Abstract
Background
Care pathways are primarily aimed at decreasing length of hospital stay (LOS) and preventing unnecessary costs while maintaining or improving the quality of care. In laparoscopic cholecystectomy, there is insufficient evidence for proving an impact upon postoperative complications.
Methods
In this retrospective study, logistic regression was used to calculate a propensity score, and, after carrying out 1:1 nearest-neighbor matching, 296 patients were analyzed in both groups with regard to postoperative complications using the Clavien-Dindo classification system as a primary aim. In addition, secondary aims were LOS, compliance to care, and deviation from the care pathway with respect to patient discharge. Relative risk of the primary outcome was calculated and compared with the e-value as sensitivity testing approach.
Results
Due to the mandatory part of the care pathway, patient record compliance was 100%. Deviation from the care pathway with respect to the planned patient discharge on postoperative day 2 was noted in 16% of the cases. After adjustment for potential factors, the relative risk when comparing Clavien-Dindo complication grades 0 versus 1–4 is 1.64 (95% CI 0.87–3.11), which did not reach significance (p = 0.127). After matching, LOS lasted 3.69 days without and 3.26 days with the care pathway, respectively.
Conclusions
Against the background of already implemented structured standard operation procedures, a care pathway is not able to reduce postoperative complications. Nevertheless, we consider our clinical pathway a highly valuable tool for the interdisciplinary management of patient hospitalization under the supervision of experienced specialized surgeons.
Keywords: Clinical pathway, Laparoscopic cholecystectomy, Clavien-Dindo complication grading, Postoperative complications
Introduction
Since the Institute of Medicine published its report “To Err is Human,” a wealth of interventions has been implemented in different health care systems reducing the burden of surgical harm [1, 2]. Thus, in a systematic review of the interventions used to reduce adverse events in surgery, the following procedures produced a significant decrease in mortality and morbidity: improving nurse/patient ratios, physician involvement in postoperative care in intensive care units, subspecialization, submission of outcome data to national audits, use of safety checklists, team training, and adherence to a care pathway [2]. Besides structural and process improvements, the impact of nontechnical skills on technical performance in surgery has also been highlighted [3].
Care pathways (synonyms: case management plans, critical pathways, clinical pathways, CPWs, care map, or integrated care pathway) were primarily aimed at decreasing length of hospital stay (LOS) and preventing unnecessary costs while maintaining or improving the quality of care [4, 5]. In an attempt to assess the effect of CPWs, Rotter et al. [6] performed a Cochrane systematic review. For this analysis, the authors defined CPWs as follows: (1) The intervention was a structured multidisciplinary plan of care. (2) The intervention was used to channel the translation of guidelines or evidence into local structures. (3) The intervention detailed the steps in a course of treatment or care in a plan, pathway, algorithm, guideline, protocol, or other inventory of actions. (4) The intervention had time frames of criteria-based progression (i.e., steps were taken if designated criteria were met). (5) The intervention aimed to standardize care for a specific clinical problem, procedure, or episode of care in a specific population. After applying these inclusion criteria, the authors concluded that CPWs are associated with reduced inhospital complications.
In a meta-analysis of 7 randomized controlled trials, Zhang et al. [7] analyzed the clinical effects of CPW implementing for laparoscopic cholecystectomy (LC). The authors came to the conclusion that CPW application for LC effectively reduced hospital stay and total costs. However, there was insufficient evidence for proving an impact upon postoperative complications. Therefore, our study addressed the question of whether CPW implementation has the potential of reducing postoperative complications using a widely used complication grading system.
Materials and Methods
The present study was created on the basis of STROBE, SQUIRE 2.0, and STaRI guidelines (Strengthening the Reporting of Observational Studies in Epidemiology; Standards for Quality Improvement Reporting Excellence; Standards for Reporting Implementation Studies) [8, 9, 10].
Study Design and Overview
This retrospective cohort study analyzed a total of 696 patients, i.e., all patients with acute cholecystitis or symptomatic cholecystolithiasis who underwent LC at the Department of General, Visceral, and Thoracic Surgery, Asklepios Hospital Langen, Germany, from 2013 through 2016. No extra patient consent was required because of the retrospective study design; data confidentiality and permission of data review were provided in the hospital admission consent.
Setting
The Asklepios Clinic in Langen is a 400-bed hospital of basic and regular care and an academic teaching hospital of the Goethe University in Frankfurt/Main.
The following departments exist at the hospital: the Department of Gynecology and Obstetrics with an Interdisciplinary Breast Center; Medical Clinic I (Cardiology) with a Chest Pain Unit; Medical Clinic II (Gastroenterology, Pneumology, Hepatology, Infectious Diseases, and Oncology); General, Visceral, and Thoracic Surgery; Orthopedics and Trauma Surgery with a certified Arthroplasty Center and a certified Trauma Center; Anesthesia and Peri-Operative Medicine with 14 interdisciplinary intensive care unit beds and equipment for acute renal dialysis, MARS (molecular adsorbent recirculating system) treatment, and ECMO (extracorporeal membrane oxygenation).
The main focus of the Department of General, Visceral, and Thoracic Surgery is minimally invasive techniques. The staff consists of 1 surgical chair, 4 senior surgeons, and 11 surgical residents. Cholecystectomy for acute cholecystitis is always performed by a senior physician or the chair. Indications for endoscopic retrograde cholangiopancreatography and for the treatment of acute cholecystitis are standardized by department policy. All cholecystectomies conform to a treatment protocol in accordance with the requirements of a peer review project of the Hesse State Chamber of Physicians, Germany. In addition, the department is a regional study center of CHIR-Net, a surgical study network, and is certified by CAMIC (Chirurgische Arbeitsgemeinschaft für Minimal-Invasive Chirurgie, i.e., surgical working group of minimally invasive surgery) of the German Association of General and Visceral Surgery.
Patients and CPWs
Patients underwent LC within a period of 2 years before CPW implementation (2013–2014) as well as a period of 2 years thereafter (2015–2016). The CPW is described in detail in online supplementary chart 1 (for all online suppl. material, see www.karger.com/doi/10.1159/000506718). It is a mandatory part of the patients' record and was developed by surgeons and nurses of the Department.
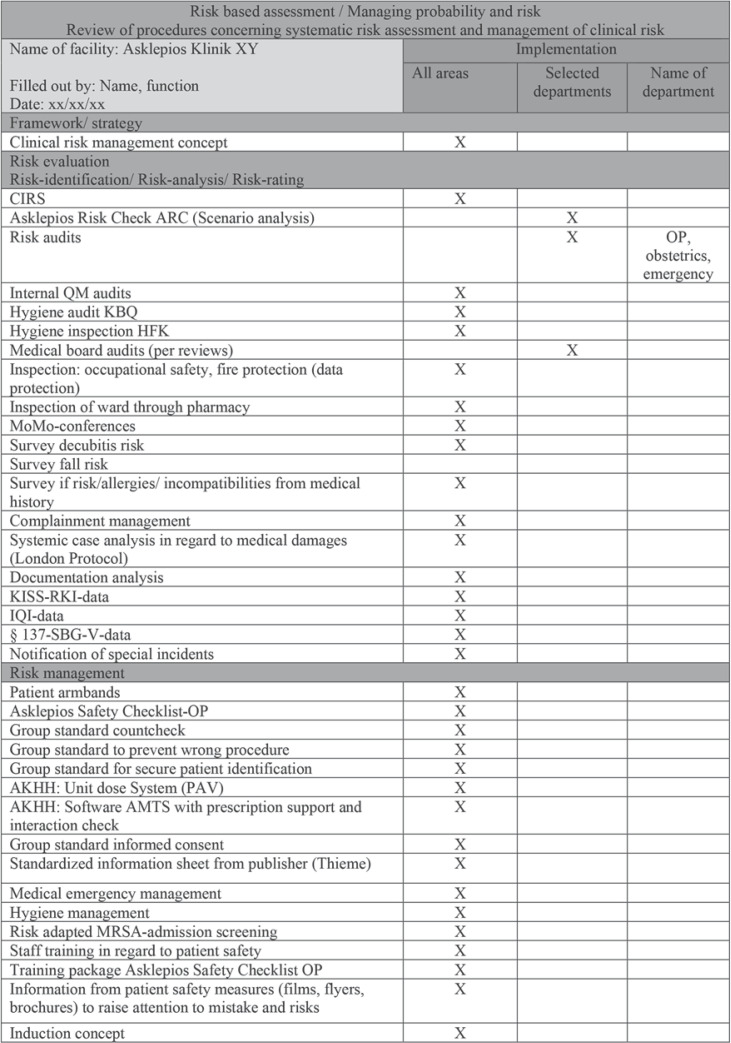
Information about implemented risk management tools is provided in Figure 1 (for the peer review protocol of the Hesse State chamber of physicians see https://tinyurl.com/y67lj6e8 and for requirements for the clinical documentation and surgical standard operation procedure see https://tinyurl.com/y6k4999o and https://tinyurl.com/y5cxgcco).
Fig. 1.
Risk-based assessment/managing probability and risk at the Asklepios Clinics in Germany.
Internal and external audits are performed on a regular basis, e.g., every 3 years the whole hospital is externally audited by a KTQ (Cooperation for Transparency and Quality) team according to PDSA (Plan, Do, Study, Act) cycles and reviews the domains patient orientation, employee orientation, security risk management, information, and communication, corporate governance, and quality management.
Statistics and Propensity Score Matching
Continuous variables were presented as means and standard deviation (SDs) and compared between groups with Wilcoxon-Mann-Whitney test. Categorical variables were presented as counts with percentages and compared with χ2 and Fisher tests, as appropriate. All tests were two sided and used a significance level of α = 5%. The relative risk of the primary outcome (Clavien-Dindo complication grades 0 vs. 1–4) was calculated and compared with the e-value as sensitivity testing approach [11].
In order to increase the comparability of both treatment groups, propensity score matching was carried out. The following parameters were used for propensity matching: age; gender; American Society of Anesthesiologists (ASA) score, and the differentiation between acute versus chronic inflammation according to the pathological report. From a pool of a total of 696 patients (296 patients without and 400 patients with CPW), logistic regression was used to calculate a propensity score for each patient. With regard to this propensity score, 1:1 nearest-neighbor matching was carried out for both treatment groups. A caliper width of 0.2 SDs of the propensity score logit was used as a basis for this. After matching, 296 patients were analyzed in both groups. The effect of the matching procedure was described by standardized mean differences [12] and the respective nonparametric tests.
Statistical analysis was performed with R (R Foundation of Statistical Computing, Vienna, Austria) and BiAS for Windows (epsilon, Frankfurt, Germany).
Results
Overall, 296 patients without and 400 patients with CPW were included in the study. CPW compliance was 100% whereas deviations from the pathway with respect to the planned discharge date of the patients were observed in 16% of the patients, i.e., patients stayed longer primarily because of pain.
Table 1 summarizes the patients' clinical characteristics before any adjustments and Table 2 the corresponding Clavien-Dindo complication grading, respectively. Here, a significant increase in unfavorable outcomes as described by the Clavien-Dindo complication grading can be observed in the CPW cohort. Nevertheless, the ASA score was also significant higher in the CPW cohort.
Table 1.
Clinical characteristics − before propensity score matching
| Without CPW (n = 296) | With CPW (n = 400) | STHiff | p value | |
|---|---|---|---|---|
| Age (mean ± SD), years | 55.03±15.90 | 53.80±15.75 | 0.077 | 0.255 |
| Sex, n (%) | 0.587 | |||
| Female | 194 (65.5) | 253 (63.2) | 0.048 | |
| Male | 102 (34.5) | 147 (36.8) | −0.048 | |
| ASA, n (%) | 0.0013 | |||
| 1 | 57 (19.3) | 47 (11.8) | 0.209 | |
| 2 | 231 (78.0) | 324 (81.0) | −0.073 | |
| 3 | 8 (2.7) | 29 (7.2) | −0.210 | |
| Pathological report, n (%) | 0.524 | |||
| Chronic | 254 (85.8) | 351 (87.8) | −0.057 | |
| Acute | 42 (14.2) | 49 (12.2) | 0.057 |
CPW, clinical pathway; STDiff, standardized mean difference; SD, standard deviation; ASA, American Society of Anesthesiologists score.
Table 2.
Clavien-Dindo complication grading − before propensity score matching
| Without CPW (n = 296) | With CPW (n = 400) | STHiff | p value | |
|---|---|---|---|---|
| 0, n (%) | 282 (95.3) | 360 (90.0) | 0.203 | 0.0003 |
| 1, n (%) | 2 (0.7) | 23 (5.8) | −0.291 | |
| 2, n (%) | 11 (3.7) | 9 (2.2) | 0.086 | |
| 3, n (%) | 1 (0.3) | 5 (1.2) | −0.103 | |
| 3, n (%) | 0 (0.0) | 3 (0.8) | −0.123 |
Furthermore, LOS (in days) was compared between both cohorts. Before matching it was significantly shorter in the group with than without CPW (mean ± SD 3.32 ± 2.60 vs. 3.69 ± 2.70 days, respectively; p < 0.0001).
To account for differences in patient characteristics, propensity score matching was performed. Table 3 summarizes the patients' clinical characteristics after propensity score matching. After adjusting, both groups were comparable with respect to age, sex, ASA classification, and pathological report. Nevertheless, a significant increase in complications could still be observed in the CPW cohort (Table 4).
Table 3.
Clinical characteristics − after propensity score matching
| Without CPW (n = 296) | With CPW (n = 296) | STHiff | p value | |
|---|---|---|---|---|
| Age (mean ± SD), years | 55.03±15.90 | 56.96±13.85 | −0.130 | 0.195 |
| Sex, n (%) | 0.931 | |||
| Female | 194 (65.5) | 196/296 (66.2) | −0.014 | |
| Male | 102 (34.5) | 100/296 (33.8) | 0.014 | |
| ASA, n (%) | 0.167 | |||
| 1 | 57 (19.3) | 40 (13.5) | 0.156 | |
| 2 | 231 (78.0) | 248 (83.8) | −0.147 | |
| 3 | 8 (2.7) | 8 (2.7) | 0.000 | |
| Pathological report, n (%) | 0.263 | |||
| Chronic | 254 (85.8) | 264 (89.2) | −0.102 | |
| Acute | 42 (14.2) | 32 (10.8) | 0.102 |
See footnote to Table 1 for abbreviations.
Table 4.
Clavien-Dindo complication grading − after propensity score matching
| Without CPW (n = 296) | With CPW (n = 296) | STHiff | p value | |
|---|---|---|---|---|
| 0, n (%) | 282 (95.3) | 273 (92.2) | 0.126 | 0.0035 |
| 1, n (%) | 2 (0.7) | 15 (5.1) | −0.265 | |
| 2, n (%) | 11 (3.7) | 6 (2.0) | 0.101 | |
| 3, n (%) | 1 (0.3) | 1 (0.3) | 0.000 | |
| 4, n (%) | 0 (0.0) | 1 (0.3) | −0.082 |
See footnote to Table 1 for abbreviations.
After adjustment, LOS was significantly shorter in the group with than without CPW (mean ± SD 3.26 ± 2.63 vs. 3.69 ± 2.70 days, respectively; p < 0.0001).
Comparing Clavien-Dindo complication grades 0 versus 1–4 between both groups results in a relative risk of 1.64 (95% CI 0.87–3.11; p = 0.127) after adjustment. The corresponding e-value is 2.67.
Discussion
Key Results
Our experience and data analysis confirm that the introduction of the pathway shortened LOS from 3.69 to 3.32 days. By the end of the study period, 84% of the patients could be discharged on the 3rd postoperative day as planned after CPW implementation. The relative risk of complications was not changed by the pathway.
Interpretation
The primary goal of the surgical care process is to provide the safest, most effective, and most efficient care possible. CPWs provide a framework for determining whether quality care is being delivered in a timely and cost-effective manner, and for defining the acceptable anticipated LOS for a specific patient population. They facilitate the coordination of patient care delivery for a specific subset of patients through the use of a standardized interdisciplinary process. Furthermore, CPWs provide directions for care by delineating clinical goals and desired patient outcomes [13]. Thus, CPWs are being developed to optimize perioperative management, systemize nursing interventions, shorten LOS, improve patient satisfaction, and reduce medical costs in general for diseases with high incidence and for those for which treatment can easily be standardized as is the case for LCs. They have the following advantages according to Pearson et al. [14]: First, the quality of medical care improves, i.e., the quality of medical treatment can be elevated at least to a certain level by presenting indispensable tasks. Our results show that by developing and implementing an explicit pathway, which dictates all aspects of patient care (including individual physician and nurses practices) would reset the expectations for what was perceived as a normal postoperative course after LC. In addition, as an CPW advantage, systematization of medical practice simplifies the tasks of medical statistics and work schedules for various professionals, such as physicians, nurses, pharmacists, and nutritionists [15]. As outlined in our pathway, it primarily details an administrative structure that provides a common, institution-wide template for the care of these patients. This pathway details the aspects of care that we sought to standardize across the institution and was directed at minimizing variance in the care provided by rotating health care providers, who may interact with these patients only intermittently (e.g., residents, nurses, and students) [16]. In effect, after a CPW training period of 2 months, an integral part of the patient's record (see online suppl. Chart 1) was considered as highly valuable tool for the management of patients during their LOS by nurses and physicians, i.e., the compliance rate, which is rarely reported in the literature, was 100%.
The 12% reduction in LOS by the CPW seems low in comparison with a reported change of −15.4% (hospital stay 6.0 days without CPW vs. 5.1 with CPW) [17]. In contrast, the mean CPW-LOS in our study was 3.26 days. Still, the hospital stay in our study seems high in comparison with other health care systems. However, epidemiological data from Germany for the year 2014 demonstrate a mean LOS of 6.8 days [18]. Deviation from our pathway with regard to the LOS occurred in 16%, i.e., 84% of patients could be discharged on the 3rd postoperative day as planned. The main cause for this CPW deviation was postoperative pain.
Although the p value indicates an increase in complications with the use of a CPW, the relative risk did not change significantly. At the same time, the e-values. a tool for measuring unknown confounders, was only slightly elevated. The observed risk ratio of 1.6 could be explained away by an unmeasured confounder that was associated with both the CPW (with/without) and Clavien-Dindo classification (0 vs. 1–4) by a 2.7-fold risk ratio each, above and beyond the measured confounders, but weaker confounding could not do so. The lowest possible e-value is 1 (i.e., no unmeasured confounding is needed to explain the observed association away). The higher the e-value, the stronger the confounder association must be to explain the effect away.
Thus, the result that our CPW had no impact upon complications seems to be valid. An explanation may be that new quality assurance measures do not have a great impact on an institution with already high safety standards (see Fig. 1; https://tinyurl.com/y67lj6e8). This is particularly evident in the comment by Vanhaecht et al. [19]: The implementation of CPWs to reduce complications in an already well-functioning team may not improve outcomes.
Limitations
The main limitation of our study is its retrospective design along with the question about the nature and quality of the baseline measurements. The existing data may be incomplete or inaccurate or measured in ways that are not ideal for answering the research question [20]. Otherwise, the comprehensive, standardized, and mandatory audits of our record documentation prove a very high adherence to preset standards by nurses and physicians (see https://tinyurl.com/y2qmbv8d). That is why we think that our data and the standardized parameters analyzed in our study are of a robust nature (e.g., surgical procedure, histopathology, and LOS duration), meaning these data are available in every case.
An additional limitation may be, against the background of a low incidence of complications (according to a 2018 analysis of 12,681 cholecystectomies in the federal state Hesse/Germany, reinterventions due to complications occurred in 2.62% [21] vs. 1.02% in our clinic), that our study (as is the case for all studies in the literature analyzing the impact of CPWs upon complications) is underpowered with respect to the number of patients. When assuming a reduction in complications from 2.62 to 1%, 1,137 patients per group are needed for an adequate statistical power of 80% with a significance level of α = 5% (calculated with PASS version 14; NCSS, LLC, Kaysville, UT, USA). Such a large sample size seems unrealistic to achieve.
In the present study, the propensity score matching was used to make both groups as comparable as possible. Another limitation may be that we used the ASA score to address the risk score of every patient for propensity score matching which could be to general, but in our opinion it is a validated score making the patient groups comparable for propensity score matching.
Standardized mean differences of clinical characteristics after matching were all in respective reference ranges indicating balanced data. Even though there remain well-known limitations because of nonobservable confounders with such an approach in comparison to a randomized clinical trial, the number of studies using propensity score matching is increasing [22]. Additionally, propensity score matching seems to be a good and reliable alternative if the implementation of a randomized controlled trial is difficult, as it is for example for CPW evaluation [17, 23].
Conclusion
By introducing CPW as a mandatory part of the patient's record, a compliance rate of 100% can be achieved. As reported in the literature for a variety of surgical procedures, a reduction in LOS could be reached, but expectations to reduce the complication rate were not met, which may be explained against the background of already implemented risk management tools. Nevertheless, we consider our CPW as a highly valuable tool for the interdisciplinary management of patient hospitalization under the supervision of experienced specialized surgeons. Overall, in the armamentarium of interventions reducing the burden of surgical harm, we consider CPWs as a tessera in hospital safety culture [2].
Statement of Ethics
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. No extra patient consent was required because of the retrospective study design.
Disclosure Statement
D. Arabacioglu, B. Albers, A. Lehn, E. Herrmann, E. Hanisch, and A. Buia declare that they have no conflicts of interest or financial ties to disclose.
Funding Sources
There were no funding sources.
Authors Contributions
D. Arabacioglu did the data acquisition and interpretation, literature research, and wrote and revised the manuscript, B. Albers revised the manuscript, A. Lehn and E. Herrmann did the statistical analyses, data interpretation, wrote the statistical part, and revised the manuscript, E. Hanisch did the literature research, and wrote and revised the manuscript, A. Buia did the literature research, contributed Tables 1, 2, 3, 4, and wrote and revised the manuscript.
Supplementary Material
Supplementary data
References
- 1.Kohn LT, Corrigan JM, Donaldson MS. To Err Is Human: Building a Safer Health System. Washington (D C): The National Academy Press; 2000. [PubMed] [Google Scholar]
- 2.Howell AM, Panesar SS, Burns EM, Donaldson LJ, Darzi A. Reducing the burden of surgical harm: a systematic review of the interventions used to reduce adverse events in surgery. Ann Surg. 2014 Apr;259((4)):630–41. doi: 10.1097/SLA.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 3.Hull L, Arora S, Aggarwal R, Darzi A, Vincent C, Sevdalis N. The impact of nontechnical skills on technical performance in surgery: a systematic review. J Am Coll Surg. 2012 Feb;214((2)):214–30. doi: 10.1016/j.jamcollsurg.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Vanhaecht K, Panella M, Van Zelm R, Sermeus W. An overview on the history and concept of care pathways as complex interventions. Int J Care Pathways. [DOI]
- 5.Rotter T, Kinsman L, James E, Machotta A, Willis J, Snow P, et al. Have we drawn the wrong conclusions about the value of care pathways? Is a Cochrane review appropriate? Response to the commentary article published by Kris Vanhaecht et al. Eval Health Prof. 2012 Mar;35((1)):43–6. doi: 10.1177/0163278711409209. [DOI] [PubMed] [Google Scholar]
- 6.Rotter T, Kinsman L, James EL, Machotta A, Gothe H, Willis J, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database of Systematic Review and meta-analysis. Eval Health Prof. 2010 Mar;3:CD006632. doi: 10.1002/14651858.CD006632.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Zhou SY, Xing MY, Xu J, Shi XX, Zheng SS. The application of clinical pathways in laparoscopic cholecystectomy. Hepatobiliary Pancreat Dis Int. 2014 Aug;13((4)):348–53. doi: 10.1016/s1499-3872(14)60279-4. [DOI] [PubMed] [Google Scholar]
- 8.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. STROBE Initiative Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration10* for the STROBE initiative. Int J Surg. 2014 Dec;12((12)):1500–24. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016 Dec;25((12)):986–92. doi: 10.1136/bmjqs-2015-004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinnock H, Barwick M, Carpenter CR, Eldridge S, Grandes G, Griffiths CJ, et al. StaRI Group Standards for Reporting Implementation Studies (StaRI): explanation and elaboration document. BMJ Open. 2017 Apr;7((4)):e013318. doi: 10.1136/bmjopen-2016-013318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Der Weele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-Value. Ann Intern Med. 2017 Aug;167((4)):268–74. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 12.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009 Nov;28((25)):3083–107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topal B, Peeters G, Verbert A, Penninckx F. Outpatient laparoscopic cholecystectomy: clinical pathway implementation is efficient and cost effective and increases hospital bed capacity. Surg Endosc. 2007 Jul;21((7)):1142–6. doi: 10.1007/s00464-006-9083-x. [DOI] [PubMed] [Google Scholar]
- 14.Pearson SD, Kleefield SF, Soukop JR, Cook EF, Lee TH. Critical pathways intervention to reduce length of hospital stay. Am J Med. 2001 Feb;110((3)):175–80. doi: 10.1016/s0002-9343(00)00705-1. [DOI] [PubMed] [Google Scholar]
- 15.Uchiyama K, Takifuji K, Tani M, Onishi H, Yamaue H. Effectiveness of the clinical pathway to decrease length of stay and cost for laparoscopic surgery. Surg Endosc. 2002 Nov;16((11)):1594–7. doi: 10.1007/s00464-002-9018-0. [DOI] [PubMed] [Google Scholar]
- 16.Calland JF, Tanaka K, Foley E, Bovbjerg VE, Markey DW, Blome S, et al. Outpatient laparoscopic cholecystectomy: patient outcomes after implementation of a clinical pathway. Ann Surg. 2001 May;233((5)):704–15. doi: 10.1097/00000658-200105000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller MK, Dedes KJ, Dindo D, Steiner S, Hahnloser D, Clavien PA. Impact of clinical pathways in surgery. Langenbecks Arch Surg. 2009 Jan;394((1)):31–9. doi: 10.1007/s00423-008-0352-0. [DOI] [PubMed] [Google Scholar]
- 18.Bundesauswertung zum Erfassungsjahr. 2014. 12/1 − Cholezystektomie. Qualitätsindikatoren. [cited 2019 May 11]. Available from: https://www.sqg.de/downloads/Bundesauswertungen/2014/bu_Gesamt_12N1-CHOL_2014.pdf.
- 19.Vanhaecht K, Ovretveit J, Elliott MJ, Sermeus W, Ellershaw J, Panella M. Have we drawn the wrong conclusions about the value of care pathways? Is a Cochrane review appropriate? Eval Health Prof. 2012 Mar;35((1)):28–42. doi: 10.1177/0163278711408293. [DOI] [PubMed] [Google Scholar]
- 20.Hulley SB, Cummings SR, Browner WS. Grady D g., Newman TB Designing clinical research. 4th ed. Philadelphia: Lippincott Williams & Wilkins, a Wolters Kluwer business; 2013. [Google Scholar]
- 21.Geschäftsstelle Qualitätssicherung Hessen Cholecystektomie - Jahresauswertung. 2018. [cited 2019 May 11]. Available from: https://www.gqhnet.de/leistungsbereiche/Cholezystektomie/12-1-cholezystektomie.
- 22.Lonjon G, Porcher R, Ergina P, Fouet M, Boutron I. Potential pitfalls of reporting and bias in observational studies with propensity score analysis assessing a surgical procedure: A Methodological Systematic Review. Ann Surg. 2017 May;265((5)):901–9. doi: 10.1097/SLA.0000000000001797. [DOI] [PubMed] [Google Scholar]
- 23.Lonjon G, Boutron I, Trinquart L, Ahmad N, Aim F, Nizard R, et al. Comparison of treatment effect estimates from prospective nonrandomized studies with propensity score analysis and randomized controlled trials of surgical procedures. Ann Surg. 2014 Jan;259((1)):18–25. doi: 10.1097/SLA.0000000000000256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data