Abstract
The high prevalence of obesity and obesity-related comorbidities has reached pandemic proportions, particularly in Western countries. Obesity increases the risk to develop several chronic noncommunicable disease, ultimately contributing to reduced survival. Recently, obesity has been recognized as major risk factor for coronavirus disease-19 (COVID-19)-related prognosis, contributing to worse outcomes in those with established COVID-19. Particularly, obesity has been associated with higher hospitalization rates in acute or intensive care and greater risk for invasive mechanical ventilation than lean people.
Obesity is characterized by metabolic impairments and chronic low-grade systemic inflammation that causes a pro-inflammatory microenvironment, further aggravating the cytokine production and risk of cytokine storm response during Sars-Cov2 sepsis or other secondary infections. Moreover, the metabolic dysregulations are closely related to an impaired immune system and altered response to viral infection that can ultimately lead to a greater susceptibility to infections, longer viral shedding and greater duration of illness and severity of the disease.
In individuals with obesity, maintaining a healthy diet, remaining physically active and reducing sedentary behaviors are particularly important during COVID-19-related quarantine to reduce metabolic and immune impairments. Moreover, such stategies are of utmost importance to reduce the risk for sarcopenia and sarcopenic obesity, and to prevent a reduction and potentially even increase cardiorespiratory fitness, a well-known independent risk factor for cardiovascular and metabolic diseases and recently found to be a risk factor also for hospitalizations secondary to COVID-19. Such lifestyle strategies may ultimately reduce morbility and mortality in patients with infectious disease, especially in those with concomitant obesity.
The aim of this review is to discuss how obesity might increase the risk of COVID-19 and potentially affect its prognosis once COVID-19 is diagnosed. We therefore advocate for implementation of strategies aimed at preventing obesity in the first place, but also to minimize the metabolic anomalies that may lead to a compromized immune response and chronic low-grade systemic inflammation, especially in patients with COVID-19.
Keywords: COVID-19, Sars-CoV-2, Obesity, Immune system, Pandemic, Low-grade inflammation
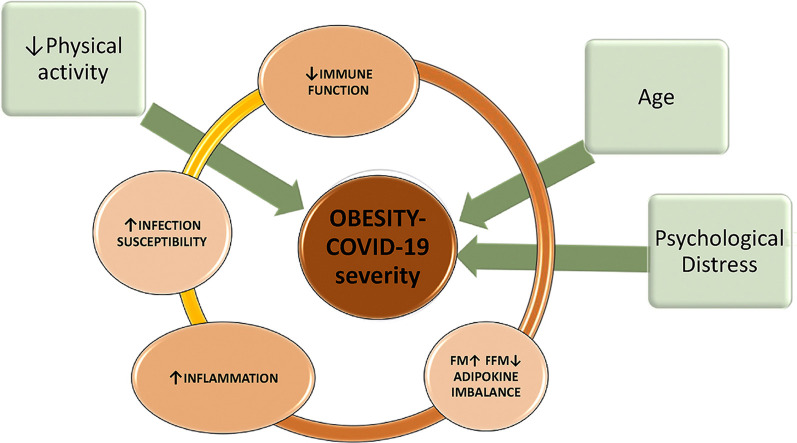
Graphical abstract
Factors associated with increased severity and risk of death for COVID-19 in obesity.
1. Introduction
In 2020 two pandemics collided: obesity, a chronic noncommunicable disease, and the corona virus disease (COVID-19), a pandemic infection caused by the virus Sars-CoV-2. Obesity and COVID-19 often share similar comorbidities, namely metabolic, cardiovascular or pulmonary. Indeed, hypertension, diabetes mellitus (DM) and chronic obstructive pulmonary disease (COPD) are highly prevalent in patients hospitalized for COVID-19. Importantly, patients affected by one of more of the above listed conditions are more likely to require invasive mechanical ventilation (IMV) in intensive care unit (ICU). In addition, clinical management of individuals with obesity during hospitalization, intubation, mechanical ventilation, imaging, positioning and nursing in general can be difficult, and those difficulties are further augmented during COVID-19, with overloaded acute and intensive care units.
Before Sars-CoV-2, obesity was constantly increasing, reaching a worldwide prevalence of 11% for men and 15% for women. In Europe and USA, which have been strongly hit by COVID-19, however, obesity reaches 25.3% (WHO data) and 42.4%, respectively [1]. Age was initially considered the major risk factor for reduced survival in patients with severe COVID-19 due to their higher likelihood of having aging-associated comorbidities, such as DM, hypertension, obstructive sleep apnea syndrome (OSAS) or respiratory conditions, without including obesity to this list [2]. However, obesity has been now recognized as a major risk factor for worse prognosis, independent of age, proposing it as therapeutic target in patients with COVID-19.
2. Methods
We have searched for “BMI” OR “Body Mass Index” OR “Obesity”, AND “COVID-19” OR “SARS-Cov-2” OR “coronavirus disease” on database as PubMed, Google Scholar, MEDLINE, EMBASE, Scopus. With the same keywords, we have also used a dedicated tool in Pubmed: "LitCovid ” (https://www.ncbi.nlm.nih.gov/research/coronavirus/). We have excluded preprint articles published on Medrixv and BioRixv, as the scope of our review is narrative and not a systematic revision of the published literature that, at this time, would be incomplete while the global pandemic is still affecting many countries and data are rapidly changing.
We included publications reporting data on obesity (e.g., prevalence) or association between BMI and clinically relevant data such as mortality, severity and outcomes of disease among laboratory confirmed COVID-19 subjects.
2.1. Obesity and COVID-19
Obesity and severe obesity are associated with a greater severity of the disease and related risk of hospitalization, worse clinical surrogate outcomes, such as lower SaO2 and PaO2 requiring IMV, longer length of hospital stay and longer time to achieve oxygen weaning [[3], [4], [5], [6], [7], [8], [9], [10], [11]].
Early data from China [12,13] showed that frequent comorbidities in patients diagnosed with COVID-19 were hypertension, DM, COPD, and coronary heart disease, without details on body mass index (BMI) [14]. Additional data including BMI became available and clearly showed that obesity was not only highly prevalent, but also associated with reduced survival. Data reported by COVID-19–Associated Hospitalization Surveillance Network (COVID-NET) show that almost 90% of patients with COVID-19 hospitalized for 1 month had at least one or more underlying conditions, being hypertension the most common (49.7%), closely followed by obesity (48.3%), chronic lung disease (34.6%), DM (28.3%), and cardiovascular disease (27.8%). In the age range 18–64 years old obesity was the most prevalent comorbidity, and within those aged 50–64 years obesity was even more prevalent than hypertension and DM [15].
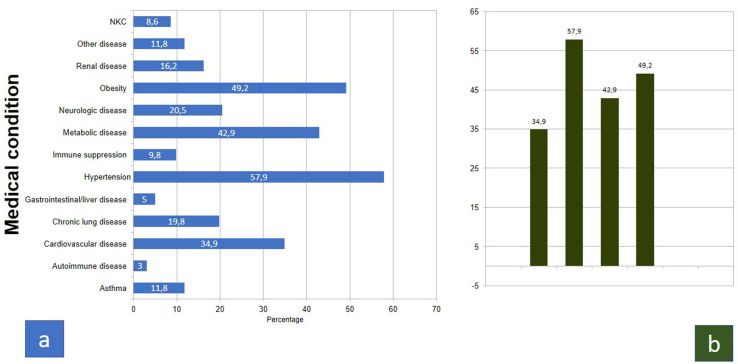
In Fig. 1 COVID-NET data are shown up to December 31, 2020 (Fig .1, a-b).
Fig. 1.
Weekly updated Data from COVID-NET [16] as of December 31, 2020, a) prevalence of medical conditions in hospitalized patients; b) focus on the most prevalent (>30%) conditions (same data showed in a).
Similar findings were confirmed in cluster of specific geographic area: Richardson reports the most common comorbidities being hypertension 56.6%, obesity 41.7%, and diabetes 33.8% in 5700 subjects hospitalized for COVID-19 in New York City (NYC) area within a month [17]. Consistently, data collected during the month of March in NYC by Petrilli's group in more than 5279 patients, confirm that age >44 years and morbid obesity (BMI ≥40 kg/m2) were strong predictors for hospitalization. Specifically, in those 75 years or older, odds ratio (OR) for hospitalization was 37.9 (95% CI, 26.1–56); age 65–74 years OR was 8.7 (95% CI, 8.7–11), and BMI ≥40 kg/m2 OR was 2.5 (95% CI, 1.8–3.4) [18].
Another report by Lighter et al. highlights the major impact of obesity in the age group younger than 60 years, in a cohort from NYC positive for COVID-19, that was fairly representative of USA population [19], with 21% of the total cohort having a BMI 30–34 kg/m2, and 16% BMI ≥35 kg/m2. Patients <60 years of age and with a BMI between 30 and 34 kg/m2 were two times more likely to be admitted to acute or critical care, while patients with a BMI ≥35 kg/m2 and aged <60 years were 2.2 times more likely to be admitted to acute care and 3 times to ICU, compared to individuals without obesity. Subjects <60 years of age in the general population without obesity were believed to be a lower risk group for COVID-19 with a more favorable prognosis. However, especially in Western countries characterized by the high prevalence of obesity, Sars-Cov-2 is spreading rapidly among the population. Obesity has been, in fact, confirmed as an independent risk factor even in younger individuals in other reports: one from an American cohort from California in which a higher level of care was required by patients with obesity (OR 2.0, P = 0.021) [4]; and one in which BMI >30 was significantly associated with higher risk for mortality, IMV and hospital admission (OR 95%CI of 6.29, 6.01, 2.61) [5].
Importantly, in a French retrospective analysis of patients admitted to ICU for SARS-CoV-2 infection, a greater BMI was positively associated with the severity of COVID-19, with nearly 90% of patients with class II obesity or greater (BMI ≥35 kg/m2) requiring IMV. The relation of obesity with SARS-CoV-2 was confirmed by using an historical ICU control group admitted for non-SARS-CoV-2 severe acute respiratory syndrome in the previous year [20]. Noteworthy, another French study reports a lower prevalence of obesity in ICU and less people requiring IMV, explaining that this difference can be due to a lower prevalence of obesity in that specific area, which was lower than a half compared to the population of the previously discussed study. Even if the overall prevalence of obesity was lower, however, in those with severe obesity the risk for IMV remained significantly greater than in the leaner counterparts (81.8% vs 41.9%, respectively) [21].
A recent analysis investigated the association between COVID-19 and metabolic-associated fatty liver disease (MAFLD) in a Chinese group of patients with obesity. Individuals with severe obesity and MALFD patients had more severe COVID-19 disease, however, the presence of obesity in those with MAFLD was associated with approximately a 6-fold increased risk of severe COVID-19. Remarkably, almost 90% of those with severe illness had obesity, compared with a lower prevalence of obesity (57%) in non-severe COVID-19. The association of obesity and COVID-19 severity remained significant, even after statistical adjustments for age, sex, smoking, DM, hypertension, and dyslipidemia [22].
In another Chinese report, Peng et al. described a statistically higher BMI in cardiovascular disease patients with a severe form of COVID-19 infection (27.0 ± 2.5 versus 22.0 ± 1.3, respectively). Furthermore, among the non-survivors, 88.2% of patients had a BMI >25 kg/m2, significantly higher than survivors (18.9%) [23].
Data available from Italy, to date, report a lower prevalence of obesity among deceased individuals, however, the overall age of Italian patients diagnosed with COVID-19 tended to be higher compared to other countries. Table 1 lists the pre-existing conditions (with a prevalence >10%) in the 27,955 deceased Italian people who tested positive for Sars-CoV-2, accessed at the institutional website on the 7th of May, 2020. Among this group, the mean number of comorbidities was 3.4 ± 1.9. Specifically, 3.9% had no comorbidity, 15% reported 1 comorbidity, 21.3% had 2 and 59.9% 3 or more comorbidities.
Table 1.
Prevalence of comorbidities in the Italian 35,563 deceased people positive for Sars-CoV-2, accessed at https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_1_march_2021.pdf [24] on the 1st of March, 2021. Modified selecting disease with a prevalence >10%.
| Women (43.9%) | Men (56.1%) | Total (n = 96141) | |
|---|---|---|---|
| Mean Age | 86 years | 80 years | 83 years |
| Hypertension | 67.9% | 64.1% | 65.7% |
| Ischemic heart disease | 23.3% | 31.1% | 27.9% |
| Atrial fibrillation | 25.5% | 23.4% | 24.3% |
| Heart failure | 17.6% | 14.2% | 15.9% |
| Stroke | 12.6% | 10.9% | 11.6% |
| Type-2 Diabetes | 27% | 30.8% | 29.3% |
| Dementia | 32.4% | 17.7% | 23.6% |
| COPD | 14.1% | 19.4% | 17.3% |
| Active cancer in last < 5 years | 15.1% | 17.7% | 16.7% |
| Chronic kidney disease | 19.8% | 22.2% | 21.2% |
| Obesity | 10.9% | 11.1% | 11% |
Legend: COPD chronic obstructive pulmonary disease.
Moreover, Table 2 summarizes the most prevalent pre-existing conditions across some countries.
Table 2.
A comparison of preexisting medical conditions between countries with the highest prevalence COVID-19 (China, USA, Italy, and France).
| Median Age (years) | Obesity (%) | Diabetes (%) | Hypertension (%) | CVD (%) | Lung-disease (%) | |
|---|---|---|---|---|---|---|
| China (Guan et al. 12) | 47 (58.1%M) | |||||
| Tot | 7.4 | 15 | 2.5 | 1.1 | ||
| Non-severe | - 5.7 | - 13.4 | - 1.8 | - 0.6 | ||
| Severe | - 16.2 | - 23.7 | - 5.8 | - 3.5 | ||
| COVID-NET (16, up to August 29th) | 47.9 | 41.5 | 56.7 | 32.5 | 18.7 | |
| COVID-NET [15] | Overall | 48.3 | 28.3 | 49.7 | 27.8 | 34.6 |
| - 50-64 | - 49 | - 32.1 | - 47.4 | - 19.6 | - 28.3 | |
| - >65 | - 41 | - 31.3 | - 72.6 | - 50.8 | - 38.7 | |
| NYC (Richardson et al., 17) | 63 (60.3%M) | 41.7 | 33.8 | 56.6 | 18 | 17.3 |
| NYC (Petrilli et al., 18) - tot | 35.3 | 22.6 | 42.7 | 52.1 | 14.9 | |
| Hospitalized | 54 (49.5%M) | 39.5 | 34.7 | 62 | 70 | 16.5 |
| Non hospitalized | (61.2%M) | 30.8 | 9.7 | 21.9 | 32.2 | 13.1 |
| NYC (Cummings et al.,3) | 62 (67% M) | 46 | 36 | 63 | ||
| France (Simonnet et al., 20) | 60 (73%M) | BMI 29.6 kg/m2 | ||||
| IMV | 31.1 kg/m2 | - 27 | - 56 | |||
| Non-IMV | 27 kg/m2 | - 13 | - 32 | |||
| China (Zheng et al., 22), tot | 47 | 24.2 | 28.8 | |||
| with obesity | - 31.1 | - 35.6 | ||||
| without obesity | - 9.5 | - 14.3 | ||||
| Italy (ISS, 24)∗ | 80 (57.6%M) | 10.4 | 29.5 | 65.8 | 44 | 17.1 |
All data are in %, except age in years.
Legend: ∗Refers to data of deceased population positive for Sars-CoV-2; NYC New York City, CHD coronary heart disease, IMV invasive mechanical ventilation.
2.1.1. Obesity and susceptibility to viral infections: the basis for a more severe COVID-19
SARS-CoV-2 binds to the receptor angiotensin converting enzyme 2 (ACE-2) to enter the cells. The expression of this receptor in particular tissues, including adipose tissue and lungs, is increased in obesity, in relation to leptin resistance and upregulation of SOCS-3 (suppressor of cytokine signaling-3), a gene involved in regulation of inflammation and inhibitor of leptin signaling. At the same time, SARS-CoV-2 affects the expression of genes related to lipid metabolism in epithelial cells, having a possible role in white fat differentiation. These pathways suggest that individuals with obesity may have a higher susceptibility to Sars-CoV-2 infection, and it could potentially also explain, at least in part, the increased risk for severe complications once COVID-19 is diagnosed [25,26].
BMI positively correlates with infectious virus shedding in aerosol of cases affected by influenza virus, and vaccination coverage is less efficient in individuals with obesity [27]. Moreover, patients with obesity show a more prolonged duration of illness as well as a greater risk for severe influenza-like illness and higher respiratory mortality during previous pandemic of viral infections, consistently with recent data on COVID-19-related mortality. This was confirmed by a large body of evidence, which has reported an impaired immune response to influenza or influenza-like viruses in obesity. Notably, subjects with obesity shed influenza A virus for a longer period of time than subjects without obesity, having a viral shedding 42% longer when symptomatic and up to 104% longer in asymptomatic or pauci-symptomatic subjects [28]. Therefore, obesity plays a crucial role in viral transmission, significantly increasing the chance to spread influenza and influenza-like diseases in countries where the prevalence of obesity is high.
Metabolic syndrome might impair the immune system efficiency, causing a chronic hyperinflammatory state in obesity that could explain these data [29,30].
Immune system dysfunction, caused by a chronic low-grade systemic inflammation in metabolic conditions such as hyperinsulinemia and hyperleptinemia, increases vulnerability to infections altering both the innate and the adaptive immune response [29,30]. Indeed, the impaired responses of B and T cell in obesity may cause an increased susceptibility and possibly a delayed resolution of viral infections [30]. Excess adipose tissue can promote this pro-inflammatory microenvironment characterized by the production of adipokines (adipose-tissue derived cytokines), with increased leptin and reduced adiponectin. This chronic imbalance between high leptin, with well-known pro-inflammatory features, and low adiponectin, an adipokine with anti-inflammatory properties, induces macrophage production of high level of interleukin (IL)-6 and tumor necrosis factor-α (TNF-α), possibly triggering and even worsening the COVID-19 inflammatory cytokine storm [30,31]. The adipokine imbalance and the lack of the adiponectin negative feedback, might also explain how the cytokine storm causes septic shock during severe sepsis, and eventually death in the setting of obesity [31].
In addition to immune and metabolic impairments, pulmonary ventilation and gas exchange in obesity may be compromised as a result of a reduced diaphragmatic excursion and relative increase in anatomical death space. COPD and OSAS are common comorbidities in obesity, and hypoxia may aggravate the pro-inflammatory state described above. Accordingly, Petrilli et al. highlighted that the degree of oxygen impairment and markers of inflammation were the strongest predictors of poor outcomes during COVID-19 hospitalization [18]. An additional condition recognized in COVID-19 that is also characteristic of obesity, is the increased risk for thromboembolism, resulting in a further increased risk for mortality [32].
2.2. Physical activity, cardiorespiratory fitness and immune system
Physical activity (PA) plays a key role in our mental and physical health. According to the World Health Organization (WHO), globally, 1 adult in 4 is not active enough and more than 80% of the adolescent population is insufficiently physically active. Insufficient PA leads to high risk of sarcopenia and reduced cardiorespiratory fitness, can cause noncommunicable disease and depression [33], as well as increasing the risk for weight gain (https://www.who.int/news-room/fact-sheets/detail/physical-activity). Of note, these conditions have been shown to correlate with a higher incidence of complications [34] and death during COVID-19 infection (https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-analysis-of-deaths). Even children are at risk of weight gain for social distancing and stay-at-home requirements, with reduced opportunities of PA and increased sedentary behaviors [35].
Regular PA is a simple and effective way to deal with stress and frustration, and bad quality of sleep, especially during the current COVID-19 lockdown [36,37]. PA exerts positive effects on insulin resistance and immune response by inhibiting inflammatory cytokines pathway and macrophage activation [[38], [39], [40]] and by modulating inflammation and improving vaccination outcomes in the elderly [41]. In addition, PA can enhance antioxidant defense and reduce oxidative stress [30], acting as a strong non-pharmacological immunomodulatory intervention and as modifier of the adipokines imbalance. Therefore, the immune system is affected by regular exercise with a clear inverse relationship between moderate exercise training and illness risk [42].
Although the lockdown is the principal strategy to contain the virus spread, it can promote sedentary behaviors, reducing regular PA and energy expenditure and increasing the risk of potential worsening of chronic health conditions and sarcopenia, potentially leading to worse outcomes in COVID-19 infections [13]. Therefore, it is strongly recommended to maintain appropriate level of PA, even at home during quarantine also to counteract the loss of muscle mass and muscle functionality and therefore the risk for sarcopenia, and to maintain adequate immune system functions during such a current difficult period [43,44]. It is possible to improve cardiorespiratory fitness at home by performing safe, simple, and easy exercises, as stretching and strengthening exercises, activities for balance and control, or a combination of them (e.g. walking in the house, alternating leg lunges, stair climbing, stand-to-sit and sit-to-stand using a chair and from the floor, chair squats, and sit-ups and pushups). The use of eHealth and exercise videos to encourage and deliver PA through the Internet, mobile technologies, and television are other strategies [45,46]. In children, the schools should plan physical education classes delivered through video to promote PA and possibly improve cardiorespiratory fitness even while staying at home [35]. Finding a personal program to follow a proper PA would be particularly important during quarantine with stay-at-home requirement, when the psychological and physical burden can be heavy, especially in inactive or sedentary subjects.
At last, metabolic improvement, reduced systemic inflammation and improved immune system achieved through exercise training, in subjects with obesity even in absence of weight loss, may reduce morbidity and mortality during influenza and influenza-like disease [47]. To maintain appropriate level of cardiorespiratory fitness, mental health, muscle mass and thus energy expenditure and body composition, Barazzoni et al. recommend to engage in exercise every day >30 min, or every other day > 1 h [48,49].
2.3. Psychoneuroimmune implication of COVID-19
The COVID-19 pandemic affects also psychological well-being, triggering a wide range of psychological problems, such as panic disorder, anxiety and depression.
Researchers at Shanghai Mental Health Center developed a Covid-19 Peritraumatic Distress Index (CPDI), a national online survey to assess Covid-19 distress level. Starting from January 31st 2020, this first nationwide large-scale survey of psychological distress in the general population of China collected 52,730 valid questionnaires by 35.27% males and 64.73% females. Almost 35% of them experienced psychological distress (50,364). In Italy numerous studies are currently ongoing (https://ec.europa.eu/eusurvey/runner/COVIDSurvey2020).
Multiple studies on psychoneuroimmunology describe how emotional distress through the nervous system can impair immunity and influence recovery from diseases [50]. Quarantined positive COVID-19 subjects may be at greater risk for depression, fear, guilt and anger related to the disease itself; however, even individuals without COVID-19 can be psychologically affected by the pandemic. The dramatic increase of public fears and decrease in social and economic activities may, in fact, trigger psychosocial distress [51]. Subjects with obesity who are already stigmatized, during self-quarantine and social distancing are, in fact, experiencing higher rates of depression [52]. Moreover, the awareness of the high risk for severe complications of COVID-19 in obesity can increase the psychological burden. Psychosocial distress in obesity can be due to multiple reasons, such as quarantine, duration of social and family distancing, social media reports, fear of infection, provision of protective-supplies, financial crisis, and stigma [49].
To reduce the negative impact of these stressors on mental health, different strategies can be implemented, including optimization of remote clinical mental support using telemedicine, promoting virtual connections with family and friends to facilitate social relationships and emotional support, and promoting entertainment activities (e.g., books, games, indoor hobbies and physical activity, phones, internet access), but also reinforcing the concept that personal commitment in social distancing and quarantine are useful strategies to reduce the risks related to Sars-Cov2 infection [53]. Finally, psychological support should be routinely implemented to enhance psychological resilience and to eventually improve psychoneuroimmunity during COVID-19 [51].
2.4. Dietary quality and vitamin supplementation
Some vitamins and micronutrients are known to have a role in the immune system and subjects with obesity are often in deficiency or insufficiency states due to unhealthy diets. Subjects with obesity often show low blood levels of Vitamin D, that is recognized to exert positive functions on inflammation and to protect against respiratory tract infection [54].
Many other micronutrients are involved metabolic and immunological pathways, therefore an assessment of trace element and vitamins such as Iron, Zinc, Selenium, Vitamin A, E, B6 and B12 might be indicated to tailor medical nutrition therapy and the related need for dietary supplementations [48]. A special attention to protein and energy intake should aim to prevent or even treat sarcopenic obesity, especially in those subjects discharged after hospitalization or in any older obese patients. Essential aminoacid supplementation may support nutritional management, if required. Importantly, reducing the consumption of foods with pro-inflammatory properties, such as added sugars and saturated fats, and possibly increasing the consumption of foods rich in unsaturated fatty acids with well-known anti-inflammatory properties, might represent a useful strategy [54].
2.5. Study limitations
Our manuscript is not without limitations, particularly it analyzed reports with a wide variability of data, including national reports on deceased subjects, as in the case of Italy [15], but also observational studies from different countries including outcomes on both hospitalized and not-hospitalized patients. Moreover, severity of disease was differently classified, as per requirement of IMV. Notably, most publications on COVID-19 at the beginning of the pandemic did not mention obesity among comorbidities. It is not clear if this missing data was less relevant at the beginning of the outbreak, or obesity relevance was just reported later during COVID-19 pandemic.
Future analysis will be necessary to compare multiple data on COVID-19 outbreak from different countries and health systems in subjects with obesity and identify the most appropriate therapeutic strategy in this population.
3. Conclusion
Taken together, this review highlights the importance of prevention of obesity in the first place. Subjects with obesity may, in fact, need to extend the quarantine period and to take extra-precautions during viral pandemic such as COVID-19, adopting preventive measures such as social distancing, appropriate hygiene, and wearing face masks in public settings. More importantly, the best practice to prevent worse outcomes and to lower the mortality of acute and chronic diseases would be to lower the burden of obesity, not only during the state of emergency, but as prevention in possible future viral pandemics.
Finally, the nutritional management after hospitalization for COVID-19 patients, especially after ICU stay or intubation, represents a major opportunity to improve post-discharge quality of life, and this remains challenging in patients with obesity. Hospitalization-related or disease-related complications may have worse outcome in obesity after a long inpatient stay, such as dysphagia, loss of muscle mass and function (sarcopenia and sarcopenic obesity) [55] and impaired mobility, or secondary infections.
Funding
Authors did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors for this manuscript.
Conflict of interest
Dr Carbone is supported by a Career Development Award 19CDA34660318 from the American Heart Association and by the Clinical and Translational Science Awards Program UL1TR002649 from National Institutes of Health to Virginia Commonwealth University. The remaining authors have nothing to disclose.
Acknowledgments
Authors would like to thank the project AIRI-CLIP (Associazione internazionale ricercatori italiani – COVID-19 Literature in pills) for inspiration, and support during the Sars-Cov-2 pandemic infection and the health emergency affecting Italy and many countries.
References
- 1.Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. National Center for Health Statistics; Hyattsville, MD: 2020. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, no 360. [PubMed] [Google Scholar]
- 2.Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 — United States, February 12–march 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebinger J.E., Achamallah N., Ji H., Sun N., Botting P., Nguyen T.T., et al. Pre-existing traits associated with Covid-19 illness severity. PloS One. 2020;15(7) doi: 10.1371/journal.pone.0236240. Published 2020 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg E., Wright E., Kushner B. Young adults with COVID-19, obesity is associated with adverse outcomes. West J Emerg Med. 2020;21(4):752–755. doi: 10.5811/westjem.2020.5.47972. Published 2020 Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S., et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moriconi D., Masi S., Rebelos E., Virdis A., Manca M.L., De Marco S., et al. Obesity prolongs the hospital stay in patients affected by COVID-19, and may impact on SARS-COV-2 shedding. Obes Res Clin Pract. 2020;14(3):205–209. doi: 10.1016/j.orcp.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suleyman G., Fadel R.A., Malette K.M., Hammond C., Abdulla H., Entz A., et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan detroit. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.12270. Published 2020 Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalligeros M., Shehadeh F., Mylona E.K., Benitez G., Beckwith C.G., Chan P.A., et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity. 2020;28(7):1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Q., Chen F., Wang T., Luo F., Liu X., Wu Q., et al. Obesity and COVID-19 severity in a designated hospital in shenzhen, China. Diabetes Care. 2020;43(7):1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 11.Ortiz-Brizuela E., Villanueva-Reza M., González-Lara M.F., Tamez-Torres K.M., Román-Montes C.M., Díaz-Mejía B.A., et al. Clinical and epidemiological characteristics of patients diagnosed with covid-19 in A tertiary care center in Mexico city: a prospective cohort study. Rev Invest Clin. 2020;72(3):165–177. doi: 10.24875/RIC.20000211. [DOI] [PubMed] [Google Scholar]
- 12.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.4683. [published online ahead of print, 2020 Mar 23] [DOI] [PubMed] [Google Scholar]
- 15.Garg S., Kim L., Whitaker M., O’Halloran A., Cummings C., Holstein R., et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. Published 2020 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.“Covid-Net COVID-19-Associated hospitalization surveillance Network, centers for disease control and prevention. WEBSITE. https://gis.cdc.gov/grasp/COVIDNet/COVID19_5.html
- 17.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. the Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.6775. Erratum in: JAMA. 2020 May 26;323(20):2098. PMID: 32320003; PMCID: PMC7177629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F., et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020 doi: 10.1002/oby.22831. Erratum in: Obesity (Silver Spring). 2020 Oct;28(10):1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caussy C., Wallet F., Laville M., Disse E. Obesity is associated with severe forms of COVID-19. Obesity. 2020 doi: 10.1002/oby.22842. 10.1002/oby.22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng K.I., Gao F., Wang X.B., Sun Q.F., Pan K.H., Wang T.Y., et al. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020:154244. doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Y.D., Meng K., Guan H.Q., Leng L., Zhu R.R., Wang B.Y., et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xinxueguanbing Zazhi. 2020;48:E004. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 24.https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_7_September_2020.pdf
- 25.Heialy Saba Al, Hachim M., Senok A., Gaudet M., Abou Tayoun A., Hamoudi R., et al. Regulation of angiotensin converting enzyme 2 (ACE2) in obesity: implications for COVID-19. Front Physiol. 2020 Sept 18;11(555039) doi: 10.3389/fphys.2020.555039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel A.B., Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? J Am Med Assoc. 2020 doi: 10.1001/jama.2020.4812. [published online ahead of print, 2020 Mar 24] 10.1001/jama.2020.4812. [DOI] [PubMed] [Google Scholar]
- 27.Honce R., Schultz-Cherry S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol. 2019;10:1071. doi: 10.3389/fimmu.2019.01071. Published 2019 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maier H.E., Lopez R., Sanchez N., Ng S., Gresh L., Ojeda S., et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis. 2018;218(9):1378–1382. doi: 10.1093/infdis/jiy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen C.J., Murphy K.E., Fernandez M.L. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7(1):66–75. doi: 10.3945/an.115.010207. Published 2016 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luzi L., Radaelli M.G. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020:1–6. doi: 10.1007/s00592-020-01522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan P.M., Caplice N.M. Is adipose tissue a reservoir for viral spread, immune activation and cytokine amplification in COVID-19 ? Obesity. 2020 doi: 10.1002/oby.22843. 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Movahed M.R., Khoubyari R., Hashemzadeh M., Hashemzadeh M. Obesity is strongly and independently associated with a higher prevalence of pulmonary embolism. Respir Investig. 2019;57(4):376–379. doi: 10.1016/j.resinv.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Lavie C.J., Ozemek C., Carbone S., Katzmarzyk P.T., Blair S.N. Sedentary behavior, exercise, and cardiovascular health. Circ Res. 2019;124(5):799–815. doi: 10.1161/CIRCRESAHA.118.312669. [DOI] [PubMed] [Google Scholar]
- 34.Rundle A.G., Park Y., Herbstman J.B., Kinsey E.W., Wang Y.C. COVID-19-Related school closings and risk of weight gain among children. Obesity. 2020 doi: 10.1002/oby.22813. 10.1002/oby.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu J., Shen B., Zhao M., Wang Z., Xie B., Xu Y. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: implications and policy recommendations. Gen Psychiatr. 2020;33(2) doi: 10.1136/gpsych-2020-100213. Published 2020 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y., Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatr Res. 2020;288:112954. doi: 10.1016/j.psychres.2020.112954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieman D.C., Wentz L.M. The compelling link between physical activity and the body's defense system. J Sport Health Sci. 2019;8(3):201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng Q., Cui G., Chen J., Gao H., Wei Y., Uede T., et al. Regular exercise enhances the immune response against microbial antigens through up-regulation of toll-like receptor signaling pathways. Cell Physiol Biochem. 2015;37(2):735–746. doi: 10.1159/000430391. [DOI] [PubMed] [Google Scholar]
- 39.Reidy P.T., Yonemura N.M., Madsen J.H., McKenzie A.l., Mahmassani Z.S., Rondina M.T., et al. An accumulation of muscle macrophages is accompanied by altered insulin sensitivity after reduced activity and recovery. Acta Physiol. 2019;226(2) doi: 10.1111/apha.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong G.C.L., Narang V., Lu Y., Camous X., Nyunt M.S.Z., Carre C., et al. Hallmarks of improved immunological responses in the vaccination of more physically active elderly females. Exerc Immunol Rev. 2019;25:20–33. [PubMed] [Google Scholar]
- 41.Nieman D.C., Wentz L.M. The compelling link between physical activity and the body's defense system. J Sport Health Sci. 2019;8(3):201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beaudart C., Dawson A., Shaw S.C., Harvey N.C., Kanis J.A., Binkley N., et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28(6):1817–1833. doi: 10.1007/s00198-017-3980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landi F., Calvani R., Picca A., Tosato M., Martone A.M., D’Angelo E., et al. Impact of habitual physical activity and type of exercise on physical performance across ages in community-living people. PloS One. 2018;13(1) doi: 10.1371/journal.pone.0191820. Published 2018 Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tate D.F., Lyons E.J., Valle C.G. High-tech tools for exercise motivation: use and role of technologies such as the internet, mobile applications, social media, and video games. Diabetes Spectr. 2015;28(1):45–54. doi: 10.2337/diaspect.28.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen P., Mao L., Nassis G.P., Harmer P., Ainsworth B.E., Li F. Coronavirus disease (COVID-19): the need to maintain regular physical activity while taking precautions. J Sport Health Sci. 2020;9(2):103–104. doi: 10.1016/j.jshs.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Codella R., Luzi L., Inverardi L., Ricordi C. The anti-inflammatory effects of exercise in the syndromic thread of diabetes and autoimmunity. Eur Rev Med Pharmacol Sci. 2015;19(19):3709–3722. [PubMed] [Google Scholar]
- 47.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. endorsed by the ESPEN Council ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. S0261-5614(20)30140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frühbeck G., Baker J.L., Busetto L., Dicker D., Goossens G.H., Halford J.C.G., et al. European association for the study of obesity position statement on the global COVID-19 pandemic. Obes Facts. 2020:1–5. doi: 10.1159/000508082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev. 2018;98(1):477–504. doi: 10.1152/physrev.00039.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim S.W., Su K.P. Using psychoneuroimmunity against COVID-19. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.03.025. S0889-1591(20)30391-30393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryan D.H., Ravussin E., Heymsfield S. COVID 19 and the patient with obesity - the editors speak out. Obesity. 2020;28(5):847. doi: 10.1002/oby.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brooks S.K., Webster R.K., Smith L.E., Woodland L., Wessely S., Greenberg N., et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. Published 2017 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Billingsley H.E., Carbone S., Lavie C.J. Dietary fats and chronic noncommunicable diseases. Nutrients. 2018;10(10):1385. doi: 10.3390/nu10101385. Published 2018 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cava E., Carbone S. Coronavirus disease 2019 pandemic and alterations of body composition. Curr Opin Clin Nutr Metab Care. 2021 Feb 10 doi: 10.1097/MCO.0000000000000740. [DOI] [PMC free article] [PubMed] [Google Scholar]