Abstract
Introduction
The aim of this study was to investigate the technical success rate of obtaining 3D-safety margin in superselective conventional transarterial chemoembolization (cTACE) using 3D images for small hepatocellular carcinoma (HCC).
Methods
Consecutive 48 HCC nodules (diameter, 1–3 cm) in 44 patients were intentionally treated by superselective cTACE in an attempt to achieve 3D-safety margin. Superselective CT during hepatic arteriography (CTHA) was obtained before cTACE. When negative 3D-safety margin was found, branches supplied into the margin area were detected by using a 3D workstation. The technical success rate to obtain 3D-safety margin was investigated by intend-to-treat analysis. Local tumor recurrence rate and adverse events were also evaluated.
Result
Nine of 48 tumors (18.8%) had 3D-safety margin in the initial superselective CTHA. After pulling back of the catheter and/or selection of another branch based on 3D images, 3D-safety margin was finally achieved in 45 (93.8%). There were 8 of 46 tumors (17.4%) with local recurrence after 5-year follow-up. Grade 3–4 of aspartate aminotransferase, alanine aminotransferase, and total bilirubin were found in 38.6, 36.4, and 2.3%, respectively. One portal vein thrombus and 3 biliary dilation or biloma were developed.
Conclusion
Superselective cTACE obtaining 3D-safety margin in small HCC was feasible with a high success rate by using 3D images, which could be tolerable and prevent local tumor recurrence.
Keywords: Hepatocelluar carcinoma, Transarterial chemoembolization, Lipiodol
Introduction
Most international guidelines suggest that surgical resection or local ablation is recommended for early stage of hepatocellular carcinoma (HCC). However, some patients with early-stage HCC cannot be treated by resection and ablation due to some causes, that is, comorbidity, tumor location, frequent recurrence, or poor liver function. For such cases, superselective conventional transarterial chemoembolization (cTACE) is one of the alternative treatment options [1].
Due to recent development of molecular targeted agents and immunotherapies for HCC, the role of TACE has been changed from palliative treatment to curative treatment [2, 3]. Previously published literature studies demonstrated that cTACE is a possible curative treatment for small HCC [4, 5]. In some reports, achieving dense lipiodol accumulation in the tumor and reflux into the adjacent portal vein improved local tumor control. However, these outcomes are not achievable in all patients [6, 7, 8]. Namely, various tumor factors, that is, tumor vascularity or hemodynamics, could influence the results. Other reports showed local recurrence occurred more frequently in cases with high serum alpha-fetoprotein level and tumors located at the segmental border zone of the liver. These are also tumor factors, which are not able to be controlled by TACE technique [9, 10].
Obtaining safety margins of TACE is a promising technical factor to improve local tumor control [11, 12, 13, 14]. Several recent retrospective studies proved that superselective TACE with obtaining 3D-safety margin could significantly improve local tumor control. Also, the survival rate was prolonged compared with the negative 3D-safety margin cases [14]. However, to date, the success rate to obtain the 3D-safety margin remains unknown due to the fact that all previous studies were retrospective analyses. Current 3D image workstation technology could be helpful to achieve the 3D-safety margin.
In this study, we evaluated the technical success rate to obtain the 3D-safety margin by using 3D-image workstation in superselective cTACE for small HCC by an intend-to-treat analysis. Local tumor recurrence rate and adverse events were also evaluated.
Materials and Methods
From March 2012 to May 2014, consecutive patients with small HCC treated by superselective cTACE were enrolled in this study. In all cases, operators attempted to achieve 3D-safety margin. The inclusion criteria were as follows: (1) tumor size 1–3 cm in diameter, (2) homogenous enhancement in tumor on dynamic enhanced CT, (3) preserved liver function of Child-Pugh score <7, (4) no previous treatments for the target tumor, and (5) no vascular invasion and arterioportal shunt. This study was approved by our Institutional Review Board. All of the included patients signed the inform consents.
TACE Procedure
A combined CT and angiography system (Angio-CT System, Infinix Activ; Canon Medical Systems, Ohtawara, Japan) was used. Digital subtraction angiography (DSA), CT during hepatic arteriography (CTHA), and CT during arterial portography were obtained. CT-maximum intensity projection (MIP) image of the hepatic artery was created using 3D CT workstation (SYNAPSE VINCENT; Fujifilm, Tokyo, Japan) to find tumor-feeding arteries. The CTHA scan protocol was as follows: diluted contrast material (270 mgL/mL) was injected at a speed of 1.5–2.0 mL/s and the CT scan started after 7 s. A 1.8- or 2.0-Fr tip microcatheter (1.8-F Carnelian Pixie; Tokai Medical, Gifu, Japan, or 2.0-F Estream; Toray Industries, Tokyo Japan) was superselectively inserted into the suspected feeding branch using this navigation image.
After obtaining a DSA, a CTHA via the microcatheter (superselective CTHA) was performed and the axial, coronal, and sagittal reconstruction images were created. Then, it was evaluated whether the distribution area of contrast material covered the whole tumor with safety margin in all 3 images (3D-safety margin). For evaluation of presence or absence of the 3D-safety margin, a fusion image of the CTHA via the common hepatic artery and superselective CTHA was created.
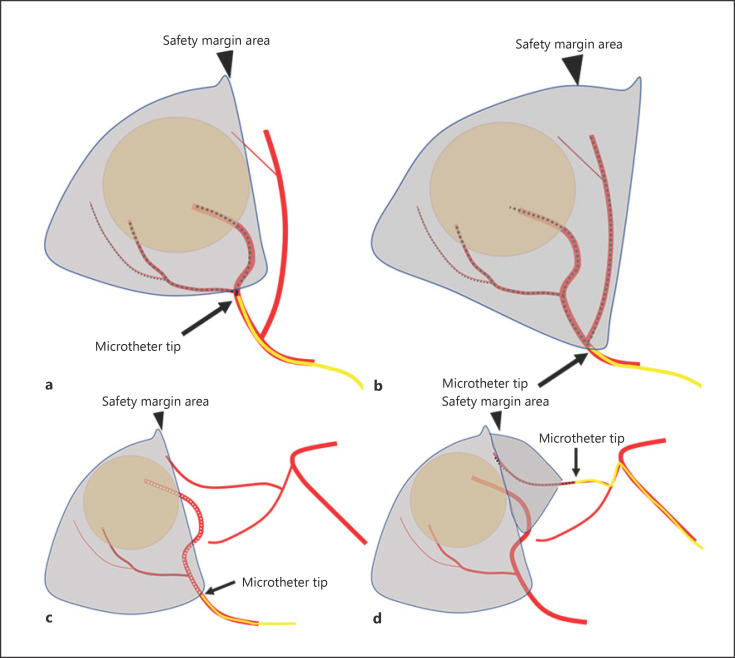
In case of the absence of 3D-safety margin, there were 2 possible techniques to achieve 3D-safety margin. First, the tip of the microcatheter was pulled back to the position including branches supplying into the lack of safety margin area. In this situation, the initial inserted microcatheter position was too deep in the feeding branch. Therefore, the catheter was repositioned at the more proximal site. Second, a catheter was inserted into another arterial branch supplying to the adjacent area. In most cases, the tumor was located at a segmental border of the liver. TACE was performed via the first-inserted branch. Then, the microcatheter was taken out and reinserted into the second branch (Fig. 1).
Fig. 1.
Techniques to achieve 3D-safety margin. a The initial inserted microcatheter tip is too deep in the feeding branch (arrow). There is no safety margin (arrowhead). b The tip of the microcatheter is pulled back to the position (arrow), including the branch supplying into the lack of safety margin area (arrowhead). c The branch that a microcatheter initially inserted (arrow) does not supply to the safety margin area (arrowhead) due to the fact that the tumor locates at a segmental border of the liver. d A catheter is inserted into another arterial branch (arrow) supplying to the safety margin area (arrowhead) after TACE via the first inserted branch. TACE, transarterial chemoembolization.
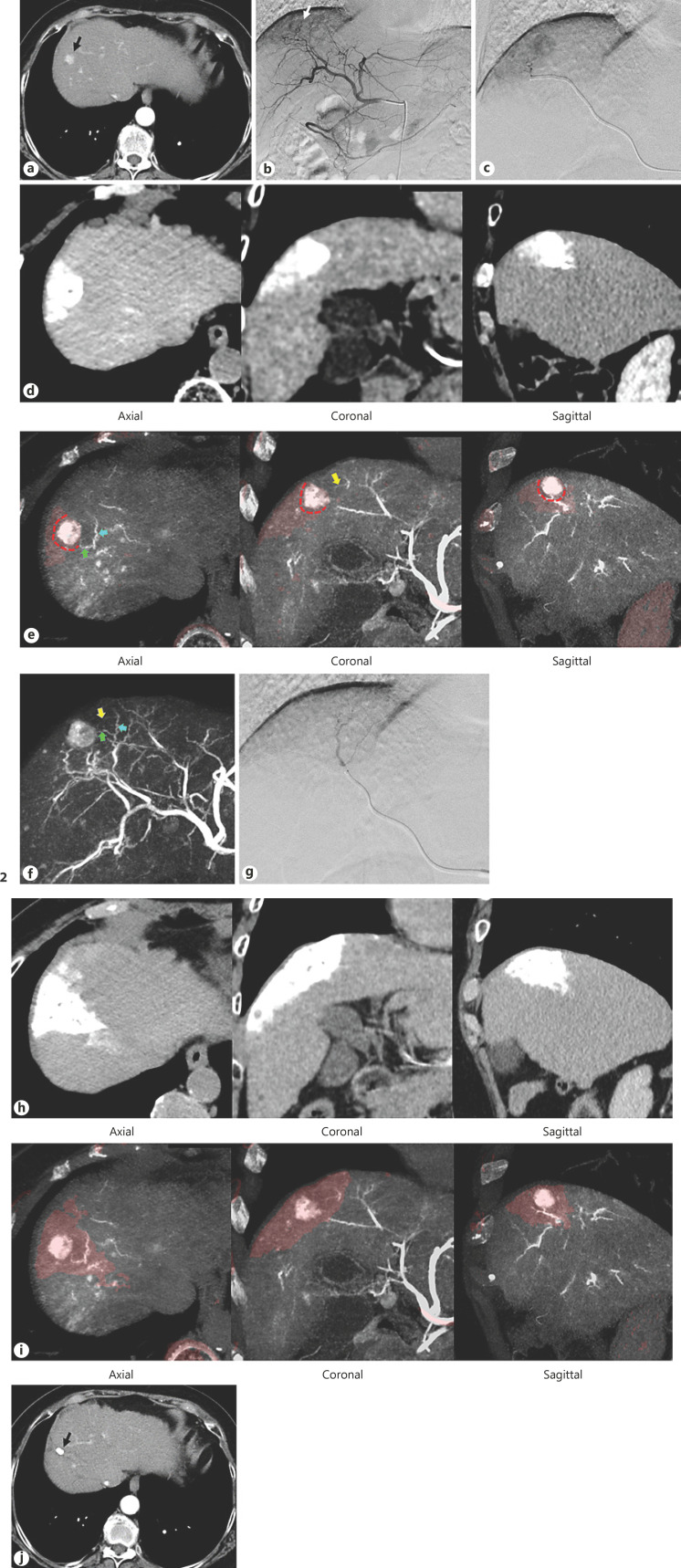
Selection of these methods was determined by using the created fusion images of the CTHA via the common hepatic artery and the superselective CTHA. A mark was placed on the arteries supplying to the negative margin area and it was displayed on a MIP image to show the corresponding branches (Fig. 2).
Fig. 2.
A case obtained 3D-safety margin using a fusion image. a CT before TACE showed a tumor located in the segment 8 (arrow), which was invisible in ultrasonography. b DSA via the common hepatic artery showed a tumor stain (arrow). c A microcatheter was superselectively inserted into the tumor-feeding artery. d Superselective CTHA via the microcatheter showed that the distribution area of contrast material covered the whole tumor. e A fusion image of CTHA via the common hepatic artery and the superselective CTHA (light red color area) showed the presence of 3D-safety margin on the axial, coronal, and sagittal images (red dotted lines). The branches suppling to the safety margin area were marked arrows (green, blue, and yellow arrows). f These arrows appeared on the MIP image of the CTHA via the common hepatic artery. g A microcatheter was inserted into the branch supplying to the safety margin area. h CT after TACE via 2 branches showed the lipiodol accumulation in the tumor and the liver parenchyma around the tumor. i A fusion image of CTHA via the common hepatic artery and the CT after TACE showed presence of 3D-safety margin on the axial, coronal and sagittal images. j CT obtained 3 years after TACE showed no local recurrence. TACE, transarterial chemoembolization; CTHA, CT during hepatic arteriography; DSA, digital subtraction angiography; MIP, maximum intensity projection.
A mixture of epirubicin solution and ethiodized oil (Lipiodol Ultra-Fluide; Guerbet, Villepinte, France) was formed by a pumping emulsification technique. The emulsion was infused into the feeding arteries followed by 1-mm gelatin sponge particles (Gelpart, Nippon Kayaku, Tokyo, Japan) to achieve complete stasis. The TACE procedures were performed by one of the 3 interventional radiologists with 10, 17, and 20 years of experience, respectively.
Evaluation of Achieving 3D-Safety Margin
Immediately after TACE, non-enhanced CT was obtained to confirm the distribution of lipiodol. The existence of 3D-safety margin was evaluated on a fusion image of the CTHA via the common hepatic artery and the post-TACE CT. Positive 3D-safety margin was defined as at least 1-mm thick lipiodol accumulation around the tumor on the multiplanar reconstruction images. When the tumor was located in the subcapsular region and no extrahepatic collateral feeding artery, the subcapsular margin was considered positive. These definitions were according to the previous report [14].
Follow-Up
Blood tests were examined 1, 3, and 5 days and 1 month after TACE. Dynamic contrast-enhanced CT or MR was performed at 4–6 weeks after TACE. When there was no evidence of recurrence, follow-up dynamic contrast-enhanced CT or MR was performed every 3 months. Local disease-free survival (DFS) was defined as the time from the date of the TACE procedure to the date of local tumor recurrence or death from any causes. Total DFS was defined as time from the date of the TACE procedure to the date of tumor recurrence including non-targeted lesions or death from any causes. Overall survival (OS) was defined as the time from the date of the TACE procedure to the date of death. Adverse events were graded according to the Common Toxicity Criteria for Adverse Events (CTCAE) version 5.0. Deterioration of liver function was evaluated by the comparison of Child-Pugh scores between pre-TACE and 1 month after TACE.
Statistical Analysis
Cumulative OS and local/total DFS curves were generated according to the Kaplan-Meier method. Fisher's exact test was used to compare the recurrence ratio. The statistical analysis was performed with commercially available software (SPSS version 22.0; SPSS, Chicago, IL, USA).
Result
There were 48 tumors in 44 patients included in this study. Demographic data of the patients and target tumors are summarized in Table 1. The reasons why TACE was chosen for the treatment were as follows: multiple nodules (n = 15), previously repeated recurrence (n = 10), tumor location (n = 16), patient's wish (n = 4), ascites (n = 1), and others (n = 2).
Table 1.
Demographic data
| Median age | 74 years (56–85 years) |
| Sex (male/female) | 34 (77.3%)/10 (22.7%) |
| Underlying liver disease (HCV/HBV/alcoholic/ | 28 (63.6%)/7 (15.9%)/3 (6.8%)/0 (0%)/6 (13.6%) |
| NASH/others) | |
| Child-Pugh (A/B) | 42 (95.4%)/2 (4.6%) |
| ALBI grade (1/2/3) | 20 (45.5%)/23 (52.3%)/1 (2.3%) |
| Prior treatments | |
| No prior treatment | 10 (22.7%) |
| Prior surgery | 2 (4.6%) |
| Prior TACE | 25 (56.8%) |
| Prior surgery and TACE | 6 (13.6%) |
| Prior TACE and RFA | 1 (2.3%) |
| Number of target tumor (1/2) | 40 (90.9%)/4 (9.1%) |
| Target tumor size (mean±SD) | 1.9±0.5 cm (1.0–3.0 cm) |
| Target tumor location (S1/S2/S3/S4/S5/S6/S7/S8) | 0 (0%)/7 (14.6%)/8 (16.7%)/6 (12.5%)/7 (14.6%)/6 (12.5%)/4 (8.3%)/10 (20.8%) |
| Subcapsular region (±) | 26/22 |
| Serum alpha-fetoprotein level (mean±SD) | 60.8±129.6 ng/mL |
The insides of the tumors were homogenously enhanced in all cases in the initial superselective CTHA. There were only 9 target tumors of 48 tumors (18.8%) that had positive 3D-safety margin. For the remaining 39 tumors having lack of margin in a part of the surrounding tumor, the operator used the pulling back of the catheter method in 16 tumors (41.0%), used the selection of another branch that fed the adjacent area in 20 tumors (51.3%), and used both methods in 3 tumors (7.7%). The mean lipiodol volume used in each target tumor was 3.0 ± 1.0 cm3. The mean amount of epirubicin in each tumor was 27.9 ± 10 mg.
Finally, after using these 2 methods, 45 of the 48 tumors achieved 3D-safety margin in CT after TACE. The technical success rate was 93.8%. In the 3 failed cases, the 3D-safety margin could not be obtained due to the following causes. First, 1 patient previously received 5 TACE procedures. The operator could not find the corresponding branch supplying to the negative safety margin area due to stenosis of the hepatic arterial branches. Second, in another patient, the microcatheter was unable to be inserted into the corresponding branch due to the angulation. Third, in the last patient, intimal injury occurred when the guide wire was inserted into the branch. Lipiodol was homogeneously accumulated in all tumors.
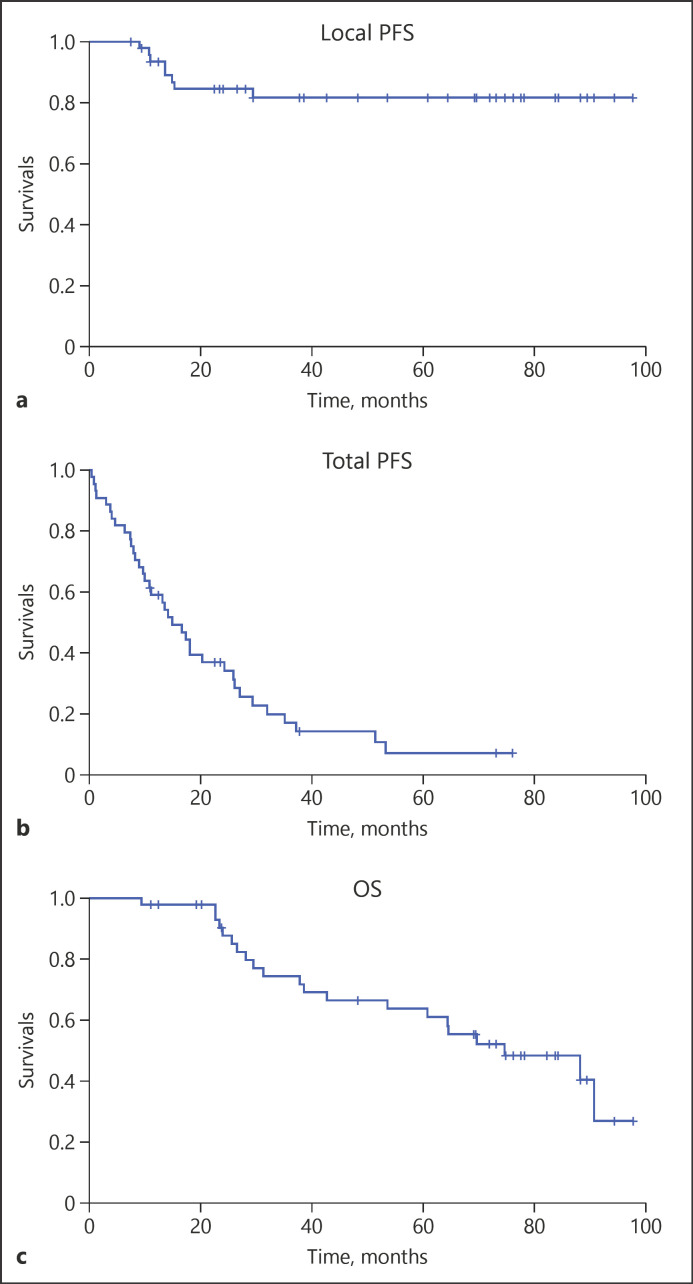
Totally, there were 9 target tumors (18.8%) that had local recurrence after 5-year follow-up. The mean local recurrent time of these 9 tumors was 13.9 ± 6.0 months. In patients who had positive 3D-safety margin, there were 8 of 45 tumors (17.8%) that had local recurrence after 5-year follow-up, while in patients who had negative 3D-safety margin, there was 1 of 3 tumors (33.3%) that had local recurrence (p = 0.47). The portal vein visualization according to Miyayama's criteria was grade 0 in 19 patients, grade 1 in 8 patients, and grade 2 in 21 patients. The local recurrence ratio in each portal vein visualization grads was 31.6% in grade 1, 12.5% in grade 2, and 9.5% in grade 3 [8]. The overall local tumor 1-, 2-, 3-, 4-, and 5-year recurrence rates were 8.3, 16.7, 18.6, 18.6, and 18.6%, respectively. The total tumor recurrence which includes local and non-local recurrence occurred in 37 of 44 patients (84.1%). The median total progression-free survival was 14.9 months (95% confidence interval, 9.8–20.0 months). The median OS was 74.7 months (95% confidence interval, 48.4–101.0 months) (Fig. 3).
Fig. 3.
a Cumulative local PFS of 48 tumors enrolled in this study, calculated by the Kaplan-Meier method. The local PFS at 1, 2, 3, 4, and 5 years was 91.7, 83.3, 81.4, 81.4, and 81.4%, respectively. b Cumulative total PFS of 44 patients who enrolled in this study. The median total PFS was 14.9 months (95% confidence interval, 9.8–20.0 months). c Cumulative OS of 44 patients who enrolled in this study. The median OS was 74.7 months (95% confidence interval, 48.4–101.0 months). PFS, progression-free survival; OS, overall survival.
The adverse events after TACE are shown in Table 2. A total of 34 over grade 3 adverse events were found. All serum aspartate aminotransferase and alanine aminotransferase elevation improved to pre-treatment baseline at 1-month follow-up. There was 1 patient with grade 3 serum bilirubin elevation who still had an abnormal level at 1-month follow-up (from 1.3 mg/dL before procedure to 2.6 mg/dL at 1-month follow-up) which caused a deterioration of Child-Pugh score from B7 to B8. This patient also had portal vein thrombus after TACE and received warfarin treatment for 5 months. Consequently, the thrombus disappeared and the liver function improved 7 months after TACE. Deterioration of liver function at 1 month after TACE was found in 4 patients in Child-Pugh score and 6 patients in albumin-bilirubin grade. Three patients with Child-Pugh score A5 before TACE became worse to A6. However, all of them recovered to A5 at 1.5, 2, and 5 months after TACE. The remaining 1 case had prolonged deterioration of B7, as described above. Six patients with albumin-bilirubin grade 1 had deterioration to grade 2 at 1 month after TACE. However, all of them recovered 1, 1.5, 2, 2.5, 4, and 5 months after TACE.
Table 2.
Adverse events
| All, n (%) | Over grade 3, n (%) | |
|---|---|---|
| Elevated aspartate aminotransferase | 44 (100) | 17 (38.6) |
| Elevated alanine aminotransferase | 42 (95.5) | 16 (36.4) |
| Increased serum bilirubin | 25 (56.8) | 1 (2.3) |
| Fever | 10 (22.7) | 0 (0.0) |
| Abdominal pain | 12 (27.3) | 0 (0.0) |
| Appetite loss | 17 (38.6) | 0 (0.0) |
| Portal vein thrombosis | 1 (2.3) | 1 (2.3) |
| Bile duct dilatation | 2 (4.5) | 0 (0.0) |
| Biloma | 1 (2.3) | 0 (0.0) |
TACE, transarterial chemoembolization.
As post-embolization symptoms, abdominal pain (27.3%), fever (22.7%), and appetite loss (38.6%) occurred. All of them were either grade 1 or 2. There were 2 patients with mild intrahepatic bile duct dilatation and 1 patient with a biloma in the liver segment in which TAC was performed. These patients had no symptoms. Therefore, any treatments were not necessary.
Discussion
Previously published literature studies showed that in retrospective studies, the ratios of positive safety margins were 47–87% [11, 12, 13, 14]. In our prospective study, the safety margin success rate was 95.8% which was higher than all of the previous studies. This result demonstrated that when the operator had intention to obtain safety margin, it could be achieved in most cases.
Hybrid angio-CT or cone beam CT is needed to evaluate safety margin. The safety margin area is not visible in DSA due to absence of tumor stain. Miyayama et al. [12] reported the success rate of safety margin was 65.3% by using only DSA whereas 87.2% by using DSA and cone beam CT. Only 18.8% of the cases had 3D-safety margin in the initial superselective CTHA. The 3D reconstruction image created by a workstation was useful. The feeding branches supplying to the margin area could be shown on the MIP images. An initially inserted catheter was pulled back and/or another branch was selected based on the 3D images. In our study, a fusion image technique was used, which could clearly show the negative safety margin area on the CTHA via the common hepatic artery. Current cone beam CT software can automatically show the tumor feeding artery including the safety margin area.
Previously, Park et al. [10] reported that tumors located in the center of the liver segment showed lower recurrence ratio of 13.3% when compared with tumors located in a segmental border zone of 44.8% during the median follow-up period of 17 months. In our results, the overall 1-year and 2-year local recurrence ratios were 8.3 and 16.7%, respectively. Our results were similar to those of Park et al. [10] in the center of the liver segment. This could be due to obtaining 3D-safety margin in an adjacent area in cases with tumors located in a segmental or subsegmental border.
The 5-year local recurrence ratio of 18.6% could be acceptable in cases with contraindications of RFA and surgical resection. Previously, the local recurrence ratios of RFA of 4.6–21% were reported [15, 16, 17]. The indication criteria of RFA and resection are various among institutions and physicians. In general, RFA obtaining adequate safety margin achieved less local recurrence. The median OS of 74.7 months in our study could also be favorable.
In our study, there were no statistically significant differences of local recurrence ratio between the safety margin-positive and margin-negative groups. The following reasons could be considered: (1) the sample size was small, and the negative group was only 3 cases; (2) In all cases, the operator attempted to obtain 3D-safety margin. Therefore, even in the negative group, the insufficient safety margin portion was small. Consequently, there was no recurrence in 2 of the 3 negative margin cases.
Our data also demonstrated the local recurrence ratio is influenced by the portal vein visualization. However, the ratio in each grade was lower than that of Miyayama's report (74% in grade 0, 42% in grade 1, and 19% in grade 2) [8]. This could be due to the fact that, in our study, only small tumors were enrolled and the safety margins were intentionally obtained in all cases. The optimal width of safety margin has not been defined yet. Tajima et al. [11] reported that at least 3 mm was needed. Miyayama et al. [12] defined that the sufficient safety margin was 5 mm for tumors smaller than 2.5 cm and 10 mm for tumors 2.5 cm or larger. Two reports by Bannangkoon et al. [13] and Kattipatanapong et al. [14] showed the safety margin in any thickness, even 1 mm, was sufficient. When treatment including occult daughter nodule is considered, a 5-mm safety margin is needed [18]. On the other hand, for the blockage of reperfusion into the tumor via non-embolized artery, even 1-mm safety margin might be sufficient.
When 3D-safety margin is intentionally obtained, care must be given to adverse events due to large embolization areas. In our study, all elevations of aspartate aminotransferase, alanine aminotransferase, and total bilirubin were temporal. In most patients, liver function improved to pre-treatment baseline within 1 month. However, there were a few patients that still had deterioration of Child-Pugh score at 1-month follow-up. Especially, the patient with Child-Pugh score 7 before TACE had prolonged deterioration with portal vein thrombosis. Therefore, the indication of obtaining safety margins should be carefully considered in poor liver function patients. Although there were only a few patients having focal intrahepatic bile duct dilatation and biloma, there were no liver abscesses or infarctions. Post embolization symptoms, that is, pain, fever, or loss of appetite were mild.
Several limitations could be considered in this study. First, only small size tumors were enrolled. The technical success ratio and local control ratio in middle size tumors should have been evaluated. Second, 95% of the patients had good liver function with Child-Pugh score A. Therefore, safety of this technique in poor liver function was unclear.
In conclusion, our study suggested that superselective TACE performed with the intention of obtaining 3D-safety margin was feasible by using 3D images, achieving a high success rate in small HCC. This treatment could be safe and tolerable in patients with good liver function and prevent local tumor recurrence.
Statement of Ethics
This study complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. This study was approved by the Nara Medical University Institutional Review Board (Approval Number, 2615). Written informed consent was obtained from all patients.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors did not receive any funding.
Author Contributions
C.C., T.S., T.M., and N.H. analyzed and interpreted the patient data. T.T., N.H., and H.A. contributed to the enrollment of patients and conducted TACE procedures. T.T. checked this study protocol. N.M. and K.K. were supervisors of this study. All authors read and approved the final manuscript.
Acknowledgement
We thank Ms. Marian Pahud for advice in submitting this article.
References
- 1.Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35((9)):2155–66. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kudo M. Extremely high objective response rate of lenvatinib: its clinical relevance and changing the treatment paradigm in hepatocellular carcinoma. Liver Cancer. 2018;7((3)):215–24. doi: 10.1159/000492533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma. Asia-Pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9((3)):245–60. doi: 10.1159/000507370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohishi H, Uchida H, Yoshimura H, Ohue S, Ueda J, Katsuragi M, et al. Hepatocellular carcinoma detected by iodized oil. Use of anticancer agents. Radiology. 1985;154((1)):25–9. doi: 10.1148/radiology.154.1.2981114. [DOI] [PubMed] [Google Scholar]
- 5.Miyayama S, Matsui O. Superselective conventional transarterial chemoembolization for hepatocellular carcinoma: rationale, technique, and outcome. J Vasc Interv Radiol. 2016;27((9)):1269–78. doi: 10.1016/j.jvir.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Nishimine K, Uchida H, Matsuo N, Sakaguchi H, Hirohashi S, Nishimura Y, et al. Segmental transarterial chemoembolization with lipiodol mixed with anticancer drugs for nonresectable hepatocellular carcinoma: follow-up CT and therapeutic results. Cancer Chemother Pharmacol. 1994;33((Suppl)):S60–8. doi: 10.1007/BF00686670. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo N, Uchida H, Sakaguchi H, Nishimine K, Nishimura Y, Hirohashi S, et al. Optimal lipiodol volume in transcatheter arterial chemotherapy for hepatocellular carcinoma: study based on lipiodol accumulayion patterns and histopathologic findings. Sem Oncol. 1997;24((Suppl 6)):61–70. [PubMed] [Google Scholar]
- 8.Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, et al. Ultraselective transcatheter arterial chemoembolization with 2-f tip microcatheter for small hepatoceullar carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodised oil. J Vasc Interv Radiol. 2007;18((3)):365–76. doi: 10.1016/j.jvir.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Ichikawa T, Machida N, Sasaki H, Tenmoku A, Kaneko H, Negishi R, et al. Early prediction of the outcome using tumor markers and mRECIST in unresectable hepatocellular carcinoma patients who underwent transarterial chemoembolization. Oncology. 2016;91((6)):317–30. doi: 10.1159/000448999. [DOI] [PubMed] [Google Scholar]
- 10.Park SH, Cho YK, Ahn YS, Park YO, Kim JK, Chung JW. Local recurrence of hepatocellular carcinoma after segmental transarterial chemoembolization: risk estimates based on multiple prognostic factors. Korean J Radiol. 2007 Mar-Apr;8((2)):111–9. doi: 10.3348/kjr.2007.8.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tajima T, Nichie A, Asayama Y, Ishigami K, Ushijima Y, Kakihara D, et al. Safety margins of hepatocellular carcinoma demonstrated by 3-dimentional fused images of computed tomographic hepatic arteriography/unenhanced computed tomography: prognostic significance in patients who underwent transcatheter arterial chemoembolization. J Comput Assist Tomogr. 2010;34((5)):712–9. doi: 10.1097/RCT.0b013e3181e1d241. [DOI] [PubMed] [Google Scholar]
- 12.Miyayama S, Yamashiro M, Hashimoto M, Hashimoto N, Ikuno M, Okumura K, et al. Comparison of local control in transcatheter arterial chemoembolization of hepatocellular carcinoma ≤6 cm with or without intraprocedural monitoring of the embolized area using cone-beam computed tomography. Cardiovasc Intervent Radiol. 2014;37((2)):388–95. doi: 10.1007/s00270-013-0667-2. [DOI] [PubMed] [Google Scholar]
- 13.Bannangkoon K, Hongsakul K, Tubtawee T, Piratvisuth T. Safety margin of embolized area can reduce local recurrence of hepatocellular carcinoma after superselective transarterial chemoembolization. Clin Mol Hepatol. 2019;25((1)):74–85. doi: 10.3350/cmh.2018.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kattipatanapong T, Nishiofuku H, Tanaka T, Sato T, Masada T, Tatsumoto S, et al. Improved local tumor control and survival rates by obtaining a 3D-safety margin in superselective transarterial chemoembolization for small hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2020 Mar;43((3)):423–33. doi: 10.1007/s00270-019-02365-9. [DOI] [PubMed] [Google Scholar]
- 15.Hori T, Nagata K, Hasuike S, Onaga M, Motoda M, Moriuchi A, et al. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38((10)):977–81. doi: 10.1007/s00535-003-1181-0. [DOI] [PubMed] [Google Scholar]
- 16.Raut CP, Izzo F, Marra P, Ellis LM, Vauthey JN, Cremona F, et al. Significant long-term survival after radiofrequency ablation of unresectable hepatocellular carcinoma in patients with cirrhosis. Ann Surg Oncol. 2005 Aug;12((8)):616–28. doi: 10.1245/ASO.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Horiike N, Iuchi H, Ninomiya T, Kawai K, Kumagi T, Michitaka K, et al. Influencing factors for recurrence of hepatocellular carcinoma treated with radiofrequency ablation. Oncol Rep. 2002 Sep-Oct;9((5)):1059–62. [PubMed] [Google Scholar]
- 18.Sasaki A, Kai S, Iwashita Y, Hirano S, Ohta M, Kitano S. Microsatellite distribution and indication for locoregional therapy in small hepatocellular carcinoma. Cancer. 2005 Jan 15;103((2)):299–306. doi: 10.1002/cncr.20798. [DOI] [PubMed] [Google Scholar]