Abstract
Major depressive disorder (MDD) affects more than cognition, having a temporal relationship with neuroinflammatory pathways of Parkinson's disease (PD). Although this association is supported by epidemiological and clinical studies, the underlying mechanisms are unclear. Microglia and astrocytes play crucial roles in the pathophysiology of both MDD and PD. In PD, these cells can be activated by misfolded forms of the protein α-synuclein to release cytokines that can interact with multiple different physiological processes to produce depressive symptoms, including monoamine transport and availability, the hypothalamus-pituitary axis, and neurogenesis. In MDD, glial cell activation can be induced by peripheral inflammatory agents that cross the blood-brain barrier and/or c-Fos signalling from neurons. The resulting neuroinflammation can cause neurodegeneration due to oxidative stress and glutamate excitotoxicity, contributing to PD pathology. Astrocytes are another major link due to their recognized role in the glymphatic clearance mechanism. Research suggesting that MDD causes astrocytic destruction or structural atrophy highlights the possibility that accumulation of α-synuclein in the brain is facilitated as the brain cannot adequately clear the protein aggregates. This review examines research into the overlapping pathophysiology of MDD and PD with particular focus on the roles of glial cells and neuroinflammation.
Keywords: Depression, Parkinson's disease, Alpha-synuclein, Aquaporin, Glymphatic flow, Neuroinflammation
Introduction
Major depressive disorder (MDD), more commonly known as depression, is an affective disorder characterized in the Diagnostic and Statistical Manual of Mental Disorders by symptoms including constant depressed moods, fatigue, reduced interest in daily activities, and/or recurrent thoughts of death among others [1]. Although it has long been viewed by society as a cognitive disorder, emerging research has focussed on physiological signs of the disorder including neuroinflammation [2]. Thus, neuroinflammation has linked the pathophysiology of MDD to that of other diseases such as Parkinson's disease (PD) as possibly a symptom, risk factor, and/or prodrome.
Depression is well established often to result from and coexist with PD as a common non-motor symptom [3]. Prevalence rates of some forms of depressive symptoms in PD are variable but generally average at around 35% even in newly diagnosed individuals [4]. However, the accuracy of these figures is difficult to assess given that depression is often missed in PD due to the high degree of overlap in symptoms [3, 5]. Depression serves as one of the largest contributors to poor quality of life in PD patients, equally as or even more important than high severity of motor impairment [5, 6]. Its effects on management have been found to include stronger indications for treatment initiation, higher treatment frequency, and increased referrals. It has also been associated with exacerbated motor impairment and faster disease progression. Although research regarding these last 2 factors has been contentious, the major impact this comorbidity has on patients cannot be denied [5].
Conversely, many epidemiological studies have suggested that MDD can increase the likelihood of developing PD in later life [7, 8]. This highlights MDD as a mental health concern that is not only debilitating through its influence on mood but also in its potential to predispose physical impairments in the form of parkinsonism. However, the mechanisms underlying the temporal relationship between MDD and PD remain poorly explained by the current literature. This review will examine recent research which has suggested overlap between MDD and PD in terms of activity-based and morphological changes in glial cells, particularly associated with neuroinflammation. If a causal link exists, it would emphasize the importance of promoting positive mental health and antidepressant treatment as a preventative measure for decreasing susceptibility to PD. Additionally, understanding the link will facilitate the development of targeted early detection methods for MDD patients potentially at risk of developing parkinsonian syndromes and prophylactic measures that target neuroinflammation or glial cell changes.
Parkinsonism and α-Synucleinopathies
Parkinsonism refers to the motor features of parkinsonian conditions, including idiopathic PD, dementia with Lewy bodies (DLB), multiple system atrophy (MSA), progressive supranuclear palsy, corticobasal degeneration, and vascular parkinsonism among other rarer conditions. They are grouped based on their similar clinical signs, such as bradykinesia, a tremor that is distinct at rest or when performing certain tasks, muscle rigidity and stiffness, an unstable gait, and other signs of motor dysfunction [9, 10]. α-Synucleinopathies refer to a group within these, which includes PD, DLB, and MSA. They are neurodegenerative diseases associated with the formation of harmful α-synuclein (α-syn) aggregates throughout the central nervous system. α-Syn is a small presynaptic protein made up of 140 amino acid residues folded to form 3 major domains: the N-terminal domain, a highly hydrophobic central domain, and the C-terminal domain [11]. In its normal state, the protein has been found to play a role in lipid membrane remodelling, vesicle trafficking, membrane curvature sensing and induction, regulation of exocytotic fusion pore size [12], and possibly neurotransmitter release and other neuronal functions linked to high concentration in nerve terminals [13]. However, the misfolded fibrillar form of α-syn rich in β-sheet has an increased tendency to spontaneously aggregate and induce neurodegeneration [13]. Secreted extracellular aggregates of α-syn are able to be taken up by cells and propagated to neighbouring cells in a prion-like manner. How and why this misfolding occurs is unclear but has been associated often with oxidative stress, metal ion dyshomeostasis, and mutations of genes such as SNCA[12, 14]. PD and DLB are associated with α-syn aggregates in the form of Lewy bodies and Lewy neurites in dopaminergic neurons in the substantia nigra pars compacta [15] and the cortex, respectively [16]. MSA is associated with glial cytoplasmic inclusions, primarily in oligodendrocytes [17].
Microglia
Microglia are myeloid cells that, under physiological conditions, have homeostatic and immune roles in the brain, but they are also central to disease processes. In response to different exogenous stimuli, microglia can be activated into either an M1 (pro-inflammatory) or M2 (anti-inflammatory) phenotype, the first being implicated in neuroinflammation [18]. Neuroinflammation is an automatic protective mechanism that the brain undertakes in response to microenvironmental disturbances such as injury, invasion of pathogens, and irradiation. Activated microglia play major roles in inducing and promoting this self-propagating process by releasing various pro-inflammatory factors [19]. Additionally, they can express antigens such as α-syn on major histocompatibility complex class II (MHCII) to stimulate an adaptive immune response, and adopt a phagocytic phenotype to directly combat stressors [20]. These responses have been exhibited in both PD models [19, 20] and in studies indicating inflammatory markers in MDD [21]. Activated microglia have also been shown to be potential mediators of spread of pathological α-syn aggregates to adjacent brain regions during disease progression [22].
Microglial Involvement in PD and the Link to Depression as a Non-Motor Symptom
Neuroinflammation in PD
The presence of neuroinflammation in PD patients has been strongly established through various methods. These include post-mortem analyses of patients' brains and ante-mortem analyses of inflammatory markers in serum and cerebrospinal fluid (CSF). Levels of these markers, including enzymes and cytokines such as IFN-γ, IL-1β, IL-6, and TNF-α, have also been found to determine the severity of non-motor disease symptoms including mild cognitive impairment and depression, hence prefacing an inflammatory link between PD and MDD [23, 24, 25]. Moreover, Williams-Gray et al. [25] have indicated pro-inflammatory markers were upregulated in early-stage (newly diagnosed) PD when compared to controls. Geriatric Depression Scale scores were also higher in the PD group when compared to controls [25]. Further evidence to the importance of neuroinflammation in PD includes animal models of PD which have demonstrated increased pro-inflammatory enzymes COX2 and iNOS resulting from α-syn aggregation [19]. Positron emission tomography has also revealed microglial activation as a significant contributor to PD progression [26, 27] and non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen have been associated with reduced risk of PD [27, 28]. In addition, both sporadic and familial PD have been linked to neuroinflammation via genome-wide association studies. Genes related to cytokine expression and signalling pathways regulating innate immune responses such as DJ-1, PINK-1, and Parkin have been implicated [29] as well as genes in the HLA region [30].
Microglial Activation in PD
Microglial activation has been observed as a physical transition from a quiescent ramified form to an amoeboid-shaped form, which has been demonstrated to increase in the substantia nigra and hippocampus of human brains as a result of α-syn aggregation, as a component of PD pathology [31]. This is thought to be due to excessive α-syn released from neurons [32]. Recent research has suggested that a mechanism of activation of microglia in response to pathogens, including aggregated proteins, is via toll-like receptors (TLRs), particularly TLR2. TLR2 upregulation has been detected in animal models of PD [33] as well as in the brain tissue of PD patients [31]. TLR2 deficiencies in mice have also led to elimination of α-syn-induced microglial activation [34]. Other membrane components in α-syn-induced microglial activation have also been identified, including CD36 and MHCII among others [19, 20].
Cytokine Release in PD and Depressive Behaviour
Activated microglia release many cytokines including those often found elevated in patients experiencing depressive symptoms such as TNF-α and IL-6 [21]. Cytokines have been suggested to be key players in the manifestation of depressive behaviour as they interact with a multitude of processes in the brain involved in MDD pathology. Such processes include the reuptake and availability of monoamine neurotransmitters, including serotonin, dopamine, and noradrenaline, that have been implicated in depression when dysregulated. Reuptake is increased and availability is decreased by IFN-γ, IL-6, IL-1β, and TNF-α in a 2-hit effect on serotonin transporters (SERT) and enzymes related to monoamine metabolism, respectively. Both mechanisms reduce synaptic serotonin levels, which is well established as a contributor to both somatic and cognitive types of depressive behaviour [35, 36]. This explains why elevated levels of inflammatory markers have been observed in patients resistant to antidepressant treatment as front-line antidepressants such as SSRIs function mainly to increase synaptic monoamines, and this is disrupted in the presence of cytokines that oppose this action [37].
Another process is the hypothalamic-pituitary-adrenal axis, the regulation of which can be impaired by TNF-α, IL-1, and IFN-α via influence of the cytokine on glucocorticoid (GC) receptor function and hence GC resistance [38]. IL-1β, via NF-κβ signalling activation, can also reduce neural plasticity in brain regions to which cognition is localized through glutamate excitotoxicity and reduced levels of brain-derived neurotrophic factor (BDNF) [39]. GCs also cause glutamate excitotoxicity through modulation of astrocytic release and uptake [40]. This is supported by serum BDNF levels being lower in PD patients compared to controls as well as in PD patients with depression compared to non-depressed PD patients. This suggests that BDNF reduction occurs in PD and may lead to depression [41]. The inhibition of neural plasticity is another speculated cause of depressive behaviour [42].
There are limitations in the current data as most of the research has involved animal models. However, cases of depressive behaviour induced in humans through cytokine therapy have been reported [39]. Moreover, TNF-α antagonism provides little benefit for generalized treatment of depressed patients possibly due to the wide range of cytokines active in neuroinflammatory depression [37]. A recent meta-analysis investigating the relationship between cytokines and GC resistance also indicated a need for more standardized and valid biochemical analysis to better enable researchers to address the gaps in knowledge of the biochemical mechanisms that still exist in the literature [43].
Catecholamine Dysfunction in PD and Depressive Behaviour
In addition to the neuroinflammatory cause of depression, mid-stage α-synucleinopathies show correlation of α-syn pathology with brainstem and limbic involvement, brain regions rich in noradrenergic and cholinergic receptors [44]. The dysfunction of catecholamine circuits as part of PD pathology may also contribute to the onset of depressive symptoms independent of cytokines. This has been demonstrated in a neural imaging study of cases with PD and depression which, using [11C]RTI-32 positron emission tomography, found decreased catecholaminergic innervation of multiple limbic regions involved in emotional processing including the locus coeruleus, amygdala, and thalamus [45]. This is further supported by observations that pramipexole, a preferential agonist of dopamine D3 receptors, is effective in reducing the severity of depression in PD patients [46]. Furthermore, Lewy body deposition has been found in serotonergic neurons during early stages of PD, resulting in reduction in serotonergic activity. SERT expression has also been suggested to increase in response to increased dopamine stimulation as serotonin is usually responsible for inhibiting dopamine release [47]. Therefore, depression may result from PD due to both the varied influences of cytokines as well as catecholamine dysregulation.
Microglial and Cytokine Involvement in MDD and the Link to PD
Neuroinflammation in MDD
Neuroinflammation has been investigated as a part of MDD pathology for many years with substantial supporting studies. MDD has been associated with significant increases in pro-inflammatory cytokines including IL-1β, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, TNF-α, and IFN-γ [21, 48, 49]. The reports are variable, however, as a meta-analysis by Dowlati et al. [21, 49] found no changes in IL-1β but increases in other cytokines and other research has found decreases in IL-10 as an anti-inflammatory cytokine. These differences may be attributable to individual variability between MDD cases but reasons for this should be further researched with larger sample sizes for reliability.
Microglial Activation in MDD
Along with systemic inflammation, microglial activation has been empirically demonstrated in MDD, such as in animal models of acute and chronic psychological stress, where increases in microglial activation were measured in brain regions linked to fear and threat appraisal including the prefrontal cortex, nucleus accumbens, bed nucleus of the stria terminalis, amygdala, hippocampus, and periaqueductal grey [50, 51, 52]. To better determine whether the microgliosis was directly related to stress, the inhibitor of microglial activation, minocycline, was administered to rats to observe the impact on stress symptoms. As this resulted in an attenuation of neuronal stress-induced activation and reversal of the animals' working memory deficits, it was evident that microglia may play an important role in the brain's response to chronic stress which can lead to mood disorders such as MDD [50]. Similar findings have been found in humans where depressed patients demonstrated significantly higher microglial activation detected through QUIN expression compared to controls [53]. As there are increased pro-inflammatory cytokines and microgliosis, and MDD can be treated effectively by NSAIDs [54], it may be that some MDD cases may be microglial disorders and may overlap with pathological processes that can result in PD. This indicates 2 possible scenarios, the first being that MDD may be a prodrome in PD through a direct link between MDD pathology and PD progression [7]. The second is that MDD may be a risk factor for PD by sensitizing the body to produce more exaggerated responses to future insults [55, 56].
Prodrome Hypothesis via Direct Link
Chronic neuroinflammation associated with severe MDD could eventually lead to the damaging inflammation-induced effects seen in PD including neurodegeneration [57, 58]. This possibility is highlighted by the extensive research that has been done on stress being able to induce degeneration of hippocampal neurons [59]. The role of neuroinflammation in neurodegeneration has also been supported by early findings that neurodegeneration can be limited by anti-inflammatory compounds. In addition, studies involving the injection of lipopolysaccharide − an inflammagen that simulates natural pro-inflammatory compounds − into rats have resulted in observable neurodegeneration, particularly in the dopaminergic neurons of the substantia nigra, likely due to high microglial density in this region [58]. Similar results have been obtained in studies of M1 activation [60]. According to mouse model studies, this may be due to nitric oxide and superoxide production, which both cause direct oxidative and nitrosative damage to neurons. They also cause glutamate excitotoxicity by triggering the release of excess glutamate and causing overstimulation [57]. This is supported by a recent meta-analysis that reported elevated markers of oxidative stress in MDD patients [61], which can lead to neurodegeneration [57]. However, there is conflicting research regarding this point [62, 63].
In MDD, microglial activation mainly appears to be dependent on c-Fos signalling as gliosis occurs primarily around c-Fos-expressing neurons such as in the hypothalamus, thalamus, and hippocampus [51]. This signalling has been demonstrated to be activated in response to peripheral inflammation, suggesting a mechanism for how MDD related to peripheral inflammation can progress to and cause central nervous system neuroinflammation and contribute towards the aetiology of PD [57]. Although these regions do not seem to overlap with those typically affected by PD, the growing body of research into microvesicles suggests that these extracellular vesicles can store and release pro-inflammatory agents to potentially propagate inflammation, and subsequently neurodegeneration, through to other brain regions. This idea is supported by animal studies as well as increased levels of microvesicles positive for myeloid markers found in the CSF of humans diagnosed with neuroinflammatory conditions [64].
Risk Factor Hypothesis via Indirect Link
Alternatively, or additionally, it may not be the direct stimulation of microglia that is the primary promoter of neurodegeneration in MDD but the increased reactivity of microglia in future inflammatory events. This is implicated by the significantly elevated pro-inflammatory cytokine levels produced from ex vivo stimulation by lipopolysaccharide in rats subject to stress compared to control rats. Thus, stress-related disorders such as MDD may increase susceptibility to PD by enhancing the inflammatory profile of microglia in response to α-syn aggregation. This could be attributed to the stress-induced increase in numbers of microglial TLR4, as well as increased numbers of CD86, a co-stimulatory protein expressed on microglia that plays a role in the presentation of antigens by MHCII. This was demonstrated by Wohleb et al. [56] who found through staining of surface antigens of microglia in rats that rats exposed to social disruption stress had a significantly higher mean percent of microglial TLR4 and CD86 compared to control rats. These increased numbers would essentially increase the sensitivity of microglia to neurological events, thereby increasing the likelihood of inducing a potentially damaging neuroinflammatory response [56].
It has also been proposed that exaggerated neuroinflammation may result from the hypothalamic-pituitary-adrenal axis hyperactivity and GC resistance characteristic of MDD. Whilst GCs have been strongly recognized as being anti-inflammatory, many studies have demonstrated that GC signalling changes in response to acute and chronic stress. More specifically, immune cells including microglia have reduced sensitivity to the anti-inflammatory action of GCs despite the presence of high circulating cortisol levels. This supports the notion that MDD primes the body to induce more severe inflammation, and subsequently PD pathology, as neuroinflammation would be readily triggered and resistant to arrest [38, 55, 56]. Other actions of chronic GCs on neurons include impairment of energy metabolism, calcium regulation, and glutamate excitotoxicity [59]. M1 microglial activation also reportedly decreases the rate of degradation of internalized extracellular protein aggregates [60]. Therefore, this evidence together emphasizes MDD as both an early risk factor for PD as well as a comorbidity that can worsen the severity of PD.
Astrocytes
Along with microglia, astrocytes, or astroglia, play a major role as immune cells in the neuroinflammatory process. Moreover, astrocytes can be activated by or cause the activation of microglia through the release of pro-inflammatory cytokines [65]. Making up 20–50% of brain volume, astrocytes possess numerous long processes, known as endfeet, that mingle through other neural cells and surround vascular walls as part of the blood-brain barrier (BBB). With a large number of receptors and transporters located on their membranes, they play key roles in monitoring the local microenvironment and extracellular concentrations of substances such as ions and glutamate [66]. Emerging research has suggested that these endfeet also make up a newly described complex cleaning system of the brain known as the glymphatic system [67].
Astroglial Involvement in PD and the Link to Depression as a Non-Motor Symptom
The link between PD and MDD as a non-motor symptom may be linked to the neuroinflammatory role of astroglia. Astrogliosis has been empirically demonstrated in PD and other α-syn pathologies including DLB and MSA but has been largely inconsistent for PD where no, mild, and marked astrogliosis has been reported. This has been attributed to variability between individual PD patients due to the multitude of factors associated with astroglial activation. However, astroglia have an acknowledged role in PD and DLB pathogenesis as pathological α-syn forms inclusion bodies within them [65]. These are thought to derive from misfolded α-syn ejected by neurons that is then endocytosed by astroglia as part of the glial cell's role in maintaining the local microenvironment [65, 68]. Although astroglia normally express α-syn, the expression is much lower when compared to neurons, implicating neurons as the primary source of pathological α-syn [11, 68]. Subsequently, α-syn interaction and uptake stimulates a neuroinflammatory response in conjunction with microglia that can induce MDD likely through similar mechanisms to those of microglia including cytokine release. In addition, astrocytes encode for important protective proteins that can be downregulated to allow sensitivity to PD pathophysiology. Some of these have also been demonstrated as direct contributors to Lewy body formation as they have been found within the core of the inclusions [68].
What Is the Glymphatic System?
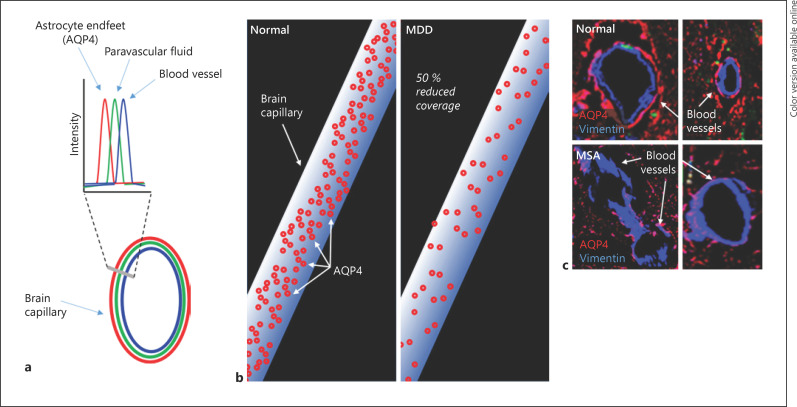
Iliff et al. [67] proposed the existence of the glymphatic system − a paravascular pathway that removes interstitial solutes from and circulates nutrients throughout the brain parenchyma. This discovery was made through the use of tracers that allowed observation of CSF flow in the brain. It was found that the tracers moved rapidly through pathways bounded by arteries/arterioles and astrocytic endfeet, through paravascular sheaths (Fig. 1) [67].
Fig. 1.
The glymphatic system and potential dysfunction in MDD and PD. a Glymphatic system structure shows solute (green) within the paravascular space [67] between the blood vessels (blue) and the AQP4-positive astrocytic endfeet (red). In normal brain, the glymphatic system likely clears solutes via the movement of CSF through these paravascular pathways. b The coverage of blood vessels by AQP4 associated with astrocytic endfeet in post-mortem brain showed a significant 50% reduction in the MDD group compared to the control group [69]. c Reduced AQP4 density around vimentin-positive blood vessels in the atypical PD, multiple system atrophy, compared to normal visual cortex (de Smet and Pountney, unpublished data). MDD, major depressive disorder; PD, Parkinson's disease; AQP4, aquaporin-4; CSF, cerebrospinal fluid; MSA, multiple system atrophy.
Astrocytic endfeet act as molecular sieves as larger solutes were observed to be excluded from the pathways. Another apparent role of the endfeet is the expression of aquaporin-4 (AQP4) water channels that are important for facilitating fluid transport between the CSF and the interstitial fluid. This means that solutes can be transported via the convective flow of fluid from the brain interstitium to the CSF for bulk flow out of the brain via the venous system (the tracers were found alongside venules at later time points after their injection). The importance of AQP4 was supported by comparison of solute clearance, namely radiolabelled [3H]mannitol and soluble amyloid β, between AQP4-null mice and wild-type control mice. As the AQP4-null mice demonstrated reduced clearance of the solutes, it was concluded that AQP4, along with arterial pulsation, drives the bulk flow of subarachnoid CSF through the para-arterial spaces into the brain interstitium and out via paravenous pathways, the bloodstream or transport mechanisms of the BBB to the cervical lymphatics [67]. Since this study, numerous rodent studies have supported the idea of an AQP4-dependent clearance system in the brain [70, 71] although human research has been scarce but consistent. For example, AQP4 mis-localization has been associated with worsened Alzheimer's disease severity and amyloid-β pathology, indicating poor amyloid-β clearance [72].
Clearance systems in the brain have been established prior to this discovery, and controversial research regarding whether it is the primary mechanism of clearance or whether the glymphatic system even exists continues to emerge. With evidence supporting and refuting both the glymphatic mechanism and BBB diffusion mechanisms, these systems undoubtedly require further research, especially because similar experiments have reported both affirmative and non-affirmative findings [73]. Table 1 summarizes the limitations in knowledge related to glymphatic clearance and BBB clearance.
Table 1.
Limitations in knowledge related to glymphatic clearance and BBB clearance (adapted [73])
| Limitations for glymphatic clearance | Limitations for BBB clearance |
|---|---|
| Fluorescent tracing has demonstrated size-dependent flow of tracers consistent with diffusion coefficients, suggesting a diffusion model of transport instead of the glymphatic model | Fluorescent tracing has demonstrated similar rate constants for the transport of solutes with large size variations, suggesting a convective model |
|
| |
| AQP4 cannot transport macromolecules and AQP4-null mice have demonstrated no qualitative or quantitative variation in solute clearance | AQP4-null mice have demonstrated impaired solute clearance |
|
| |
| Bulk flow is unlikely with the high hydraulic resistance of such narrow pathways, even with a major pressure difference | Diffusion transport is only short range and thus cannot explain how solutes are cleared over the long distance between IF and the BBB |
|
| |
| There may be insufficient coverage of cerebral blood vessels by astrocytic endfeet to justify their role in “sieving” interstitial solutes | Diffusion of solutes into the CSF for clearance is exclusive to small and/or lipophilic molecules, suggesting the sieving effect of astrocytic endfeet |
BBB, blood-brain barrier; AQP4, aquaporin-4; IF, interstitial fluid; CSF, cerebrospinal fluid.
It is unclear what the balance is between the glymphatic system and BBB in the removal of extracellular waste products in the human brain, particularly in light of the limited research conducted on human samples as opposed to animal brains. However, considering the substantial research supporting the existence and role of the glymphatic system in protein aggregation-induced pathologies, the glymphatic system may be key to explaining a potential link between MDD and PD [74].
Astroglial Involvement in MDD and the Link to PD
Astroglial involvement has been implicated in MDD as immunofluorescent staining followed by confocal image analysis of post-mortem brain tissue has demonstrated that the coverage of blood vessels in the brain by astrocytic endfeet is significantly reduced in MDD patients when compared to control participants [69] (Fig. 1b).
This potentially results in an impaired ability of the glymphatic system to clear solutes like α-syn since its main driver, AQP4, is scarce along with the astrocytic endfeet on which it is located. This has been exhibited in immunofluorescent staining in mice which displayed decreased penetration of tracers into the brain parenchyma after being subject to chronic unpredictable mild stress, indicating stress-induced poor glymphatic flow. This was attributed to reduced AQP4 expression in astrocytic endfeet [75], which is supported by human studies demonstrating reduced AQP4 mRNA transcripts in the hippocampal tissue of patients with MDD compared to controls [76]. Another potential cause of compromised glymphatic flow is reduced astrocyte counts in patients with MDD [69]. Other studies have also suggested that structural atrophy − reduced process length, branching, and volume − occurs as opposed to cell reduction. The exact cause of these phenomena in MDD is not established but it may be that the GCs released during chronic stress reduce the receptor expression in astrocytes, and reduce astrocyte numbers [52]. Alternatively, it could result from the damaging neuroinflammatory response in MDD as the anti-inflammatory antidepressant fluoxetine has been demonstrated to limit stress-induced astrocyte reduction [77, 78].
Thus, the current data suggest a dysfunctional glymphatic system may contribute to accumulation of α-syn within the central nervous system, in particular the brain. As solutes including proteins like α-syn are not cleared properly, pathological forms of the protein are more likely to accumulate and cause PD. This impaired glymphatic clearance as an α-synucleinopathy-related pathology is supported by measurements of low α-syn levels in CSF, despite high levels in plasma and in the brains of MSA and DLB patients [79, 80, 81]. As further support, lower CSF α-syn levels have been correlated with increased severity of DLB [79], but PD studies remain inconsistent with reports of higher, lower, and no differences in CSF α-syn levels compared to control groups [82] although there was a correlation with age in PD cases [83]. In atypical PD such as MSA, extracellular α-syn aggregates induce astrocyte activation [84] and this correlated with reduced AQP4 density associated with vimentin-positive blood vessels in close proximity to α-syn aggregate pathology (De Smet and Pountney, unpublished data; Fig. 1c). Another consideration in light of this impaired glymphatic flow is the ability to clear peripherally sourced α-syn, which has garnered more attention with new research demonstrating that α-syn from the gut may contribute to PD pathology [85]. Recent research has demonstrated that depressed patients have higher serum α-syn, suggesting a possible peripheral effect of MDD pathology on α-syn metabolism, such as high-circulating GCs [86]. Extracellular fluid could then potentially serve as a peripheral source of α-syn that can be taken up if inadequately cleared. However, glymphatic flow was able to improve in mice with the administration of the SSRI fluoxetine, suggesting that this antidepressant may be an effective therapy for both relieving MDD symptoms and preventing pathological protein accumulation [75].
Besides impairing glymphatic clearance, AQP4 downregulation has further consequences as it has been demonstrated to increase TNF-α and IL-1β production. This suggests the potential to trigger and propagate a hyperactive immune response that may exacerbate neurodegeneration in PD; however the research on this is limited and thus requires further testing [87, 88]. From these deductions, it is evident that astrocytes and the neuroinflammatory response they produce are key contributors to PD pathophysiology by possibly allowing α-syn to accumulate and spread throughout the brain, as well as encouraging neurodegeneration by releasing pro-inflammatory cytokines similar to the case of microglia [88].
Interestingly, existing research suggests that astrocyte density is lower in younger MDD patients compared to patients above 60 years of age with late-onset depression. The higher astrocyte densities in older patients is believed to be compensation for the neurodegeneration that occurs in these older patients. Thus, this is an intriguing explorable area to determine how the compensatory effect may influence the pathogenesis of PD in MDD patients [77]. For example, perhaps it is a determining factor for why MDD patients do not all go on to develop PD in later life, as genetic or environmental factors could facilitate increased astrocyte density as a compensatory effect to allow for a reversal or improvement of the impairment in glymphatic clearance.
Although it is clear that MDD and PD have overlapping molecular pathways, it remains unclear whether depression is a molecular consequence of PD, a molecular trigger for PD, or a co-occurring phenomenon with PD that together may constitute a separate syndrome or disease subtype. Figure 2 summarizes the interplay between the molecular aetiology of MDD and PD elaborating on the cytokine, GC, and glymphatic hypotheses.
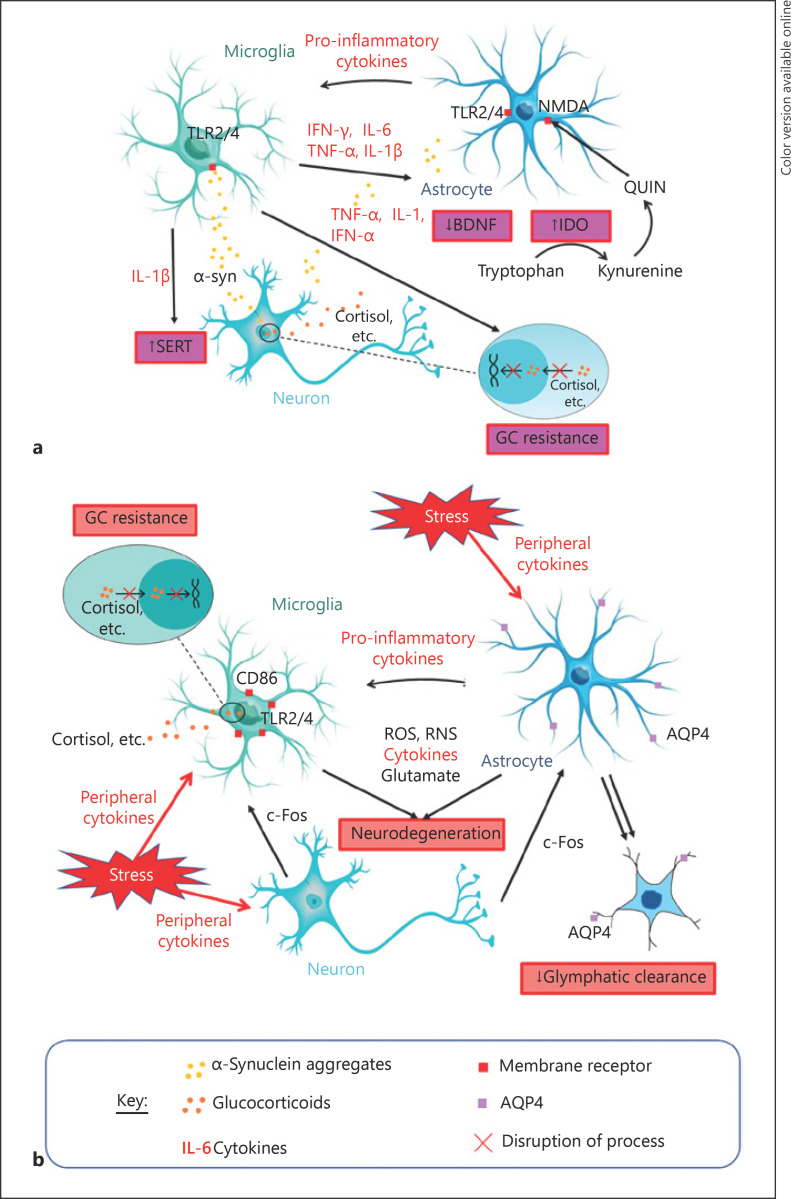
Fig. 2.
Summary of potential mechanisms linking PD and MDD. a MDD as a non-motor symptom of PD: oligomeric misfolded α-syn released by neurons is detected by microglia and astrocytes mainly via TLRs. Activated microglia release various pro-inflammatory markers, propagating neuroinflammation to other microglia and astrocytes and interacting with different processes that can contribute to the manifestation of depressive behaviours. IL-1β acts via IL-1β receptors and the p38 MAPK inflammatory pathway to increase serotonin reuptake from synapses through SERT, reducing synaptic serotonin levels to produce depressive behaviour [57]. TNF-α, IL-1, and IFN-α disrupt HPA axis function and cause GC resistance, which are hallmarks of depression. IFN-γ, IL-6, and TNF-α induce IDO, an enzyme that catabolizes the monoamine precursor tryptophan into kynurenine, which reduces capacity for monoamine synthesis by reducing concentrations of their precursor [35]. The kynurenine can be converted to an NMDA receptor agonist (QUIN) which stimulates increased glutamate release and reduces its uptake by astrocytes (an effect also observed due to increased circulating GCs), resulting in glutamate excitotoxicity [39, 40]. This excess glutamate combined with neuroinflammation reduce BDNF, impairing neural plasticity and subsequently contributing to depressive behaviour [39]. Inhibition of neural plasticity may also be caused by IL-1β through NF-κβ signalling activation [42]. b MDD as a prodrome or risk factor of PD: microglia and astrocytes respond to stress potentially via c-Fos signalling from neurons [51] and/or peripheral cytokines that have travelled past the BBB [57]. These activated glial cells propagate neuroinflammation to activate other glial cells, all of which may produce ROS, RNS, cytokines, and glutamate, which can lead to neurodegeneration through glutamate excitotoxicity and/or direct oxidative and nitrative damage [57, 61]. Stress also increases neuroinflammatory signalling proteins, including TLR4 and CD86, on microglial membranes, leading to the “priming” of microglia to subsequent pathogens to produce more vigorous α-syn-induced microglial activation in future [56]. Astrocyte activation results in pro-inflammatory cytokine secretion and impairs glymphatic clearance which further increases the risk of α-syn accumulation and PD pathology, possibly through reduced AQP4 distribution, structural atrophy of astrocytes, and/or physical reduction in astrocytes. The combination of these processes may increase the risk of PD as glymphatic disruption means that any misfolded fibrillar α-syn present is more likely to accumulate in the brain parenchyma. Following this, neuroinflammatory responses are able to do more significant damage as the microglia being activated have been primed [52, 69, 75, 76]. AQP, aquaporin; BDNF, brain-derived neurotrophic factor; CD, cluster of differentiation; GC, glucocorticoid; IDO, indoleamine 2,3-dioxygenase; IFN, interferon; IL, interleukin; NMDA, N-methyl-D-aspartate; QUIN, quinolinic acid; ROS, reactive oxygen species; RNS, reactive nitrogen species; SERT, serotonin transporter; TLR, toll-like receptor; TNF, tumour necrosis factor; α-syn, α-synuclein; MDD, major depressive disorder; PD, Parkinson's disease; MAPK, mitogen-activated protein kinase; HPA, hypothalamic-pituitary-adrenal; BBB, blood-brain barrier.
Implications for Therapeutic Strategies
From this review, it is apparent that inflammation and glial cells play important roles in the temporal relationship between, and resulting coexistence of, MDD and PD. As such, these factors are major potential targets for treatment − and possibly prevention − of both disorders due to their overlapping pathology in regard to neuroinflammatory pathways and gliosis. Some potential therapeutic anti-inflammatory agents are depicted in Table 2.
Table 2.
Potential anti-inflammatory agents for treating and/or preventing PD and/or MDD [78, 89–95]
| Potential therapeutic agent | Study | Empirically derived effect |
|---|---|---|
| Lenalidomide | [78] | Targets NOX2 activation to inhibit microglial activation and cytokine release, reducing dopaminergic neurodegeneration in transgenic mice |
|
| ||
| Diphenyleneiodonium | [91] | Targets NOX2 activation to reduce microglial activation, reducing dopaminergic neurodegeneration and α-syn aggregation in mice |
|
| ||
| Taurine | [92] | Targets NOX2 activation to reduce microglial activation, reducing dopaminergic neurodegeneration and α-syn aggregation in mice |
|
| ||
| α-Mangostin | [93] | Reduces NOX2 signalling and release of pro-inflammatory agents, including iNOS and ROS, by microglia in primary microglial cultures prepared from rats |
|
| ||
| Dimethyl fumarate | [90] | Reduces α-syn aggregation, reverses oxidative stress in neurons, reduces levels of COX2 and IL-1β, and induces natural antioxidant response in mice |
|
| ||
| AZD480 | [94] | Inhibits JAK1/2 in the JAK/STAT pathway (a cytokine signalling pathway) that can activate in response to α-syn overexpression to produce a neuroinflammatory response |
|
| ||
| Semapimod | [89] | Inhibits MAPK, attenuating microglial activation and dopaminergic neuron degeneration |
|
| ||
| Fluoxetine olanzapine amitriptyline | [95] | Antidepressants that attenuate astrocyte reduction and hence limit impairment in glymphatic clearance |
MDD, major depressive disorder; PD, Parkinson's disease; ROS, reactive oxygen species; IL, interleukin; MAPK, mitogen-activated protein kinase.
Following further efficacy studies to establish higher reliability, it is particularly important for future human research to be conducted to determine effectiveness and side effects [89]. This is especially crucial because anti-inflammatory agents are naturally associated with more severe adverse effects due to some degree of immune suppression. Hence, further research to determine best practice and risk benefit when prescribing such medications is necessary [90]. For example, Di Benedetto et al. [96] reported that response to fluoxetine treatment relies on the presence of AQP4, indicating that best practice for fluoxetine administration would be to increase AQP4 polarization to astrocytic endfeet beforehand. Another avenue for further treatment development may also be blood glutamate scavenging to oppose glutamate excitotoxicity, which has shown some promise for other neurological diseases [39].
Although there are Movement Disorder Society Task Force recommendations for the treatment of depression in PD, clinical practice often sees the usual antidepressants being administered. These include SSRIs, monoamine oxidase type B inhibitors, and tricyclic antidepressants. However, their efficacy remains controversial and SSRIs are often preferred simply for the reduced risk of adverse effects [97]. Besides regulating catecholamines, it has been proposed that the effectiveness of antidepressants may also rely on their antioxidant activity as understandings of neuroinflammatory processes in MDD have improved. This indicates the likelihood that anti-inflammatory agents may have a place in depressive treatment if administration technicalities (e.g., dosage) are devised and adverse effects are sufficiently reduced through continued research. These would then possibly have the added benefit, beyond treating depression, of preventing and/or managing PD as well as neuroinflammatory disorders [62]. However, it is important to note that anti-inflammatory agents such as NSAIDs that simply inhibit pro-inflammatory processes may not be as effective as agents that promote anti-inflammatory processes. This is because agents like NSAIDs can uncontrollably suppress both M1- and M2-activated microglia, whilst promoting M2 activation is itself favourable as it inhibits M1 activation in more targeted ways, as observed in interferon treatment for multiple sclerosis [18].
Glial cells may also provide an avenue for early detection and targeting of PD before onset in people diagnosed with MDD. For example, Iliff et al. [98] found that contrast-enhanced MRI, an already common-place clinical tool, was able to inform glymphatic flow and the rates of CSF-ISF exchange in the paravascular pathways. Thus, this could become an invaluable tool in detecting impaired glymphatic clearance in glial-related MDD, and subsequently administering treatment. The pursuit of improved understanding of AQP4 polarization to astrocytic endfeet would also pave the way to treatments targeting impaired glymphatic flow [96].
Limitations in the Research
As clinical imaging does not provide sufficient detail to inform physiological processes at cellular levels, and post-mortem tissue is difficult to obtain, most studies on neurological diseases like PD and MDD are conducted on animals. This means that results derived from these models must be validated in human studies due to species-specific variation [50]. Some studies have also been conducted in vitro and whilst these have provided insightful findings, physiological environments are never perfectly replicable, and the time frames used cannot demonstrate the true extent of disease processes [64]. In addition, experimental conditions can produce misleading findings such as in models of neuroinflammation using lipopolysaccharide whereby the glial activation measured following administration may be a result of tissue damage caused during administration rather than the pro-inflammatory chemical itself [50]. Furthermore, there has been emphasis on the preparation of α-syn oligomers and fibrillar aggregates being an important source of variation in observations of PD pathology, due to strain specificity [99].
Issues have also been identified in the use of protein expression to determine cell numbers as experimental factors have been observed to cause selective reduction of certain proteins such as glial fibrillary acidic protein but not the density of the astrocytes on which they are located [52]. In psychiatric disorders such as MDD, it is also challenging to determine the directionality of associations between pathological signs and the condition. For example, it is unclear whether neuroinflammation causes or is a result of MDD and thus whether treatments targeting neuroinflammation would only relieve symptoms or eliminate the cause. In addition, it should be recognized that although this review focussed on the biochemical aspects of MDD, social and mental factors should not be ignored and depression in patients should be managed with both pharmacological and non-pharmacological treatment such as psychotherapy [97]. This relates to another limitation related to MDD research whereby studies that assess depression as a whole may miss symptom-specific associations, particularly distinguishing between somatic symptoms such as fatigue and cognitive-affective symptoms such as poor mood. This may also serve as an explanation for varying results and effect magnitudes [100].
With the many errors that can arise in different studies and the unpredictability of neurological disease processes, it is inevitable that conflicting research has arisen, as has been mentioned multiple times throughout this review. For example, astrogliosis in PD remains poorly characterized with research suggesting varying levels of activation to no activation. This could be due to issues with internal validity or a result of variation between individual cases of disease, but this is difficult to determine [65]. The glymphatic system is another topic of major controversy requiring further investigation [73].
Conclusion
While there have been multitudes of epidemiological studies relating MDD to PD, the exact mechanisms underlying their temporal relationship require further investigation. However, the overlaps uncovered between neurodegenerative pathologies, particularly in terms of glial cells and neuroinflammation, form the basis of the links made in this review. Indeed, Khoo et al. [101] have illustrated a higher degree of non-motor symptom burden in the postural instability gait difficulty motor phenotype. The postural instability gait difficulty phenotype is thought to have a higher degree of neurotransmitter imbalances involving dopaminergic and non-dopaminergic systems, the latter includes cholinergic, serotoninergic, and noradrenergic systems which have also been implicated in limbic system and affective disorders. This accentuates the necessity for mental health awareness and allocation of health resources to this area to not only limit depression but possibly also PD. Landau et al. [102] suggested psychopathological phenotypes in PD which pointed at a need for increased understanding of mechanisms to improve management. Such approaches have been mentioned in this review but there are still many issues to consider as anti-inflammatory treatments can have damaging adverse effects. Further avenues for exploration include how propagative mechanisms such as microvesicles and microglia can spread pro-inflammatory agents throughout the brain and whether they may spread cytokines from MDD-affected brain regions to PD-affected regions specifically. More prospective studies focussing on biomarkers directly linking PD and MDD and experimental research into the mechanisms of their temporal relationship should be conducted. Additionally, although this review has focussed on PD and MDD specifically, much of the pathophysiology discussed is not unique to these disorders. Hence, links between depression and other neuroinflammatory disorders or protein aggregation pathologies, such as Alzheimer's disease, potentially involving similar impaired systems, including glymphatic clearance, or between other stress-related disorders, such as post-traumatic stress disorder, and PD will serve as interesting areas for further research.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors gratefully acknowledge the financial support of Griffith University.
Author Contributions
A.A.T. and D.L.P. provided the principle contributions to writing. G.D.G., T.K.K., and M.D.S. provided intellectual input and editing.
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65((9)):732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson's disease. Mov Disord. 2009;24((15)):2175–86. doi: 10.1002/mds.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reijnders J, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson's disease. Mov Disord. 2008;232((2)):183–313. doi: 10.1002/mds.21803. quiz 313. [DOI] [PubMed] [Google Scholar]
- 5.Ravina B, Camicioli R, Como PG, Marsh L, Jankovic J, Weintraub D, et al. The impact of depressive symptoms in early Parkinson disease. Neurology. 2007;69((4)):342–7. doi: 10.1212/01.wnl.0000268695.63392.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menon B, Nayar R, Kumar S, Cherkil S, Venkatachalam A, Surendran K, et al. Parkinson's disease, depression, and quality-of-Life. Indian J Psychol Med. 2015;37((2)):144–8. doi: 10.4103/0253-7176.155611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang F, Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, et al. Depression and the subsequent risk of Parkinson's disease in the NIH-AARP Diet and Health Study. Mov Disord. 2010;25((9)):1157–62. doi: 10.1002/mds.23092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dlay JK, Duncan GW, Khoo TK, Williams-Gray CH, Breen DP, Barker RA, et al. Progression of neuropsychiatric symptoms over time in an incident Parkinson's disease cohort (ICICLE-PD) Brain Sci. 2020;10((2)):78. doi: 10.3390/brainsci10020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams DR, Litvan I. Parkinsonian syndromes. Continuum. 2013;19((5 Movement Disorders)):1189–212. doi: 10.1212/01.CON.0000436152.24038.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radford R, Wong M, Pountney DL. Neurodegenerative aspects of multiple system atrophy. In: Kostrzewa R, editor. Handbook of neurotoxicity. New York, NY: Springer; 2014. [Google Scholar]
- 11.Villar-Piqué A, Lopes da Fonseca T, Outeiro TF. Structure, function and toxicity of alpha-synuclein: the Bermuda triangle in synucleinopathies. J Neurochem. 2015;139:240–55. doi: 10.1111/jnc.13249. [DOI] [PubMed] [Google Scholar]
- 12.Melki R. Alpha-synuclein and the prion hypothesis in Parkinson's disease. Rev Neurol. 2018;174((9)):644–52. doi: 10.1016/j.neurol.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Breydo L, Wu JW, Uversky VN. Α-synuclein misfolding and Parkinson's disease. Biochim Biophys Acta. 2012;1822((2)):261–85. doi: 10.1016/j.bbadis.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 14.McLeary F, Rcom-H'cheo-Gauthier A, Goulding M, Radford R, Okita Y, Faller P, et al. Switching on endogenous metal binding proteins in Parkinson's disease. Cells. 2019;8((2)):179. doi: 10.3390/cells8020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surmeier DJ. Determinants of dopaminergic neuron loss in Parkinson's disease. FEBS J. 2018;285((19)):3657–68. doi: 10.1111/febs.14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donaghy PC, McKeith IG. The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res Ther. 2014;6((4)):46. doi: 10.1186/alzrt274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenning GK, Stefanova N, Jellinger KA, Poewe W, Schlossmacher MG. Multiple system atrophy: a primary oligodendrogliopathy. Ann Neurol. 2008;64((3)):239–46. doi: 10.1002/ana.21465. [DOI] [PubMed] [Google Scholar]
- 18.Moehle MS, West AB. M1 and M2 immune activation in Parkinson's disease: foe and ally? Neuroscience. 2015;302:59–73. doi: 10.1016/j.neuroscience.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson's disease. Neurobiol Aging. 2008;29((11)):1690–701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, et al. MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci. 2013;33((23)):9592–600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67((5)):446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Valdinocci D, Grant GD, Dickson TC, Pountney DL. Epothilone D inhibits microglia-mediated spread of alpha-synuclein aggregates. Mol Cell Neurosci. 2018;89:80–94. doi: 10.1016/j.mcn.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, et al. Cerebrospinal fluid inflammatory markers in Parkinson's disease: associations with depression, fatigue, and cognitive impairment. Brain Behav Immun. 2013;33:183–9. doi: 10.1016/j.bbi.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 24.King E, O'Brien J, Donaghy P, Williams-Gray CH, Lawson RA, Morris CM, et al. Inflammation in mild cognitive impairment due to Parkinson's disease, Lewy body disease, and Alzheimer's disease. Int J Geriatr Psychiatry. 2019;34((8)):1244–50. doi: 10.1002/gps.5124. [DOI] [PubMed] [Google Scholar]
- 25.Williams-Gray CH, Wijeyekoon R, Yarnall AJ, Lawson RA, Breen DP, Evans JR, et al. Serum immune markers and disease progression in an incident Parkinson's disease cohort (ICICLE-PD) Mov Disord. 2016;31((7)):995–1003. doi: 10.1002/mds.26563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edison P, Ahmed I, Fan Z, Hinz R, Gelosa G, Ray Chaudhuri K, et al. Microglia, amyloid, and glucose metabolism in Parkinson's disease with and without dementia. Neuropsychopharmacology. 2013;38((6)):938–49. doi: 10.1038/npp.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis. 2006;21((2)):404–12. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology. 2011;76((10)):863–9. doi: 10.1212/WNL.0b013e31820f2d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang T, Li G, Xu J, Gao S, Chen X. The challenge of the pathogenesis of Parkinson's disease: is autoimmunity the culprit? Front Immunol. 2018;9:2047. doi: 10.3389/fimmu.2018.02047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill-Burns EM, Factor SA, Zabetian CP, Thomson G, Payami H. Evidence for more than one Parkinson's disease-associated variant within the HLA region. PLoS One. 2011;6((11)):e27109. doi: 10.1371/journal.pone.0027109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doorn KJ, Goudriaan A, Blits-Huizinga C, Bol JG, Rozemuller AJ, Hoogland PV, et al. Increased amoeboid microglial density in the olfactory bulb of Parkinson's and Alzheimer's patients. Brain Pathol. 2013;24((2)):152–65. doi: 10.1111/bpa.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285((12)):9262–72. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, et al. Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol Aging. 2009;30((5)):759–68. doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, et al. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35((3)):702–21. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. 2010;35((13)):2510–20. doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70((1)):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21((1)):9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dantzer R, Walker AK. Is there a role for glutamate-mediated excitotoxicity in inflammation-induced depression? J Neural Transm. 2014;121((8)):925–32. doi: 10.1007/s00702-014-1187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13((1)):22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Liu H, Du XD, Zhang Y, Yin G, Zhang BS, et al. Association of low serum BDNF with depression in patients with Parkinson's disease. Parkinsonism Relat Disord. 2017;41:73–8. doi: 10.1016/j.parkreldis.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107((6)):2669–74. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perrin AJ, Horowitz MA, Roelofs J, Zunszain PA, Pariante CM. Glucocorticoid resistance: is it a requisite for increased cytokine production in depression? A systematic review and meta-analysis. Front Psychiatry. 2019;10((423)):423. doi: 10.3389/fpsyt.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adler CH, Beach TG, Zhang N, Shill HA, Driver-Dunckley E, Caviness JN, et al. Unified staging system for Lewy body disorders: clinicopathologic correlations and comparison to Braak staging. J Neuropathol Exp Neurol. 2019;78((10)):891–9. doi: 10.1093/jnen/nlz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson's disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128((Pt 6)):1314–22. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- 46.Kano O, Ikeda K, Kiyozuka T, Iwamoto K, Ito H, Kawase Y, et al. Beneficial effect of pramipexole for motor function and depression in Parkinson's disease. Neuropsychiatr Dis Treat. 2008;4((4)):707–10. [PMC free article] [PubMed] [Google Scholar]
- 47.Grosch J, Winkler J, Kohl Z. Early degeneration of both dopaminergic and serotonergic axons: a common mechanism in Parkinson's disease. Front Cell Neurosci. 2016;10:293. doi: 10.3389/fncel.2016.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dahl J, Ormstad H, Aass HC, Malt UF, Bendz LT, Sandvik L, et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. 2014;45:77–86. doi: 10.1016/j.psyneuen.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 49.Song C, Halbreich U, Han C, Leonard BE, Luo H. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry. 2009;42((5)):182–8. doi: 10.1055/s-0029-1202263. [DOI] [PubMed] [Google Scholar]
- 50.Hinwood M, Morandini J, Day TA, Walker FR. Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex. 2012;22((6)):1442–54. doi: 10.1093/cercor/bhr229. [DOI] [PubMed] [Google Scholar]
- 51.Sugama S, Takenouchi T, Fujita M, Conti B, Hashimoto M. Differential microglial activation between acute stress and lipopolysaccharide treatment. J Neuroimmunol. 2009;207((1–2)):24–31. doi: 10.1016/j.jneuroim.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, et al. Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol. 2013;126((1)):75–91. doi: 10.1007/s00401-013-1102-0. [DOI] [PubMed] [Google Scholar]
- 53.Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. 2011;8((1)):94. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71((12)):1381–91. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 55.Bellavance MA, Rivest S. The HPA: immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front Immunol. 2014;5:136. doi: 10.3389/fimmu.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31((17)):6277–88. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28((30)):7687–98. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Machado A, Herrera AJ, Venero JL, Santiago M, de Pablos RM, Villarán RF, et al. Inflammatory animal model for Parkinson's disease: the intranigral injection of LPS induced the inflammatory process along with the selective degeneration of nigrostriatal dopaminergic neurons. ISRN Neurol. 2011;2011:476158. doi: 10.5402/2011/476158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villarán A, Drenjancević-Perić I. Impact of glucocorticoids and chronic stress on progression of Parkinson's disease. Med Hypotheses. 2008;71((6)):952–6. doi: 10.1016/j.mehy.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 60.Lee HJ, Suk JE, Bae EJ, Lee SJ. Clearance and deposition of extracellular alpha-synuclein aggregates in microglia. Biochem Biophys Res Commun. 2008;372((3)):423–8. doi: 10.1016/j.bbrc.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 61.Palta P, Samuel LJ, Miller ER, Szanton SL. Depression and oxidative stress: results from a meta-analysis of observational studies. Psychosom Med. 2014;76((1)):12–9. doi: 10.1097/PSY.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Black CN, Bot M, Scheffer PG, Penninx BW. Oxidative stress in major depressive and anxiety disorders, and the association with antidepressant use; results from a large adult cohort. Psychol Med. 2017;47((5)):936–48. doi: 10.1017/S0033291716002828. [DOI] [PubMed] [Google Scholar]
- 63.Tsai MC, Huang TL. Increased activities of both superoxide dismutase and catalase were indicators of acute depressive episodes in patients with major depressive disorder. Psychiatry Res. 2016;235:38–42. doi: 10.1016/j.psychres.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 64.Verderio C, Muzio L, Turola E, Bergami A, Novellino L, Ruffini F, et al. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann Neurol. 2012;72((4)):610–24. doi: 10.1002/ana.23627. [DOI] [PubMed] [Google Scholar]
- 65.Brück D, Wenning GK, Stefanova N, Fellner L. Glia and alpha-synuclein in neurodegeneration: a complex interaction. Neurobiol Dis. 2016;85:262–74. doi: 10.1016/j.nbd.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seth P, Koul N. Astrocyte, the star avatar: redefined. J Biosci. 2008;33((3)):405–21. doi: 10.1007/s12038-008-0060-5. [DOI] [PubMed] [Google Scholar]
- 67.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4((147)):147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson's disease. Mov Disord. 2011;26((1)):6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- 69.Rajkowska G, Hughes J, Stockmeier CA, Javier Miguel-Hidalgo J, Maciag D. Coverage of blood vessels by astrocytic endfeet Is reduced in major depressive disorder. Biol Psychiatry. 2013;73((7)):613–21. doi: 10.1016/j.biopsych.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, Pu T, et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife. 2018;7:e40070. doi: 10.7554/eLife.40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teng Z, Wang A, Wang P, Wang R, Wang W, Han H. The effect of aquaporin-4 knockout on interstitial fluid flow and the structure of the extracellular space in the deep brain. Aging Dis. 2018;9((5)):808–16. doi: 10.14336/AD.2017.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zeppenfeld DM, Simon M, Haswell JD, D'Abreo D, Murchison C, Quinn JF, et al. Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. 2017;74((1)):91–9. doi: 10.1001/jamaneurol.2016.4370. [DOI] [PubMed] [Google Scholar]
- 73.Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG. The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathologica. 2018;135:387–407. doi: 10.1007/s00401-018-1812-4. [DOI] [PubMed] [Google Scholar]
- 74.Benveniste H, Liu X, Koundal S, Sanggaard S, Lee H, Wardlaw J. The glymphatic system and waste clearance with brain aging: a review. Gerontology. 2019;65((2)):106–19. doi: 10.1159/000490349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xia M, Yang L, Sun G, Qi S, Li B. Mechanism of depression as a risk factor in the development of Alzheimer's disease: the function of AQP4 and the glymphatic system. Psychopharmacology. 2017;234:365–79. doi: 10.1007/s00213-016-4473-9. [DOI] [PubMed] [Google Scholar]
- 76.Medina A, Watson SJ, Bunney W, Jr, Myers RM, Schatzberg A, Barchas J, et al. Evidence for alterations of the glial syncytial function in major depressive disorder. J Psychiatr Res. 2016;72:15–21. doi: 10.1016/j.jpsychires.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets. 2013;14((11)):1225–36. doi: 10.2174/13894501113149990156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valera E, Mante M, Anderson S, Rockenstein E, Masliah E. Lenalidomide reduces microglial activation and behavioral deficits in a transgenic model of Parkinson's disease. J Neuroinflammation. 2015;12:93. doi: 10.1186/s12974-015-0320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noguchi-Shinohara M, Tokuda T, Yoshita M, Kasai T, Ono K, Nakagawa M, et al. CSF alpha-synuclein levels in dementia with Lewy bodies and Alzheimer's disease. Brain Res. 2009;1251:1–6. doi: 10.1016/j.brainres.2008.11.055. [DOI] [PubMed] [Google Scholar]
- 80.Reesink FE, Lemstra AW, van Dijk KD, van de Berg WD, Klein M, et al. CSF α-synuclein does not discriminate dementia with Lewy bodies from Alzheimer's disease. J Alzheimers Dis. 2010;22((1)):87–95. doi: 10.3233/JAD-2010-100186. [DOI] [PubMed] [Google Scholar]
- 81.Yang F, Li WJ, Huang XS. Alpha-synuclein levels in patients with multiple system atrophy: a meta-analysis. Int J Neurosci. 2017;128:1–27. doi: 10.1080/00207454.2017.1394851. [DOI] [PubMed] [Google Scholar]
- 82.Kim D, Paik JH, Shin DW, Kim HS, Park CS, Kang JH. What is the clinical significance of cerebrospinal fluid biomarkers in Parkinson's disease? Is the significance diagnostic or prognostic? Exp Neurobiol. 2014;23((4)):352–64. doi: 10.5607/en.2014.23.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yarnall AJ, Breen DP, Duncan GW, Khoo TK, Coleman SY, Firbank MJ, et al. Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology. 2014;82((4)):308–16. doi: 10.1212/WNL.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Radford R, Rcom-H'cheo-Gauthier A, Wong MB, Eaton ED, Quilty M, Blizzard C, et al. The degree of astrocyte activation in multiple system atrophy is inversely proportional to the distance to α-synuclein inclusions. Mol Cell Neurosci. 2015;65:68–81. doi: 10.1016/j.mcn.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 85.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167((6)):1469–80.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ishiguro M, Baba H, Maeshima H, Shimano T, Inoue M, Ichikawa T, et al. Increased serum levels of α-synuclein in patients with major depressive disorder. Am J Geriatr Psychiatry. 2019;27((3)):280–6. doi: 10.1016/j.jagp.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 87.Chi Y, Fan Y, He L, Liu W, Wen X, Zhou S, et al. Novel role of aquaporin-4 in CD4+ CD25+ T regulatory cell development and severity of Parkinson's disease. Aging Cell. 2011;10((3)):368–82. doi: 10.1111/j.1474-9726.2011.00677.x. [DOI] [PubMed] [Google Scholar]
- 88.Sun H, Liang R, Yang B, Zhou Y, Liu M, Fang F, et al. Aquaporin-4 mediates communication between astrocyte and microglia: implications of neuroinflammation in experimental Parkinson's disease. Neuroscience. 2016;317:65–75. doi: 10.1016/j.neuroscience.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 89.Ferreira SA, Romero-Ramos M. Microglia response during Parkinson's disease: alpha-synuclein intervention. Front Cell Neurosci. 2018;12:247. doi: 10.3389/fncel.2018.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Campolo M, Casili G, Biundo F, Crupi R, Cordaro M, Cuzzocrea S, et al. The neuroprotective effect of dimethyl fumarate in an MPTP-mouse model of Parkinson's disease: involvement of reactive oxygen species/nuclear factor-κB/nuclear transcription factor related to NF-E2. Antioxid Redox Signal. 2017;27:453–71. doi: 10.1089/ars.2016.6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Q, Qian L, Chen SH, Chu CH, Wilson B, Oyarzabal E, et al. Post-treatment with an ultra-low dose of NADPH oxidase inhibitor diphenyleneiodonium attenuates disease progression in multiple Parkinson's disease models. Brain. 2015;138((Pt 5)):1247–62. doi: 10.1093/brain/awv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Che Y, Hou L, Sun F, Zhang C, Liu X, Piao F, et al. Taurine protects dopaminergic neurons in a mouse Parkinson's disease model through inhibition of microglial M1 polarization. Cell Death Dis. 2018;9((4)):435. doi: 10.1038/s41419-018-0468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu Z, Wang W, Ling J, Jiang C. α-Mangostin inhibits α-synuclein-induced microglial neuroinflammation and neurotoxicity. Cell Mol Neurobiol. 2016;36((5)):811–20. doi: 10.1007/s10571-015-0264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qin H, Buckley JA, Li X, Liu Y, Fox TH, 3rd, Meares GP, et al. Inhibition of the JAK/STAT pathway protects against α-synuclein-induced neuroinflammation and dopaminergic neurodegeneration. J Neurosci. 2016;36((18)):5144–59. doi: 10.1523/JNEUROSCI.4658-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valera E, Ubhi K, Mante M, Rockenstein E, Masliah E. Antidepressants reduce neuroinflammatory responses and astroglial alpha-synuclein accumulation in a transgenic mouse model of multiple system atrophy. Glia. 2014;62((2)):317–37. doi: 10.1002/glia.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Di Benedetto B, Malik VA, Begum S, Jablonowski L, Gómez-González GB, Neumann ID, et al. Fluoxetine requires the endfeet protein aquaporin-4 to enhance plasticity of astrocyte processes. Front Cell Neurosci. 2016;10:8. doi: 10.3389/fncel.2016.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frenklach A. Management of depression in Parkinson's disease. Am J Psychiatry Resid J. 2016;11((4)):8–11. [Google Scholar]
- 98.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123((3)):1299–309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, et al. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522((7556)):340–4. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- 100.Iob E, Kirschbaum C, Steptoe A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: the role of cognitive-affective and somatic symptoms. Mol Psychiatry. 2020 May;25((5)):1130–40. doi: 10.1038/s41380-019-0501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khoo TK, Yarnall AJ, Duncan GW, Coleman S, O'Brien JT, Brooks DJ, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology. 2013;80((3)):276–81. doi: 10.1212/WNL.0b013e31827deb74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Landau S, Harris V, Burn DJ, Hindle JV, Hurt CS, Samuel M, et al. Anxiety and anxious-depression in Parkinson's disease over a 4-year period: a latent transition analysis. Psychol Med. 2016;46((3)):657–67. doi: 10.1017/S0033291715002196. [DOI] [PMC free article] [PubMed] [Google Scholar]