Abstract
Background
Coronavirus disease-19 (COVID-19) infection is associated with an uncontrolled systemic inflammatory response. Statins, given their anti-inflammatory properties, may reduce the associated morbidity and mortality. This study aimed to determine the association between statin use prior to hospitalization and in-hospital mortality in COVID-19 patients.
Methods
In this retrospective study, clinical data were collected from the electronic medical records of patients admitted to the hospital with confirmed COVID-19 infection from March 1, 2020 to April 24, 2020. A multivariate regression analysis was performed to study the association of pre-admission statin use with in-hospital mortality.
Results
Of 255 patients, 116 (45.5%) patients were on statins prior to admission and 139 (54.5%) were not. The statin group had a higher proportion of end stage renal disease (ESRD) (13.8% vs. 2.9%, p = 0.001), diabetes mellitus (63.8% vs. 35.2%, p<0.001), hypertension (87.9% vs. 61.1%, p < 0.001) and coronary artery disease (CAD) (33.6% vs. 5%, p < 0.001). On multivariate analysis, we found a statistically significant decrease in the odds of in-hospital mortality in patients on statins before admission (OR 0.14, 95% CI 0.03- 0.61, p = 0.008). In the subgroup analysis, statins were associated with a decrease in mortality in those with CAD (OR 0.02, 95% CI 0.0003–0.92 p = 0.045) and those without CAD (OR 0.05, 95% CI 0.005–0.43, p = 0.007).
Conclusions
Our study suggests that statins are associated with reduced in-hospital mortality among patients with COVID-19, regardless of CAD status. More comprehensive epidemiological and molecular studies are needed to establish the role of statins in COVID-19.
Key Indexing Terms: Statins, Covid-19, Mortality
Introduction
Coronavirus disease-19 (COVID-19), caused by SARS-CoV-2, was first reported in December 2019 in Wuhan, China, and subsequently spread to over 200 countries, leading to a worldwide pandemic.1 At present, there is no specific treatment or vaccine for this virus, and efforts to understand risks factors, susceptibilities and therapeutics have been going on across the world. SARS-CoV-2 infection has been shown to trigger a cytokine storm with resultant uncontrolled systemic inflammatory response, causing acute respiratory distress syndrome (ARDS), multiple organ dysfunction and eventually death in severe cases.2 , 3 Underlying cardiovascular disease has been shown to be associated with increased mortality among patients with COVID-19.4, 5, 6, 7, 8 There exists an urgent need to find safe and effective therapies to reduce the morbidity and mortality associated with COVID-19. Already existing drugs that target the host immune response to mitigate the effect of COVID-19 are of broad interest.
Statins decrease low-density lipoprotein (LDL) levels,9 , 10 and are well established in the primary11 , 12 and secondary prevention13 , 14 of cardiovascular events and mortality. Besides these well-known effects, statins also reduce C-reactive protein (CRP) and pro-inflammatory cytokine levels15, 16, 17 and demonstrate various anti-inflammatory18 and immunomodulatory19 , 20 effects. These pleotropic effects of statins are evidence of beneficial effects in a variety of diseases like inflammatory bowel diseases,21, 22, 23 autoimmune diseases,24 chronic obstructive pulmonary disease (COPD),25 , 26 cancer27, 28, 29 and various infections.30, 31, 32 Furthermore, several observational studies in patients with influenza have shown a mortality benefit with statin therapy.26 , 33 , 34
Given these multifaceted benefits of statins, we aimed to study the effects of statins on the outcomes of patients admitted to the hospital with COVID-19 infection.
Methods
We performed a retrospective review of patients above 18 years of age admitted to Albert Einstein Medical Center in Philadelphia with confirmed COVID-19 infection from March 1, 2020 to April 24, 2020. A diagnosis of COVID-19 infection was made using a standardized RT-PCR nasal swab test.
The following data was collected retrospectively from the electronic medical record (EMR)- patients’ demographic characteristics, including age, gender, race and body mass index (BMI); comorbidities, presenting symptoms, relevant laboratory values on admission and home medications at the time of admission. We also collected data regarding length of hospital stay, need for intubation, duration of mechanical ventilation and in-hospital mortality via chart review.
Statistical analysis
We compared baseline characteristics, laboratory values and presenting symptoms among those who were on statins before admission to those who were not on statins. We reported categorical variables as numbers (percentages) and continuous variables as means (standard deviation). We performed a multivariable regression analysis to study association of statin use with in-hospital mortality. We adjusted for known confounders of mortality from prior literature and also variables accounting for differences among statin users and non-users. The model with the lowest Akaike's Information Criterion was selected and used to estimate the odds ratio (OR) for the association. We defined statistical significance as p value<0.05. We used Stata, version 12.1 (Stat Corp, College Station, Texas) to perform statistical analysis.
Results
This study included 255 inpatients admitted with COVID-19 with a mean age of 65.4 ± 15.2 years, 51% (n = 100) were male and 60% (n = 153) were African American. The mean body mass index (BMI) was 29.5 ± 9.1. The most common comorbidities in this cohort were hypertension (n = 187, 73.3%), diabetes mellitus (n = 123, 48.2%) and obstructive lung disease (n = 51, 19.9%). (Table 1 )
Table 1.
Baseline characteristics comparing participants who were on statins at admission with those who were not on statins.
| Not on statins at admission | On statins at admission | Overall | p-value | |
|---|---|---|---|---|
| Characteristics | ||||
| Number of participants, n (%) | 139 (54.5) | 116 (45.5) | 255 | |
| Age in years, mean (SD) | 62.4 (17.7) | 69 (10.6) | 65.4 (15.2) | <0.001 |
| Female gender, n (%) | 68 (48.9) | 57 (49.1) | 125 (49) | 0.972 |
| African American race, n (%) | 76 (54.7) | 77 (66.4) | 153 (60) | 0.057 |
| Body mass index in kg/m2, mean (SD) | 29.7 (8.3) | 29.4 (9.9) | 29.5 (9.1) | 0.811 |
| Asthma, n (%) | 9 (6.5) | 10 (8.6) | 19 (7.4) | 0.516 |
| COPD, n (%) | 15 (10.8) | 17 (14.7) | 32 (12.5) | 0.354 |
| Cirrhosis, n (%) | 6 (4.3) | 3 (2.6) | 9 (3.5) | 0.456 |
| Diabetes mellitus, n (%) | 49 (35.2) | 74 (63.8) | 123 (48.2) | <0.001 |
| End stage renal disease, n (%) | 4 (2.9) | 16 (13.8) | 20 (7.8) | 0.001 |
| Coronary artery disease, n (%) | 7 (5) | 39 (33.6) | 46 (18) | <0.001 |
| Hypertension, n (%) | 85 (61.1) | 102 (87.9) | 187 (73.3) | <0.001 |
| SOFA on admission, mean (SD) | 3.4 (3.6) | 4.4 (3.5) | 3.9 (3.6) | 0.13 |
| PaO2: FiO2 ratio | 278.6 (153.3) | 268 (164.6) | 274.3 (157.5) | 0.704 |
| Home Medications | ||||
| Antiplatelets, n (%) | 29 (20.8) | 66 (56.9) | 95 (37.2) | <0.001 |
| ACEI/ARB, n (%) | 33 (23.7) | 54 (46.5) | 87 (34.1) | <0.001 |
| NSAIDs, n (%) | 11 (7.9) | 10 (8.7) | 21 (8.3) | 0.822 |
| Home anticoagulation, n (%) | 18 (12.9) | 19 (16.4) | 37 (14.5) | 0.439 |
| Prednisone, n (%) | 13 (9.3) | 5 (4.3) | 18 (7.1) | 0.117 |
Abbreviations: SOFA, Sequential Organ Failure Assessment; ACEI, Angiotensin converting enzyme inhibitor; ARB, Angiotensin receptor blocker; NSAID, Non-steroidal anti-inflammatory drug.
There were 116 (45.5%) patients who were on statins before admission and 139 (54.5%) patients who were not on statins. The mean age of those on statins was higher (69 ± 10.6 years) than those not on statins (62.4 ± 17.7 years, p <0.001). The statin group had a higher proportion of end stage renal disease (ESRD) (13.8% vs. 2.9%, p = 0.001), diabetes mellitus (63.8% vs. 35.2%, p<0.001), hypertension (87.9% vs. 61.1%, p < 0.001) and coronary artery disease (CAD) (33.6% vs. 5%, p < 0.001) than the non-statin group. There were also more patients in the statin group on an angiotensin converting enzyme inhibitor (ACEI) / Angiotensin receptor blocker (ARB) (46.5% vs. 23.7%, p<0.001) and an anti-platelet medication (56.9% vs. 20.8%, p<0.001). Laboratory parameters on admission did not significantly differ between the two groups as shown in Table 2 . The statin group had a trend towards higher ferritin (p = 0.056) and higher fibrinogen (p = 0.056). Sequential Organ Failure Assessment (SOFA) score on admission was available for 134 (52.5%) patients did not differ significantly between the two groups (4.4 ± 3.5 vs. 3.5 ± 3.6, p = 0.13). PaO2/Fio2 (PF ratio) on admission was available in 132 (51.8%) patients and did not differ significantly between the two groups (268 ± 164.6 vs. 278.6 ± 153, p = 0.704).
Table 2.
Baseline laboratory values at admission.
| Not on statins at admission | On statins at admission | Total | Number of patients | p-value | |
|---|---|---|---|---|---|
| Total leukocyte count (x 103 cells/microliter), mean (SD) | 8.1 (4.3) | 8.0 (5.4) | 8.1 (4.8) | 253 | 0.83 |
| Segmented leukocytes (%), mean (SD) | 70.95 (13.3) | 80.4 (75.5) | 75.1 (50.9) | 213 | 0.179 |
| Lymphocytes (%), mean (SD) | 15.9 (9.8) | 16.0 (8.0) | 15.9 (9.0) | 212 | 0.892 |
| Neutrophil to lymphocyte ratio (NLR), mean (SD) | 8.7 (14.4) | 7.0 (8.9) | 7.9 (12.3) | 209 | 0.317 |
| Lactate (mmol/L), mean (SD) | 2.4 (2.2) | 2.0 (1.5) | 2.2 (1.9) | 165 | 0.2 |
| Creatinine (mg/dL), mean (SD) | 1.8 (2.3) | 2.4 (2.3) | 2.1 (2.4) | 250 | 0.054 |
| CRP (mg/dL), mean (SD) | 160.3 (123.8) | 128.4 (104.8) | 145.3 (115.8) | 104 | 0.161 |
| Fibrinogen (mg/dL), mean (SD) | 547.2 (188.3) | 633.2 (164.9) | 578.3 (183.8) | 72 | 0.056 |
| Procalcitonin (ng/ml), mean (SD) | 2.7 (8.8) | 1.4 (3.9) | 2.1 (7.0) | 137 | 0.322 |
| Lactate dehydrogenase (U/L), mean (SD) | 481.1 (359) | 452.1 (270.0) | 467.6 (320.1) | 155 | 0.576 |
| Ferritin (mcg/L), mean (SD) | 1456 (2311) | 2308 (3257) | 1842 (2803) | 159 | 0.056 |
| B-type natriuretic peptide (pg/ml), mean (SD) | 267.9 (676.4) | 400.6 (704.3) | 340.8 (691.2) | 91 | 0.365 |
During the course of hospitalization, 22.3% (n = 54) of the study population required mechanical ventilation and 28.8% (n = 65) required intensive care unit (ICU) admission. A total of 53 patients (20.1%) died and 38 patients (14.9%) required hospice care. There was no difference between the two groups in terms of requirement of mechanical ventilation (p = 0.353), days on the ventilator (p = 0.253), days on vasopressors (p = 0.787), requirement of continuous renal replacement therapy (CRRT) (p = 0.5) and in-hospital mortality (Table 3 ). Steroids were given in 9.3% of patients in the statin group as compared to 7.1% of patients in the non-statin group. (p = 0.117).
Table 3.
Measures of health care utilization comparing participants who were on statins at admission with those who were not on statins.
| Not on statins at admission | On statins at admission | Total | p-value | |
|---|---|---|---|---|
| CRRT, % (n) | 6.5 (9) | 13.8 (16) | 9.8 (25) | 0.5 |
| Mechanical Ventilation, % (n) | 21.5 (28) | 23.2 (26) | 22.3 (54) | 0.755 |
| Days on mechanical ventilation, mean (SD) | 2.8 (5.1) | 2.0 (3.3) | 2.4 (4.4) | 0.253 |
| Days in the ICU, mean (SD) | 2.4 (4.7) | 1.8 (3.3) | 2.1 (4.1) | 0.343 |
| Death, % (n) | 23 (32) | 18.3 (21) | 20.1 (53) | 0.353 |
| Hospice, % (n) | 17.3 (24) | 12.1 (14) | 14.9 (38) | 0.246 |
Abbreviations: CRRT, Continuous Renal Replacement Therapy; ICU, Intensive Care Unit.
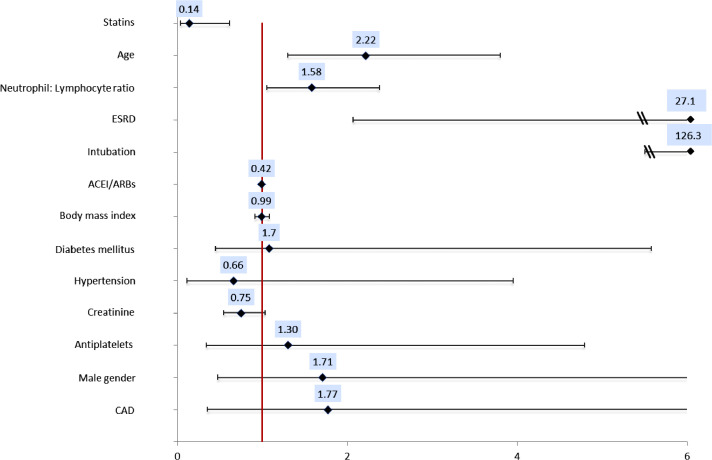
On multivariate analysis, we found a statistically significant decrease in the odds of in-hospital mortality in patients on statins before admission (OR 0.14, 95% CI 0.03- 0.61, p = 0.008), after adjusting for age, gender, BMI, ESRD, intubation during hospitalization, creatinine, neutrophil: lymphocyte ratio (NLR), hypertension, diabetes mellitus, CAD, anti-platelets and ACEI/ARBs (Fig. 1 and Table 4 ). Other factors significantly associated with in-hospital mortality as seen in Fig. 1 were age (OR 2.2, 95% CI 1.3- 3.8, p = 0.004), ESRD (OR 27.1, 95% CI 2.08- 353.6, p = 0.012) intubation (OR 126.30, 95% CI 28.20- 565.73, p<0.001) and NLR (OR 1.58, 95% CI 1.05–2.38, p = 0.028).
Fig. 1.
Forest plot based on the results of the multivariate analysis. This figure depicts the of odds ratio (OR) and confidence interval for in-hospital mortality in COVID-19. OR for age and NLR is shown for increments of 10. Increase in age, neutrophil: lymphocyte ratio, presence of ESRD and intubation during hospitalization were all associated with an increased risk of in-hospital mortality. Use of statins pre-admission was associated with decrease in the odds of in-hospital mortality (OR 0.14, 95% CI 0.03- 0.61, p = 0.008). All other variables including gender, body mass index, hypertension, diabetes, coronary artery disease and antiplatelets or ACEI/ARBs before admission were not associated with in hospital mortality. Abbreviations: ESRD, End Stage Renal Disease; ACEI, Angiotensin Converting Enzyme Inhibitor; ARB, Angiotensin Receptor Blocker.
Table 4.
Results of multivariable regression analysis for association of statin use with in hospital mortality in patients with COVID 19.
| Odds ratios | 95% confidence interval | P-value | |
|---|---|---|---|
| Statins | 0.14 | 0.03 - 0.61 | 0.008 |
| Age | 2.2a | 1.3 - 3.8 | 0.004 |
| Neutrophil: lymphocyte ratio (NLR) | 1.58a | 1.05 - 2.38 | 0.028 |
| End stage renal disease | 27.1 | 2.08 - 353.6 | 0.012 |
| Intubation | 126.3 | 28.2 - 565.73 | <0.001 |
| Male gender | 1.6 | 0.45 - 5.49 | 0.482 |
| BMI | 0.99 | 0.91 - 1.08 | 0.908 |
| Hypertension | 0.66 | 0.11 - 3.95 | 0.654 |
| Diabetes mellitus | 1.7 | 0.47 - 6.24 | 0.412 |
| Coronary artery disease | 1.7 | 0.35 - 8.8 | 0.486 |
| Anti-platelets | 1.3 | 0.34 - 4.98 | 0.698 |
| ACEI/ARBs | 0.42 | 0.09–1.87 | 0.256 |
| Creatinine at admission | 0.7 | 0.54–1.03 | 0.079 |
Abbreviations: ACEI, Angiotensin converting enzyme inhibitor; ARB, Angiotensin receptor blocker; BMI, Body Mass Index.
Odds ratios for age and NLR are shown for increments of 10 for clinical relevance and ease of depiction.
We performed subgroup analysis to study the effect of statins on mortality among patients with and without CAD separately. In the adjusted analysis, statins were associated with a decrease in mortality in those with CAD (OR 0.02, 95% CI 0.0003–0.92 p = 0.045) and also among those without CAD (OR 0.05, 95% CI 0.005–0.43, p = 0.007). Similarly, statin use was significantly associated with reduction in mortality in separate analysis for patients with diabetes (p value=0.01) and a trend towards reduction in mortality in those without diabetes mellitus (p value=0.07). There was no effect modification of ESRD and hypertension on the association of statin use with mortality.
Among the 54% of cohort who had SOFA score available at the time of admission, statins were associated with decrease in mortality (OR 0.03 95% CI 0.002–0.38, p = 0.006) after adjusting for SOFA score in addition to the previous adjusted confounders, including, age, sex, gender, BMI, ESRD, intubation during hospitalization, creatinine, NLR, hypertension, diabetes mellitus, CAD, anti-platelets and ACEI/ARBs.
Discussion
Some observational studies have reported an association of statins with reduction in adverse cardiovascular outcomes and mortality in patients admitted with influenza and/or pneumonia.26 , 34 , 35 It is therefore conceivable that statins can offer a protective effect in acute viral illness of COVID-19. Progress in the development of effective vaccines and antiviral drugs, although the focus of much research, has been disappointing and time consuming. Therefore, utilizing statins to mitigate the inducing effect of COVID-19 on the immune system merits exploration. Additionally, an in-silico molecular modeling study by Wang et al. to identify FDA approved drugs targeting SARS-CoV-2 identified rosuvastatin as the sixth potentially usable drug that may have clinical utility in COVID-19.36
Our study suggests that statins are associated with reduced in-hospital mortality among patients with COVID-19 infection. Patients in the statin group were older in age and had a higher prevalence of end stage renal disease (ESRD), diabetes mellitus, hypertension and coronary artery disease (CAD). These co-morbidities are known to contribute a high mortality in SARS-CoV-2 patients.6 , 37, 38, 39, 40, 41, 42, 43, 44 Hence, this group represents a higher risk group and would be expected to have worse outcomes. On the contrary, multivariate analysis found statin use to be associated with lower odds of mortality in patients with COVID-19, compared to those not on statins.
There are several proposed mechanisms for the effect of statins on disease caused by SARS-CoV-2. In addition to the indirect effect of statins on decreasing cardiovascular complications by anti-inflammatory and immunomodulatory effect, various studies have shown direct effect on viral particles.45 It is postulated that some of the pleiotropic effects of statins such as the downregulation of CD147 expression and function, lipid raft disruption, autophagy activation, and attenuation of both the inflammatory response and the coagulation activation are relevant in the infection and replication of SARS-CoV-2 in host cells.46 However it is unknown if any of these mechanisms are responsible for the observed association or it is an epiphenomenon.
The same dilemma was faced by Kruger et al., who at a time when it was recommended that statins be stopped during an acute infection, described a significantly lower mortality rate in patients with bacteremia who continued statin therapy during the hospital admission.47 In the study those who continued statins had the lowest mortality, followed by those who stopped statins on admission and highest mortality was seen in the no statin group.47 They considered the possibility that statins had a synergistic effect with antibiotic therapy or that they played an immunomodulatory role.
We did not find any difference in levels of inflammatory markers at admission between the statin and no statin group. Based on our findings, the reduction in mortality by statins was unlikely to be mediated by the level of inflammatory markers on admission.
To minimize any bias by indication, we separately analyzed those with CAD and those without CAD. We found that, on multivariable analysis, statin use before admission was associated with reduction in mortality in both these groups.
Our study has several limitations common to retrospective studies. Data regarding the duration of statin therapy prior to presentation, specific type and dose of statins, and whether they were continued or stopped during the hospitalization was not available. Also given the retrospective nature of this study, we are unable to eliminate hidden confounding. We also could not compare the interaction of statins with some of the other potential treatments of COVID-19 like antiviral drugs. However, the protective effect of statins in our study was consistent among all subgroups and after adjusting for all available confounders.
Our study builds evidence that statins may be helpful in mitigating effects of COVID-19. Given their low cost, great safety profile and worldwide availability, they portend great potential. More comprehensive epidemiological and molecular studies are needed to establish the role of statins in COVID-19 including role of continuation of therapy, de novo initiation of therapy and potential harms associated with statin use in those with COVID-19.
Funding source
None.
Author contributions
All authors contributed equally to the manuscript.
Declaration of Competing Interest
No conflict of interest for any of the authors.
References
- 1.Guan W-j, Ni Z-y, Hu Y. Clinical characteristics of Coronavirus disease 2019 in China. New Eng J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X., Geng M., Peng Y. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with Coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehra M.R., Desai S.S., Kuy S. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382:e102. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Ruan Q., Yang K., Wang W. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones P., Kafonek S., Laurora I. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study) Am J Cardiol. 1998;81(5):582–587. doi: 10.1016/s0002-9149(97)00965-x. [DOI] [PubMed] [Google Scholar]
- 10.Rosenson R.S. Rosuvastatin: a new inhibitor of HMG-coA reductase for the treatment of dyslipidemia. Expert Rev Cardiovasc Ther. 2003;1(4):495–505. doi: 10.1586/14779072.1.4.495. [DOI] [PubMed] [Google Scholar]
- 11.Downs J.R., Clearfield M., Weis S. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 12.Montori V.M., Devereaux P.J., Adhikari N.K. Randomized trials stopped early for benefit: a systematic review. JAMA. 2005;294(17):2203–2209. doi: 10.1001/jama.294.17.2203. [DOI] [PubMed] [Google Scholar]
- 13.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 14.Baigent C., Blackwell L., Emberson J. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musial J., Undas A., Gajewski P. Anti-inflammatory effects of simvastatin in subjects with hypercholesterolemia. Int J Cardiol. 2001;77(2–3):247–253. doi: 10.1016/s0167-5273(00)00439-3. [DOI] [PubMed] [Google Scholar]
- 16.Ridker P.M., Rifai N., Lowenthal S.P. Rapid reduction in C-reactive protein with cerivastatin among 785 patients with primary hypercholesterolemia. Circulation. 2001;103(9):1191–1193. doi: 10.1161/01.cir.103.9.1191. [DOI] [PubMed] [Google Scholar]
- 17.Ridker P.M., Rifai N., Pfeffer M.A. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100(3):230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan C.J., Gotto A.M., Jr., Basson C.T. The evolving role of statins in the management of atherosclerosis. J Am Coll Cardiol. 2000;35(1):1–10. doi: 10.1016/s0735-1097(99)00525-2. [DOI] [PubMed] [Google Scholar]
- 19.Kwak B., Mulhaupt F., Myit S. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6(12):1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 20.Mulhaupt F., Matter C.M., Kwak B.R. Statins (HMG-CoA reductase inhibitors) reduce CD40 expression in human vascular cells. Cardiovasc Res. 2003;59(3):755–766. doi: 10.1016/s0008-6363(03)00515-7. [DOI] [PubMed] [Google Scholar]
- 21.Côté-Daigneault J., Mehandru S., Ungaro R. Potential Immunomodulatory Effects of Statins in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22(3):724–732. doi: 10.1097/MIB.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 22.Crockett S.D., Hansen R.A., Stürmer T. Statins are associated with reduced use of steroids in inflammatory bowel disease: a retrospective cohort study. Inflamm Bowel Dis. 2012;18(6):1048–1056. doi: 10.1002/ibd.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ungaro R., Chang H.L., Côté-Daigneault J. Statins associated with decreased risk of new onset inflammatory bowel disease. Am J Gastroenterol. 2016;111(10):1416–1423. doi: 10.1038/ajg.2016.233. [DOI] [PubMed] [Google Scholar]
- 24.Khattri S., Zandman-Goddard G. Statins and autoimmunity. Immunol Res. 2013;56(2–3):348–357. doi: 10.1007/s12026-013-8409-8. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W., Zhang Y., Li C.W. Effect of statins on COPD: a Meta-analysis of randomized controlled trials. Chest. 2017;152(6):1159–1168. doi: 10.1016/j.chest.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Frost F.J., Petersen H., Tollestrup K. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest. 2007;131(4):1006–1012. doi: 10.1378/chest.06-1997. [DOI] [PubMed] [Google Scholar]
- 27.Beckwitt C.H., Brufsky A., Oltvai Z.N. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res. 2018;20(1):144. doi: 10.1186/s13058-018-1066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fatehi Hassanabad A. Current perspectives on statins as potential anti-cancer therapeutics: clinical outcomes and underlying molecular mechanisms. Transl Lung Cancer Res. 2019;8(5):692–699. doi: 10.21037/tlcr.2019.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stryjkowska-Góra A., Karczmarek-Borowska B., Góra T. Statins and cancers. Contemp Oncol (Pozn) 2015;19(3):167–175. doi: 10.5114/wo.2014.44294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almog Y., Novack V., Eisinger M. The effect of statin therapy on infection-related mortality in patients with atherosclerotic diseases. Crit Care Med. 2007;35(2):372–378. doi: 10.1097/01.CCM.0000253397.42079.D5. [DOI] [PubMed] [Google Scholar]
- 31.Björkhem-Bergman L., Bergman P., Andersson J. Statin treatment and mortality in bacterial infections–a systematic review and meta-analysis. PLoS ONE. 2010;5(5):e10702. doi: 10.1371/journal.pone.0010702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan Y.D., Sun T.W., Kan Q.C. Effect of statin therapy on mortality from infection and sepsis: a meta-analysis of randomized and observational studies. Crit Care. 2014;18(2):R71. doi: 10.1186/cc13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwong J.C., Li P., Redelmeier D.A. Influenza morbidity and mortality in elderly patients receiving statins: a cohort study. PLoS ONE. 2009;4(11):e8087. doi: 10.1371/journal.pone.0008087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandermeer M.L., Thomas A.R., Kamimoto L. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis. 2012;205(1):13–19. doi: 10.1093/infdis/jir695. [DOI] [PubMed] [Google Scholar]
- 35.Douglas I., Evans S., Smeeth L. Effect of statin treatment on short term mortality after pneumonia episode: cohort study. BMJ. 2011;342:d1642. doi: 10.1136/bmj.d1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farag A., Wang P., Boys I. ChemRxiv; 2020. Identification of Atovaquone, Ouabain and Mebendazole as FDA Approved Drugs Tar-geting SARS-CoV-2 (Version 4)https://chemrxiv.org/articles/preprint/Identification_of_FDA_Approved_Drugs_Targeting_COVID-19_Virus_by_Structure-Based_Drug_Repositioning/12003930 [Google Scholar]
- 37.Henry B.M., Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52(6):1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar A., Arora A., Sharma P. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leiva Sisnieguez C.E., Espeche W.G., Salazar M.R. Arterial hypertension and the risk of severity and mortality of COVID-19. Eur Respir J. 2020;55(6):1148–2020. doi: 10.1183/13993003.01148-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H., Wang S., Zhong F. Age-dependent risks of incidence and mortality of COVID-19 in Hubei Province and other parts of China. Front Med (Lausanne) 2020;7:190. doi: 10.3389/fmed.2020.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lippi G., Wong J., Henry B.M. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130(4):304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 44.Yamada T., Mikami T., Chopra N. Patients with chronic kidney disease have a poorer prognosis of coronavirus disease 2019 (COVID-19): an experience in New York City. Int Urol Nephrol. 2020;52(7):1405–1406. doi: 10.1007/s11255-020-02494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reiner Ž., Hatamipour M., Banach M. Statins and the COVID-19 main protease: in silico evidence on direct interaction. Arch Med Sci. 2020;16(3):490–496. doi: 10.5114/aoms.2020.94655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodrigues-Diez R.R., Tejera-Muñoz A., Marquez-Exposito L. Statins: could an old friend help in the fight against COVID-19? Br J Pharmacol. 2020;177:4873–4886. doi: 10.1111/bph.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kruger P., Fitzsimmons K., Cook D. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med. 2006;32(1):75–79. doi: 10.1007/s00134-005-2859-y. [DOI] [PubMed] [Google Scholar]