Abstract
Background
Cardiac injury is frequently encountered in patients with coronavirus disease 2019 (COVID-19) and is associated with increased risk of mortality. Elevated troponin may signify myocardial damage and is predictive of mortality. This study aimed to assess the prognostic value of troponin above the 99th percentile upper reference limit (URL) for mortality, and factors affecting the relationship.
Methods
A comprehensive literature search of PubMed (MEDLINE), Scopus and Embase was undertaken, from inception of the databases until 16 December 2020. The key exposure was elevated serum troponin, defined as troponin (of any type) above the 99th percentile URL. The outcome was mortality due to any cause.
Results
In total, 12,262 patients from 13 studies were included in this systematic review and meta-analysis. The mortality rate was 23% (20–26%). Elevated troponin was observed in 31% (23–38%) of patients. Elevated troponin was associated with increased mortality [odds ratio (OR) 4.75, 95% confidence interval (CI) 4.07–5.53; P < 0.001; I2 = 19.9%]. Meta-regression showed that the association did not vary with age (P = 0.218), male gender (P = 0.707), hypertension (P = 0.182), diabetes (P = 0.906) or coronary artery disease (P = 0864). The association between elevated troponin and mortality had sensitivity of 0.55 (0.44–0.66), specificity of 0.80 (0.71–0.86), positive likelihood ratio of 2.7 (2.2–3.3), negative likelihood ratio of 0.56 (0.49–0.65), diagnosis odds ratio of 5 (4–5) and area under the curve of 0.73 (0.69–0.77). The probability of mortality was 45% in patients with elevated troponin and 14% in patients with non-elevated troponin.
Conclusion
Elevated troponin was associated with mortality in patients with COVID-19 with 55% sensitivity and 80% specificity.
Keywords: Troponin, COVID-19, Cardiac injury, Heart, Prognosis
Background
Coronavirus disease 2019 (COVID-19) has affected more than 105 million people globally and resulted in at least 2.3 million deaths (World Health Organization, 2021). Although most patients with COVID-19 have mild symptoms or are asymptomatic, a significant proportion of patients will experience multiple complications, potentially resulting in death (Lim et al., 2020). Biomarkers are crucial in decision-making in order to facilitate efficient resource allocation (Huang et al., 2020).
Cardiac injury is frequently encountered in patients with COVID-19 and is associated with increased risk of death (Nishiga et al., 2020, Shi et al., 2020). Elevated troponin may signify myocardial damage and is predictive of mortality. However, the prognostic performance of troponin and whether its value is affected by various comorbidities that may be present in patients with COVID-19 are not known. This study aimed to assess the prognostic value of troponin above the 99th percentile upper reference limit (URL) for mortality, and factors affecting this relationship.
Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Eligibility criteria
This study included both prospective and retrospective observational studies containing primary data on: (1) confirmed cases of COVID-19; (2) troponin with cut-off point above the 99th percentile URL; and (3) mortality rate based on the troponin cut-off point. Preprints, abstract-only publications, letters without primary data, case reports, editorials, commentaries and non-English-language articles were excluded from this study.
Search strategy and study selection
A comprehensive literature search of PubMed (MEDLINE), Scopus and Embase was undertaken using the keywords ‘covid-19’ OR ‘sars-cov-2’ OR ‘2019-ncov’ AND ‘troponin’ AND ‘mortality’ OR ‘death’ OR ‘non-survivor’ from inception of the search databases until 16 December 2020. Following the initial search, duplicates were excluded. Titles and abstracts of the identified articles were screened for eligibility by two independent authors, and the full-text of potentially eligible studies was assessed based on the inclusion and exclusion criteria.
Data extraction
Two authors extracted data independently using a form. Data of interest included first author, year of publication, sample size, study design, type of troponin, cut-off point for elevated troponin, age, sex, hypertension, diabetes, coronary artery disease and mortality rate.
The key exposure in this study was elevated serum troponin, defined as troponin (of any type) above the 99th percentile URL. The outcome was mortality, defined as clinically validated death/non-survival due to any cause.
The association between key exposure and outcome has been reported as odds ratio (OR) and 95% confidence interval (CI). To assess the prognostic value of elevated troponin, pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR) and area under the curve (AUC) were calculated.
Risk-of-bias assessment
The Newcastle–Ottawa Scale (NOS) was used by two independent authors to assess the risk of bias of the included studies. Discrepancies that arose were resolved by discussion.
Statistical analysis
STATA 16 (Stata Corp, College Station, TX, USA) was used to perform the meta-analysis. Meta-analysis of proportion was performed to pool the prevalence of elevated troponin and mortality. The OR and 95% CI of the main outcome was calculated using the DerSimonian and Laird random-effects model. P < 0.05 was considered to indicate significance. Heterogeneity among the studies was assessed using the I 2 and Cochran Q tests, in which a value <50% or P < 0.10, respectively, indicates significant heterogeneity. Meta-regression analysis was performed using age, gender, hypertension, diabetes and coronary artery disease as moderators. Funnel plot analysis, Egger’s test and Deek’s asymmetry test were used to assess the risk of publication bias and small study effects. Trim-and-fill analysis was performed to ‘normalize’ the asymmetric funnel plot. Pooled sensitivity, specificity, summary receiver operating characteristic (SROC) curve, Fagan’s nomogram and Deek’s asymmetry plot were generated.
Results
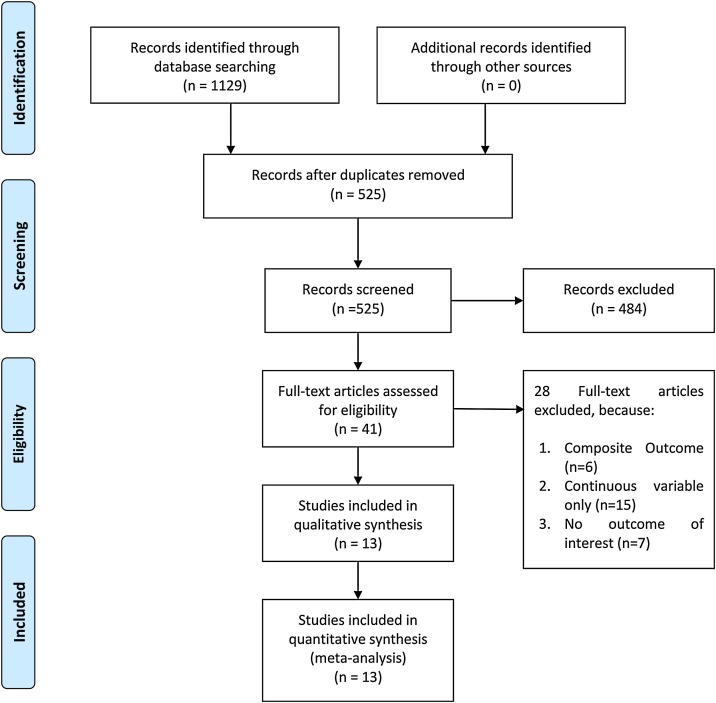
In total, this systematic review and meta-analysis included 12,262 patients from 13 studies (Figure 1 ) (Al Abbasi et al., 2020, Arcari et al., 2020, Connor-Schuler et al., 2020, Du et al., 2020, Heron and Chiu, 2020, Li et al., 2020, Lombardi et al., 2020, Maeda et al., 2020, Raad et al., 2020, Shah et al., 2020, Harmouch et al., 2021, Majure et al., 2021, Metkus et al., 2021) The mortality rate was 23% (20–26%). Thirty-one percent (23–38%) of patients had elevated troponin. The risk-of-bias assessment using the NOS indicates low–moderate risk of bias.
Figure 1.
PRISMA flowchart.
Elevated troponin and mortality
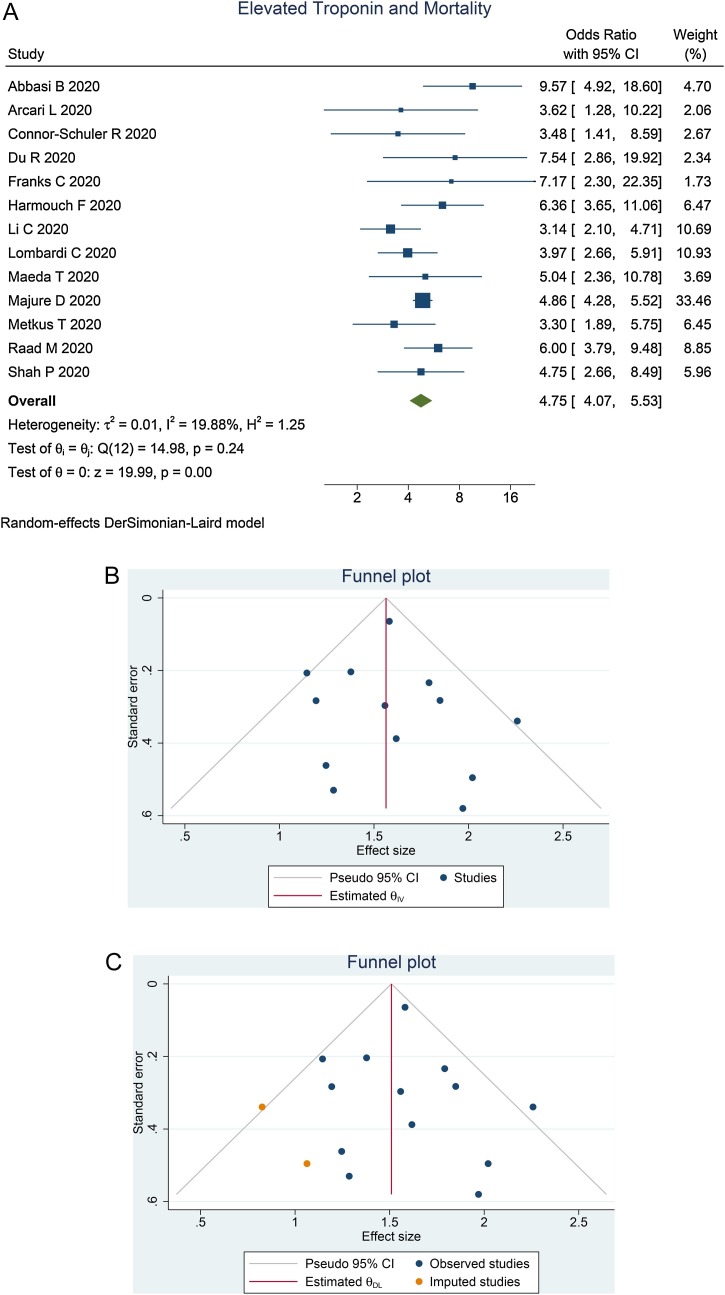
Elevated troponin was associated with increased mortality [OR 4.75 (95% CI 4.07–5.53], P < 0.001; I 2 = 19.9%, P = 0.242] (Figure 2 ). Meta-regression showed that the association did not vary with age (P = 0.218), male gender (P = 0.707), hypertension (P = 0.182), diabetes (P = 0.906) or coronary artery disease (P = 0.864). Egger’s test showed no indication of a small study effect (P = 0.536). The funnel plot was asymmetric (Figure 2B). Trim-and-fill analysis by imputation of two studies resulted in OR of 4.52 (95% CI 3.82–5.36) (Figure 2C).
Figure 2.
Elevated troponin and mortality. (A) Forest plot. (B) Funnel plot. (C) Trim-and-fill analysis. CI, confidence interval.
Prognostic value
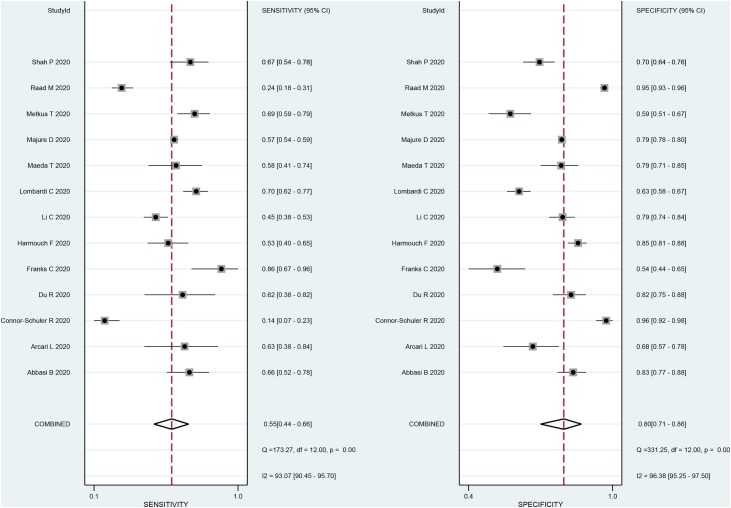
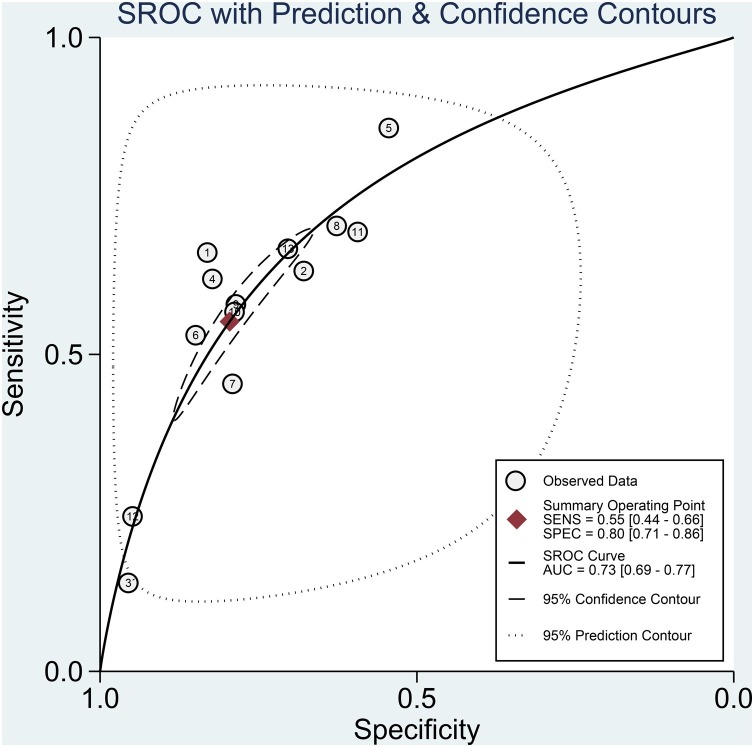
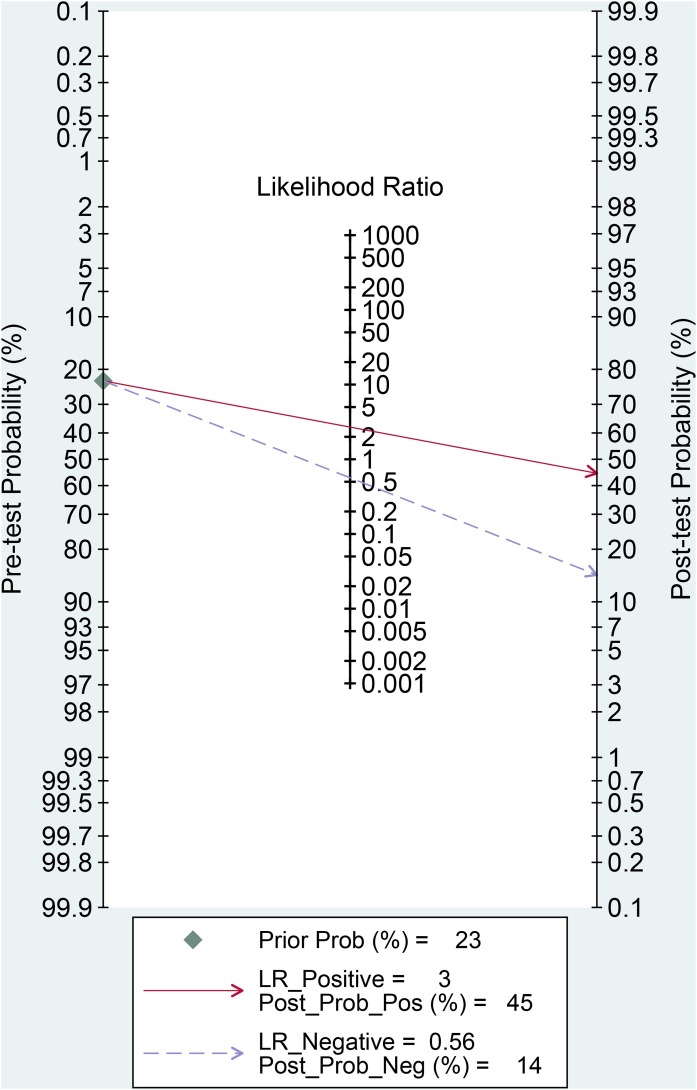
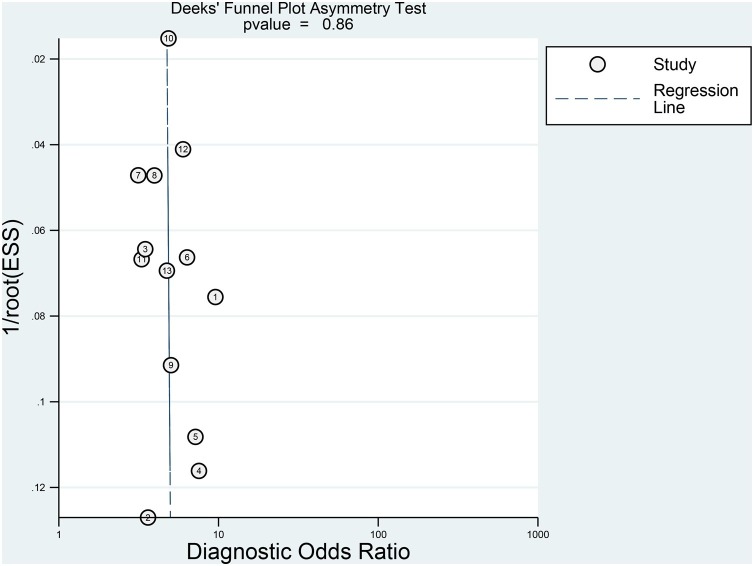
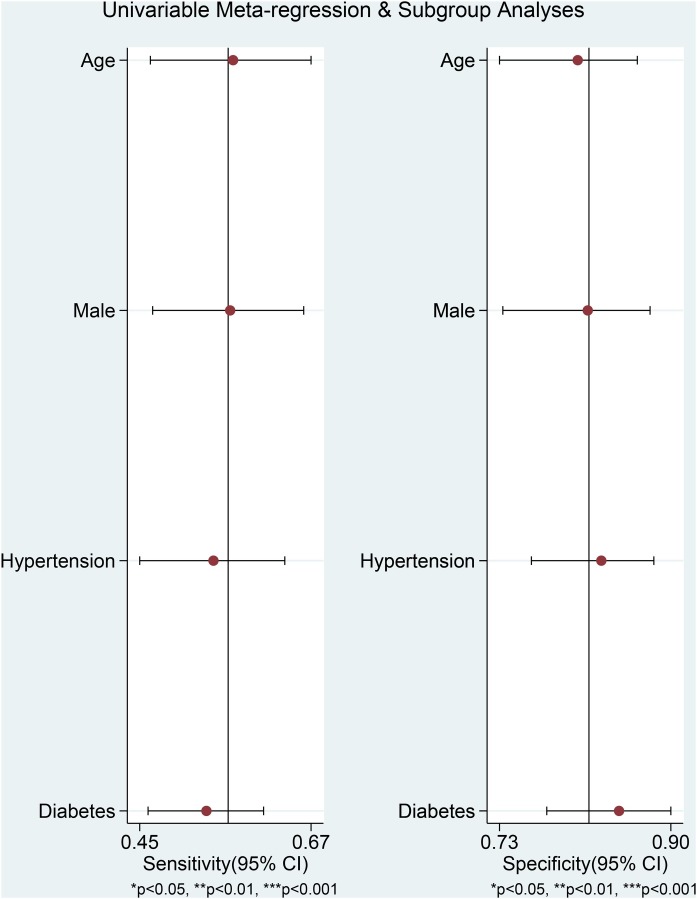
The association between elevated troponin and mortality had sensitivity of 0.55 (0.44–0.66), specificity of 0.80 (0.71–0.86) (Figure 3 ), PLR of 2.7 (2.2–3.3), NLR of 0.56 (0.49–0.65), DOR of 5 (4–5) and AUC of 0.73 (0.69–0.77) (Figure 4 ). Fagan’s nomogram indicated that elevated troponin resulted in 45% post-test probability of mortality, and non-elevated troponin resulted in 14% post-test probability of mortality (Figure 5 ). Deek’s funnel plot indicated symmetry with respect to the regression line, and the quantitative plot asymmetry test was not significant (P = 0.86) (Figure 6 ). Univariate meta-regression and subgroup analyses indicated that age, male gender, hypertension and diabetes did not significantly affect pooled sensitivity or specificity (Figure 7 ).
Figure 3.
Sensitivity and specificity. CI, confidence interval.
Figure 4.
Summary receiver operating characteristics (SROC) curve. SENS, sensitivity; SPEC, specificity; AUC, area under the curve.
Figure 5.
Fagan’s nomogram. LR, likelihood ratio.
Figure 6.
Deek’s asymmetry plot.
Figure 7.
Univariate meta-regression and subgroup analyses. CI, confidence interval.
Discussion
Elevated troponin was associated with an almost five-fold increase in mortality compared with patients without elevated troponin, with 55% sensitivity and 80% specificity.
Meta-regression indicated that the association between elevated troponin and mortality did not vary with age, male gender, hypertension, diabetes or coronary artery disease, indicating that although some of the abovementioned factors are associated with mortality, myocardial damage/dysfunction (Zethelius et al., 2006, Pattanshetty et al., 2012, Mcevoy et al., 2015, Segre et al., 2015, Cavender et al., 2017, Whelton et al., 2017, Lombardi et al., 2020) and mortality in patients with COVID-19 (Huang et al., 2020, Pranata et al., 2020c, Pranata et al., 2020d, Pranata et al., 2020g), these factors did not modify the troponin–mortality relationship. However, other crucial variables such as chronic kidney disease and chronic obstructive pulmonary disease cannot be assessed because they were not reported in most of the studies (Pranata et al., 2020e, Pranata et al., 2020f).
Elevated troponin was associated with 80% specificity and 45% chance of mortality with 23% pre-test probability of mortality. However, it had low sensitivity (50%), so elevated troponin is best used for ruling in the risk of mortality rather than ruling it out. The funnel plot was slightly asymmetric, indicating possible publication bias; a trim-and-fill analysis was performed which slightly reduced the effect estimate. Thus, it is improbable that publication bias has altered the positive association between elevated troponin and mortality. Furthermore, Egger’s test and Deek’s asymmetry test did not detect possible publication bias. The pooled effect estimate had low heterogeneity, indicating consistency despite different types of troponin and populations. This low heterogeneity is likely due to the use of a cut-off point above the 99th percentile URL instead of a specific cut-off point, as the result was less likely to be altered by laboratory reference values and calibrations.
Cardiac injury is frequently encountered in patients with COVID-19. Although the mechanism is only vaguely understood, the interaction between the S protein and angiotensin-converting enzyme 2 is likely to be essential (Nishiga et al., 2020, Shi et al., 2020). In addition to cardiac injury, COVID-19 may also cause arrhythmia, myocardial ischaemia and thromboembolism (Pranata et al., 2020b). Other cardiac biomarkers, such as natriuretic peptide, are often elevated in patients with COVID-19 and signify poor prognosis (Pranata et al., 2020a).
Additionally, echocardiographic parameters such as global longitudinal strain, right ventricular strain and tricuspid annular plane systolic excursion have been shown to be valuable tools in patients with COVID-19 (Martha et al., 2021, Wibowo et al., 2021). These parameters may signify cardiac injury and right ventricular performance, which may be affected by ongoing lung pathologies in patients with COVID-19.
Clinical implications
This meta-analysis found that troponin above the 99th percentile URL increases mortality five-fold with 55% sensitivity and 80% specificity. It has a PLR of 2.7, NLR of 0.56 and AUC of 0.73. However, sensitivity is low. To enhance performance, echocardiographic and laboratory parameters such as natriuretic peptides need to be integrated into the prognostic model. This may result in greater sensitivity and specificity, which may increase its clinical usefulness.
Limitations
The main limitation of this review is that most studies were retrospective. Additionally, a significant proportion of the studies did not report crucial variables such as chronic kidney disease, chronic obstructive pulmonary disease and heart failure; thus, these variables could not be included in the meta-regression. Nevertheless, the low heterogeneity means that, despite variation in these comorbidities, the association between elevated troponin and mortality was consistent.
Conclusion
Elevated troponin was associated with mortality with 55% sensitivity and 80% specificity. The association did not vary with age, male gender, hypertension, diabetes or coronary artery disease.
Conflict of interest
None.
Funding
None.
Ethical approval
Not required.
CRediT authorship contribution statement
Arief Wibowo: Conceptualization, Data curation, Investigation, Writing - original draft, Writing - review & editing. Raymond Pranata: Conceptualization, Methodology, Software, Data curation, Formal analysis, Investigation, Validation, Writing - original draft, Writing - review & editing. Mohammad Rizki Akbar: Conceptualization, Investigation, Writing - original draft, Supervision. Augustine Purnomowati: Investigation, Writing - review & editing. Januar Wibawa Martha: Investigation, Writing - review & editing.
References
- Al Abbasi B., Torres P., Ramos-Tuarez F., Dewaswala N., Abdallah A., Chen K. Cardiac troponin-I and COVID-19: a prognostic tool for in-hospital mortality. Cardiol Res. 2020;11:398–404. doi: 10.14740/cr1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcari L., Luciani M., Cacciotti L., Musumeci M.B., Spuntarelli V., Pistella E. Incidence and determinants of high-sensitivity troponin and natriuretic peptides elevation at admission in hospitalized COVID-19 pneumonia patients. Intern Emerg Med. 2020;15:1467–1476. doi: 10.1007/s11739-020-02498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender M.A., White W.B., Jarolim P., Bakris G.L., Cushman W.C., Kupfer S. Serial measurement of high-sensitivity troponin I and cardiovascular outcomes in patients with type 2 diabetes mellitus in the EXAMINE trial (Examination of Cardiovascular Outcomes with Alogliptin Versus Standard of Care) Circulation. 2017;135:1911–1921. doi: 10.1161/CIRCULATIONAHA.116.024632. [DOI] [PubMed] [Google Scholar]
- Connor-Schuler R., Wong A.I., Shah A., Fiza B., Lyle M., Ramonell R. Experience with cardiology-oriented outcomes in critically ill patients with coronavirus disease 2019. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M. Predictors of mortality for patients with COVID-19 pneumonia caused by SARSCoV-2: a prospective cohort study. Eur Respir J. 2020;55 doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmouch F., Shah K., Hippen J.T., Kumar A., Goel H. Is it all in the heart? Myocardial injury as major predictor of mortality among hospitalized COVID-19 patients. J Med Virol. 2021;93:973–982. doi: 10.1002/jmv.26347. [DOI] [PubMed] [Google Scholar]
- Heron R.C., Chiu W.W. Hydroxy-chloroquine interference in common biochemistry laboratory assays. J Appl Lab Med. 2020;5:1130–1137. doi: 10.1093/jalm/jfaa099. [DOI] [PubMed] [Google Scholar]
- Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression: diabetes and COVID-19. Diabetes Metab Syndr Clin Res Rev. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Jiang J., Wang F., Zhou N., Veronese G., Moslehi J.J. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J Mol Cell Cardiol. 2020;147:74–87. doi: 10.1016/j.yjmcc.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M.A., Pranata R., Huang I., Yonas E., Soeroto A.Y., Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Health Dis. 2020;7 doi: 10.1177/2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi C.M., Carubelli V., Iorio A., Inciardi R.M., Bellasi A., Canale C. Association of troponin levels with mortality in Italian patients hospitalized with coronavirus disease 2019: results of a multicenter study. JAMA Cardiol. 2020;5:1274–1280. doi: 10.1001/jamacardio.2020.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Obata R., Rizk D., Kuno T. Cardiac injury and outcomes of patients with COVID-19 in New York City. Hear Lung Circ. 2020 doi: 10.1016/j.hlc.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majure D.T., Gruberg L., Saba S.G., Kvasnovsky C., Hirsch J.S., Jauhar R. Usefulness of elevated troponin to predict death in patients with COVID-19 and myocardial injury. Am J Cardiol. 2021;138:100–106. doi: 10.1016/j.amjcard.2020.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martha J.W., Pranata R., Wibowo A., Lim M.A. Tricuspid annular plane systolic excursion (TAPSE) measured by echocardiography and mortality in COVID-19: a systematic review and meta-analysis. Int J Infect Dis. 2021;1:1–8. doi: 10.1016/j.ijid.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcevoy J.W., Chen Y., Nambi V., Ballantyne C.M., Sharrett A.R., Appel L.J. High-sensitivity cardiac troponin T and risk of hypertension. Circulation. 2015;132:825–833. doi: 10.1161/CIRCULATIONAHA.114.014364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metkus T.S., Sokoll L.J., Barth A.S., Czarny M.J., Hays A.G., Lowenstein C.J. Myocardial injury in severe COVID-19 compared to non-COVID acute respiratory distress syndrome. Circulation. 2021;143:553–565. doi: 10.1161/CIRCULATIONAHA.120.050543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanshetty D.J., Bhat P.K., Aneja A., Pillai D.P. Elevated troponin predicts long-term adverse cardiovascular outcomes in hypertensive crisis: a retrospective study. J Hypertens. 2012;30:2410–2415. doi: 10.1097/HJH.0b013e3283599b4f. [DOI] [PubMed] [Google Scholar]
- Pranata R., Huang I., Lukito A.A., Raharjo S.B. Elevated N-terminal pro-brain natriuretic peptide is associated with increased mortality in patients with COVID-19: systematic review and meta-analysis. Postgrad Med J. 2020;96:387–391. doi: 10.1136/postgradmedj-2020-137884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Huang I., Raharjo S.B. Incidence and impact of cardiac arrhythmias in coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Indian Pacing Electrophysiol J. 2020;20:193–198. doi: 10.1016/j.ipej.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin-Angiotensin-Aldosterone Syst. 2020;21 doi: 10.1177/1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Lim M.A., Huang I., Raharjo S.B., Lukito A.A. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin-Angiotensin-Aldosterone Syst. 2020;21 doi: 10.1177/1470320320926899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Soeroto A.Y., Huang I., Lim M.A., Santoso P., Permana H. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tuberc Lung Dis. 2020;24:838–843. doi: 10.5588/ijtld.20.0278. [DOI] [PubMed] [Google Scholar]
- Pranata R., Supriyadi R., Huang I., Permana H., Lim M.A., Yonas E. The association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients: a meta-analysis. Clin Med Insights Circ Respir Pulm Med. 2020;14 doi: 10.1177/1179548420959165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranata R., Lim M.A., Yonas E., Vania R., Lukito A.A., Siswanto B.B. Body mass index and outcome in patients with COVID-19: a dose–response meta-analysis. Diabetes Metab. 2020;47(2):101178. doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raad M., Dabbagh M., Gorgis S., Yan J., Chehab O., Dagher C. Cardiac injury patterns and inpatient outcomes among patients admitted with COVID-19. Am J Cardiol. 2020;133:154–161. doi: 10.1016/j.amjcard.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre C.A.W., Hueb W., Garcia R.M.R., Rezende P.C., Favarato D., Strunz C.M.C. Troponin in diabetic patients with and without chronic coronary artery disease. BMC Cardiovasc Disord. 2015;15:72. doi: 10.1186/s12872-015-0051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P., Doshi R., Chenna A., Owens R., Cobb A., Ivey H. Prognostic value of elevated cardiac troponin I in hospitalized Covid-19 patients. Am J Cardiol. 2020;135:150–153. doi: 10.1016/j.amjcard.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton S.P., McEvoy J.W., Lazo M., Coresh J., Ballantyne C.M., Selvin E. High-sensitivity cardiac troponin T (hs-cTnT) as a predictor of incident diabetes in the atherosclerosis risk in communities study. Diabetes Care. 2017;40:261–269. doi: 10.2337/dc16-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibowo A., Pranata R., Astuti A., Tiksnadi B.B., Martanto E., Martha J.W. Left and right ventricular longitudinal strains are associated with poor outcome in COVID-19: a systematic review and meta-analysis. J Intensive Care. 2021;9:9. doi: 10.1186/s40560-020-00519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2021. Weekly epidemiological update – 9 February 2021. [Google Scholar]
- Zethelius B., Johnston N., Venge P. Troponin I as a predictor of coronary heart disease and mortality in 70-year-old men: a community-based cohort study. Circulation. 2006;113:1071–1078. doi: 10.1161/CIRCULATIONAHA.105.570762. [DOI] [PubMed] [Google Scholar]