Introduction
We present 4 cases of delayed inflammatory reaction (DIR) to facial dermal hyaluronic acid filler rapidly following vaccination for COVID-19. All DIRs occurred after a hyaluronic acid filler had been placed more than 1 year before vaccination. All patients responded rapidly to therapy with a low dose of oral lisinopril, an angiotensin-converting enzyme inhibitor (ACE-I), which decreases the cutaneous filler-related inflammatory reaction and edema by a novel proposed mechanism.
Case series
Case 1
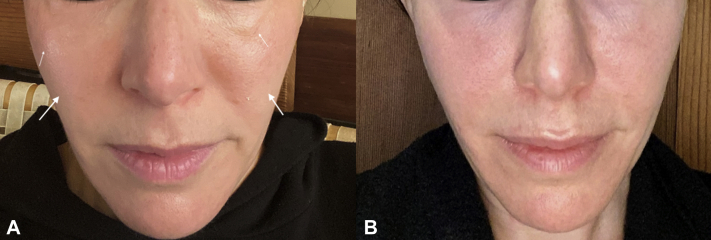
A 43-year-old woman had a prior medical history significant for idiopathic urticaria, which was well controlled by daily cetirizine (10 mg). She had no known drug allergies and no prior history of angioedema or inflammatory filler reaction. Approximately 2 years before vaccination, 1 syringe of Restylane Lyft (Galderma) was injected into her temples, and 1 syringe of Juvéderm Voluma (Allergan) was injected into the lateral parts of her cheeks. The first dose of the Pfizer BNT162b2 vaccine (Pfizer) was well tolerated except for 2 days of mild injection-site pain. Two days after the second dose of the Pfizer vaccine (given 3 weeks after the first), moderate swelling was noted in the periorbital area, which extended to involve extended to involve the medial and lateral cheeks. On the evening of the third day, the patient took 5 mg of lisinopril. On the morning of the next day, she was markedly improved. Cheek lateral and medial swelling and discomfort had reduced to near normal levels, prior to vaccine dose. She was back to baseline by the following day but continued ACE-I therapy for 2 more days without incident (Fig 1, A and B).
Fig 1.
Improvement in periorbital and mid-cheek edema. A, Filler reaction 24 hours after vaccination and before angiotensin-converting enzyme inhibitor treatment (arrows). B, 30 hours after initiation of angiotensin-converting enzyme inhibitor treatment.
Case 2
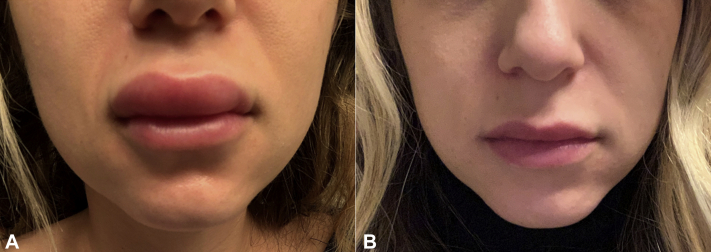
A 31-year-old woman had no known drug allergies and no history of angioedema. She had the following history of use of dermal fillers: In fall 2018, her lips were treated with Juvéderm Volbella (Allergan). In October 2018, her earlobes were treated with Juvéderm Voluma. In June 2019 and June 2020, her nasolabial folds were treated with Juvéderm Ultra (Allergan). In June 2019, her bilateral malar cheeks were treated with Juvéderm Voluma. In the past, she had a reaction to filler with mild prolonged edema in the filler treatment areas lasting more than a week following an upper respiratory tract infection, which was treated with antihistamines and oral prednisone. She received the first dose of the Moderna COVID-19 messenger RNA (mRNA)-1273 vaccine in early January 2021 without incident. Twenty-four hours after the second dose of the Moderna vaccine, she had swelling of the upper mucosal lip, which progressed to involve the left earlobe and bilateral zygomas throughout the evening. She took 5 mg of lisinopril, 48 hours after the vaccine dose, which had minimal effect on the swelling. The lisinopril dose was increased to 10 mg daily, and after 72 hours on the 10-mg daily dose, she returned back to baseline (Fig 2, A and B).
Fig 2.
Improvement in mid-cheek and lips. A, Swelling 24 hours after vaccination. B, 30 hours after initiation of therapy with 10 mg lisinopril.
Case 3
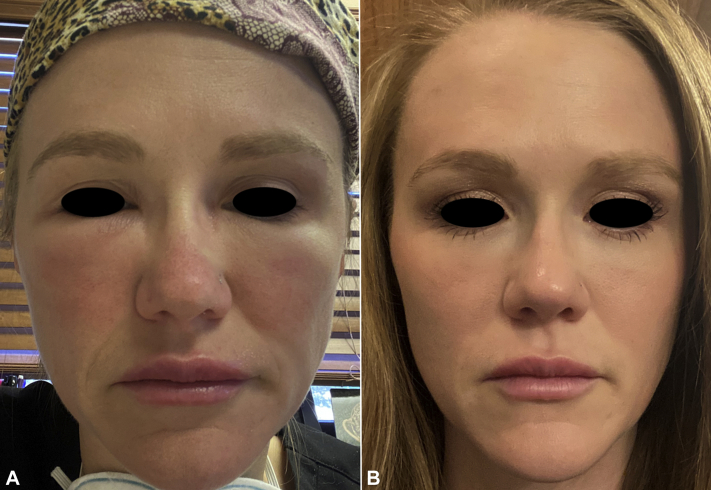
A healthy 36-year-old woman had no known drug allergies and no history of angioedema. In November 2019, she was injected with 1 mL of Voluma 1 in the bilateral tear troughs and 1 mL of Juvéderm Ultra in the upper and lower part of the lips. In December 2020, she developed fever (>38°C), malaise, fatigue, and myalgias 13 hours after administration of the Moderna vaccine. She also noted worsening bilateral infraorbital and perioral edema beginning 18 hours after vaccination. By 48 hours after vaccination, the right infraorbital edema worsened, making it difficult to open the right eye. Subsequently, edema worsened in the upper lip and swelling and inflammation expanded to include the mid-cheeks. The patient refused oral corticosteroids because of previous feelings of intense jitteriness with that medication. Cetirizine (20 mg) did not provide relief. At this time Cetirizine was stopped, all other drugs were stopped, and oral lisinopril 5 mg once daily was initiated. Arrest of left tear trough swelling was noted within 5 to 7 hours of lisinopril treatment along with visual improvement of right tear trough swelling. Over a period of 24 hours, periorbital edema was markedly improved, and the patient returned to normal pre-vaccine appearance.1 Lisinopril was discontinued 2 days after swelling disappeared. The second Moderna vaccine dose was administered 1 month later, as scheduled. Lisinopril at a dose of 10 mg daily was started 2 days before vaccination. Mild fatigue was noted 12 hours after vaccination, but there were no other constitutional symptoms. Mild perioral and periorbital edema occurred but was noticeably less involved than that after the first dose (Fig 3). Residual swelling resolved completely by 72 hours after vaccination.
Fig 3.
Facial swelling on the right. A, 48 hours after the first vaccine dose before lisinopril therapy was initiated. B, 48 hours after the second vaccine dose, with lisinopril already on board, started 48 hours before vaccine.
Case 4
A healthy 76-year-old woman had no known drug allergies and no history of angioedema. She had a prior history of a filler reaction consisting of mild firmness and tenderness occurring 12 months after injection with Juvéderm Ultra in the cheeks in 2019, which responded to hyaluronidase and intralesional corticosteroid combined with 5-fluorouracil. Ten days after the first dose of the Pfizer vaccine, the filler-treated areas became inflamed, along with panfacial and periorbital swelling. She was administered lisinopril (5 mg) alone and improved within 4 days, with nearly complete resolution 7 days after starting the ACE-I. She had not received her second injection before submission of the manuscript of this article.
Discussion
The worldwide incidence of DIR occurring from any triggering event following filler injection is thought to be 0.8%.2,3 It is unknown why DIR affects some filler-treated patients and not others. Decates et al postulated that some human leukocyte antigen subtypes could predispose to immune-related adverse filler reactions. They found a fourfold increase in the odds of immune-mediated adverse events in subjects with human leukocyte antigen subtypes B∗08 or DRB1∗03.4 From our experience to date, it is conceivable that as vaccination rolls out to members of all eligible adult demographics, vaccination for COVID-19 could become among the most common triggers of hyaluronic acid filler–associated DIR.
In the cases described above, DIR occurred rapidly within 24 to 48 hours after COVID-19 mRNA vaccination (either the first or the second dose). This is a more rapid time course than that of classic immune-mediated hypersensitivity. ACE-I therapy provided rapid clinical improvement, without the need for potentially immunosuppressive doses of oral corticosteroids, which may blunt the immunoglobulin response to SARS-COV-2 spike glycoprotein or cause other untoward side effects. ACE-I has been previously reported to have therapeutic value in the treatment of other cutaneous disorders, including hypertrophic scars, keloids, systemic sclerosis, and recessive dystrophic epidermolysis bullosa.5
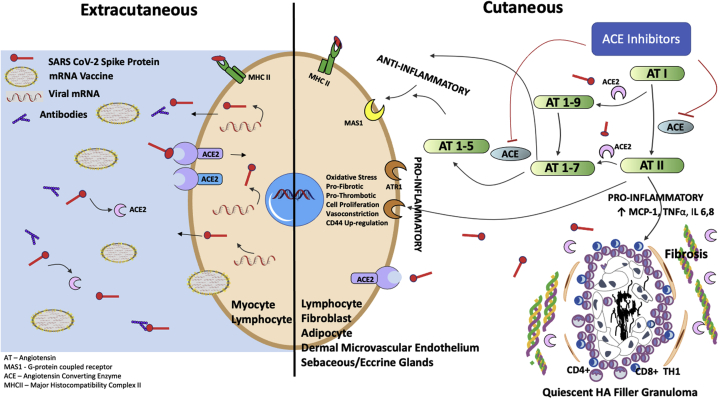
As detailed in Figure 4, enveloped COVID-19 mRNA from the inoculum actively and passively enters extracutaneous cell types, directing the cells' cytosolic machinery to produce spike protein. Resident cutaneous cell types, including lymphocytes, fibroblasts, and adipocytes, are replete with angiotensin-converting enzyme receptor (ACE2), the target ligand for SARS-CoV-2 spike protein.6 Membrane-bound and soluble ACE2 catalyzes conversion of the proinflammatory angiotensin II (AngII) to metabolites angiotensin 1-7, serving a protective function.7 The receptor-binding domain of the COVID spike protein irreversibly binds to membrane-bound ACE2, initiating membrane fusion and entry into the cell, effectively down-regulating ACE2 and the ability to locally control AngII.8 Soluble ACE2 also interacts with spike protein, resulting in its inactivation.9,10 In other tissues, including cardiac, renal, and vascular tissue, the loss of ACE2 leads to functional deterioration.11
Fig 4.
Proposed mechanism of action of ACE-I acting on the cutaneous RAS to decrease AngII, in the setting of post COVID-19 vaccination, with reduction of AngII-induced proinflammatory pathway stimulation of DIR to residual hyaluronic acid filler. ACE, Angiotensin-converting enzyme; ACE-I, angiotensin-converting enzyme inhibitor; ACE2, angiotensin-converting enzyme receptor; AngII, angiotensin II; AT, angiotensin; ATR1, angiotensin II receptor type 1; DIR, delayed inflammatory reaction; HA, hyaluronic acid; IL, interleukin; MAS1, G-coupled protein receptor; MCP-1, monocyte chemoattractant protein type 1; MHCII, major histocompatibility complex I/II; RAS, renin-angiotensin system; TNFα, tumor necrosis factor α. (A portion of this figure was adapted from Jaume Alijotas-Reig et al. Clin Rev Allergy Immunol. 2013; Aug;45(1):97-108.)
A functional renin-angiotensin system (RAS) is mirrored in the skin, just as in other organs of the body. RAS has a critical role in maintaining keratinocyte differentiation, wound healing, and sodium balance.5,12 Accumulation of AngII up-regulates the expression of monocyte chemoattractant protein type 1, tumor necrosis factor α, interleukin 6, and interleukin 8, which are potent chemoattractants and activators of neutrophils.13 Intracellularly, binding of AngII to AT1R (Angiotensin II Receptor type 1) results in vasoconstriction, along with a proliferative, prothrombotic, and profibrotic response. Finally, AngII has been shown to up-regulate CD44 glycoprotein, which is expressed on the surface of many mammalian cells, including lymphocytes, endothelial cells, macrophages, fibroblasts, and keratinocytes. CD44 glycoprotein has an affinity for binding free extracellular hyaluronic acid, especially low-molecular-weight hyaluronic acid,13, 14, 15 providing another possible nidus for inflammatory reaction targeted to the quiescent hyaluronic acid granuloma.
Residual hyaluronic acid filler was present in the cases described above for 8 months or more. Beleznay et al noted that residual filler begins to break down in 3 to 5 months.3 DIR is thought to result from catabolism of filler, with generation of short-chain, low-molecular-weight hyaluronic acid molecules, perhaps accompanied by other inciting factors, such as biofilms.16,17 Figure 4 shows residual and partially degraded dermal hyaluronic acid chains in the center of a subclinical, quiescent granuloma containing CD8+ and CD4+ T cells and macrophages and surrounded by fibroblasts, fibrosis (after wound healing), and dermal microcirculation (from angiogenesis).16 This type of environment has been reported, in some models, to contain a high ACE2 concentration18 and can be potential modulated by inhibiting the RAS. The authors postulate that high levels of AngII can stimulate inflammation in this perigranuloma milieu, inciting a CD8+, TH1 immune response (Fig 4) that results in the clinical findings seen with DIR.16
ACE-I exerts its effects by blocking the production of AngII and reducing the substrate for ACE2,19 thereby compensating for a down-regulation of ACE2 secondary to spike protein binding. The therapeutic benefits of ACE-I in the reduction of inflammation could be explained by such a shift toward an anti-inflammatory response driven by angiotensin 1-7. Additionally, utilization of ACE-I, such as lisinopril (with rapid onset of action), decreases angiotensin II–induced aldosterone secretion by the adrenal cortex, which leads to an increase in sodium excretion and subsequently increases water outflow.19 As a consequence, there is a reduction in interstitial swelling and rapid resolution of facial edema associated with DIR.
Lisinopril is generally well tolerated, with minimal side effects.19 Along with its use for alleviation of the acute condition, we raise the possibility of using ACE-I prophylactically before vaccination, for example, to prevent DIR in an anxious patient with a history of longstanding filler placement. As seen in case 3, ACE-I can be used to minimize the risk of DIR after a second dose of vaccine, if DIR occurred after the first dose.
Approximately 35% of prescriptions for antihypertensive drugs written in the United States are for ACE-Is. The starting dose of lisinopril for treatment of hypertension is usually 10 mg once daily, titrated up to 20 to 40 mg once daily. Lisinopril usually reaches peak effectiveness in 6 hours after an oral dose, with peak serum concentrations occurring about 7 hours after a single dose. The mean half-life is approximately 12 hours, with clearance by kidney filtration. Blood pressure is usually checked in the office 1 to 2 weeks after therapy is initiated. We have found that 5 mg of lisinopril is a good starting point for DIR treatment, escalating to 10 mg if minimal improvement occurs. Caution should be taken when lisinopril is used with concurrent drugs capable of inducing hyperkalemia, such as spironolactone. In this case, a short discontinuance of concurrent drugs should be considered. Suggested therapy is for 3 to 5 days, or at least 1 to 2 days after reduction of visible swelling. Prescribers should be familiar with other side effects of lisinopril, including hypotension, cough, and head and neck angioedema. The patient in case 3 experienced very mild postural hypotension after initiation of therapy, which resolved quickly with drug discontinuation. Baseline blood pressure should be considered when counseling patients. Cough is usually only a consideration with long-term ACE-I therapy. Angioedema is a potentially life-threatening reaction that can occur in 0.1% to 0.7% of recipients, with data suggesting a persistent and relatively constant risk over time.20 Notably, the incidence of ACE-I–induced angioedema is up to 5 times greater in people of African descent.20 Alternatively, captopril, which has a much shorter half-life, can be used at a dose of 6.25 mg, 3 times daily.
It should be noted that mild to severe DIR reactions will likely self-resolve without therapy over the course of 3 days to several weeks (authors' observation). Antihistamines could be used, although their effectiveness is limited (authors' observation). Proper evaluation of the natural history of COVID-related DIR would require a prospective, controlled study. The duration of COVID-related DIR is likely related to the amount of initial filler placed; the filler technique (bolus vs. nonbolus), which can affect the dermal transit time; and the duration of filler placement. Because of their history of use in other dermal fibrotic skin diseases and their suppressive effect on tissue levels of AngII, ACE-I could also be tested as therapy for other chronic or non-COVID cases of DIR.
Conflicts of interest
Dr Munavalli is an investigator for Allergan, Galderma, and Merz. Dr Geronemus is an investigator for Allergan and Galderma. Dr Lupo is a consultant and investigator for Allergan, Galderma, and Merz. The authors have no conflicts of interest regarding the data presented in this article. No funding was used for the writing or preparation of the manuscript.
Acknowledgments
The authors thank Jill Javahery, MD, Alex Zeitany, MD, Ric White, MD, and especially Mahesh Devarasetty, PhD, for their assistance with cases and concepts that appear in this article.
Footnotes
Dr Munavalli is the principal author.
Funding sources: None.
IRB approval status: Not applicable.
Institution where the work was performed: Dermatology, Laser and Vein Specialists of the Carolinas, Charlotte.
References
- 1.Munavalli G.G., Guthridge R., Knutsen-Larson S., Brodsky A., Matthew E., Landau M. COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment. Arch Dermatol Res. 2021:1–15. doi: 10.1007/s00403-021-02190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice S.M., Ferree S.D., Mesinkovska N.A., Kourosh A.S. The art of prevention: COVID-19 vaccine preparedness for the dermatologist. Int J Womens Dermatol. 2021 doi: 10.1016/j.ijwd.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beleznay K., Carruthers J., Carruthers A., Mummert M.E., Humphrey S. Delayed-onset nodules secondary to a smooth cohesive 20mg/ml hyaluronic acid filler: cause and management. Dermatol Surg. 2015;41(8):929–939. doi: 10.1097/DSS.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 4.Decates T.S., Velthuis P.J., Schelke L.W. Increased risk of late-onset, immune-mediated, adverse reactions related to dermal fillers in patients bearing HLA-B∗08 and DRB1∗03 haplotypes. Dermatol Ther. 2021;34:e14644. doi: 10.1111/dth.14644. [DOI] [PubMed] [Google Scholar]
- 5.Silva I.M.S., Assersen K.B., Willadsen N.N., Jepsen J., Artuc M., Steckelings U.M. The role of the renin-angiotensin system in skin physiology and pathophysiology. Exp Dermatol. 2020;29(9):891–901. doi: 10.1111/exd.14159. [DOI] [PubMed] [Google Scholar]
- 6.Steckelings U.M., Wollschläger T., Peters J., Henz B.M., Hermes B., Artuc M. Human skin: source of and target organ for angiotensin II. Exp Dermatol. 2004;13(3):148–154. doi: 10.1111/j.0906-6705.2004.0139.x. [DOI] [PubMed] [Google Scholar]
- 7.Behl T., Kaur I., Bungau S. The dual impact of ACE2 in COVID-19 and ironical actions in geriatrics and pediatrics with possible therapeutic solutions. Life Sci. 2020;257:118075. doi: 10.1016/j.lfs.2020.118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J., Petitjean S.J.L., Koehler M. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat Commun. 2020;11:4541. doi: 10.1038/s41467-020-18319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao L., Sakagami H., Miwa N. ACE2: the key molecule for understanding the pathophysiology of severe and critical conditions of COVID-19: demon or angel? Viruses. 2020;12(5):491. doi: 10.3390/v12050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roshanravan N., Ghaffari S., Hedayati M. Angiotensin converting enzyme-2 as therapeutic target in COVID-19. Diabetes Metab Syndr. 2020;14(4):637–639. doi: 10.1016/j.dsx.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuba K., Imai Y., Penninger J.M. Multiple functions of angiotensin-converting enzyme 2 and its relevance in cardiovascular diseases. Circ J. 2013;77(2):301–308. doi: 10.1253/circj.cj-12-1544. [DOI] [PubMed] [Google Scholar]
- 12.Liao X., Xiao J., Li S.H. Critical role of the endogenous renin-angiotensin system in maintaining self-renewal and regeneration potential of epidermal stem cells. Biochim Biophys Acta Mol Basis Dis. 2019;1865(10):2647–2656. doi: 10.1016/j.bbadis.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Chang Y., Wei W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin Exp Immunol. 2015;179(2):137–145. doi: 10.1111/cei.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y., Eom S., Park D., Kim H., Jeoung D. The hyaluronic acid-HDAC3-miRNA network in allergic inflammation. Front Immunol. 2015;6:210. doi: 10.3389/fimmu.2015.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naor D. Editorial: Interaction between hyaluronic acid and its receptors (CD44, RHAMM) regulates the activity of inflammation and cancer. Front Immunol. 2016;7:39. doi: 10.3389/fimmu.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alijotas-Reig J., Fernandez-Figueras M.T., Puig L. Late-onset inflammatory adverse reactions related to soft tissue filler injections. Clin Rev Allergy Immunol. 2013;45(1):97–108. doi: 10.1007/s12016-012-8348-5. [DOI] [PubMed] [Google Scholar]
- 17.Artzi O., Cohen J.L., Dover J.S. Delayed inflammatory reactions to hyaluronic acid fillers: a literature review and proposed treatment algorithm. Clin Cosmet Investig Dermatol. 2020;13:371–378. doi: 10.2147/CCID.S247171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scholzen T.E., Ständer S., Riemann H., Brzoska T., Luger T.A. Modulation of cutaneous inflammation by angiotensin-converting enzyme. J Immunol. 2003;170:3866–3873. doi: 10.4049/jimmunol.170.7.3866. [DOI] [PubMed] [Google Scholar]
- 19.https://pubchem.ncbi.nlm.nih.gov/compound/Lisinopril#section=MeSH-Pharmacological-Classification Accessed January 20, 2021. Available at:
- 20.https://www.uptodate.com/contents/ace-inhibitor-induced-angioedema/print Accessed January 22, 2021. Available at: