M., Forshee R.A., Kumar S., McKean S., Warnock R., Izurieta H.S., Gondalia R., Johnson C., Mintz P.D., Walderhaug M.O., et al. Babesiosis Occurrence among the Elderly in the United States, as Recorded in Large Medicare Databases during 2006–2013. PLoS ONE. 2015;10:e0140332. doi: 10.1371/journal.pone.0140332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arsuaga M., González L.M., Padial E.S., Dinkessa A.W., Sevilla E., Trigo E., Puente S., Gray J., Montero E. Misdiagnosis of Babesiosis as Malaria, Equatorial Guinea, 2014. Emerg. Infect. Dis. 2018;24:1588. doi: 10.3201/eid2408.180180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y., Li S.-G., Jiang J.-F., Wang X., Zhang Y., Wang H., Cao W.-C. Babesia venatorum Infection in Child, China. Emerg. Infect. Dis. 2014;20:896–897. doi: 10.3201/eid2005.121034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang J.-F., Zheng Y.-C., Jiang R.-R., Li H., Huo Q.-B., Jiang B.-G., Sun Y., Jia N., Wang Y.-W., Ma L., et al. Epidemiological, clinical, and laboratory characteristics of 48 cases of “Babesia venatorum” infection in China: A descriptive study. Lancet. Inf. Dis. 2015;15:196–203. doi: 10.1016/S1473-3099(14)71046-1. [DOI] [PubMed] [Google Scholar]
- 11.Jia N., Zheng Y.-C., Jiang J.-F., Jiang R.-R., Jiang B.-G., Wei R., Liu H.-B., Huo Q.-B., Sun Y., Chu Y.-L., et al. Human Babesiosis Caused by a Babesia crassa-like Pathogen: A Case Series. Clin. Infect. Dis. 2018:ciy212. doi: 10.1093/cid/ciy212. [DOI] [PubMed] [Google Scholar]
- 12.Man S.-Q., Qiao K., Cui J., Feng M., Fu Y.-F., Cheng X.-J. A case of human infection with a novel Babesia species in China. Infect. Dis. Poverty. 2016;5:28. doi: 10.1186/s40249-016-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou X., Li S.-G., Wang J.-Z., Huang J.-l., Zhou H.-J., Chen J.-H., Zhou X.-N. Emergence of human babesiosis along the border of China with Myanmar: Detection by PCR and confirmation by sequencing. Emerg. Microbes Infect. 2014;3:e55. doi: 10.1038/emi.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang S., Zhang L., Yao L., Li J., Chen H., Ni Q., Pan C., Jin L. Human babesiosis in Southeast China: A case report. Int. J. Inf. Dis. 2018;68:36–38. doi: 10.1016/j.ijid.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Wang H., Huang F. Babesia Infection in the Southwest of China, A Case Report. Jundishapur J. Microbiol. 2014;7:e13504. doi: 10.5812/jjm.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qi C., Zhou D., Liu J., Cheng Z., Zhang L., Wang L., Wang Z., Yang D., Wang S., Chai T. Detection of Babesia divergens using molecular methods in anemic patients in Shandong Province, China. Parasitol Res. 2011;109:241–245. doi: 10.1007/s00436-011-2382-8. [DOI] [PubMed] [Google Scholar]
- 17.Kim J.-Y., Cho S.-H., Joo H.-N., Tsuji M., Cho S.-R., Park I.-J., Chung G.-T., Ju J.-W., Cheun H.-I., Lee H.-W., et al. First Case of Human Babesiosis in Korea: Detection and Characterization of a Novel Type of Babesia sp. (KO1) Similar to Ovine Babesia. J. Clin. Microbiol. 2007;45:2084–2087. doi: 10.1128/JCM.01334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Q., Tsuji M., Zamoto A., Kohsaki M., Matsui T., Shiota T., Telford S.R., 3rd, Ishihara C. Human babesiosis in Japan: Isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J. Clin. Microbiol. 2001;39:2178–2183. doi: 10.1128/JCM.39.6.2178-2183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong S.H., Anu D., Jeong Y.I., Abmed D., Cho S.H., Lee W.J., Lee S.E. Molecular detection and seroprevalence of Babesia microti among stock farmers in Khutul City, Selenge Province, Mongolia. Korean J. Parasitol. 2014;52:443–447. doi: 10.3347/kjp.2014.52.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foppa I.M., Krause P.J., Spielman A., Goethert H., Gern L., Brand B., Telford S.R., 3rd Entomologic and serologic evidence of zoonotic transmission of Babesia microti, eastern Switzerland. Emerg. Infect. Dis. 2002;8:722–726. doi: 10.3201/eid0807.010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paparini A., Senanayake S.N., Ryan U.M., Irwin P.J. Molecular confirmation of the first autochthonous case of human babesiosis in Australia using a novel primer set for the beta-tubulin gene. Exp. Parasitol. 2014;141:93–97. doi: 10.1016/j.exppara.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Ramharter M., Walochnik J., Lagler H., Winkler S., Wernsdorfer W.H., Stoiser B., Graninger W. Clinical and Molecular Characterization of a Near Fatal Case of Human Babesiosis in Austria. J. Travel Med. 2010;17:416–418. doi: 10.1111/j.1708-8305.2010.00446.x. [DOI] [PubMed] [Google Scholar]
- 23.Herwaldt B.L., Caccio S., Gherlinzoni F., Aspock H., Slemenda S.B., Piccaluga P., Martinelli G., Edelhofer R., Hollenstein U., Poletti G., et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect. Dis. 2003;9:942–948. doi: 10.3201/eid0908.020748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blum S., Gattringer R., Haschke E., Walochnik J., Tschurtschenthaler G., Lang F., Oberbauer R. The Case | Hemolysis and acute renal failure. Kidney Int. 2011;80:681–683. doi: 10.1038/ki.2011.184. [DOI] [PubMed] [Google Scholar]
- 25.Gorenflot A., Moubri K., Precigout E., Carcy B., Schetters T.P. Human babesiosis. Ann. Trop Med. Parasitol. 1998;92:489–501. doi: 10.1080/00034983.1998.11813307. [DOI] [PubMed] [Google Scholar]
- 26.Strizova Z., Havlova K., Patek O., Smrz D., Bartunkova J. The first human case of babesiosis mimicking Reiter’s syndrome. Folia Parasitol. 2020;67:031. doi: 10.14411/fp.2020.031. [DOI] [PubMed] [Google Scholar]
- 27.Karita H., Pekka S., Antti S., Heli S., Jokiranta T.S. Fatal Babesiosis in Man, Finland, 2004. Emerg. Infect. Dis. 2010;16:1116. doi: 10.3201/eid1607.091905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinot M., Zadeh M.M., Hansmann Y., Grawey I., Christmann D., Aguillon S., Jouglin M., Chauvin A., Briel D.D. Babesiosis in Immunocompetent Patients, Europe. Emerg. Infect. Dis. 2011;17:114. doi: 10.3201/eid1701.100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hildebrandt A., Hunfeld K.P., Baier M., Krumbholz A., Sachse S., Lorenzen T., Kiehntopf M., Fricke H.J., Straube E. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2007;26:595–601. doi: 10.1007/s10096-007-0333-1. [DOI] [PubMed] [Google Scholar]
- 30.Häselbarth K., Tenter A.M., Brade V., Krieger G., Hunfeld K.-P. First case of human babesiosis in Germany–Clinical presentation and molecular characterisation of the pathogen. Int. J. Med. Microbiol. 2007;297:197–204. doi: 10.1016/j.ijmm.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Mørch K., Holmaas G., Frolander P.S., Kristoffersen E.K. Severe human Babesia divergens infection in Norway. Int. J. Inf. Dis. 2015;33:37–38. doi: 10.1016/j.ijid.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 32.Kukina I.V., Guzeeva T.M., Zelya O.P., Ganushkina L.A. Fatal human babesiosis caused by Babesia divergens in an asplenic host. IDCases. 2018;13:e00414. doi: 10.1016/j.idcr.2018.e00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strasek-Smrdel K., Korva M., Pal E., Rajter M., Skvarc M., Avsic-Zupanc T. Case of Babesia crassa-Like Infection, Slovenia, 2014. Emerg. Infect. Dis. 2020;26:1038–1040. doi: 10.3201/eid2605.191201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arsuaga M., Gonzalez L.M., Lobo C.A., de la Calle F., Bautista J.M., Azcarate I.G., Puente S., Montero E. First Report of Babesia microti-Caused Babesiosis in Spain. Vector Borne Zoonotic Dis. 2016;16:677–679. doi: 10.1089/vbz.2016.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González L.M., Castro E., Lobo C.A., Richart A., Ramiro R., González-Camacho F., Luque D., Velasco A.C., Montero E. First report of Babesia divergens infection in an HIV patient. Int. J. Inf. Dis. 2015;33:202–204. doi: 10.1016/j.ijid.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez L.M., Rojo S., Gonzalez-Camacho F., Luque D., Lobo C.A., Montero E. Severe Babesiosis in Immunocompetent Man, Spain, 2011. Emerg. Infect. Dis. 2014;20:724. doi: 10.3201/eid2004.131409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svensson J., Hunfeld K.-P., Persson K.E.M. High seroprevalence of Babesia antibodies among Borrelia burgdorferi-infected humans in Sweden. Ticks Tick-Borne Dis. 2018 doi: 10.1016/j.ttbdis.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Tanyel E., Guler N., Hokelek M., Ulger F., Sunbul M. A case of severe babesiosis treated successfully with exchange transfusion. Int. J. Inf. Dis. 2015;38:83–85. doi: 10.1016/j.ijid.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 39.Bullard J.M.P., Ahsanuddin A.N., Perry A.M., Lindsay L.R., Iranpour M., Dibernardo A., Van Caeseele P.G. The first case of locally acquired tick-borne Babesia microti infection in Canada. Can. J. Inf. Dis. Med. Microbiol. 2014;25:e87–e89. doi: 10.1155/2014/209521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott J.D. First record of locally acquired human babesiosis in Canada caused by Babesia duncani: A case report. SAGE Open Med. Case Rep. 2017;5:2050313×17725645. doi: 10.1177/2050313×17725645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott J., Scott C. Human Babesiosis Caused by Babesia duncani Has Widespread Distribution across Canada. Healthcare. 2018;6:49. doi: 10.3390/healthcare6020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaspar P.-L., Lucero B., Carlos P.-O., Claudia M.-Z. Human Babesiosis, Yucatán State, Mexico, 2015. Emerg. Infect. Dis. 2018;24:2061. doi: 10.3201/eid2411.170512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swei A., O’Connor K.E., Couper L.I., Thekkiniath J., Conrad P.A., Padgett K.A., Burns J., Yoshimizu M.H., Gonzales B., Munk B., et al. Evidence for transmission of the zoonotic apicomplexan parasite Babesia duncani by the tick Dermacentor albipictus. Int. J. Parasitol. 2018 doi: 10.1016/j.ijpara.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prince H.E., Lapé-Nixon M., Patel H., Yeh C. Comparison of the Babesia duncani (WA1) IgG Detection Rates among Clinical Sera Submitted to a Reference Laboratory for WA1 IgG Testing and Blood Donor Specimens from Diverse Geographic Areas of the United States. Clin. Vaccine Immunol. 2010;17:1729–1733. doi: 10.1128/CVI.00256-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Primus S., Akoolo L., Schlachter S., Gedroic K., Rojtman A.D., Parveen N. Efficient detection of symptomatic and asymptomatic patient samples for Babesia microti and Borrelia burgdorferi infection by multiplex qPCR. PLoS ONE. 2018;13:e0196748. doi: 10.1371/journal.pone.0196748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herwaldt B.L., de Bruyn G., Pieniazek N.J., Homer M., Lofy K.H., Slemenda S.B., Fritsche T.R., Persing D.H., Limaye A.P. Babesia divergens-like infection, Washington State. Emerg. Infect. Dis. 2004;10:622–629. doi: 10.3201/eid1004.030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beattie J.F., Michelson M.L., Holman P.J. Acute Babesiosis Caused by Babesia divergens in a Resident of Kentucky. N. Engl. J. Med. 2002;347:697–698. doi: 10.1056/NEJM200208293470921. [DOI] [PubMed] [Google Scholar]
- 48.Herc E., Pritt B., Huizenga T., Douce T., Hysell M., Newton D., Sidge J., Losman E., Sherbeck J., Kaul D.R. Probable Locally Acquired Babesia divergens–Like Infection in Woman, Michigan, USA. Emerg. Infect. Dis. 2018;24:1558. doi: 10.3201/eid2408.180309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herwaldt B., Persing D.H., Precigout E.A., Goff W.L., Mathiesen D.A., Taylor P.W., Eberhard M.L., Gorenflot A.F. A fatal case of babesiosis in Missouri: Identification of another piroplasm that infects humans. Ann. Intern. Med. 1996;124:643–650. doi: 10.7326/0003-4819-124-7-199604010-00004. [DOI] [PubMed] [Google Scholar]
- 50.Gabrielli S., Totino V., Macchioni F., Zuniga F., Rojas P., Lara Y., Roselli M., Bartoloni A., Cancrini G. Human Babesiosis, Bolivia, 2013. Emerg. Infect. Dis. 2016;22:1445–1447. doi: 10.3201/eid2208.150195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al Zoubi M., Kwak T., Patel J., Kulkarni M., Kallal C.A. Atypical challenging and first case report of babesiosis in Ecuador. IDCases. 2016;4:15–17. doi: 10.1016/j.idcr.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tijsse-Klasen E., Jacobs J.J., Swart A., Fonville M., Reimerink J.H., Brandenburg A.H., van der Giessen J.W., Hofhuis A., Sprong H. Small risk of developing symptomatic tick-borne diseases following a tick bite in The Netherlands. Parasit Vectors. 2011;4:17. doi: 10.1186/1756-3305-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michelet L., Delannoy S., Devillers E., Umhang G., Aspan A., Juremalm M., Chirico J., van der Wal F.J., Sprong H., Boye Pihl T.P., et al. High-throughput screening of tick-borne pathogens in Europe. Front. Cell Infect. Microbiol. 2014;4:103. doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Øines Ø., Radzijevskaja J., Paulauskas A., Rosef O. Prevalence and diversity of Babesia spp. in questing Ixodes ricinus ticks from Norway. Parasit. Vectors. 2012;5:156. doi: 10.1186/1756-3305-5-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonnet S., Brisseau N., Hermouet A., Jouglin M., Chauvin A. Experimental in vitro transmission of Babesia sp. (EU1) by Ixodes ricinus. Vet. Res. 2009;40:21. doi: 10.1051/vetres/2009004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oechslin C.P., Heutschi D., Lenz N., Tischhauser W., Péter O., Rais O., Beuret C.M., Leib S.L., Bankoul S., Ackermann-Gäumann R. Prevalence of tick-borne pathogens in questing Ixodes ricinus ticks in urban and suburban areas of Switzerland. Parasit. Vectors. 2017;10:558. doi: 10.1186/s13071-017-2500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Otranto D., Dantas-Torres F., Giannelli A., Latrofa M.S., Cascio A., Cazzin S., Ravagnan S., Montarsi F., Zanzani S.A., Manfredi M.T., et al. Ticks infesting humans in Italy and associated pathogens. Parasit. Vectors. 2014;7:328. doi: 10.1186/1756-3305-7-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Becker C.A.M., Bouju-Albert A., Jouglin M., Chauvin A., Malandrin L. Natural Transmission of Zoonotic Babesia spp. by Ixodes ricinus Ticks. Emerg. Infect. Dis. 2009;15:320–322. doi: 10.3201/eid1502.081247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venclikova K., Mendel J., Betasova L., Hubalek Z., Rudolf I. First evidence of Babesia venatorum and Babesia capreoli in questing Ixodes ricinus ticks in the Czech Republic. Ann. Agric. Environ. Med. 2015;22:212–214. doi: 10.5604/12321966.1152067. [DOI] [PubMed] [Google Scholar]
- 60.Tuvshintulga B., Battsetseg B., Battur B., Myagmarsuren P., Narantsatsral S., Sivakumar T., Takemae H., Igarashi I., Inoue N., Yokoyama N. First detection of Babesia venatorum (EU1) in Ixodes persulcatus ticks in Mongolia. J. Protozool. Res. 2015;25:29–37. [Google Scholar]
- 61.Karnath C., Obiegala A., Speck S., Essbauer S., Derschum H., Scholz H., Kiefer D., Tserennorov D., Dashdavaa O., Tsogbadrakh N., et al. Detection of Babesia venatorum, Anaplasma phagocytophilum and Candidatus Neoehrlichia mikurensis in Ixodes persulcatus ticks from Mongolia. Ticks Tick-Borne Dis. 2016;7:357–360. doi: 10.1016/j.ttbdis.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 62.Mancini F., Di Luca M., Toma L., Vescio F., Bianchi R., Khoury C., Marini L., Rezza G., Ciervo A. Prevalence of tick-borne pathogens in an urban park in Rome, Italy. Ann. Agric. Environ. Med. 2014;21:723–727. doi: 10.5604/12321966.1129922. [DOI] [PubMed] [Google Scholar]
- 63.Aureli S., Galuppi R., Ostanello F., Foley J.E., Bonoli C., Rejmanek D., Rocchi G., Orlandi E., Tampieri M.P. Abundanceof questing ticks and molecular evidence for pathogens in ticks in three parks of Emilia-Romagnaregion of Northern Italy. Ann. Agric. Environ. Med. 2015;22:459–466. doi: 10.5604/12321966.1167714. [DOI] [PubMed] [Google Scholar]
- 64.Stanczak J., Cieniuch S., Lass A., Biernat B., Racewicz M. Detection and quantification of Anaplasma phagocytophilum and Babesia spp. in Ixodes ricinus ticks from urban and rural environment, northern Poland, by real-time polymerase chain reaction. Exp. Appl. Acarol. 2015;66:63–81. doi: 10.1007/s10493-015-9887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamšíková Z., Kazimírová M., Haruštiaková D., Mahríková L., Slovák M., Berthová L., Kocianová E., Schnittger L. Babesia spp. in ticks and wildlife in different habitat types of Slovakia. Parasit. Vectors. 2016;9:292. doi: 10.1186/s13071-016-1560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reis C., Cote M., Paul R.E., Bonnet S. Questing ticks in suburban forest are infected by at least six tick-borne pathogens. Vector Borne Zoonotic Dis. 2011;11:907–916. doi: 10.1089/vbz.2010.0103. [DOI] [PubMed] [Google Scholar]
- 67.Wang J.L., Li T.T., Liu G.H., Zhu X.Q., Yao C. Two Tales of Cytauxzoon felis Infections in Domestic Cats. Clin. Microbiol. Rev. 2017;30:861–885. doi: 10.1128/CMR.00010-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bloch E.M., Yang Y., He M., Tonnetti L., Liu Y., Wang J., Guo Y., Li H., Leiby D.A., Shan H., et al. A pilot serosurvey of Babesia microti in Chinese blood donors. Vox Sang. 2018 doi: 10.1111/vox.12648. [DOI] [PubMed] [Google Scholar]
- 70.Gabrielli S., Otasevic S., Ignjatovic A., Savic S., Fraulo M., Arsic-Arsenijevic V., Momcilovic S., Cancrini G. Canine Babesioses in Noninvestigated Areas of Serbia. Vector Borne Zoonotic Dis. 2015;15:535–538. doi: 10.1089/vbz.2015.1797. [DOI] [PubMed] [Google Scholar]
- 71.Abdullah S., Helps C., Tasker S., Newbury H., Wall R. Prevalence and distribution of Borrelia and Babesia species in ticks feeding on dogs in the U.K. Med. Vet. Entomol. 2018;32:14–22. doi: 10.1111/mve.12257. [DOI] [PubMed] [Google Scholar]
- 72.Inokuma H., Yoshizaki Y., Shimada Y., Sakata Y., Okuda M., Onishi T. Epidemiological Survey of Babesia Species in Japan Performed with Specimens from Ticks Collected from Dogs and Detection of New Babesia DNA Closely Related to Babesia odocoilei and Babesia divergens DNA. J. Clin. Microbiol. 2003;41:3494–3498. doi: 10.1128/JCM.41.8.3494-3498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Król N., Obiegala A., Pfeffer M., Lonc E., Kiewra D. Detection of selected pathogens in ticks collected from cats and dogs in the Wrocław Agglomeration, South-West Poland. Parasit. Vectors. 2016;9:351. doi: 10.1186/s13071-016-1632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lempereur L., De Cat A., Caron Y., Madder M., Claerebout E., Saegerman C., Losson B. First molecular evidence of potentially zoonotic Babesia microti and Babesia sp. EU1 in Ixodes ricinus ticks in Belgium. Vector. Borne Zoonotic Dis. 2011;11:125–130. doi: 10.1089/vbz.2009.0189. [DOI] [PubMed] [Google Scholar]
- 75.Potkonjak A., Gutiérrez R., Savić S., Vračar V., Nachum-Biala Y., Jurišić A., Kleinerman G., Rojas A., Petrović A., Baneth G., et al. Molecular detection of emerging tick-borne pathogens in Vojvodina, Serbia. Ticks Tick-Borne Dis. 2016;7:199–203. doi: 10.1016/j.ttbdis.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 76.Schreiber C., Krücken J., Beck S., Maaz D., Pachnicke S., Krieger K., Gross M., Kohn B., von Samson-Himmelstjerna G. Pathogens in ticks collected from dogs in Berlin/Brandenburg, Germany. Parasit Vectors. 2014;7:535. doi: 10.1186/s13071-014-0535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith F.D., Wall L.E. Prevalence of Babesia and Anaplasma in ticks infesting dogs in Great Britain. Vet. Parasitol. 2013;198:18–23. doi: 10.1016/j.vetpar.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 78.Stensvold C.R., Al Marai D., Andersen L.O.B., Krogfelt K.A., Jensen J.S., Larsen K.S., Nielsen H.V. Babesia spp. and other pathogens in ticks recovered from domestic dogs in Denmark. Parasit. Vectors. 2015;8:262. doi: 10.1186/s13071-015-0843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zygner W., Baska P., Winsniewski M., Halina W. The Molecular Evidence of Babesia microti in Hard Ticks Removed from Dogs in Warsaw (central Poland) Pol. J. Microbiol. 2010;59:95–97. doi: 10.33073/pjm-2010-014. [DOI] [PubMed] [Google Scholar]
- 80.Chan J.M., Tsang K.Y., Chik T., Leung W.S., Tsang O.T. Babesiosis acquired from a pet dog: A second reported case in Hong Kong. Hong Kong Med. J. 2016;22:393–395. doi: 10.12809/hkmj144390. [DOI] [PubMed] [Google Scholar]
- 81.El-Bahnasawy M.M., Khalil H.H., Morsy T.A. Babesiosis in an Egyptian boy aquired from pet dog, and a general review. J. Egypt Soc. Parasitol. 2011;41:99–108. [PubMed] [Google Scholar]
- 82.Klevens R.M., Cumming M.A., Caten E., Stramer S.L., Townsend R.L., Tonnetti L., Rios J., Young C.T., Soliva S., DeMaria A. Transfusion-transmitted babesiosis: One state’s experience. Transfusion. 2018;58:2611–2616. doi: 10.1111/trf.14943. [DOI] [PubMed] [Google Scholar]
- 83.Iyer S., Goodman K. Congenital Babesiosis from Maternal Exposure: A Case Report. J. Emerg. Med. 2019 doi: 10.1016/j.jemermed.2018.12.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.