Abstract
Background
A serology testing for infectious diseases (HIV, hepatitis B and C, syphilis) is mandatory in tissue donors. In many donors postmortem blood is the only sample available. Even though serological tests and nucleic acid amplification tests (NAT) used are validated for postmortem blood, a characterization of those blood samples is not yet established. We therefore investigated the total immunoglobulin G (IgG) content in postmortem blood of tissue donors and compared it to a corresponding antemortem blood sample.
Methods
Ante- and postmortem blood samples were obtained from 100 consecutive tissue donors. The total IgG of all samples was measured using an immune-turbidometric test on the AU 480 Chemistry Analyzer (Beckman Coulter).
Results
The mean total IgG concentration of antemortem blood samples from all 100 donors was 8.9 g/L ± 3.7 g/L (median 8.9 g/L, range 1.5 to 23.8 g/L). In 80 donors the IgG concentration in the antemortem blood sample was within the normal range with values ≥6 g/L (mean 10.0 g/L ± 3.3 g/L, median 9.3 g/L,). The total IgG concentration of the postmortem blood samples was lower with 7.2 g/L ± 3.2 g/L (median 6.7 g/L, range 0.6 to 18.2 g/L). The difference between the values of the antemortem and postmortem blood samples was 1.7 g/L ± 2.6 g/L (16.3%) (median 1.6 g/L, range −7.7 to 10.1 g/L). In 36 donors this difference was less than 10%, in 23 it was between 10 and 25%, in 33 between 26 and 50%, and in 8 over 50%. In 57 donors the total IgG in the postmortem blood sample was within the normal range with ≥6 g/L, in 53 of them also the value of the antemortem blood sample was within the normal range. No correlation for total IgG was found regarding the donor characteristics (age, sex, disease) and the sample characteristics (hemolysis, postmortem time).
Conclusion
Total IgG values in antemortem samples were below the lower limit of 6 g/L in 20% of the cases. Total IgG was significantly lower in the postmortem samples compared to the antemortem samples, while 57% were still above the lower limit. No correlation with the postmortem time could be found. This lowered IgG levels should be payed attention to when using postmortem blood for infectious serology testing. Additional NAT testing should be considered.
Keywords: Postmortem blood, Immunoglobulin, Donor screening, Tissue donation, Tissue bank
Introduction
The demand for tissue transplants is still far behind meeting the needs. For example, Gain et al. [1] showed in their global survey in 2016 that only 1 cornea can be provided for 70 needed. With an aging population in developed countries, the need for corneas and other tissue transplants is expected to continue to grow. Last but not least, improved surgical techniques with better postoperative functional results and thus an extended indication, such as Descemet membrane endothelial keratoplasty, play a major role in the increasing demand [2].
In order to minimize the risk of transmitting a disease from the donor to the recipient of a tissue transplant, the donor's medical history is reviewed by a physician, physical examination is performed, and infectious serological testing is carried out. According to current guidelines, at least the determination of antibodies against the human immunodeficiency virus (anti-HIV-1 and −2), the hepatitis B and C virus (anti-HBc and anti-HCV) as well as against Treponema pallidum (TPHA and TPPA) is carried out. In addition, the donor blood is tested for the surface antigen of the hepatitis B virus (HBsAg). Furthermore, testing for hepatitis C virus RNA is mandatory in Germany [3]. In addition, it is not uncommon for tissue donors to be tested for hepatitis B, hepatitis A, and HIV by nucleic acid amplification test (NAT), in analogy to blood donors, before the donated tissue is released for transplantation [4]. On the one hand, this is done to avoid a second serological blood test of the donor who donates tissue during lifetime (e.g., placenta for the extraction of amniotic membrane, femoral head donation in the context of a femoral head endoprosthesis), which is mandatory due to the diagnostic window of these infectious diseases, as this is associated with a certain amount of effort and would also mean a second blood sample collection from the tissue donor. On the other hand for tissue donors who donate tissue after their death (e.g., cornea, bones), NAT testing is also useful, since severe diseases and their treatment, e.g., malignancies, autoimmune diseases, or septicemia, of the donor can lead to immunosuppression and therefore a false negative serological test result. In order not to have to make an individual medical decision in the sense of a risk assessment, many tissue establishments carry out an extended diagnosis by means of NAT as standard.
The use of postmortem blood samples for infectious serology and NAT testing is permitted, as long as the tests used for this purpose are validated accordingly and the postmortem blood sample was obtained within a certain time window after death. In the member states of the European Union this time is limited to a maximum of 24 h. Although scientific studies have been taken into account in setting this 24-hour period, there are studies that could allow a significantly longer period for obtaining postmortem blood samples [5]. While the infectious serological testing of live blood samples in clinical routine, e.g., in the testing of blood donors, has been firmly established for many years with automated and validated procedures, the testing of postmortem blood samples continues to be rather the exception. Against the background that false negative test results must strictly be avoided, the establishment of the 24-hour limit for postmortem blood samples can certainly be seen as a regulatory compromise decision. Unfortunately, this results in a relevant loss of tissue donors for whom neither a live blood sample is available nor a blood sample can be obtained within 24 h after death. In the background of a significant shortage of tissue transplants described above, this is a relevant problem in the recruitment of tissue donors in many countries. It would therefore be desirable to have one or more objective laboratory parameters that would allow the suitability of a postmortem blood sample to be determined independently of time. Immunoglobulins are protein molecules with antibody activity and are produced by the terminal cells of B-cell differentiation known as “plasma cells.” There are five classes of immunoglobulin: immunoglobulin G (IgG), IgM, IgA, IgD, and IgE. In normal serum, about 80% is IgG, 15% is IgA, 5% is IgM, 0.2% is IgD, and a trace is IgE [6]. The infectious serology testing is targeting especially IgG. Therefore, this study was performed to determine and compare the total IgG in ante- and postmortem blood samples from the same tissue donor.
Materials and Methods
From January to June 2020, ante- and postmortem blood samples could be obtained from 100 consecutive tissue donors from the university tissue bank of the Charité − Universitätsmedizin Berlin. For some of the donors, a second postmortem blood sample was obtained at a later date.
Antemortem Blood Samples
The antemortem blood samples are samples that are examined in clinical diagnostics − the tissue donors were all hospitalized before their death − and afterwards routinely kept refrigerated at 4 ± 2°C for several days in the laboratory (Labor Berlin GmbH, Berlin, Germany). These samples are EDTA and lithium heparin blood samples (BD Vacutainer®, Becton, Dickinson and Company, Franklin Lakes, NJ, USA), which are stored upright after centrifugation and testing. At the time of tissue donation, these samples were collected from the laboratory, transported refrigerated, and centrifuged again at 5,000 rpm for 5 min. The heparin or EDTA plasma was transferred into a sterile tube without additives (BD Vacutainer®) and stored below −20°C until the determination of total IgG, which in the majority of cases was performed together with the infectious serological testing. The samples were stored frozen for 1 to 4 days and thawed at room temperature on the morning of the day of measurement. For all samples, the time between blood collection and preparation in the university tissue bank was recorded in hours. This time corresponds to the antemortem time of these blood samples.
Postmortem Blood Samples
The postmortem blood samples were taken at the time of tissue explantation. The subclavian vein was punctured with a large-lumen cannula. Postmortem coagulation and sedimentation phenomena of the blood usually occur. As a result, it is not uncommon to obtain serum and not whole blood postmortem. Therefore, it cannot be excluded that pleural effusion is aspirated and mistaken for serum. In this study, therefore, only donors were included in whom it was certain that the blood or serum could be obtained by means of the collection technique (e.g., depth of puncture). The blood samples were taken by experienced and qualified staff of the university tissue bank.
The blood was then transferred into serum tubes (S-Monovette® Serum Gel, Nümbrecht, Germany) and transported cooled together with the donor tissues to the university tissue bank. There, centrifugation and storage were performed, analogous to the antemortem blood samples. The samples were stored in frozen condition for 7 to 30 days and thawed at room temperature on the morning of the day of measurement. For all samples, the time between patient death (irreversible cardiac arrest) and preparation in the university tissue bank was recorded in hours. This time corresponds to the postmortem time of these blood samples. The collection of the second postmortem blood sample and its preparation took place at a later time, otherwise identical as described above. The hemolysis of the postmortem blood sample was classified by an experienced staff member by visual inspection of the supernatant after centrifugation into three grades: no (1), low to moderate (2), severe hemolysis (3).
IgG Measurement
Total IgG was determined in the laboratory for infectious serology of the Center for Transfusion Medicine and Cell Therapy Berlin (ZTB − Zentrum für Transfusionsmedizin und Zelltherapie Berlin, Germany) at the AU480 Chemistry Analyzer (Beckman Coulter Ireland Inc., Clare, Ireland) with reagents of the test specification OSR64472. The principle is an immune-turbidometric test for the quantitative determination of IgG in human serum or plasma (EDTA or heparinized). Human IgG reacts specifically with anti-human IgG antibodies to yield insoluble aggregates. The absorbance of these aggregates is proportional to the IgG concentration in the sample.
Tissue Donors
The 100 donors included in this study were postmortem tissue donors who all donated cornea and in 6 cases also bone. For all donors, medical contraindications for tissue donation were excluded before explantation. These include the presence of a hematological neoplasia (e.g., leukemia, lymphoma), a hemodilution of more than 50% by transfusion or infusion, the presence or increased risk of transmissible infectious diseases, neurodegenerative diseases of unclear etiology, and unclear cause of death or underlying diseases. Carcinoma and septicemia are no contraindications for cornea donation.
For this study, the causes of death of tissue donors were divided into the following categories:
pulmonary/respiratory (e.g., pneumonia),
cardiac/cardiocirculatory (e.g., heart failure, cardiovascular failure, myocardial infarction),
cerebral (e.g., apoplex, intracerebral hemorrhage),
multi-organ failure (e.g., metastatic carcinoma),
and sepsis.
In addition, an assessment was made for a possible disease-related immunosuppression: presence of a carcinoma, chemotherapy administered in the last 3 months, or presence of an autoimmune disease treated with immunosuppressive drugs.
Statistical Analysis
The data were collected for analysis using Excel and evaluated for descriptive statistics. All statistical analysis was performed using R 3.63 (ggplot2 for plotting), SPSS 26, and SAS 9.4M5.
To compare the mean difference of the samples we used the independent samples t test as well as the paired sample t test. The significance level was set to 0.05, which means p values ≤0.05 were considered to be significant. The data was generally corrected for outliers. The assumption of normality was tested by Shapiro-Wilk (p > 0.05). In cases were the assumption of normality was violated, we checked whether the sample size was big enough to compensate for that − in order to reach an approximate normal distribution of the sample mean. The assumption of homogeneity of variances was tested with Levene's test for equality of variances (p ≤ 0.05). We calculated the exact p values and confidence intervals (CI) with corresponding t values.
In order to investigate the effect of time (postmortem time of measurement) on the size of difference in IgG we used a linear regression model. In general, the assumption of homoscedasticity was checked via a scatterplot of standardized predicted values versus standardized residuals. Whether residuals were normally distributed was assessed by checking the corresponding Q-Q plot. Model fit was assessed via R2 and outlier detection was based on Cook's distance.
The difference was defined as “difference IgG (antemortem) − IgG (postmortem)” and consequently a positive difference meant a reduction and a negative difference an increase in the IgG level. More precisely, we used a (simple) linear regression model: “difference” was the dependent while time (postmortem time of measurement) was the (only) independent variable.
We tested this model based on four different pre-selections of the samples (1st: consider all 100 samples, given the first postmortem time; 2nd: consider only non-negative differences, given the first postmortem time; 3rd: consider all 100 samples, given the first and where possible the second postmortem time; 4th: consider only non-negative differences, given the first and where possible the second postmortem time).
Results
The donor characteristics are shown in Table 1. The mean total IgG concentration of antemortem blood samples from all 100 donors was 8.9 ± 3.7 g/L (median 8.9 g/L, range 1.5 to 23.8 g/L). In 80 donors the IgG concentration in the antemortem blood sample was above the lower reference value of ≥6 g/L (mean 10.0 ± 3.3 g/L, median 9.3 g/L). In 20 donors the mean value was reduced with 4.5 ± 1.2 g/L (median 4.8 g/L). There was no correlation between the IgG values and the antemortem time of these blood samples. In the blood samples with IgG values above the lower reference value the mean antemortem time was 46.7 ± 28.6 h (median 40.5 h), while in the blood samples with diminished total IgG value it was 36.3 ± 28 h (median 33 h).
Table 1.
Donor characteristics (n = 100)
| Age, years | |
| Mean ± SD | 75.2±9.3 |
| Median (IQR) | 77.0 (69–82.5) |
| Postmortem time, h | |
| Mean ± SD | 25.9±12.3 |
| Median (range) | 24 (6–64) |
| Sex | |
| Female | 32 |
| Male | 68 |
| Cause of death | |
| Pulmonal/respiratory | 26 |
| Cardiac/cardiocirculatory | 23 |
| Cerebral | 14 |
| Multi-organ failure | 12 |
| Septicemia | 25 |
| Carcinoma | 43 |
| Chemotherapy up to 12 weeks before death | 9 |
| Autoimmune disease | 6 |
The mean total IgG concentration of the postmortem blood samples was 7.2 ± 3.2 g/L (median 6.7 g/L, range 0.6 to 18.2 g/L), which is significantly lower than in the antemortem blood samples (p < 0.0001, CI 1.18 to 2.24, t value = 6.46). The difference between the values of the antemortem and postmortem blood samples was on average 1.7 g/L ± 2.6 g/L (reduction by 16.3%) (median 1.6 g/L; range −7.7 to 10.1 g/L). In 36 donors this difference was less than 10%, in 23 it was between 10 and 25%, in 33 between 25 and 50%, and in 8 over 50%.
In 56 donors the total IgG in the postmortem blood sample was within the normal range with ≥6 g/L, in 53 of them the value of the antemortem blood sample was above the lower reference value. In the samples with normal IgG values the mean postmortem time was 24.8 ± 11.9 h (median 23.5 h, range 6 to 58 h). In the group of 44 donors with a reduced IgG value in the postmortem blood sample, the mean postmortem time was 27.1 ± 12.9 h (median 24 h, range 9 to 64 h). There was no statistically significant difference in the postmortem time (p = 0.143, CI −1.1 to 7.5, t value = 1.48).
Likewise, no correlation was found with the degree of hemolysis of the postmortem sample (in the group with normal values on average 1.9, in the group with reduced values 2.3).
In the group of 29 donors in whom a second postmortem blood sample was obtained at a mean interval of 26.9 h from the first postmortem sample, the mean total IgG value (i) in the antemortem blood sample was 8 ± 3.4 g/L (median 8.1 g/L), (ii) in the first postmortem blood sample was 6.9 ± 3.1 g/L (median 5.9 g/L), and (iii) in the second postmortem blood sample was 5.5 ± 2.7 g/L (median 5.4 g/L). In all cases the IgG concentration in the second postmortem blood sample was lower than in the first postmortem sample. This difference was on average 1.8 g/L (25.4%). However, no correlation with postmortem time could be found: in the 7 donors where the difference in total IgG between the first and second postmortem blood samples was less than 10%, the second sampling took place on average 44.6 ± 8.9 h after irreversible cardiac arrest, in the other 22 donors with more than 10% reduction in total IgG this was the case after 51.6 ± 19.4 h.
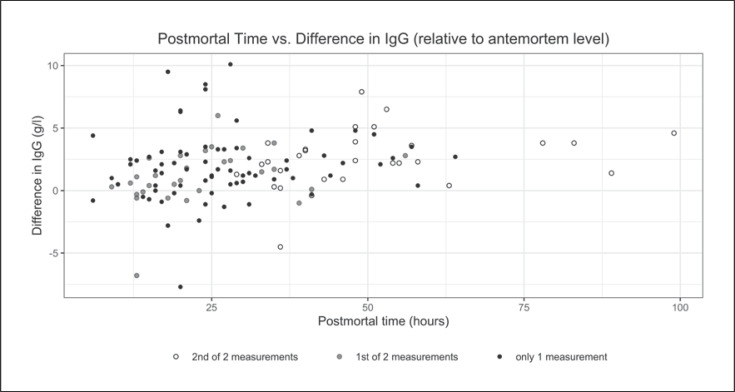
Figure 1 illustrates the distribution of IgG level differences (“difference IgG [antemortem] − IgG [postmortem]”) versus the postmortem time of all blood samples (scatter plot). According to the results of the 1st setting of our model, the postmortem time did not significantly predict the difference in IgG concentration (F(1,94) = 3.76, p = 0.056, R2 = 0.04). Table 2 compares the total IgG values of ante- and postmortem blood samples with donor characteristics such as age, cause of death, and the presence of carcinomas or autoimmune diseases. Multiple regression analysis to investigate these possible factors influencing the postmortem drop in IgG levels was not performed (a) since we were not able to establish a significant linear relationship between time and the difference in IgG and (b) due to the small number of cases in the respective groups (causes of death) and the considerable variance in IgG levels measured in the postmortem specimen.
Fig. 1.
Postmortem time (in hours) in terms of the difference between ante- and postmortem IgG levels (in g/L) for all samples. The results for the 71 donors with only one postmortem blood sample are shown with a black dot. For the 29 donors with two postmortem blood samples, the differences are displayed with a grey dot for the first sample and a white dot for the second sample.
Table 2.
Total IgG content in g/L (±SD)
| Antemortem blood | Postmortem blood | p value | |
|---|---|---|---|
| Total concentration | 8.9±3.7 | 7.2±3.2 | <0.0001 |
| Age | |||
| ≤60 years (n = 11) | 8.5±2.9 | 5.8±2.6 | <0.0002 |
| 60 to 80 years (n = 49) | 8.5±3.7 | 7.0±3.3 | <0.0007 |
| ≥80 years (n = 40) | 9.4±3.9 | 7.8±3.2 | <0.0001 |
| Cause of death | |||
| Pulmonary/respiratory (n = 26) | 9.2±3.5 | 8.0±3.4 | 0.005 |
| Cardiac/cardiocirculatory (n = 23) | 7.9±2.7 | 7.2±3.1 | >0.3 |
| Cerebral (n = 14) | 8.5±3.6 | 7.1±3.4 | <0.007 |
| Multi-organ failure (n = 12) | 11.7±5.2 | 8.2±3.1 | − |
| Septicemia (n = 25) | 8.3±3.6 | 5.9±2.8 | <0.0001 |
| Carcinoma | |||
| No carcinoma (n = 57) | 8.3±3.3 | 7.2±3.2 | <0.0008 |
| Carcinoma (n = 43) | 9.6±4.1 | 7.2±3.2 | <0.0001 |
| Chemotherapy up to 12 weeks before death (n = 9) | 9.6±4.2 | 8.0±3.7 | <0.022 |
| Autoimmune disease | |||
| No autoimmune disease (n = 94) | 8.9±3.7 | 7.2±3.3 | <0.0001 |
| Autoimmune disease (n = 6) | 9.0±4.4 | 7.4±2.0 | 0.2 |
As the antemortem blood samples were samples for routine clinical diagnostic, only EDTA and heparin plasma was available. No statistically significant difference for the type of antemortem plasma could be found comparing the IgG values in EDTA and heparin samples in total and in the groups with values < and ≥6 g/L (p = 0.128, CI −0.27 to 2.08, t value = 1.5; p = 0.632, CI −0.87 to 1.42, t value = 0.5; p = 0.515, CI −0.51 to 0.98, t value = 0.67).
Discussion
To the best of our knowledge, this is the first study that quantitatively examines and compares total IgG in ante- and postmortem blood samples from tissue donors. In 80 and thus the majority of the 100 donors examined, the value of the antemortem blood sample was within the normal range. In 53 of them, the value of the corresponding postmortem blood samples was also within the normal range. A correlation between donor characteristics and the age of the blood sample was not recognizable. We think it is therefore not possible to draw any reliable conclusions about the immunoglobulin value from the diseases of the donors, their age, sex, or age of the blood samples. The total IgG values were lower in the postmortem samples. In the donors in whom a second blood sample was obtained postmortem, the IgG concentration was further reduced. The postmortem time was not the decisive influencing factor. Consequently, there must be other influencing factors, but these remain unclear to us after this first examination.
The total IgG values of the examined tissue donors, who mainly died after a longer-lasting and severe course of a disease, are comparable to the distribution found in patients with severe sepsis or septic shock by Dietz et al. [7] and could therefore be considered representative for severely ill patients. The serum IgG levels of the septic patients in the work of Dietz et al. [7] were not correlated with mortality, which would suggest that we have not found a correlation with cause of death.
Freezing and storage at −20°C of the plasmas in our study most likely has no negative effect on the IgG level. Siennicka et al. [8] were able to show that even repeated freezing and thawing of serum samples up to 10 times had no negative effect on IgG stability.
Edler et al. [5] found in their 2011 study a reliable detection of IgG antibodies against HCV, HBV, and HIV up to 48 h postmortem without false negative test results. They therefore assumed a high stability of the corresponding immunoglobulins. This is in line with the results of our work with slightly more than 50% of the donors with normal IgG in the first postmortem blood sample after 24.8 h on average and the average value of 5.5 g/L close to the lower limit in a second postmortem blood sample after about 42 h.
In 2006 Challine et al. [9] described that the macroscopic aspect of postmortem blood samples is the best predictor of the specificity of serological testing in cornea donors. We found no correlation between the degree of hemolysis and the IgG value in postmortem blood samples. The phenomenon of false positive serological test results seems to be related with the degree of hemolysis, but not directly with the IgG content [9].
The antemortem blood samples used from the tissue donors came from the routine clinical laboratory, which mainly uses EDTA and heparin plasma for its diagnostics. Serum samples were therefore not available. The measurement method used to determine IgG is suitable and validated for the use of such materials. Accordingly, we found no statistically significant difference in IgG concentrations for these different plasma types. From the results of our study we conclude that the IgG level is basically a suitable parameter to assess the suitability of a blood sample for serological testing. If its total IgG value is within the normal range, specific fractions should also be detected with the infectious serology tests without any problems. If this value is lowered, also by hemodilution, it is more likely that specific IgG antibodies are present but below the detection limit of the test.
With regard to false negative serological test results, which must be strictly avoided in order to ensure the safety of the tissue transplant recipients, the average lowered total IgG in the postmortem blood sample compared to the antemortem value and the only 50% normal values in the donors in our study is remarkable. The problem of false negative serological test results and thus possible transmission is well known for hepatitis C in tissue donors [10, 11]. This is one of the reasons why the determination of HCV RNA by NAT is mandatory in tissue donors in Germany.
Due to the possible and unpredictable lowered total IgG levels in pre- and postmortem blood samples in our study, routine NAT testing for HCV, HBV, and HIV seems to be reasonable. Already in 2010, a review paper by Pruß et al. [12] came to the recommendation to test tissue donors more frequently by NAT. Even if antemortem blood samples remain the preferred method for donor testing, validated NAT systems are now also available for postmortem blood samples [13, 14].
We think that further studies are useful investigating the IgG content to find donor-related influencing factors, since the number of subjects in our study is rather low. Additionally the investigation of other parameters characterizing the protein content of the plasma sample seems to be of interest to us.
Statement of Ethics
An ethics approval was not required.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding.
Author Contributions
Gudrun Larscheid contributed to the conception of this study, analyzed the data of this study, and wrote the manuscript. Tino Schulz and Tina Trögel realized the acquisition of the blood samples and planning the tests. They also critically revised the manuscript. Sascha Eulert contributed to the conception of the statistical calculations of the study and conducted them. He also critically revised the manuscript. Axel Pruß and Hermann Herbst contributed to the conception of this study and the interpretation of the results and critically revised the manuscript. Jan Schroeter contributed to the conception and design of this study. He critically revised the analysis and interpretation of the data and the manuscript. All authors approved the final version of the manuscript for publication.
Acknowledgement
The authors would like to thank the staff of the Center for Transfusion Medicine and Cell Therapy Berlin (ZTB − Zentrum für Transfusionsmedizin und Zelltherapie Berlin), the Labor Berlin GmbH, and Sven Schurig for providing and processing the blood samples.
References
- 1.Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, et al. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016 Feb;134((2)):167–73. doi: 10.1001/jamaophthalmol.2015.4776. [DOI] [PubMed] [Google Scholar]
- 2.Flockerzi E, Maier P, Böhringer D, Reinshagen H, Kruse F, Cursiefen C, et al. all German Keratoplasty Registry Contributors Trends in Corneal Transplantation from 2001 to 2016 in Germany: A Report of the DOG-Section Cornea and its Keratoplasty Registry. Am J Ophthalmol. 2018 Apr;188:91–8. doi: 10.1016/j.ajo.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Schroeter J, Maier P, Bednarz J, Blüthner K, Quenzel M, Pruss A, et al. [Procedural guidelines. Good tissue practice for cornea banks] Ophthalmologe. 2009 Mar;106((3)):265–74. doi: 10.1007/s00347-008-1913-x. [DOI] [PubMed] [Google Scholar]
- 4.Heck E, Brown A, Cavanagh HD. Nucleic acid testing and tissue safety: an eye bank's five-year review of HIV and hepatitis testing for donor corneas. Cornea. 2013 Apr;32((4)):503–5. doi: 10.1097/ICO.0b013e3182653a7a. [DOI] [PubMed] [Google Scholar]
- 5.Edler C, Wulff B, Schröder A, Wilkemeyer I, Polywka S, Meyer T, et al. A prospective time-course study on serological testing for human immunodeficiency virus, hepatitis B virus and hepatitis C virus with blood samples taken up to 48 h after death. J Med Microbiol. 2011;60:920–26. doi: 10.1099/jmm.0.027763-0. [DOI] [PubMed] [Google Scholar]
- 6.Edler C, Stoop JW, Zegers BJM, Sander PC, Ballieux RE. Serum immunoglobulin levels in healthy children and adults. Clin Exp Immunol. 1969;4:101–12. [PMC free article] [PubMed] [Google Scholar]
- 7.Dietz S, Lautenschläger C, Müller-Werdan U, Pilz G, Fraunberger P, Päsler M, et al. Serum IgG levels and mortality in patients with severe sepsis and septic shock : the SBITS data. Med Klin Intensivmed Notf Med. 2017 Jun;112((5)):462–70. doi: 10.1007/s00063-016-0220-6. [DOI] [PubMed] [Google Scholar]
- 8.Siennicka J, Laskowska A, Trzcińska A. [Evaluating of influence of repeated thaw/freeze cycles on IgG and IgM stability] Med Dosw Mikrobiol. 2010;62((3)):281–3. [PubMed] [Google Scholar]
- 9.Challine D, Roudot-Thoraval F, Sabatier P, Dubernet F, Larderie P, Rigot P, et al. Serological viral testing of cadaveric cornea donors. Transplantation. 2006 Sep;82((6)):788–93. doi: 10.1097/01.tp.0000236572.27197.08. [DOI] [PubMed] [Google Scholar]
- 10.Challine D, Pellegrin B, Bouvier-Alias M, Rigot P, Laperche L, Pawlotsky JM. HIV and hepatitis C virus RNA in seronegative organ and tissue donors. Lancet. 2004 Oct;364((9445)):1611–2. doi: 10.1016/S0140-6736(04)17315-7. [DOI] [PubMed] [Google Scholar]
- 11.Tugwell BD, Patel PR, Williams IT, Hedberg K, Chai F, Nainan OV, et al. Transmission of hepatitis C virus to several organ and tissue recipients from an antibody-negative donor. Ann Intern Med. 2005 Nov;143((9)):648–54. doi: 10.7326/0003-4819-143-9-200511010-00008. [DOI] [PubMed] [Google Scholar]
- 12.Pruss A, Caspari G, Krüger DH, Blümel J, Nübling CM, Gürtler L, et al. Tissue donation and virus safety: more nucleic acid amplification testing is needed. Transpl Infect Dis. 2010 Oct;12((5)):375–86. doi: 10.1111/j.1399-3062.2010.00505.x. [DOI] [PubMed] [Google Scholar]
- 13.Gubbe K, Scharnagl Y, Grosch S, Tonn T, Schmidt M, Hourfar KM, et al. Validation of Virus NAT for HIV, HCV, HBV and HAV Using Post-Mortal Blood Samples. Transfus Med Hemother. 2012 Dec;39((6)):381–5. doi: 10.1159/000345319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Victer TN, Dos Santos CS, Báo SN, Sampaio TL. Deceased tissue donor serology and molecular testing for HIV, hepatitis B and hepatitis C viruses: a lack of cadaveric validated tests. Cell Tissue Bank. 2016 Dec;17((4)):543–53. doi: 10.1007/s10561-016-9564-7. [DOI] [PubMed] [Google Scholar]