Abstract
Coronavirus Disease 2019 (COVID-19) can present with different grades of severity from mild to critical. Evaluation of biomarkers predicting severity is crucial to identify patients at high risk of disease progression and poor prognosis. Serum Amyloid A (SAA) is an acute-phase protein mainly produced by the liver in response to pro-inflammatory cytokines. In this study, we investigated SAA levels at admission (T1) and after 15 days (T2) of hospitalization in two groups of patients: survivors and non-survivors. At T1, the non-survivors showed higher SAA level than survivors (74 mg/dL vs 48.75 mg/dL). At T2, the survivor group value decreased to 6.55 mg/dL, the non-survivor group still showed high levels (51.1 mg/dL). The SAA level in control group was 0.35 mg/dL.
Furthermore, a cut-off value of 63 mg/dL able to discriminate survivors from non-survivors was established by ROC curve analysis at T1. At T2, the cut-off decreased to 30.9 mg/dL.
A similar decreasing trend was observed for D-Dimer, hsCRP, IL-6 and procalcitonin levels.
The results of this retrospective study suggest that SAA is a good marker of COVID-19 disease alone and/or in combination with other inflammatory biomarkers. Identification of reliable prognostic analytes is of great clinical relevance, as it would improve patient management besides being costs saving.
Keywords: SARS-CoV-2, Covid-19, Serum Amyloid A, Biomarkers
1. Introduction
In the late 2019 a novel human coronavirus emerged that caused a severe acute respiratory syndrome (SARS) responsible for the disease known as coronavirus disease 19 (COVID-19) [1]. Based on the sequence analysis, this virus was classified as a β-coronavirus and named SARS-CoV-2 [2]. Patients infected by SARS-CoV-2 may present a clinical picture ranging from mild to severe with a large part of the infected individuals being asymptomatic carriers.
In 15% of infected patients the clinical course of this pathology can be complicated by the onset of a serious form of interstitial pneumonia, which can therefore progress towards acute respiratory distress syndrome (ARDS), multi-organ failure (MOF) and death [3]. This virus has led to the deaths of 1.760.000 people around the world to date [4].
Laboratory findings in COVID-19 patients show alterations of several parameters including decrease in lymphocyte and eosinophils counts, lower median hemoglobin values along with increases in white blood cells (WBC), neutrophil counts, and serum levels of high sensitivity C-Reactive Protein (hsCRP), lactate dehydrogenase (LDH), aspartate aminotransferaese (AST) and alanine aminotransferase (ALT) [5]. Initial hsCRP and neutrophil levels have been reported to be independent predictors for the development of severe COVID-19 [6], [7]. D-dimer levels have also been associated with the severity of COVID-19: patients with severe COVID-19 have higher value of D-dimer than those with milder disease (weighted mean difference 2.97 mg/L; 95% CI: 2.47–3.46 mg/L) with an increased risk of disseminated coagulopathy [8]. However, predictors for severe COVID-19 are not well defined yet. Macrophages activation can trigger the cytokine cascade by releasing tumor necrosis factor α (TNF-α), interleukin 1 (IL-1), nitric oxide (NO), and reactive oxygen species (ROS) to induce a severe inflammatory response, which boost liver cells to produce Serum Amyloid A (SAA) [9]. Most cytokines and CRP ere associated with variability of sex steroid hormones including both endogenous and exogenous molecules which could explain the pro-inflammatory states that most postmenopausal women suffer from [10], [11], [12].
SAA is an acute phase protein produced by hepatocytes in response to pro-inflammatory cytokines, released during virus infection [13]. Recent investigations found that SAA might be a useful indicator of disease severity in COVID-19 patients; descending levels of SAA correlated with a better prognosis compared to patients with an ascending trend. Furthermore, patients with initial high levels of SAA are more likely to have worst chest computed tomography (CT) imaging [14]. The utility of monitoring SAA levels as predictor of prognosis in COVID-19 patients is supported also by other observations where high levels of SAA have been associated with unfavorable outcome [15].
The aim of this study intends to explore the possibility to use SAA in the diagnosis of COVID-19 infection, in particular as predictor of disease severity. The levels of serum amyloid A (SAA) were investigated in two groups of patients diagnosed with COVID-19 and classified as survivors or non-survivors, as well as in a control group (healthcare workers screened for internal surveillance). The levels of SAA were monitored in the patients on admission and on at least two weeks after hospital admission. Preliminary data support SAA as a good potential biomarker for predicting COVID-19 severity and prognosis.
2. Materials and methods
2.1. Patients
We conducted a retrospective study focusing on the significance of SAA in evaluating the severity and prognosis of COVID-19 patients admitted to our Institution (University Hospital Tor Vergata, PTV, Rome, Italy) from March 1 to April 30, 2020. The study was approved by the Hospital Ethics Committee (Registration Number: R.S.44.20) and conducted according to the revised Declaration of Helsinki. At admission, all patients provided written informed consent to anonymous data collection and analysis for research purposes. Confirmed COVID-19 patients were divided into two groups according to the outcomes of survival (n = 20; mean age 63.5 years ± 16.3 years) and death (n = 23; mean age 66.4 years ± 12.5 years), namely: survivors and non-survivors. Control group (n = 30; mean age 46.4 years ± 11.6 years) consists of healthcare workers screened for internal surveillance with negative nasopharyngeal swab.
2.2. Laboratory investigations
Suspected COVID-19 patients presenting clinical symptoms of cough, fever, dyspnoea and/or anosmia), and imaging tests (chest X-ray and/or computed tomography) suggestive of viral pneumonia were confirmed by molecular test using the Allplex™ 2019-nCoV assay (Seegene, Seoul, South Korea) run on the CFX96TMDx platform (Bio-Rad Laboratories, Inc., CA, USA) and interpreted by Seegene Viewer Software. RNA extraction and PCR set-up were performed on the NIMBUS platform, an automated liquid handling workstation (Seegene, Soul, South Korea).
Serum levels of hsCRP (reference range: 0.0–5.0 mg/L), were measured by immunoturbidimetric method (Abbott, Architect C-16000, Illinois, USA). Serum levels of interleukin-6 (IL-6; reference range: 0–50 pg/mL), were measured by chemiluminescent immunoassay (IMMULITE 2000XPi Immunoassay System, Siemens Healthcare Srl, Milan, Italy). Serum levels of ferritin (reference range: 21.81–274.66 ng/mL) and procalcitonin (PCT; reference range: 0.01–0.50 ng/mL) were measured by chemiluminescent methods (Abbott, Architect C-16000, Illinois, USA). Plasma fibrinogen concentrations (reference range: 200–400 mg/dL) were measured by the Clauss method (ACL-TOP instrumentation, Werfen, Milan, Italy). Plasma D-dimer levels (reference range: 0–500 ng/mL) were measured by latex enhanced immunoassay ACL-TOP instrumentation (Werfen, Milan, Italy). Serum Amyloid A (SAA) (reference range: <0.64 mg/dL) was measured by Siemens BN ProSpec nefelometer, using the Siemens N Latex SAA assay (Siemens Healthcare Srl, Milan, Italy).
All biochemical parameters were measured on survivors and non-survivors groups at two different time points: hospital admission (T1) and after 15 days from admission (T2).
2.3. Statistical analysis
Descriptive statistics such as frequency, percentage, mean and standard deviation (SD), median and percentiles were calculated. The normality of all the data were determined by Shapiro-Wilk normality test. In case of normal distribution, parametric tests were used such as Anova with Bonferroni post hoc test in case of more than two variables, or t-test in case of two variables. Hence, non-parametric tests, such as Kruskal–Wallis test and Mann–Whitney U test were used to test differences in groups. A p-value lower than 0.05 (p-value < 0.05) was considered statistically significant. ROC curve analysis was performed to assess sensitivity and specificity of different biochemical tests in survivors vs non-survivors COVID-19 patients.
All analyses were performed using Med Calc Ver.18.2.18 (MedCalc Software Ltd, Ostend, Belgium).
3. Results
We compared SAA levels in two groups of patients diagnosed with COVID-19: survivors and non-survivors. SAA levels were monitored upon admission to the hospital and two weeks after admission. Serum Amyloid A levels were also assessed in a control group of physicians and healthcare professionals with negative SARS-CoV-2 RT-PCR collected for internal surveillance.
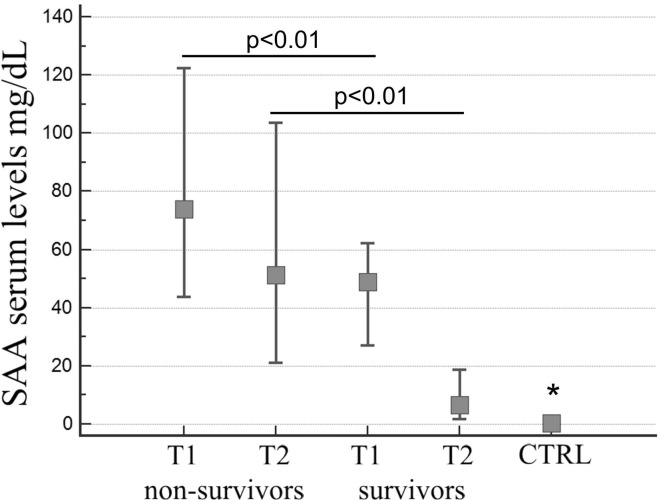
Data show a great increase of the SAA values compared to the normal reference range (<0.64 mg/dL) in the two groups of patients at admission (T1), along with a significant difference in concentration between them: the level of SAA in non-survivor group was significantly higher than survivor group, median value 74 mg/dL vs 48.75 mg/dL, respectively (Kruskal–Wallis test, p < 0.01; Fig. 1 ). The difference in SAA levels was even more evident after 15 days (T2) of hospitalization: while in the survivor group there was a reduction to a median value of 6.55 mg/dL, the non-survivor group still showed high median value levels 51.1 mg/dL (Kruskal–Wallis test, p < 0.01; Fig. 1). The SAA different levels observed in the two groups of patients at admission (T1) and after 15 days (T2) were statistically significant (Kruskal–Wallis test, p < 0.01; Fig. 1). The SAA levels in control group were in the reference range and statistically different from all the other groups with a median value of 0.35 mg/dL.
Fig. 1.
SAA serum levels in control group and in non-survivors and survivors groups at two different time points: hospital admission (T1), 15 days after admission (T2) (p < 0.01; Kruskal–Wallis test). Control group value was significantly different from all the other groups (* Kruskal–Wallis test).
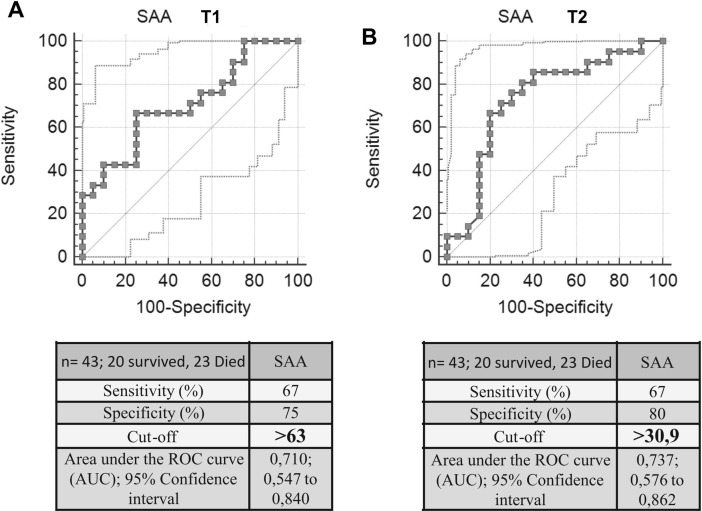
Furthermore, by ROC curves analysis was established a cut-off value for SAA levels able to differentiate the two groups of patients and therefore to predict patients’ prognosis. At T1, the cut-off value was 63 mg/dL with a sensitivity of 67% and a specificity of 75%. At T2, the group of survivors showed a marked decrease in SSA levels, confirmed by a lower cut-off of 30.9 mg/dL with a sensitivity of 67% and a specificity of 80% (Fig. 2 ).
Fig. 2.
ROC curve analysis of serum SAA in non-survivors and survivors groups. Panel A: hospital admission (T1). Panel B: 15 days after admission (T2).
Finally, the cut-offs of other biochemical parameters such as hsCRP, D-Dimer, IL-6, ferritin and procalcitonin were calculated. Data obtained are shown in Table 1 . Interestingly, even these parameters showed a significant decrease in the cut-off values between T1 (hospital admission) and T2 (15 days after admission), with the exception of ferritin.
Table 1.
Sensitivity, specificity, cut-off and area under curve (AUC) values of SAA, hsCRP, D-dimer, IL-6, Ferritin and Procalcitonin at two different time points: time of hospital admission (T1) and after 15 days from admission (T2).
| Time of hospital admission (T1) |
||||||
|---|---|---|---|---|---|---|
| n = 43; 20 survived, 23 Died | SAA | hsCRP | D-Dimer | IL-6 | Ferritin | Procalcitonin |
| Sensitivity (%) | 67 | 61 | 44 | 28 | 78 | 52 |
| Specificity (%) | 75 | 80 | 100 | 92 | 70 | 80 |
| Cut-off | >63 | >154,3 | >5598 | >151 | >360 | >0,23 |
| Area under the ROC curve (AUC); 95% Confidence interval | 0,710; 0,547 to 0,840 | 0,707; 0,548 to 0,835 | 0,746; 0,590 to 0,866 | 0,505; 0,332 to 0,678 | 0,754; 0,599 to 0,873 | 0,653; 0,493 to 0,792 |
| After 15 days from admission (T2) | ||||||
| n = 43; 20 survived, 23 Died | SAA | hsCRP | D-Dimer | IL-6 | Ferritin | Procalcitonin |
| Sensitivity (%) | 67 | 83 | 53 | 84 | 70 | 100 |
| Specificity (%) | 80 | 75 | 75 | 62 | 90 | 48 |
| Cut-off | >30,9 | >34,2 | >1732 | >31,4 | >390 | >0,09 |
| Area under the ROC curve (AUC); 95% Confidence interval | 0,737; 0,576 to 0,862 | 0,798; 0,648 to 0,905 | 0,707; 0,548 to 0,835 | 0,749; 0,565 to 0,885 | 0,870; 0,732 to 0,953 | 0,781; 0,627 to 0,894 |
4. Discussion
Several biochemical parameters have been assessed as possible predictors of disease severity since the beginning of the COVID-19 pandemic. Elevated levels of IL-6, D-Dimer, hsCRP, neutrophils, and low lymphocyte count have been associated with severe forms of COVID-19 as well as high neutrophil-lymphocyte ratio [16]. Nevertheless, a clear and useful predictor of disease severity still needs to be defined.
The clinical value of SAA, a marker of inflammation, has been attracting increasing attention during COVID-19 pandemic. Several studies showed that SAA levels in patients with severe respiratory syndrome are significantly high [17], [18], suggesting that SAA may be an important biomarker in monitoring respiratory diseases.
Macrophages recognize pathogen-associated molecular patterns and trigger innate immunity and host defences. Macrophages activation can release tumor necrosis factor α (TNF-α), interleukin 1 (IL-1), nitric oxide (NO), and reactive oxygen species (ROS) to induce a severe inflammatory response, which boost liver cells to produce SAA [9]. Patients with severe acute respiratory syndrome had significantly increased level of SAA, suggesting SAA could be used as a biomarker to monitor the progression of respiratory diseases [14]. SAA is able to promote inflammatory response through chemokines activation; thus, when SAA is activated, even at low concentrations, it could promote inflammation by activating chemokines and inducing chemotaxis [19], [20].
Therefore, SAA appears as a promising biomarker for predicting disease severity [14], [15]. Here, we show that SAA level can be informative since the admission of COVID-19 patients in the hospital. Actually, differences in the level of SAA between the groups of survivors and non-survivors analyzed were found at the moment of their admission with the last ones having a statistically significant higher level (74 mg/dL vs 48.75 mg/dL). Afterwards, the level of SAA decreased in both patient groups but the non-survivors continued to have high levels (51.1 mg/dL vs 6.55 mg/dL) respect to the survivor group. ROC curve analysis confirmed this trend in the groups of patients as demonstrated by the cut-off values of SAA determined at T1 and T2. During this 15 days period, the SAA cut-off value dropped from 63 mg/dl to 30.9 mg/dl corresponding to a sensitivity of 67% and a specificity of 80%. A similar decreasing trend was observed for hsCRP (154.3 mg/L vs 34.2 mg/L), D-Dimer (5598 ng/mL vs 1732 ng/mL), IL-6 (151 pg/mL vs 31.4 pg/mL) and procalcitonin (0.23 ng/mL vs 0.09 ng/mL) values, except for ferritin (360 ng/mL vs 390 ng/mL). Thus, a decline in the SAA levels after hospital admission is suggestive of a favorable prognosis, in addition to the decrease in D-Dimer, hsCRP, IL-6 and procalcitonin levels observed in our patients.
The study has some limitations. The small sample size reduces the power of the study; however, considering the data as whole they suggest that the levels of SSA, D-Dimer, hsCRP, IL-6 and procalcitonin might be used as a predictive algorithm of COVID-19 prognosis since the admission of the patients in the hospital. SAA predicts the severity of COVID-19 and can distinguish critically ill patients from mild ones as already reported [14], [21]. SAA might be a useful biomarker to monitor the complicated clinical course of the disease.
5. Conclusions
SAA might be a good prognostic marker in COVID-19 disease alone and/or in combination with other inflammatory biomarkers such as D-Dimer, hsCRP, IL-6 and procalcitonin. Identification of reliable prognostic analytes is of great clinical relevance, as it would improve patient management besides being costs saving.
CRediT authorship contribution statement
Massimo Pieri: Conceptualization, Investigation, Formal analysis, Methodology, Visualization, Writing - original draft, Writing - review & editing. Marco Ciotti: Conceptualization, Investigation, Formal analysis, Methodology, Visualization, Writing - original draft, Writing - review & editing. Marzia Nuccetelli: Conceptualization, Investigation, Formal analysis, Methodology, Visualization, Writing - original draft, Writing - review & editing. Marco Alfonso Perrone: Formal analysis, Methodology, Visualization. Maria Teresa Caliò: Formal analysis, Methodology, Visualization. Maria Stella Lia: Formal analysis, Visualization, Investigation, Methodology. Marilena Minieri: Conceptualization, Visualization. Sergio Bernardini: Conceptualization, Visualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The Authors would like to thank Rosanna Di Maio for her support; Siemens Healthcare for kindly providing kits and instrument for this study.
References
- 1.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.of the International, C.S.G., The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2, Nat. Microbiol. 5(4), 536. [DOI] [PMC free article] [PubMed]
- 3.Akhmerov A., Marban E. COVID-19 and the Heart. Circ. Res. 2020;126:1443–1455. doi: 10.1161/CIRCRESAHA.120.317055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization, Coronavirus disease 2019 (COVID-19) Situation Report-29 December 2020, 2020.
- 5.Lippi G., Plebani M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin. Chem. Lab. Med. 2019;58(2020):1063–1069. doi: 10.1515/cclm-2020-0240. [DOI] [PubMed] [Google Scholar]
- 6.Bhargava A., Fukushima E.A., Levine M., Zhao W., Tanveer F., Szpunar S.M., Saravolatz L. Predictors for Severe COVID-19 Infection. Clin. Infect. Dis. 2020;71:1962–1968. doi: 10.1093/cid/ciaa674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C.Z., Hu S.L., Wang L., Li M., Li H.T. Early risk factors of the exacerbation of coronavirus disease 2019 pneumonia. J. Med. Virol. 2019;92(2020):2593–2599. doi: 10.1002/jmv.26071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippi G., Favaloro E.J. D-dimer is Associated with Severity of Coronavirus Disease 2019: A Pooled Analysis. Thromb. Haemost. 2020;120:876–878. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X., Tang J., Shuai W., Meng J., Feng J., Han Z. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm. Res. 2020;69:883–895. doi: 10.1007/s00011-020-01378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Lami R.A., Urban R.J., Volpi E., Algburi A.M.A., Baillargeon J. Sex Hormones and Novel Corona Virus Infectious Disease (COVID-19) Mayo Clin. Proc. 2020;95:1710–1714. doi: 10.1016/j.mayocp.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaskins A.J., Wilchesky M., Mumford S.L., Whitcomb B.W., Browne R.W., Wactawski-Wende J., Perkins N.J., Schisterman E.F. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am. J. Epidemiol. 2012;175:423–431. doi: 10.1093/aje/kwr343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caricchio R., Gallucci M., Dass C., Zhang X., Gallucci S., Fleece D., Bromberg M., Criner G.J. Preliminary predictive criteria for COVID-19 cytokine storm. Ann. Rheum. Dis. 2021;80:88–95. doi: 10.1136/annrheumdis-2020-218323. [DOI] [PubMed] [Google Scholar]
- 13.Jensen L.E., Whitehead A.S. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem. J. 1998;334(Pt 3):489–503. doi: 10.1042/bj3340489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H., Xiang X., Ren H., Xu L., Zhao L., Chen X., Long H., Wang Q., Wu Q. Serum Amyloid A is a biomarker of severe Coronavirus Disease and poor prognosis. J. Infect. 2020;80:646–655. doi: 10.1016/j.jinf.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu J., Huang P.P., Zhang S., Yao Q.D., Han R., Liu H.F., Yang Y., Zhang D.Y. The value of serum amyloid A for predicting the severity and recovery of COVID-19. Exp. Ther. Med. 2020;20:3571–3577. doi: 10.3892/etm.2020.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q., Dai Y., Feng M., Wang X., Liang W., Yang F. Associations between serum amyloid A, interleukin-6, and COVID-19: A cross-sectional study. J. Clin. Lab. Anal. 2020;34 doi: 10.1002/jcla.23527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yip T.T., Chan J.W., Cho W.C., Wang Z., Kwan T.L., Law S.C., Tsang D.N., Chan J.K., Lee K.C., Cheng W.W., Ma V.W., Yip C., Lim C.K., Ngan R.K., Au J.S., Chan A., Lim W.W. Protein chip array profiling analysis in patients with severe acute respiratory syndrome identified serum amyloid a protein as a biomarker potentially useful in monitoring the extent of pneumonia. Clin. Chem. 2005;51:47–55. doi: 10.1373/clinchem.2004.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S.L., Wang S.Y., Sun Y.F., Jia Q.Y., Yang C.L., Cai P.J., Li J.Y., Wang L., Chen Y. Expressions of SAA, CRP, and FERR in different severities of COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2020;24:11386–11394. doi: 10.26355/eurrev_202011_23631. [DOI] [PubMed] [Google Scholar]
- 19.De Buck M., Gouwy M., Wang J.M., Van Snick J., Proost P., Struyf S., Van Damme J. The cytokine-serum amyloid A-chemokine network. Cytokine Growth Factor Rev. 2016;30:55–69. doi: 10.1016/j.cytogfr.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connolly M., Rooney P.R., McGarry T., Maratha A.X., McCormick J., Miggin S.M., Veale D.J., Fearon U. Acute serum amyloid A is an endogenous TLR2 ligand that mediates inflammatory and angiogenic mechanisms. Ann. Rheum. Dis. 2016;75:1392–1398. doi: 10.1136/annrheumdis-2015-207655. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Chang Y., Su P., Wang X. A Pair-Wise Meta-Analysis Highlights Serum Amyloid Has A Potential Clinical Value for Diagnosis and Prognosis Prediction of Gastric Cancer. Clin. Lab. 2020;66 doi: 10.7754/Clin.Lab.2019.190808. [DOI] [PubMed] [Google Scholar]