Abstract
Background
Treatment outcomes of laparoscopic liver resection (LLR) and percutaneous radiofrequency ablation (p-RFA) for small single hepatocellular carcinomas (HCCs) have not yet been fully compared. The aim of this study was to compare LLR and p-RFA as first-line treatment options in patients with single nodular HCCs ≤3 cm.
Methods
From January 2014 to December 2016, a total of 566 patients with single nodular HCC ≤3 cm treated by either LLR (n = 251) or p-RFA (n = 315) were included. The recurrence-free survival (RFS) and cumulative incidence of local tumor progression (LTP) were estimated using Kaplan-Meier methods and compared using the log-rank test. Treatment outcome of 2 treatment modalities was compared in the subgroup of patients according to the tumor location.
Results
There were no significant differences in overall survival between LLR and p-RFA (p = 0.160); however, 3-year RFS was demonstrated to be significantly higher after LLR (74.4%) than after p-RFA (66.0%) (p = 0.013), owing to its significantly lower cumulative incidence of LTP (2.1% at 3 years after LLR vs. 10.0% after p-RFA, p < 0.001). The complication rate of p-RFA was significantly lower than that of LLR (5.1 vs. 10.0%, p = 0.026). LLR also provided significantly better local tumor control than p-RFA for subscapular tumors (3-year LTP rates: 1.9 vs. 8.8%, p = 0.012), perivascular tumors (3-year LTP rates: 0.0 vs. 17.2%, p = 0.007), and tumors located in anteroinfero-lateral liver portions (3-year LTP rates: 0.0 vs. 10.7%, p < 0.001). However, there were no significant differences in LTP rates between LLR and p-RFA for non-subcapsular and non-perivascular tumors (p = 0.482) and for tumors in postero-superior liver portions (p = 0.380).
Conclusions
LLR can provide significantly better local tumor control than p-RFA for small single HCCs in subcapsular, perivascular, and anteroinferolateral liver portions and thus may be the preferred treatment option for these tumors.
Keywords: Hepatocellular carcinoma, Radiofrequency ablation, Laparoscopic liver resection, Local tumor progression
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors and the third most common cause of cancer-related death worldwide [1]. For the management of this disease, liver resection has been demonstrated to be the most effective and curative treatment option, particularly in patients with well-preserved liver function and early-stage HCCs [2, 3, 4, 5], while percutaneous radiofrequency ablation (p-RFA) has emerged as another curative local treatment modality that can provide comparable overall survival outcomes to liver resection, particularly for small HCCs ≤3 cm in size [6, 7, 8]. Thus, the recently updated HCC management guideline proposed by the European Association for the Study of the Liver (EASL) currently recommends both liver resection and p-RFA for patients with very early-stage HCCs [4]. However, several investigators have since demonstrated that the clinical outcomes of liver resection have vastly improved along with several important advances in surgical techniques [9, 10, 11], and thus an investigation into which of the 2 modalities would be the most appropriate treatment option in patients with early-stage HCCs is warranted.
One of the most important recent technological innovations in liver resection is laparoscopic liver resection (LLR). LLR is considered to be a safe and effective treatment modality for HCC, and recent studies have reported that its overall survival rates as well as recurrence-free survival (RFS) rates were nearly identical to that of conventional open liver resection while providing a significantly lower rate of complications and hospital stay durations [12, 13, 14]. In LLR, however, tumor location is known to be a very important factor for its therapeutic success with superficial or anteroinfero-lateral positions of the liver regarded to be particularly good for LLR, while the postero-superior portion of the liver has been shown to be a difficult area for treatment [4, 15]. Although there has been 1 previous study comparing the clinical outcomes of LLR to p-RFA for HCCs located at the liver surface, reporting a significantly lower rate of local recurrence after LLR than p-RFA [16], the number of patients in the aforementioned study was small, and only superficial tumors were assessed. Therefore, further studies with a larger number of patients including tumors from various locations are warranted to better determine which modality among LLR or p-RFA would most benefit patients with small single nodular HCCs. Indeed, clinical outcomes including overall survival and RFS as well as complication rate of LLR have not been fully compared with those of p-RFA. The purpose of this study, therefore, was to compare the clinical outcomes of LLR and p-RFA for single nodular HCCs ≤3 cm in size, variously located across the liver, in a large-scale multicenter study.
Patients and Methods
Patients
The Institutional Review Boards of each of the 3 participating centers approved this retrospective study, and the requirement for written informed consent was waived. The inclusion criteria for this study were as follows: (a) single nodular HCCs ≤3 cm in size treated by either LLR or p-RFA; (b) no previous treatment history for HCC; (c) no evidence of macrovascular invasion nor extrahepatic metastasis (EM); (d) Child-Pugh class A liver function; (e) no concomitant serious medical illnesses such as malignancies other than HCC; and (f) available medical records and/or imaging as well as laboratory studies before and after treatment. Through a search of the databases maintained by the departments of surgery and radiology in 3 university-affiliated hospitals between January 2014 and December 2016, we initially found 625 patients with Child-Pugh class A liver function who underwent LLR or p-RFA for a single nodular HCC ≤3 cm in size. The exclusion criteria for this study were as follows: (a) multiple HCCs or single HCC >3 cm in size; (b) presence of macrovascular invasion and/or EM; (c) Child-Pugh class B or C liver function; (d) concomitant serious comorbidities including congestive heart failure, chronic kidney disease needing dialysis, and a previous history of malignancies other than HCC; and (e) no available medical records or laboratory/imaging studies before and after treatment. Among 625 patients initially enrolled, 59 patients were excluded from this study for the following reasons: (a) presence of concomitant serious comorbidities (n = 21 [congestive heart failure, n = 3; chronic kidney disease on hemodialysis, n = 5; previous history of gastric cancer, n = 12; and previous history of lung cancer, n = 1]); (b) lack of pretreatment alpha-fetoprotein (AFP) level (n = 12); (c) presence of subsegmental portal vein tumor invasion (n = 17); and (d) immediate follow-up loss after treatment (n = 9). Therefore, the remaining 566 patients (LLR, n = 251, and p-RFA, n = 315) constituted our final study population (Fig. 1). The baseline characteristics of all of the study patients are summarized in Table 1. All HCCs treated by LLR were diagnosed by histopathology, and HCCs treated by p-RFA were diagnosed by either histopathology via percutaneous liver biopsy (n = 6) or noninvasive imaging criteria (n = 309) according to the Korean Liver Cancer Association-National Cancer Center Korea Guidelines [17].
Fig. 1.
Flow diagram summarizing the patient enrollment process of this study. HCC, hepatocellular carcinoma; p-RFA, percutaneous radiofrequency ablation; AFP, alpha-fetoprotein.
Table 1.
Baseline patient characteristics of the 2 treatment methods
| Parameters | Laparoscopic resection (n = 251) | p-RFA (n = 315) | p value |
|---|---|---|---|
| Age, years (mean ± SD) | 57.5±9.3 | 60.8±9.6 | <0.001 |
| Gender (n, M:F) | 199:52 | 227:88 | 0.050 |
| Etiology, n (%) | |||
| HBV | 196 (78.1) | 234 (74.3) | 0.730 |
| HCV | 21 (8.4) | 34 (10.8) | |
| Alcoholic | 29 (11.5) | 40 (12.7) | |
| Others | 5 (2.0) | 7 (2.2) | |
| Tumor size, cm (mean ± SD) | 2.13±1.44 | 1.69±0.50 | <0.001 |
| Albumin, mg/dL (mean ± SD) | 4.36±0.38 | 4.11±0.45 | <0.001 |
| Total bilirubin, mg/dL (mean ± SD) | 0.76±0.37 | 0.81±0.44 | 0.146 |
| Prothrombin activity (INR, mean ± SD) | 1.04±0.08 | 1.10±0.11 | <0.001 |
| AFP, ng/mL (mean ± SD) | 218.4±904.2 | 72.9±211.6 | 0.013 |
| Platelet count, K/mm3 (mean ± SD) | 168.4±53.5 | 130.2±50.9 | <0.001 |
| Tumor location, n (%) | |||
| Perivascular alone | 33 (13.1) | 64 (20.3) | <0.001 |
| Subcapsular alone | 135 (53.8) | 93 (29.5) | |
| Non-subcapsular and non-perivascular | 52 (20.7) | 144 (45.7) | |
| Perivascular and subcapsular | 31 (12.4) | 14 (4.5) | |
| Tumor in postero-superior portion, n (%) | 94 (37.5) | 162 (51.4) | 0.001 |
p-RFA, percutaneous radiofrequency ablation; SD, standard deviation; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; AFP, alpha-fetoprotein. Tumor in postero-superior portion of the liver includes tumors in segments 1, 7, 8, and 4a.
Procedure and Follow-Up after Treatment
Selection of treatment modality for small single HCC between LLR and p-RFA was done by clinicians including the liver surgeons and hepatologists through the discussion. In general, for patients with well-preserved liver function and small peripheral HCC, LLR was firstly considered. Patient preference for certain treatment modality was also considered for selection of treatment modality. All LLR procedures were performed by surgeons with >10 years of experience in liver surgery. The decision regarding the type and extent of LLR was generally made according to the patient's history of previous abdominal operations, tumor location, and the patient's liver function, as well as medical condition. All RFA procedures were done percutaneously under conscious sedation by radiologists with >5 years of experience in ultrasound-guided interventions. Immediately after RFA, all patients underwent contrast-enhanced multiphasic liver CT to evaluate successful complete ablation which was defined as complete coverage of index tumor by hypoattenuated area without contrast enhancement. Among the 315 patients treated by p-RFA, 6 patients (1.9%, 6/315) showed incomplete ablation and were treated by repeated ablation (n = 3) or transarterial chemoembolization (n = 3) to achieve complete tumor necrosis. After the second session of treatment, complete tumor necrosis was achieved in all of these 6 patients. The detailed description for LLR and p-RFA is given in online suppl. data (for all online suppl. material, see www.karger.com/doi/10.1159/000510909). We also evaluated the development of complications as well as the duration of their hospital stay after treatment using our collective medical records and imaging studies. Complication after LLR and p-RFA was graded using the Clavein-Dindo classification.
One month after treatment, follow-up contrast-enhanced CT or MRI and biochemical tests including liver function tests and serum AFP levels were performed in all patients. When a patient's 1-month follow-up imaging study showed no residual tumor, follow-up examinations were done every 3 months in the first year and every 3–6 months in the second year. If there was no tumor recurrence during the 2-year follow-up period after treatment, the follow-up schedule was set to be the same as that of the surveillance program for liver cirrhosis, that is, ultrasound at 6-month intervals [18].
Development of tumor recurrence during the follow-up period was assessed and further defined into 3 categories including local tumor progression (LTP), intrahepatic distant recurrence (IDR), and EM. LTP was defined as the appearance of any arterial enhancing tumor tissue showing washout on the portal venous and/or delayed phase adjacent to the treated area (i.e., resection margin in the case of LLR and ablation zone in the case of p-RFA), and IDR was defined as the development of one or more HCCs apart from the treated area [19]. Development of recurrence exceeding Milan criteria was also assessed and compared between LLR and p-RFA.
Statistical Analysis
To compare the baseline characteristics between LLR and p-RFA, we used the χ2 or Fisher's exact test for categorical variables and the Mann-Whitney U test for continuous variables. Overall survival was defined as the interval between HCC treatment and death or the date of their last follow-up visit prior to August 31, 2018. RFS was defined as the interval between HCC treatment and the first date of any type of recurrence (either local and/or distant) or the last follow-up date, if there was no recurrence. Patients who underwent liver transplantation during the follow-up period after HCC treatment were censored from the study at the date of their transplantation. Overall survival and RFS were estimated using the Kaplan-Meier method and were compared between LLR and p-RFA using the log-rank test. The cumulative incidence of each type of recurrence (i.e., LTP, IDR, and EM) and cumulative incidence of recurrence exceeding Milan criteria were also estimated using the competing risk model and were compared between LLR and p-RFA using the Gray test. After the initial analysis, subgroup analyses for RFS as well as the cumulative incidence of each type of recurrence were performed according to the tumor location, and their results were compared between LLR and p-RFA. Tumor location was classified into 4 categories: subcapsular; perivascular; non-subcapsular and non-perivascular; and subcapsular and perivascular. Subcapsular HCCs were defined as index tumors located within 0.1 cm of the liver capsule [20, 21]. Perivascular tumors were defined as index tumors contacting the first- or second-degree branches of portal or hepatic veins 3 mm or greater in axial diameter [21]. In addition, tumors located in the postero-superior portion of the liver were defined as tumors in liver segments 1, 7, 8, and 4a, which was considered to be a difficult location for LLR [15, 22]. Subsequently, tumors located in the anteroinfero-lateral portion of the liver were defined as tumors in liver segments 2, 3, 4b, 5, and 6.
After the first analysis, we additionally performed propensity score analysis so as to reduce potential biases that may have originated from differences in the baseline characteristics of those who underwent LLR and p-RFA [23]. Using multivariate logistic regression models, location, serum albumin level, serum total bilirubin level, serum AFP level, and platelet count were evaluated. The resultant propensity score model was then used to create a one-to-one match using the nearest neighbor matching method with a caliper width of 0.1 [24, 25]. After propensity score matching, the McNemar test for categorical variables and the paired t test for continuous variables were used to compare the baseline characteristics between LLR and p-RFA. RFS, as well as the cumulative incidence of each type of recurrence, was then compared between LLR and p-RFA in the one-to-one matched cohort, consisting of 118 patients each. All statistical analyses were performed using SPSS version 25 (SPSS, Chicago, IL, USA).
Results
Baseline Patient Characteristics of the 2 Treatment Methods
Baseline characteristics of the study population according to the treatment method are summarized in Table 1. Distribution of tumor locations in 251 patients treated by LLR was perivascular in 33 patients, subcapsular in 135 patients, non-subcapsular and non-perivascular in 52 patients, and perivascular and subcapsular in 31 patients. Regarding the 315 patients treated by p-RFA, tumor locations were perivascular in 64 patients, subcapsular in 93 patients, non-subcapsular and non-perivascular in 144 patients, and perivascular and subcapsular in 14 patients; this difference was statistically significant (p < 0.001). The frequency of tumors in the postero-superior portion of the liver was also significantly different between the 2 treatment modalities (37.5% [94/215] in the LLR group vs. 51.4% [162/315] in the p-RFA group, p = 0.001).
Survival and Complication Outcomes of Each Treatment Modality
After a mean and median follow-up period of 30.0 ± 12.5 (standard deviation [SD]) months and 28.0 months, respectively, the estimated 1-, 2-, and 3-year overall survival rates of the 251 patients who underwent LLR were 100, 99.5, and 97.9%, respectively. The estimated 1-, 2-, and 3-year overall survival rates of the 315 patients who underwent p-RFA were 99.0, 98.3, and 97.2%, respectively. There were no significant differences in overall survival between LLR and p-RFA (p = 0.160). Among the 251 patients who underwent LLR, 3 patients underwent liver transplantation during the follow-up period due to HCC recurrence (n = 1) and liver failure (n = 2). Liver transplantation was also done in 9 of 315 patients treated by p-RFA for HCC recurrence (n = 6) and liver failure (n = 3).
Among the 251 patients treated by LLR, 25 patients experienced complication (10.0%, 25/251): grade I in 10 patients (fever, n = 8; wound infection, n = 2); gade II in 12 patients (ascites, n = 8; minor bile leakage, n = 2; hematoma formation, n = 2); and grade IIIa in 3 patients (bile leakage needing percutaneous drainage, n = 2; abscess needing drainage, n = 1). After p-RFA, 16 patients experienced complication (5.1%, 16/315): grade I in 5 patients (fever, n = 5); grade II in 8 patients (hemorrhage needing transfusion, n = 3; segmental liver infarction, n = 5); and grade III in 3 patients (active bleeding requiring angiographic embolization). The complication rate was significantly lower in the p-RFA group than in the LLR group (5.1 vs. 10.0%, p = 0.026). Mean and median hospital stays were 8.6 ± 4.8 (SD) days (range; 4–67 days) and 8 days, respectively, after LLR in 251 patients, and 3.9 ± 1.7 (SD) days (range; 1–12 days) and 3 days, respectively, after p-RFA in 315 patients. This difference was statistically significant (p < 0.001).
Recurrence Outcomes
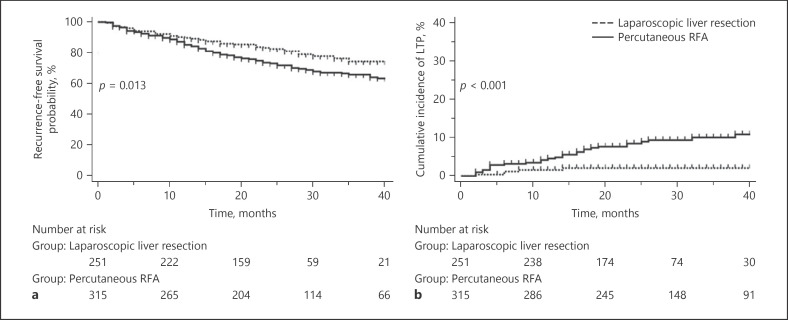
The estimated 1-, 2-, and 3-year RFS rates of the 251 patients who underwent LLR were 89.9, 82.7, and 74.4%, respectively, and were significantly higher than 85.4, 73.2, and 66.0% observed in the 315 patients who underwent p-RFA (p = 0.013) (Fig. 2a). The mean number of interventional procedures to treat recurred HCC during the follow-up period was 0.6 session (range; 0–8 sessions) in 251 patients who underwent LLR and 1.1 sessions (range; 0–9 sessions) in 315 patients who underwent p-RFA; this difference was statistically significant (p = 0.004). The estimated 1-, 2-, and 3-year cumulative incidences of LTP were 1.6, 2.1, and 2.1%, respectively, after LLR, while they were observed to be 4.5, 8.4, and 9.9%, respectively, after p-RFA; this difference was statistically significant (p < 0.001) (Fig. 2b). The cumulative incidences of IDR (p = 0.176), EM (p = 0.064), and recurrence exceeding Milan criteria (p = 0.153) were not significantly different between LLR and p-RFA.
Fig. 2.
Estimation of RFS and cumulative incidence of LTP. a Kaplan-Meier estimation of RFS after LLR in 251 patients was compared with p-RFA in 315 patients. b Cumulative incidence of LTP after LLR in 251 patients was compared with p-RFA in 315 patients. RFS, recurrence-free survival; LTP, local tumor progression; LLR, laparoscopic liver resection; p-RFA, percutaneous radiofrequency ablation.
Among the 566 patients, 362 patients had BCLC stage 0 HCC treated by either LLR (n = 120) or p-RFA (n = 242). The estimated 1-, 2-, and 3-year RFS rate of the 120 patients who underwent LLR for BCLC stage 0 HCC was 91.6, 84.1, and 77.8%, respectively, and was significantly better than 84.7, 72.6, and 67.7% observed in the 242 patients who underwent p-RFA (p = 0.027). The estimated 1-, 2-, and 3-year cumulative incidences of LTP were 0.8, 0.8, and 0.8%, respectively, after LLR for BCLC stage 0 HCC, while they were observed to be 4.2, 8.9, and 9.5%, respectively, after p-RFA; this difference was statistically significant (p = 0.003). The cumulative incidences of IDR (p = 0.153) and EM (p = 0.650) were not significantly different between LLR and p-RFA for BCLC stage 0 HCC.
Recurrence Outcomes according to Tumor Location
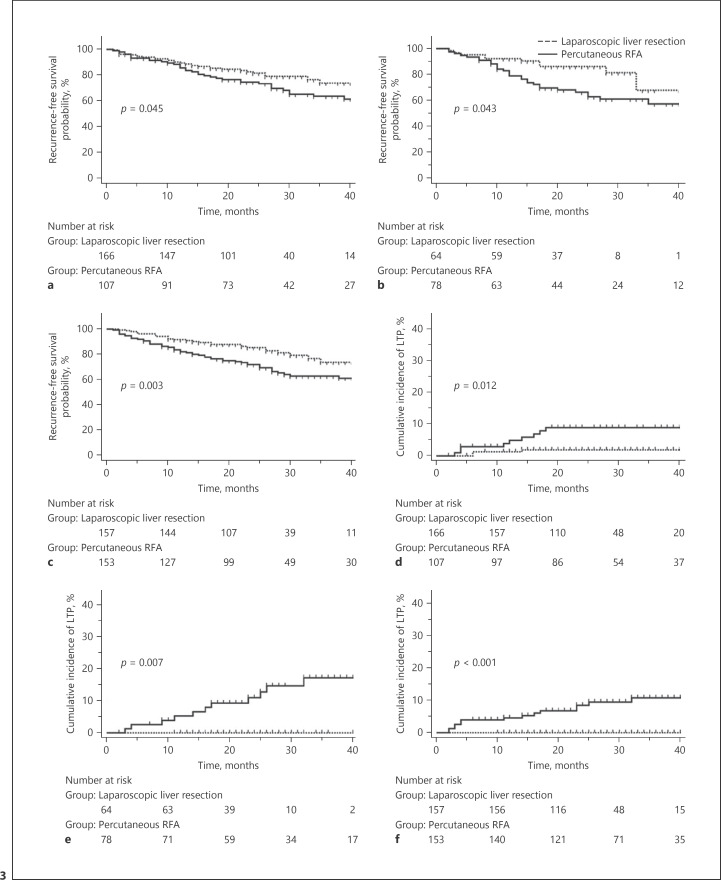
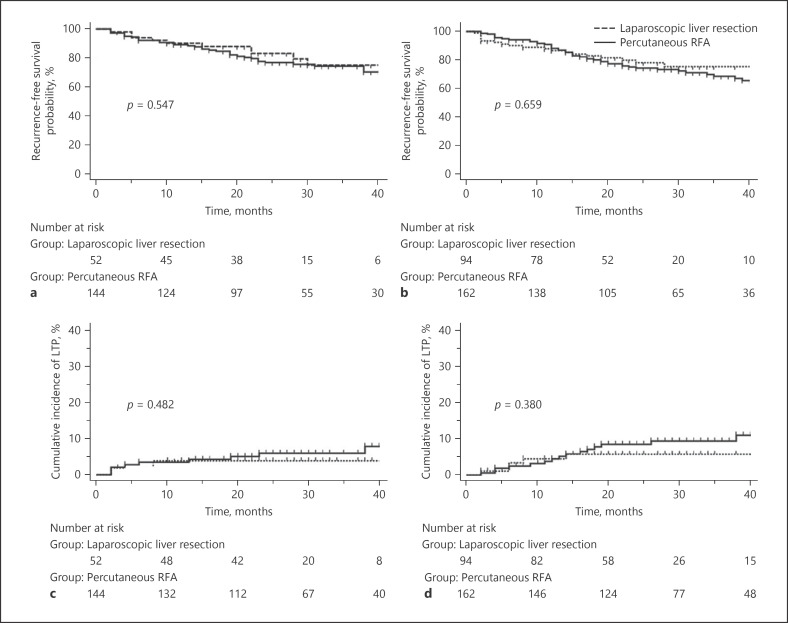
The estimated RFS and cumulative incidences of LTP according to tumor location are summarized in Table 2 and Figures 3 and 4. LLR provided significantly better RFS than p-RFA for subscapular (3-year RFS: 73.9 vs. 63.7%, p = 0.045) (Fig. 3a) and perivascular (3-year RFS: 68.0 vs. 57.6%, p = 0.043) (Fig. 3b) tumors, as well as for tumors located in the anteroinfero-lateral portion of the liver (3-year RFS: 73.8 vs. 63.1%, p = 0.003) (Fig. 3c). The cumulative incidence of LTP after LLR was also demonstrated to be significantly lower than that after p-RFA for subscapular (3-year cumulative incidence of LTP: 1.9 vs. 8.8%, p = 0.012) (Fig. 3d) and perivascular (3-year cumulative incidence of LTP: 0.0 vs. 17.2%, p = 0.007) (Fig. 3e) tumors, as well as for tumors located in the anteroinfero-lateral portion of the liver (3-year cumulative incidence of LTP: 0.0 vs. 10.7%, p < 0.001) (Fig. 3f). However, there were no significant differences in RFS between LLR and p-RFA for non-subcapsular and non-perivascular tumors (3-year RFS: 75.4 vs. 74.6%, p = 0.547) (Fig. 4a) and for tumors located in the postero-superior portion of the liver (3-year RFS: 75.5 vs. 68.8%, p = 0.659) (Fig. 4b). There were also no significant differences in the cumulative incidence of LTP between LLR and p-RFA for non-subcapsular and non-perivascular tumors (3-year cumulative incidence of LTP: 3.9 vs. 6.0%, p = 0.482) (Fig. 4c) as well as for tumors located in the postero-superior portion of the liver (3-year cumulative incidence of LTP: 5.7 vs. 9.4%, p = 0.380) (Fig. 4d). Finally, there were no significant differences in the cumulative incidences of IDR and EM between the 2 treatment modalities, regardless of the tumor location.
Table 2.
Estimated 1-, 2-, and 3-year RFS and cumulative incidence of LTP according to treatment modality
| LLR | p-RFA | p value | |
|---|---|---|---|
| 1-, 2-, and 3-year RFS | |||
| Subcapsulara (n = 273) | 89.6, 81.7, 73.9% | 85.6, 74.6, 63.7% | 0.045 |
| Perivascularb (n = 142) | 92.2, 86.4, 68.0% | 79.1, 66.6, 57.6% | 0.043 |
| Non-subcapsular and non-perivascular (n = 196) | 90.2, 83.4, 75.4% | 89.4, 77.1, 74.6% | 0.547 |
| Anteroinfero-lateral position (n = 310) | 91.7, 85.4, 73.8% | 82.2, 72.0, 63.1% | 0.003 |
| Postero-superior portion (n = 256) | 86.8, 78.1, 75.5% | 88.4, 74.4, 68.8% | 0.659 |
| 1-, 2-, and 3-year cumulative incidence of LTP | |||
| Subcapsulara (n = 273) | 1.2, 1.9, 1.9% | 4.8, 8.8, 8.8% | 0.012 |
| Perivascularb (n = 142) | 0.0, 0.0, 0.0% | 5.2, 11.0, 17.2% | 0.007 |
| Non-subcapsular and non-perivascular (n = 196) | 3.9, 3.9, 3.9% | 3.5, 6.0, 6.0% | 0.482 |
| Anteroinfero-lateral position (n = 310) | 0.0, 0.0, 0.0% | 4.6, 8.5, 10.7% | <0.001 |
| Postero-superior portion (n = 256) | 4.5, 5.7, 5.7% | 4.5, 8.6, 9.4% | 0.380 |
RFS, recurrence-free survival; LLR, laparoscopic liver resection; p-RFA, percutaneous radiofrequency ablation; LTP, local tumor progression. Tumor in anteroinfero-lateral portion of the liver includes tumors in segments 2, 3, 4b, 5, and 6; tumor in postero-superior portion of the liver includes tumors in segments 1, 7, 8, and 4a. p values <0.05 are shown in bold and italics.
Subcapular tumors included both subcapsular alone tumors and subcapsular and perivascular tumors.
Perivascular tumors included both perivascular alone tumors and subcapsular and perivascular tumors.
Fig. 3.
Estimation of RFS and cumulative incidence of LTP according to the tumor location showing significant difference. a Kaplan-Meier estimation of RFS after LLR for subcapsular tumor was compared with p-RFA. b Kaplan-Meier estimation of RFS after LLR for perivascular tumor was compared with p-RFA. c Kaplan-Meier estimation of RFS after LLR for tumor in anteroinfero-lateral portion of the liver was compared with p-RFA. d Cumulative incidence of LTP after LLR for subcapsular tumor was compared with p-RFA. e Cumulative incidence of LTP after LLR for perivascular tumor was compared with p-RFA. f Cumulative incidence of LTP after LLR for tumor in anteroinfero-lateral portion of the liver was compared with p-RFA. RFS, recurrence-free survival; LTP, local tumor progression; LLR, laparoscopic liver resection; p-RFA, percutaneous radiofrequency ablation.
Fig. 4.
Estimation of RFS and cumulative incidence of LTP according to the tumor location showing no significant difference. a Kaplan-Meier estimation of RFS after LLR for non-subcapsular and non-perivascular tumor was compared with p-RFA. b Kaplan-Meier estimation of RFS after LLR for tumor in postero-superior portion of the liver was compared with p-RFA. c Cumulative incidence of LTP after LLR for non-subcapsular and non-perivascular tumor was compared with p-RFA. d Cumulative incidence of LTP after LLR for tumor in postero-superior portion of the liver was compared with p-RFA. RFS, recurrence-free survival; LTP, local tumor progression; LLR, laparoscopic liver resection; p-RFA, percutaneous radiofrequency ablation.
Results after Propensity Score Matching
After propensity score matching, we confirmed that there were no significant differences in baseline characteristics between the patients in the 2 treatment modality groups (Table 3). With this matched cohort, our analysis revealed that there were no significant differences in overall survival between the LLR and p-RFA groups (p = 0.496). The estimated 1-, 2-, and 3-year RFS rates were 87.0, 77.7, and 65.5%, respectively, in the 118 patients who underwent LLR, which were also not significantly different from that of 85.1, 73.6, and 62.9%, respectively, in the 118 patients who underwent p-RFA (p = 0.339) (Fig. 5a). However, the cumulative incidence of LTP was significantly lower in the 118 patients who underwent LLR compared to those who underwent p-RFA (2.6% 3 years after LLR vs. 11.6% 3 years after p-RFA, p = 0.017) even after propensity score matching (Fig. 5b). Finally, although the cumulative incidences of IDR (p = 0.864), EM (p = 0.320), and recurrence exceeding Milan criteria (p = 0.454) were not significantly different between the LLR and p-RFA groups, LLR provided a significantly lower cumulative incidence of LTP than p-RFA for subcapsular tumors (Fig. 5c) and for tumors located in the anteroinfero-lateral portion of the liver (Fig. 5d) even after propensity score matching (Table 4).
Table 3.
Baseline patient characteristics between the 2 treatment methods after propensity score matching
| Parameters | Laparoscopic resection (n = 118) | p-RFA (n = 118) | p value* |
|---|---|---|---|
| Age, years (mean ± SD) | 59.5±8.7 | 60.5±10.3 | 0.378 |
| Gender, n (M:F) | 91:27 | 88:30 | 0.761 |
| Etiology, n (%) | |||
| HBV | 90 (76.3) | 84 (71.2) | |
| HCV | 10 (8.5) | 12 (10.2) | 0.783 |
| Alcoholic | 16 (13.5) | 19 (16.1) | |
| Others | 2 (1.7) | 3 (2.5) | |
| Tumor size, cm (mean ± SD) | 1.84±0.56 | 1.87±0.51 | 0.727 |
| Albumin, mg/dL (mean ± SD) | 4.27±0.37 | 4.23±0.37 | 0.486 |
| Total bilirubin, mg/dL (mean ± SD) | 0.76±0.36 | 0.79±0.45 | 0.614 |
| Prothrombin activity (INR, mean ± SD) | 1.05±0.08 | 1.07±0.10 | 0.269 |
| AFP, ng/mL (mean ± SD) | 90.2±309.0 | 67.6±173.4 | 0.496 |
| Platelet count, K/mm3 (mean ± SD) | 148.8±49.4 | 145.7±55.8 | 0.622 |
| Tumor location, n (%) | |||
| Subcapsular alone | 65 (55.1) | 65 (55.1) | |
| Perivascular alone | 14 (11.9) | 14 (11.9) | 1.000 |
| Non-subcapsular and non-perivascular | 39 (33.0) | 39 (33.0) | |
| Perivascular and subcapsular | 0 (0.0) | 0 (0.0) | |
| Tumor in postero-superior portion, n (%) | 59 (50.0) | 48 (40.7) | 0.152 |
p-RFA, percutaneous radiofrequency ablation; SD, standard deviation; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; AFP, alpha-fetoprotein. Tumor in postero-superior portion of the liver includes tumors in segments 1, 7, 8, and 4a.
p value was obtained using the McNemar test for categorical variables and the paired t test for continuous variables.
Fig. 5.
Estimation of RFS and cumulative incidence of LTP after propensity score matching. a Kaplan-Meier estimation of RFS after LLR in 118 patients was compared with p-RFA in 118 patients after propensity score matching. b Cumulative incidence of LTP after LLR in 118 patients was compared with p-RFA in 118 patients after propensity score matching. c Cumulative incidence of LTP after LLR for subcapsular tumor was compared with p-RFA after propensity score matching. d Cumulative incidence of LTP after LLR for tumor in anteroinfero-lateral portion of the liver was compared with p-RFA after propensity score matching. RFS, recurrence-free survival; LTP, local tumor progression; LLR, laparoscopic liver resection; p-RFA, percutaneous radiofrequency ablation.
Table 4.
The estimated cumulative incidence of LTP according to treatment modalities after propensity score matching
| 1-, 2-, and 3-year cumulative incidence of LTP | LLR | p-RFA | p value |
|---|---|---|---|
| Subcapsular alone (n = 130) | 1.6, 1.6, 1.6% | 8.0, 11.3, 11.3% | 0.033 |
| Perivascular alone (n = 28) | 0.0, 0.0, 0.0% | 5.2, 7.1, 20.4% | 0.279 |
| Non-subcapsular and non-perivascular (n = 78) | 5.2, 5.2, 5.2% | 2.6, 9.9, 9.9% | 0.407 |
| Anteroinfero-lateral position (n = 129) | 0.0, 0.0, 0.0% | 5.8, 10.8, 13.5% | 0.011 |
| Postero-superior portion (n = 107) | 5.3, 5.3, 5.3% | 4.5, 9.2, 9.2% | 0.376 |
p-RFA, percutaneous radiofrequency ablation; LTP, local tumor progression; LLR, laparoscopic liver resection. Tumor in anteroinfero-lateral portion of the liver includes tumors in segments 2, 3, 4b, 5, and 6; tumor in postero-superior portion of the liver includes tumors in segments 1, 7, 8, and 4a. p values <0.05 are shown in bold and italics.
Discussion
Our study demonstrated that both LLR and p-RFA were effective and safe treatment modalities for small single nodular HCCs ≤3 cm in size, providing >90% 3-year overall survival after treatment. However, in contrast to the lack of a significant difference in overall survival between the 2 treatment modalities, RFS was shown to be significantly higher after LLR than after p-RFA (74.4% 3 years after LLR vs. 66.0% after p-RFA, p = 0.013) along with a significantly lower LTP rate (2.1% 3 years after LLR vs. 10.0% after p-RFA, p < 0.001). Owing to the lower rate of recurrence, the mean number of interventional procedure to treat recurred HCC after initial therapy was significantly lower in the LLR group than in the p-RFA group (0.6 session in the LLR group vs. 1.1 sessions in the p-RFA group, p = 0.004). Our study results are in good concordance with the results of previous studies regarding LLR [16] as well as conventional open liver resection [6, 26, 27], in which they reported comparable overall survival after p-RFA to LLR, albeit with higher recurrence. The complication rate of p-RFA was significantly lower than that of LLR (5.1 vs. 10.0%), and the hospital stay duration after p-RFA was significantly shorter than LLR (8.6 days after LLR vs. 3.9 days after p-RFA, p < 0.001), indicating the less invasiveness of p-RFA compared to LLR. Furthermore, our subgroup analyses revealed that LLR was able to provide superior results regarding RFS and LTP than p-RFA for tumors in subcapsular, perivascular, and anteroinfero-lateral locations, while there were no significant differences for tumors in postero-superior portions of the liver. Thus, based on our study results, we suggest that although p-RFA can provide comparable treatment effectiveness to LLR for small single nodular HCC ≤3 cm, albeit with a higher LTP rate, LLR would be the preferred treatment option for tumors in subcapsular, perivascular, or anteroinfero-lateral portions of the liver.
Our study also revealed the effect of tumor location on local tumor control outcomes of both LLR and p-RFA, which may help clinicians in selecting the most appropriate therapy for early-stage HCCs. According to our results, all LTP cases after LLR occurred in tumors located in the postero-superior portion of the liver although when we focused on the tumors, we found that the cumulative incidence of LTP and RFS rates after LLR were not significantly different from those after p-RFA. This may be attributed to the difficulty in obtaining a sufficient free tumor resection margin compared to tumors located in the anteroinfero-lateral tumor portion owing to the limited trocar sites in the postero-superior portion of the liver and the laparoscopic instruments' lack of flexion, resulting in LTP after treatment. To the contrary, however, LLR was able to provide significantly better RFS and lower cumulative incidence of LTP than p-RFA for subcapsular and perivascular tumors. The major reasons for the higher LTP rate after p-RFA for subcapsular tumors have previously been described as stemming from the difficulty in inserting the electrode as well as in obtaining a sufficient ablative margin along the liver capsule [28, 29]. In addition, perivascular tumor location has been another well-known risk factor for the development of LTP after RFA mainly due to the insufficient ablative margin created with RFA resulting from the heat-sink effect [30, 31, 32]. Indeed, previous surgical studies have shown that subcapsular tumor location was particularly favorable for LLR [4, 15]. Our study results are well matched with those of a recent study published by Lee et al. [32], showing that liver resection would provide better long-term tumor control than p-RFA for small perivascular tumors.
After open liver resection of small HCCs, the mean hospital stay duration has been reported to range from 11.4 to 19.7 days and its complication rate from 11.1 to 27.8% [33]. In our study, the mean hospital stay after LLR was 8.6 days, and the major complication rate with equal to or more than grade II after LLR was 6.0%. Interpreting these results, we can infer that LLR appears to be a less invasive treatment modality for HCCs than open liver resection. In comparison, the mean hospital stay after p-RFA was only 3.9 days, which is even significantly shorter than that after LLR in our study. Furthermore, the major complication rate after p-RFA for HCC was only 3.5%, which would also be significantly lower than after open liver resection. In addition, the overall survival rate after LLR or p-RFA for single nodular HCCs ≤3 cm in size is comparable to that after open liver resection. Therefore, either LLR or p-RFA should be preferred to open liver resection for small HCCs as it would provide comparable survival outcomes, yet with less invasiveness.
There are several limitations in our study that warrant mention. First, our study was of retrospective design, and therefore the possibility of selection bias cannot be easily ruled out. Especially, the distribution of tumor location between LLR and p-RFA was significantly different, and this difference in tumor location might affect the result of our study. However, to reduce the potentiality of bias resulting from the different distribution of baseline characteristics between the 2 treatment modalities, we performed propensity score matching and found that local tumor control was consistent even after propensity score matching. Nevertheless, our study results need to be validated by other studies using a prospective design and a larger number of patients, especially in Western populations, as the main etiology of HCC would be different from that of our study population (i.e., hepatitis B virus related in our study vs. alcohol or hepatitis C virus related in Western countries). Second, we were able to evaluate only the midterm clinical outcomes of both LLR and p-RFA, and the median follow-up period of this study was only 28.0 months, as LLR has been regularly used for small HCCs in our hospitals since only 2014. Therefore, the long-term results of LLR and p-RFA for small single nodular HCCs need to be evaluated and compared in future studies. Third, we did not analyze cost-effectiveness of p-RFA and LLR for management of small single HCC. Although LLR provided significantly lower cumulative incidence of LTP than p-RFA for small HCCs in subcapsular, perivascular, and anteroinfero-lateral portion of the liver, the complication rate and hospital stay were significantly lower in the p-RFA group than in the LLR group. Therefore, further studies with large number of patients including meta-analyses are warranted to address the issue of cost-effectiveness of p-RFA and LLR for small single HCC. In conclusion, our study demonstrated that LLR provided significantly better local tumor control than p-RFA for small single nodular HCCs in subcapsular, perivascular, and anterolateral portions of the liver and thus may be the preferred treatment option for these tumors.
Statement of Ethics
This study was done in accordance with the World Medical Association Declaration of Helsinki. The Institutional Review Boards (IRB) of each of the 3 participating centers approved this retrospective study (H-1810-101-981 from Seoul National University Hospital, CNUHH-2019-145 from Chonnam National University Hwasun Hospital, and 2019-09-063-003 from Samsung Medical Center), and the requirement for written informed consent was waived by the IRB because of retrospective design.
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Funding Sources
The authors received no financial support for this study.
Author Contributions
All authors approved the final version of this manuscript and contributed to the editing process. Dong Ho Lee and Jin Woong Kim: analysis and interpretation of data, drafting of the manuscript, and statistical analysis. Jeong Min Lee and Jong Man Kim: study concept and design, acquisition of data, critical revision of the manuscript, and study supervision. Min Woo Lee, Hyunchul Rhim, Young Hoe Hur, and Kyung-Suk Suh: acquisition of clinical data and critical revision of the manuscript.
Supplementary Material
Supplementary data
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94((2)):153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Mazzaferro V. Results of liver transplantation: with or without Milan criteria? Liver Transpl. 2007;13((11 Suppl 2)):S44–7. doi: 10.1002/lt.21330. [DOI] [PubMed] [Google Scholar]
- 3.Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010–2016. Clin Mol Hepatol. 2016;22((1)):7–17. doi: 10.3350/cmh.2016.22.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver. Electronic address easloffice@easloffice.eu; European Association for the Study of the Liver EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67((1)):358–80. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 6.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243((3)):321–8. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.N'Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50((5)):1475–83. doi: 10.1002/hep.23181. [DOI] [PubMed] [Google Scholar]
- 8.Lee DH, Lee JM, Lee JY, Kim SH, Yoon JH, Kim YJ, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270((3)):900–9. doi: 10.1148/radiol.13130940. [DOI] [PubMed] [Google Scholar]
- 9.Lai EC, Tang CN, Ha JP, Li MK. Laparoscopic liver resection for hepatocellular carcinoma: ten-year experience in a single center. Arch Surg. 2009;144((2)):143–8. doi: 10.1001/archsurg.2008.536. [DOI] [PubMed] [Google Scholar]
- 10.Dagher I, Belli G, Fantini C, Laurent A, Tayar C, Lainas P, et al. Laparoscopic hepatectomy for hepatocellular carcinoma: a European experience. J Am Coll Surg. 2010;211((1)):16–23. doi: 10.1016/j.jamcollsurg.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Guro H, Cho JY, Han HS, Yoon YS, Choi Y, Periyasamy M. Current status of laparoscopic liver resection for hepatocellular carcinoma. Clin Mol Hepatol. 2016;22((2)):212–8. doi: 10.3350/cmh.2016.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JM, Kwon CHD, Yoo H, Kim KS, Lee J, Kim K, et al. Which approach is preferred in left hepatocellular carcinoma? Laparoscopic versus open hepatectomy using propensity score matching. BMC cancer. 2018;18((1)):668. doi: 10.1186/s12885-018-4506-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasai M, Cipriani F, Gayet B, Aldrighetti L, Ratti F, Sarmiento JM, et al. Laparoscopic versus open major hepatectomy: a systematic review and meta-analysis of individual patient data. Surgery. 2018;163((5)):985–95. doi: 10.1016/j.surg.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Yoon YI, Kim KH, Kang SH, Kim WJ, Shin MH, Lee SK, et al. Pure laparoscopic versus open right hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score matched analysis. Ann Surg. 2017;265((5)):856–63. doi: 10.1097/SLA.0000000000002072. [DOI] [PubMed] [Google Scholar]
- 15.Takahara T, Wakabayashi G, Beppu T, Aihara A, Hasegawa K, Gotohda N, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22((10)):721–7. doi: 10.1002/jhbp.276. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Tanaka S, Iwai S, Takemura S, Hagihara A, Uchida-Kobayashi S, et al. Outcomes of laparoscopic hepatic resection versus percutaneous radiofrequency ablation for hepatocellular carcinoma located at the liver surface: a case-control study with propensity score matching. Hepatol Res. 2016;46((6)):565–74. doi: 10.1111/hepr.12592. [DOI] [PubMed] [Google Scholar]
- 17.Korean Liver Cancer A, National Cancer Center GK. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2019 Jul;20((7)):1042–113. doi: 10.3348/kjr.2019.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DH, Lee JM, Lee JY, Kim SH, Kim JH, Yoon JH, et al. Non-hypervascular hepatobiliary phase hypointense nodules on gadoxetic acid-enhanced MRI: risk of HCC recurrence after radiofrequency ablation. J Hepatol. 2015;62((5)):1122–30. doi: 10.1016/j.jhep.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235:728–39. doi: 10.1148/radiol.2353042205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang TW, Lim HK, Lee MW, Kim YS, Rhim H, Lee WJ, et al. Long-term therapeutic outcomes of radiofrequency ablation for subcapsular versus nonsubcapsular hepatocellular carcinoma: a propensity score matched study. Radiology. 2016;280((1)):300–12. doi: 10.1148/radiol.2016151243. [DOI] [PubMed] [Google Scholar]
- 21.Lee DH, Lee JM, Kang TW, Rhim H, Kim SY, Shin YM, et al. Clinical outcomes of radiofrequency ablation for early hypovascular HCC: a multicenter retrospective study. Radiology. 2018;286((1)):338–49. doi: 10.1148/radiol.2017162452. [DOI] [PubMed] [Google Scholar]
- 22.Cho JY, Han HS, Yoon YS, Shin SH. Experiences of laparoscopic liver resection including lesions in the posterosuperior segments of the liver. Surg Endosc. 2008;22((11)):2344–9. doi: 10.1007/s00464-008-9966-0. [DOI] [PubMed] [Google Scholar]
- 23.Zinsmeister AR, Connor JT. Ten common statistical errors and how to avoid them. Am J Gastroenterol. 2008;103((2)):262–6. doi: 10.1111/j.1572-0241.2007.01590.x. [DOI] [PubMed] [Google Scholar]
- 24.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127((8 Pt 2)):757–63. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 25.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17((19)):2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57((4)):794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Luo Q, Li Y, Deng S, Wei S, Li X. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. PLoS One. 2014;9((1)):e84484. doi: 10.1371/journal.pone.0084484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hori T, Nagata K, Hasuike S, Onaga M, Motoda M, Moriuchi A, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38((10)):977–81. doi: 10.1007/s00535-003-1181-0. [DOI] [PubMed] [Google Scholar]
- 29.Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97((5)):1253–62. doi: 10.1002/cncr.11168. [DOI] [PubMed] [Google Scholar]
- 30.Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol. 2002;178((1)):47–51. doi: 10.2214/ajr.178.1.1780047. [DOI] [PubMed] [Google Scholar]
- 31.Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14((10)):1267–74. doi: 10.1097/01.rvi.0000092666.72261.6b. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Kang TW, Cha DI, Song KD, Lee MW, Rhim H, et al. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: propensity score analyses of long-term outcomes. J Hepatol. 2018;69((1)):70–8. doi: 10.1016/j.jhep.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 33.Vigano L, Laurenzi A, Solbiati L, Procopio F, Cherqui D, Torzilli G. Open liver resection, laparoscopic liver resection, and percutaneous thermal ablation for patients with solitary small hepatocellular carcinoma (</=30 mm): review of the literature and proposal for a therapeutic strategy. Digestive surgery. 2018;35:359–71. doi: 10.1159/000489836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data