Abstract
We describe 3 cases of coronavirus disease 2019 in health care workers in France involving presumed clinical and microbiological recurrence after recovery. All patients were immunocompetent with clinical mild form. These cases highlight the possibility of coronavirus disease–recurrence.
Keywords: COVID-19, SARS-CoV-2, Health care worker, Recurrence, Recovery
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In hospitals, there is a considerable risk of contracting COVID-19 infection among health care workers (HCWs) [1] with a high incidence of mild to moderate forms in this population [2]. The possibility of a reactivation of COVID-19 raises a major public health concern since it could contribute to the spread of the virus in the population, especially in hospitals. In this rapidly emerging epidemic, several case reports describe clinical and/or microbiological recurrences in SARS-CoV-2–infected patients [[3], [4], [5], [6], [7], [8]]. We report a case series of presumed clinical and microbiological recurrence after recovery in 3 HCWs with mild forms of COVID-19.
1. Materials and methods
In our facility, 312 HCWs were infected during the first wave [9] between March 1st and May, 30th, 2020. During the second wave [10] (from October 1st, to December 7th, 2020), 219 of our HCWs have tested positive with SARS-CoV-2 until December, 03rd. Among these 219 HCWs, 3 had already been infected with SARS-CoV-2 during the first wave.
2. Results
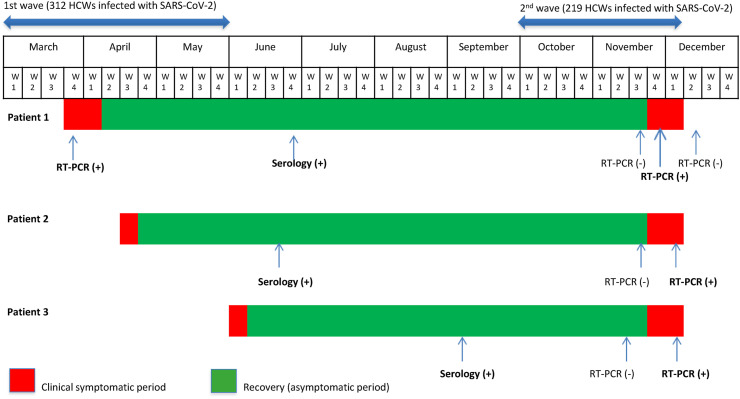
These 3 patients were females working in different departments with a median age of 43 [32–57] years. They had no comorbidities, except one patient with asthma. During the first episode, the median duration of symptoms was 10 [7–14] days with a complete recovery. All had a negative RT-PCR between the two episodes. All patients returned to work in COVID units and had possible COVID-19 re-exposure. The median interval between the two episodes was 213 [155–236] days. Clinical and microbiological data were summarized in the Fig. 1 and Table 1 . All RT-PCR testing were administered in our laboratory and all of the samples were subjected to the same assays.
Fig. 1.
Timeline in COVID-19 patients with recurrent infection after recovery. Abbrevations: HCW: health care workers; W: week.
Table 1.
Demographic, clinical characteristics and laboratory findings of COVID-19 first and 2nd episodes, from onset of first episode (D1) to last follow-up, Nord Franche-Comte Hospital, France, 2020.
| Patient 1 | Patient 2 | Patient 3 | ||
|---|---|---|---|---|
| Patients characteristics | ||||
| Age, y | 57 | 40 | 32 | |
| Sex | F | F | F | |
| Comorbidities | Asthma (HCW) | None (HCW) | None (HCW) | |
| First episode (onset = D1) | ||||
| Clinical presentation | ILI without fever-AO-DG-cough-sputum production-diarrhea | ILI, chills | ILI | |
| RT-PCRa SARS-CoV-2 | Days from onset | D6 | NA | NA |
| CT if available | POSITIVE (E 27.66 – N NA RdRP 25.62) | |||
| Serology | Days from 1st onset | 59 | 61 | 94 |
| Results | POSITIVE IgG IgM | POSITIVE IgG | POSITIVE IgG (20.2 U/mL) | |
| RT-PCRa SARS-CoV-2 (follow-up) | Days from onset | 236 | 212 | 155 |
| Results | NEGATIVE | NEGATIVE | NEGATIVE | |
| Treatment | None | None | None | |
| Duration of symptoms | 14 | 8 | 7 | |
| Second episode | ||||
| Clinical presentation | Myalgia-fatigue-dyspnea | ILI-chills-headache-tearing-AO-DG-cough-chest pain-dyspnea- | ILI- headache- AO-DG cough-diarrhea | |
| Days from 1st onset (clinical recurrence) | 243 | 219 | 176 | |
| RT-PCR SARS-CoV-2 | Days from 2nd onset | 8 | 7 | 10 |
| CT if available | POSITIVE (E NA – N 40 RdRP NA) | POSITIVE (E 36.93) ORF1 36.4 |
POSITIVE (E 0 – N 38 RdRP 0) | |
| Treatment | None | None | None | |
Abbreviations: AO: anosmia; COVID-19: coronavirus disease 2019; D: day; DG: dysgeusia; ILI: influenza-like illness (fever + myalgia/arthralgia + fatigue + sore throat + nasal congestion); HCW: Health care worker; NA: not available; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
RT-PCR: real-time reverse transcription Polymerase Chain Reaction: cycle threshold (CT), envelope gene (E), nucleocapsid gene (N), ARN polymerase gene (RdRP), specific Open Reading Frame (ORF)1.
On March 25th, 2020, a 57-year-old female with a past history of well-controlled asthma (treated with salbutamol) sought care for influenza-like illness (ILI) including myalgia, fatigue and sore throat associated to a productive cough with anosmia, dysgeusia and diarrhea. Real-time reverse transcription PCR (RT-PCR) on a nasopharyngeal swab specimen confirmed COVID-19. Fever and other symptoms disappeared after 2 weeks. On June 23rd, her SARS-CoV-2 serology (obtained upon patient's request) was positive for IgG and IgM. SARS-CoV-2 RT-PCR follow-up test (carried out on November 20th) was negative. On November 26th, the patient was presented for myalgia and fatigue. She also complained of dyspnea. On November 27th, RT-PCR was positive; RNA nucleocapsid gene (N) of SARS-CoV-2 was detected with a cycle threshold (Ct) value of 40. On December 3rd, a follow up RT-PCR was negative.
On April 14th, 2020, a 40-year-old female with no past history sought care for fever at 38.5C°, chills, myalgia and fatigue. All symptoms regressed after 8 days. COVID-19 was diagnosed from clinical presentation and serology testing (performed on June 18th, which was positive for IgG). SARS-CoV-2 RT-PCR testing was not performed initially. She only performed a follow-up nasopharyngeal swab, which proved to be negative. On November 26th, the patient was represented with ILI, retro-orbital headache, anosmia and dysgeusia associated to respiratory symptoms such as non-productive cough, chest pain and dyspnea with a normal pulmonary auscultation. She specified that she had recently been in contact with a known case of SARS-CoV-2. On December 03rd, SARS-CoV-2 RT-PCR was positive with detection of RNA envelope gene (E) and specific Open Reading Frame (ORF)1 at Ct values of 36.4 and 36.93, respectively.
On June 1st, a 32 -year-old female with no past medical history sought care for ILI related to COVID-19. On September 9th, her serology (obtained upon patient's request) was compatible with a previous SARS-CoV-2 infection (IgG 20.2 U/mL). On November 11th, she was tested during a collective screening in her hospital department after an outbreak with several cases among the HCWs. SARS-CoV-2 RT-PCR was negative. On November 27th, the patient developed similar clinical presentation to the first episode associated to anosmia, dysgeusia and gastro-intestinal symptoms such as vomiting and diarrhea. On December 04th, SARS-CoV-2 RT-PCR was positive with detection of RNA gene and (N) at Ct values of 38.
3. Discussion
In our case series, all patients presented two episodes of SARS-CoV-2 infection separated by a symptom-free interval with a median duration between the two episodes of about 8 months. During this interval, RT-PCR follow-up was negative in all cases. All of our patients have strong clinical and microbiological evidence that it is indeed a COVID-19 recurrence, more than the hypothesis of prolonged nucleic acid conversion in COVID-19 or traces of viral RNA. To explain this, several hypothesis were put forward such as viral relapse or inflammatory rebound [3,11]. Immunity protective role from re-infection along with definitive viral clearance is uncertain [12,13]. One hypothesis would be that these episodes are linked to the persistence of the virus in a reservoir (sanctuary site) with viral rebound, as previously suggested for other viral infections [14]. However, our cases are less likely to reflect persistent viral RNA shedding, including shedding related to non-viable virus. Recent studies reported that the median duration of prolonged SARS-CoV-2 RNA shedding in COVID-19 patients was about 30 days [15], unlike our HCWs' presentation with a long median interval between the two episodes (more than 7 months) and a confirmed clinical and microbiological recovery. In addition to that, risk factors of reactivation would probably include immunosuppression [3,5,6]. In a French cohort including 11 COVID-19 patients with clinical recurrences, 3 of them had received recent chemotherapy and/or rituximab and didn't develop SARS-CoV-2 antibodies more than 3 weeks after severe symptoms [3]. This probably contributes to impair viral clearance and favors reactivation. In contrast to these cases, our patients were immunocompetent and developed SARS-CoV-2 antibodies, after the first episode. Several authors have described recurrence of COVID-19 in HCWs [3,7]. Fernandes et al. have described six cases of healthcare professionals in Brazil who recovered but again presented symptoms of COVID-19 with mild-to-moderate forms, with new RT-PCR positive results [7]. Goussef et al. have reported 4 cases of HCWs with separate mild COVID-19 forms. They suggested that ‘re-infection’ is due to the prolonged exposure given the fact that the immune response may faint [3]. Finally, some authors have suggested that recurrence may be explained by inflammatory rebound [3,11]. By using mathematical models to study the pathogenic features of SARS-CoV-2 infection, cells and immune responses, Wang et al. have demonstrated that when the initiation of seroconversion is late or slow, the model predicts viral rebound and prolonged viral persistence [11].
Finally, we thought that these data are fairly strong with clinical confirmation and serological evidence of prior infection of all three cases. However, the laboratory confirmation of that re-infection is a bit weak (Ct's very high); although the Ct values may be expected to be high on reinfection with a potentially waning antigen response. Another possible limitation is that without genomic analyses of the isolates from the initial and putative reinfection episodes, it cannot be determined with certainty that SARS-CoV-2 reinfection occurred in these patients [16,17]. Unfortunately, the different strains responsible for the first and second episodes in our patients were not available for sequencing. The occurrence of these cases one month before first description of the New UK (20I/501Y.V1) and South African (20H/501Y.V2) SARS-CoV-2 variants in France [18] rules out the hypothesis of a reinfection with a new SARS-CoV-2 variant.
To conclude, the recurrence of the SARS-CoV-2 in patients who have recovered from COVID-19 is possible, but the mechanism leading to these re-positive cases is still unclear. These cases emphasize the importance of active surveillance of SARS-CoV-2 RNA for infectivity assessment, particularly in HCWs to reduce in-hospital transmissions.
Contributors
SZ and PYR collected the epidemiological and clinical data. SZ drafted the figure. SZ and TK drafted the manuscript. TK, AP, LT and VG revised the final manuscript.
We thank all patients involved in the study and especially Dr Quentin Lepiller for his support and valuable feedback.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
All authors declare no competing interests.
References
- 1.Sahu A.K., Amrithanand V.T., Mathew R., Aggarwal P., Nayer J., Bhoi S. COVID-19 in health care workers - a systematic review and meta-analysis. Am J Emerg Med. 2020;38(9):1727–1731. doi: 10.1016/j.ajem.2020.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadiane-Oussou N.J., Klopfenstein T., Royer P.-Y., Toko L., Gendrin V., Zayet S. COVID-19: comparative clinical features and outcome in 114 patients with or without pneumonia (Nord Franche-Comte Hospital, France) Microb Infect. 2020 Oct 10;22(10):622–625. doi: 10.1016/j.micinf.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gousseff M., Penot P., Gallay L., Batisse D., Benech N., Bouiller K. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020 Nov;81(5):816–846. doi: 10.1016/j.jinf.2020.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dao T.L., Hoang V.T., Gautret P. Recurrence of SARS-CoV-2 viral RNA in recovered COVID-19 patients: a narrative review. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2020 Oct 28;40(1):13–25. doi: 10.1007/s10096-020-04088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He F., Luo Q., Lei M., Fan L., Shao X., Hu K. Successful recovery of recurrence of positive SARS-CoV-2 RNA in COVID-19 patient with systemic lupus erythematosus: a case report and review. Clin Rheumatol. 2020 Sep;39(9):2803–2810. doi: 10.1007/s10067-020-05230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luciani M., Bentivegna E., Spuntarelli V., Lamberti P.A., Cacioli G., Del Porto F. Recurrent Covid-19 Pneumonia in the course of chemotherapy: consequence of a weakened immune system? J Med Virol. 2020 Nov 28;93(4):1882–1884. doi: 10.1002/jmv.26701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes Valente Takeda C., Moura de Almeida M., Gonçalves de Aguiar Gomes R., Cisne Souza T., Alves de Lima Mota M., Pamplona de Góes Cavalcanti L. Case report: recurrent clinical symptoms of COVID-19 in healthcare professionals: a series of cases from Brazil. Am J Trop Med Hyg. 2020;103(5):1993–1996. doi: 10.4269/ajtmh.20-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim A.Y., Gandhi R.T. Re-infection with SARS-CoV-2: what goes around may come back around. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 Oct 9 doi: 10.1093/cid/ciaa1541. Online ahead of print. [DOI] [Google Scholar]
- 9.Fouillet A., Pontais I., Caserio Schönemann C. Excess all-cause mortality during the first wave of the COVID-19 epidemic in France, March to May 2020. Eurosurveillance. 2020 Aug 27;25(34) doi: 10.2807/1560-7917.ES.2020.25.34.2001485. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nørgaard S.K., Vestergaard L.S., Nielsen J., Richter L., Schmid D., Bustos N. Real-time monitoring shows substantial excess all-cause mortality during second wave of COVID-19 in Europe, October to December 2020. Eurosurveillance. 2021 Jan 14;26(2) doi: 10.2807/1560-7917.ES.2021.26.1.2002023. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S., Pan Y., Wang Q., Miao H., Brown A.N., Rong L. Modeling the viral dynamics of SARS-CoV-2 infection. Math Biosci. 2020 Oct;328:108438. doi: 10.1016/j.mbs.2020.108438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casanova J.-L., Su H.C. COVID human genetic effort. A global effort to define the human genetics of protective immunity to SARS-CoV-2 infection. Cell. 2020;181(6):1194–1199. doi: 10.1016/j.cell.2020.05.016. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R. Targets of T Cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. 25. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malvy D., McElroy A.K., de Clerck H., Günther S., van Griensven J. Ebola virus disease. Lancet Lond Engl. 2019;393(10174):936–948. doi: 10.1016/S0140-6736(18)33132-5. 02. [DOI] [PubMed] [Google Scholar]
- 15.Li N., Wang X., Lv T. Prolonged SARS-CoV-2 RNA shedding: not a rare phenomenon. J Med Virol. 2020 Nov;92(11):2286–2287. doi: 10.1002/jmv.25952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta V., Bhoyar R.C., Jain A., Srivastava S., Upadhayay R., Imran M. Asymptomatic reinfection in two healthcare workers from India with genetically distinct SARS-CoV-2. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 Sep 23 doi: 10.1093/cid/ciaa1451. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shastri J., Parikh S., Agarwal S. 2020 Sep 21. Whole genome sequencing confirmed SARS-CoV-2 reinfections among healthcare workers in India with increased severity in the second episode.https://europepmc.org/article/ppr/ppr244433 [cited 2021 Feb 17]; Available from: [Google Scholar]
- 18.Eurosurveillance editorial team Updated rapid risk assessment from ECDC on the risk related to the spread of new SARS-CoV-2 variants of concern in the EU/EEA - first update. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2021 Jan;26(3) doi: 10.2807/1560-7917.ES.2021.26.3.2101211. [DOI] [PMC free article] [PubMed] [Google Scholar]