Abstract
Coronavirus disease-19 (COVID-19)-induced severe acute respiratory syndrome is a global pandemic. As a preventive measure, human movement is restricted in most of the world. The Centers for Disease Control and Prevention (CDC), the National Institutes of Health (NIH), along with the World Health Organization (WHO) have laid out some therapeutic guidelines for the infected patients. However, other than handwashing and vigilance surrounding commonly encountered oronasal symptoms and fever, no universally available prophylactic measure has yet been established. In a pandemic, the accessibility of a prophylactic biologically active substance is crucial. Ideally, it would be something readily available at a low price to a larger percentage of the population with minimal risk. Studies have demonstrated that zinc may reduce viral replication and increase immune responses. While consuming zinc (within the recommended upper safety limits), as a prophylactic might provide an additional shield against the initiation and progression of COVID-19 would need clinical studies, the potential clearly exists. Even after vaccination, low zinc status may affect the vaccination responses.
Keywords: Zinc, Antiviral, COVID-19, Pandemic, Host resistance
1. Zinc: an essential trace element
Maintaining the adequate zinc balance is essential for the normal functionality of various human systems [1]. Zinc is mostly present in muscles (60%), bones (30%), and skin (5%) in humans [2], [3], [4]. Zinc is involved in the synthesis process of various proteins and is involved in activating enzymes necessary for normal cellular functions. Zinc facilitates the absorption of vitamin A, vitamin E, and folate [1]. Zinc deficiency is associated with a higher rate of infections, degenerative diseases, oral diseases, and behavioral disorders in humans [1], [4]. Available information suggests that zinc deficiency is associated with increased disease severity of the COVID-19 [5]. In a comprehensive metal(loid)s analysis, compared to the non‐severe COVID‐19 patients, the whole blood chromium, calcium, and copper levels were higher, while the levels of zinc, magnesium, manganese, iron, lead, arsenic, and thallium were lower in the severe patients with COVID-19 [6]. Depending on age and gender, the recommended daily allowance for zinc varies from 2 and 13 mg/day, with the tolerable upper intake level for zinc is set at 40 mg/day by the Institute of Medicine (IOM).
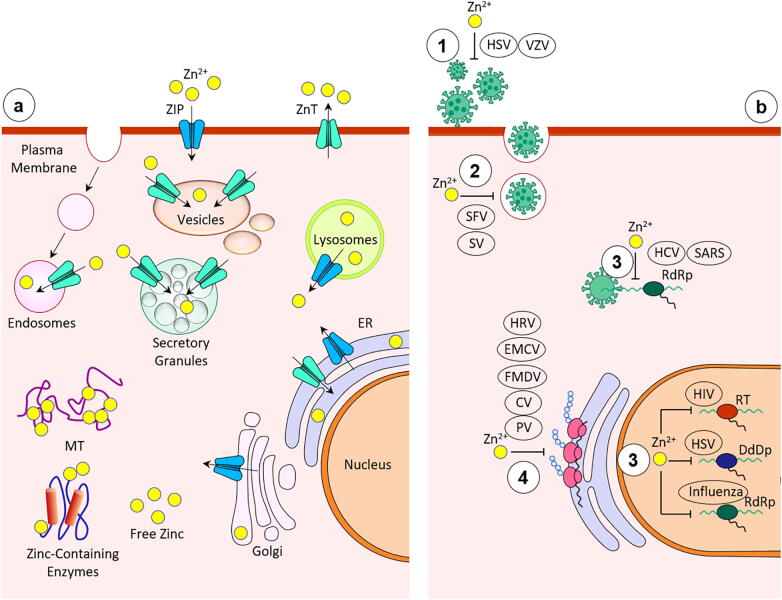
Around 25–66% of the consumed zinc is absorbed from the jejunum and ileum, and is present all over the body (in tissues, cells and fluids) [7], although the highest content of zinc is found in the muscles and bones. Zinc is bound to serum proteins (albumin, globulin, transferrin) and amino acids, which disperse zinc throughout the body; 95% of the total body zinc is present in the intracellular compartments [3]. The cellular zinc homeostasis is partly maintained by the zinc importers family (Zip) that lets the zinc accumulate into the cytosol, and by the zinc exporters family (ZnT), which transport the zinc out of the cytosol [2], [7] (Fig. 1). In addition, zinc-binding proteins, metallothioneins (MT) can also contribute to maintaining intracellular zinc homeostasis [2]. To keep the homeostatic balance in the body, zinc is excreted or eliminated through the kidneys, skin, and intestines when necessary. The possible effects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on Zip, ZnT and MT will need additional studies. Of importance, in addition to inadequate consumption of zinc, reduced intestinal absorption is a common cause of zinc inadequacy. Phytate (present in cereals, corn, and rice) has a strong inhibitory effect on zinc absorption from consumed meals. Casein can also exert a modest inhibitory effect on zinc absorption. Besides, iron supplementation may negatively influence zinc absorption, while cadmium can reduce zinc absorption [8].
Fig. 1.
a. Subcellular localization and transport of zinc b. Inhibition of viral replication by zinc 1. Free virus inactivation 2. Viral uncoating inhibition 3. Inhibition of viral genome transcription 4. Inhibition of viral protein translation and polyprotein processing Abbreviations: CV, coronavirus; DdDp, DNA-dependent DNA polymerase; EMCV, encephalomyocarditis virus; FMDV, foot and mouth disease virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPV, human papilloma virus; HRV, human rhinovirus; HSV, herpes simplex virus; PV, polio virus; RdRp, RNA-dependent RNA polymerase; RT, reverse transcriptase; SARS, severe acute respiratory syndrome coronavirus; SFV, Semliki Forest virus; SV, sindbis virus; VZV, varicella-zoster virus [15].
2. Zinc: an antiviral nutrient
SARS-CoV-2, the novel virus that causes COVID-19 infection, is estimated to have infected over 109 million people worldwide causing more than 2.4 million deaths as of February 16, 2021 (data collected from Coronavirus resource center of Johns Hopkins University, Maryland, U.S.). With heterogeneous clinical presentations [9], [10], [11], [12], it is a global priority to encourage collaborative efforts toward developing meaningful uniform therapeutic strategies to treat and reduce disease mortality [13], [14], [15]. One strategy may include zinc supplementation. The zinc compound has been speculated to reduce influenza viral infection through its antiviral effects in humans [13], [16]. Using cultured chorion cells prepared from human fetal membranes, a reduced replication of the influenza virus was noted with a zinc ionophore (pyrrolidine dithiocarbamate) [17]. Similarly, in vitro studies on Madin-Darby canine kidney (MDCK)-SIAT1 cells, zinc oxide nanoparticles exert antiviral effects against the H1N1 influenza virus infection [18]. In a related study, polyethylene glycol-coated zinc oxide nanoparticles inhibited more than 90% of H1N1 influenza virus loads [18]. Zinc has also been shown to inhibit the replication of severe acute respiratory syndrome (SARS) coronavirus by cell culture studies on Vero-E6 cells [19]. Using both zinc ions and zinc-ionophore (pyrithione), the replication of SARS coronavirus was reduced when intracellular concentration of zinc was high. The zinc-mediated inactivation of the RNA-dependent RNA polymerase (RdRp; core replication enzyme) is a possible mechanism of inhibition of SARS coronavirus replication [19]. Of relevance, replication of SARS-CoV-2 also needs two essential enzymes, RdRp and 3C-like proteinase (3CLpro). The zinc-binding sites were shown to be conserved in RdRp and 3CLpro by molecular modeling [20]. Though exact mechanisms are not yet defined, zinc-binding can reduce the enzymatic activities of 3CLpro and RdRp to inhibit viral replication [19]. In vitro studies have claimed that low zinc levels favor viral multiplication in SARS-CoV-2 infected cells [21]. Similarly, zinc could markedly inhibit the replication potential of the respiratory syncytial virus [22]. Investigators had shown an 800-fold reduction in the respiratory syncytial virus when 10 μM concentration of zinc was present during preincubation, adsorption, penetration, and egress [22]. In the clinical scenario, zinc supplementation resulted in a marked reduction in pneumonia prevalence in children [23], [24]. Hepatitis C virus (HCV) replication has also been suppressed by zinc [25], and zinc supplementation has enhanced the response of antiviral therapy for HCV-induced hepatitis patients [26], [27], [28]. The exact underlying mechanism of zinc-induced antiviral response is not well-understood; however, it has been demonstrated that zinc has the potential to inhibit viral binding to the mucosal cells, and eventual replication, possibly by generating antiviral interferon (IFN)-α and IFN-γ [29], [30]. In another study, zinc supplementation taken for 24 weeks, enhanced the response to INF-α therapy in patients with intractable chronic hepatitis C, as clinically determined by the serum level of aminotransferase and the presence of RNA for HCV [31]. SARS-CoV-2 can bind to the cell surface angiotensin-converting enzyme 2 (ACE2) through its spike proteins for entering into the cells to initiate viral replication and transcription [32]. Earlier studies have shown that ACE-2 expression is regulated by Sirtuin 1 (SIRT1), and zinc can reduce SIRT-1-mediated ACE2 expression [33], [34]. Experimental studies conducted on human lung cell lines, and treated with zinc in combination with triclabendazole (anthelmintic drug) or emetine (antiprotozoal drug), have shown to suppress ACE2 expression without producing cytotoxicity [35]. Monotherapy with triclabendazole or emetine failed to suppress ACE2 levels, suggesting the importance of zinc on the expression of ACE2 [35].
In this pandemic, chloroquine has been used to treat COVID-19 infection, with some early data suggestive of therapeutic potentials [36], [37], [38], [39], [40]. Although there are also studies that reported to have no benefit for using chloroquine on COVID-19 patients and documented increased risk of cardiotoxicity [41], [42], [43], [44], [45]. Notably, chloroquine is an ionophore for zinc and could increase the cellular entry of zinc [46]. Additionally, intracellular antiviral effects of zinc might be partly related to the chloroquine-induced beneficial effects documented in COVID-19 patients. In a recent clinical observation, zinc sulfate, in combination with hydroxychloroquine and azithromycin, has shown to provide better therapeutic benefits to the COVID-19 infected patients than the patients who received hydroxychloroquine and azithromycin, without zinc; an increased frequency of hospital discharge and reduced mortality are documented in zinc sulfate added-COVID-19 patients [47]. It needs to mention that hydroxychloroquine's potential utility to treat COVID-19 patients is debated, particularly the side effects of this drug beyond the acceptable range, and will require careful clinical consideration [48], [49], [50], [51], [52]. Additional controlled studies will also be required to gain better insight into the role and applications of zinc in COVID-19 infection. The rationale for using azithromycin, an antibiotic, against a viral infection was not clearly explained in the aforementioned study [47]. Recent in vitro studies on human airway cell lines have shown that zinc and azithromycin can suppress the expression of ACE2, and the investigators have speculated the potential prophylactic and therapeutic value of this combination for COVID-19 patients [53]. Exacerbation of antimicrobial resistance appears to be another casualty of the COVID-19 pandemic [54], and implementation of antimicrobial stewardship to reduce antimicrobial drug resistance in this pandemic should be a medical priority [55], [56].
3. Zinc: an immune-boosting nutrient
A serum zinc level of 80–130 μg/dl is considered as a normal range [57], and <70 μg/dl is regarded as clinical zinc deficiency [58], [59]. Impaired zinc homeostasis adversely affects immune cells by multiple mechanisms that result in the abnormal formation of lymphocytes, impaired intercellular cytokine communication, and diminished phagocytosis that cause an inadequate host defense [60]. Improved zinc intake may also reduce the risk of bacterial pneumonia co-infection by improving ciliary length and movement that affects viral particle removal and improves mucociliary clearance. There are many ways to supplement zinc through usual food consumption. Meat (lamb, beef, and chicken) and seafood (oysters, and lobster) are zinc-containing food. In addition, black sesame, soy foods, mushrooms, lentils, celery, legumes, nuts, almonds, and sunflower seeds are good sources of zinc [4]. Zinc can also influence the functionality of several immune cells [61], [62], and an inadequate zinc microenvironment can impair host‐defense systems [63], thus increasing the susceptibility to various microorganisms [64]. In vitro studies have shown a higher rate of mouse CD4 + CD8 + thymocyte death by apoptosis in those with low zinc concentrations [65], while apoptosis was shown to be reduced by adding zinc [66]. In a study of human children, zinc supplementation has been shown to provide T‐cell‐mediated immunity by increasing the numbers of CD4 + CD3 + cells in peripheral blood [67]. By contrast, zinc deficiency has been shown to impair B‐cell development [68], with low IgG production [69], leading to higher rates of infection and subsequent mortality [70]. Experimentally induced maternal zinc deficiency caused a lower level of antibody generation in the offspring, while zinc supplementation could restore the impaired antibody‐mediated responses [71]. Although the zinc supplementation can increase CD3 + CD4 + cells in the peripheral blood, to better understand T cell-mediated immunity, potential effects of zinc on T cell subsets, including the balance between regulatory T (Treg) cells and T helper type 17 (Th17), are needed. Of relevance, Treg cells can reduce or resolve inflammation, while Th17 cells can promote inflammation in various human diseases with immune dysregulation [72]. Studies have shown that zinc deficiency can drive Th17 polarization and promote the loss of Treg cell function [73]. More importantly, zinc supplementation can suppress Th17 cell development to provide an additional shield against the infection [74]. Cytotoxic CD8 + cells can kill virally infected cells, and experimental studies have shown that a zinc-deficient diet resulted in reduced population of CD8 + cells, thereby contributing to the exacerbation of the inflammatory responses [75], [76]. Of clinical importance, a low numbers of lymphocyte, including CD8 + cells are shown to be associated with poor prognosis of COVID-19 patients, and increasing lymphocyte counts resulted in clinical improvements [77], [78]. Macrophages showed reduced phagocytic ability against the parasites in a low zinc microenvironment, and the phagocytic activity of the macrophages can be restored by increasing zinc concentration [67].
A low zinc intake by elderly individuals has been documented in the National Health and Nutrition Examination Survey III (NHANES III); 35%–45% of elderly individuals (≥60 years) were projected to be consuming zinc below the estimated average requirements (6.8 mg/day for elderly females; 9.4 mg/day for elderly males). Even after adjusting the consumption from both food sources and dietary supplements, 20%–25% of elderly individuals were estimated to have inadequate zinc intakes [79], [80]. Reduced dietary zinc intake in elderly individuals is associated with a low intracellular concentration of zinc [81]. Of clinical significance, altered level of intracellular ionic zinc could exist, even when the plasma levels of zinc are within the normal range. This suggests that the plasma level of zinc might not always reflect the overall zinc status and could be misleading, particularly in elderly individuals [82], [83], [84]. When zinc level was measured in serum and the same individual's skin biopsy, despite low serum level of zinc, not much change in zinc content was noted in the biopsy site of the patients with leprosy when compared with the control tissue content [85]. In a similar line of study, when zinc was measured in serum and thigh skin in patients with chronic venous leg ulceration, the skin zinc concentration was elevated in patients with ulceration, as compared to the healthy controls; although the serum zinc level was lower in patients with chronic venous leg ulceration [86]. Moreover, commonly prescribed drugs, including hydrochlorothiazide, angiotensin 2 receptor antagonists, and angiotensin-converting-enzyme inhibitors that are used for the treatment of hypertension and cardiovascular disease patients can cause increased urinary excretion of zinc to induce systemic zinc deficiency [87]. In studies of zinc-deficient individuals, exogenous zinc supplementation resulted in higher INF (type I and II) production and response, along with improved immune cell survival, maturation, and function [88], [89].
Elderly individuals commonly suffer from an inadequate immune system [63], and are generally more susceptible to COVID-19 infection [90], [91]. Again, elderly individuals with comorbidity, including hypertension and diabetes, are usually zinc deficient [92]. Studies have shown that elderly individuals who consumed 45 mg elemental zinc/day for a year had a significantly reduced infection occurrence [89]. Other reports suggest zinc supplements up to 150 mg/daily are needed, especially during viral infections [93]. This seems to point to the idea that maintaining optimal zinc balance is essential in protecting against infection. The mechanism of higher intracellular zinc concentration could affect the replicative cycle of the RNA viruses to reduce viral replication. It is noteworthy that COVID-19 is an RNA virus.
4. Zinc: effects on COVID-19 patients
In a recently concluded Conference on Coronavirus Disease of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Dr. Güerri-Fernández and colleagues presented the retrospective analysis data on the impact of zinc on the mortality of COVID-19 patients among the hospital admitted patients in Spain. Out of 249 studied patients, there was 8% (21 patients) mortality. The investigators found a significantly lower plasma level of zinc in COVID-19 patients who died (43 μg/dl) than the patients who survived (63.1 μg/dl). After adjusting different variables, the investigators showed each unit increase of plasma zinc at the time of hospital admission resulted in a 7% reduced risk of in-hospital mortality. Also, less than 50 μg/dl of a plasma zinc level at the hospital admission was associated with a 2.3-fold increased risk of in-hospital deaths as compared to the patients with a plasma zinc level of 50 μg/dl or higher, suggesting the importance of maintaining adequate zinc balance [21]. In a similar line of study, serum zinc level was found to be relatively low in samples collected from COVID-19 patients compared to the healthy counterparts. The average serum zinc level was remarkably low in the samples collected from the non-surviving COVID-19 patients (n = 6) as compared to the surviving COVID-19 patients (n = 29) [94]. In another uncontrolled case series study with COVID-19 patients, a high dose of oral zinc salt resulted in clinical recovery, improved oxygenation, and less shortness of breath among those patients [95]. A study conducted in Japan on 62 patients with COVID-19 showed a close association between low serum zinc level and severity of the disease [5]. The investigators also found serum zinc level as a predictive factor for a critical illness of patients with COVID-19 [5]. In a different study, the fasting zinc level in blood in patients with COVID-19 (n = 47) was significantly lower than the healthy controls (n = 45). The median zinc level in patients with COVID-19 was 74.5 μg/dl, while in the control group, the level was 105.8 μg/dl [96]. The zinc-deficient COVID-19 patients were found to develop more complications, along with extended hospital stay and higher mortality [96]. In a trial conducted on COVID-19 patients (n = 62), treated with combination therapy of Nitazoxanide, Ribavirin, Ivermectin and zinc, showed a more rapid nasopharyngeal clearence of SARS-CoV-2 as compared to the patients (n = 51) who revived symptomatic treatment [97]. In a multicenter cohort study on 3473 hospitalized COVID-19 patients (in New York, U.S.), treatment with zinc and an ionophore resulted in a 24% reduced risk of ‘in-hospital’ mortality [98]; it was an observational study, and zinc sulfate (220 mg) was given orally, once or twice daily for four days or until discharge. As a zinc ionophore, hydroxychloroquine (400 mg twice daily for one day then 200 mg twice daily for four days) was used in patients whose oxygen saturation was <94% on room air, and whose QTc interval was <500 ms [98]. Among the zinc and ionophore treated group of 1005 hospitalized COVID-19 patients, 121 patients (12%) died, while among 2467 patients who did not receive zinc and ionophore, 424 patients (17%) died [98]. However, it needs to be mentioned that not all the studies found an association between zinc consumption and disease severity in COVID-19 patients [99]. In a retrospective analysis of 242 (Zinc-treated group: n = 196; Control group: n = 46) hospitalized patients with COVID-19, treated with zinc sulfate at a total daily dose of 440 mg (100 mg elemental zinc) showed neither additional benefit nor harmful health effects; since overall zinc status of the patients was not analyzed, no definitive conclusion could be drawn from this study [100]; also the retrospective nature of the study prevented in adjusting the uniform dose and duration of the study [100].
It is important to note that physicians’ recommendations should be sought before consuming zinc to minimize potential adverse effects. Acute exposure to high doses of zinc may induce gastrointestinal tract disorders, including nausea, vomiting, loss of appetite, epigastric pain, diarrhea, along with headache and fatigue [7]. Chronic zinc toxicity may include lethargy, copper deficiency, and severe iron deficiency anemia [101]. Excessive zinc levels are cytotoxic and shown to induce higher mortality in experimental studies [102]. The risk of developing adverse effects may limit the tolerability and long-term use of zinc.
5. Conclusion
The holistic approach of maintaining an adequate nutritional balance with healthy eating habits and keeping an active lifestyle are likely to reduce the disease burden of the COVID-19 pandemic [103], [104], [105], [106], [107], [108], [109], [110]. Without the mass availability of effective vaccines or specific drugs to treat or control COVID-19 infection, social distancing and home isolation are the most recommended measures employed to minimize the spread of COVID-19-associated infection. However, consuming zinc has the potential to provide an additional shield against the illness [111], [112], [113], [114], possibly by reducing viral load and enhancing the immunity of the COVID-19 patients (Fig. 2). The elevated intracellular concentration of zinc could inactivate the RNA-dependent RNA polymerase, the core viral replication enzyme [19], which could reduce viral replication and might have the potential to minimize the disease burden. Further studies will determine the relevance of experimental studies with zinc on human viral diseases. Of relevance, 40 mg of zinc per day is considered as the tolerable upper intake level and is unlikely to induce toxicity. Whether this same level of zinc intake can provide added protection against COVID-19 infection, perhaps by enhancing the host resistance, is an area that needs additional studies; the results of the ongoing clinical trials around the world will shed further light [115]. Another added benefit of zinc is that it is generally considered very safe to consume without harmful effects, even when consumed well above the daily recommended dietary intake [101]. The potential benefits of taking zinc in an effort to stave off COVID-19 infection will require carefully designed research studies and clinical trials to be universally recommended and would need to be a prospective, randomized placebo-controlled trial [116]. Of clinical significance, zinc deficiency has a deleterious association with severely ill patients beyond COVID-19. For instance, a South Korean study has reported that severely ill patients, who died after an intensive care unit (ICU) transfer, showed hypozincemia [117]. Even after treatment, the serum level of zinc did not change in these patients [117].
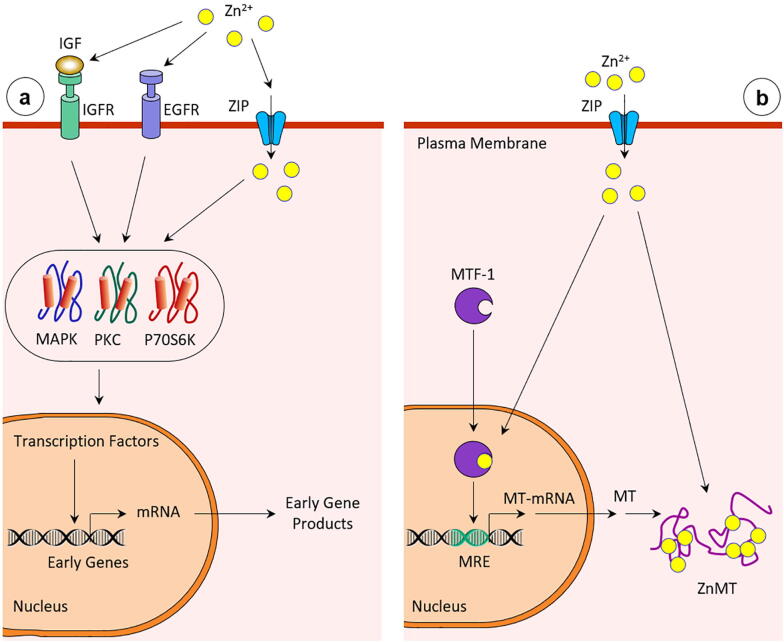
Fig. 2.
a. Zinc in a selected signal transduction pathway (IGF, insulin-like growth factor; IGFR, IGF receptors; EGFR, epidermis growth factor-receptor; MAPK, mitogen-activated protein kinase; PKC, protein kinase C; P70S6K, P70S6 kinase). b. Activation of the transcription factor MTF-1 by zinc and induction of MT [121], [122].
The double-blinded randomized controlled trials continue to be the gold standard of clinical studies. ClinicalTrials.gov and the WHO's International Clinical Trials Registry Platform (WHO ICTRP) have enlisted around 50 clinical trials to test the effects of various doses of zinc on the initiation and the progression of COVID-19 patients. Ongoing clinical trials on COVID-19 patients, either with zinc or with zinc and ionophores (quercetin or epigallocatechin gallate) are likely to extract additional information on its clinical utility [118], [119], [120]. The results of these ongoing studies in different parts of the world, particularly double-blinded randomized controlled trials, would provide information on the therapeutic value of zinc, either as a prophylactic or as an adjuvant therapy to minimize the disease burdens of COVID-19 patients. Available clinical studies with zinc supplementation on COVID-19 patients, although sparse, suggest promising prospects. Finally, the success of the ongoing COVID-19 vaccination program may be partly dependent on zinc sufficiency, and that low zinc availability may affect the vaccination responses.
Declaration of Competing Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
I want to express my sincere gratitude to Dr. Nuraly Akimbekov (Al-Farabi Kazakh National University, Kazakhstan) for his help in drawing the illustrations. Thanks to Dr. Sarah Erem, Dr. Margo Wolfe, Mr. M. Muhit Razzaque and Ms. Peace Uwambaye for carefully reading the manuscript and providing useful suggestions.
References
- 1.Gaur S., Agnihotri R. Trace mineral micronutrients and chronic periodontitis-a review. Biol Trace Elem Res. 2017;176:225–238. doi: 10.1007/s12011-016-0832-y. [DOI] [PubMed] [Google Scholar]
- 2.Kambe T., Tsuji T., Hashimoto A., Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev. 2015;95:749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 3.Kaur K., Gupta R., Saraf S.A., Saraf S.K. Zinc: the metal of life. Compr Rev Food Sci Food Saf. 2014;13:358–376. doi: 10.1111/1541-4337.12067. [DOI] [PubMed] [Google Scholar]
- 4.Uwitonze A.M., Ojeh N., Murererehe J., Atfi A., Razzaque M.S. Zinc adequacy is essential for the maintenance of optimal oral health. Nutrients. 2020;12:949. doi: 10.3390/nu12040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasui Y., Yasui H., Suzuki K., Saitou T., Yamamoto Y., Ishizaka T. Analysis of the predictive factors for a critical illness of COVID-19 during treatment – relationship between serum zinc level and critical illness of COVID-19. Int J Infect Dis. 2020;100:230–236. doi: 10.1016/j.ijid.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng H.-L., Yang Q., Yuan P., Wang X., Cheng L. Associations of essential and toxic metals/metalloids in whole blood with both disease severity and mortality in patients with COVID-19. FASEB J. 2021;35 doi: 10.1096/fj.202002346RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plum L.M., Rink L., Haase H. The essential toxin: impact of zinc on human health. Int J Environ Res Public Health. 2010;7:1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lönnerdal B. Dietary factors influencing zinc absorption. J Nutr. 2000;130:1378S–1383S. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- 9.Sattar Y., Connerney M., Rauf H., Saini M., Ullah W., Mamtani S. Three cases of COVID-19 disease with colonic manifestations. Am J Gastroenterol. 2020;115:948–950. doi: 10.14309/ajg.0000000000000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derwand R., Scholz M., Zelenko V. COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capone S., Abramyan S., Ross B., Rosenberg J., Zeibeq J., Vasudevan V. Characterization of critically Ill COVID-19 patients at a brooklyn safety-net hospital. Cureus. 2020;12 doi: 10.7759/cureus.9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alam M.M., Mahmud S., Rahman M.M., Simpson J., Aggarwal S., Ahmed Z. Clinical outcomes of early treatment with doxycycline for 89 high-risk COVID-19 patients in long-term care facilities in New York. Cureus. 2020;12 doi: 10.7759/cureus.9658. e9658-e9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eby G.A. Zinc ion availability–the determinant of efficacy in zinc lozenge treatment of common colds. J Antimicrob Chemother. 1997;40:483–493. doi: 10.1093/oxfordjournals.jac.a020864. [DOI] [PubMed] [Google Scholar]
- 14.Hemila H. Common cold treatment using zinc. JAMA. 2015;314:730. doi: 10.1001/jama.2015.8174. [DOI] [PubMed] [Google Scholar]
- 15.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eby G.A., 3rd Zinc lozenges as cure for the common cold–a review and hypothesis. Med Hypotheses. 2010;74:482–492. doi: 10.1016/j.mehy.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchide N., Ohyama K., Bessho T., Yuan B., Yamakawa T. Effect of antioxidants on apoptosis induced by influenza virus infection: inhibition of viral gene replication and transcription with pyrrolidine dithiocarbamate. Antiviral Res. 2002;56:207–217. doi: 10.1016/s0166-3542(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 18.Ghaffari H., Tavakoli A., Moradi A., Tabarraei A., Bokharaei-Salim F., Zahmatkeshan M. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J Biomed Sci. 2019;26:70. doi: 10.1186/s12929-019-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pormohammad A., Monych N.K., Turner R.J. Zinc and SARS-CoV-2: a molecular modeling study of Zn interactions with RNA-dependent RNA-polymerase and 3C-like proteinase enzymes. Int J Mol Med. 2021;47:326–334. doi: 10.3892/ijmm.2020.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel-González M., Talló-Parra M., Herrera-Fernández V., Pérez-Vilaró G., Chillón M., Nogués X., Gómez-Zorrilla S., López-Montesinos I., Villar J., Sorli-Redó M.L., Horcajada J.P., García-Giralt N., Pascual J., Díez J., Vicente R., Güerri-Fernández R. Low zinc levels at clinical admission associates with poor outcomes in COVID-19. medRxiv. 2020 doi: 10.3390/nu13020562. 2020.2010.2007.20208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suara R.O., Crowe J.E., Jr. Effect of zinc salts on respiratory syncytial virus replication. Antimicrob Agents Chemother. 2004;48:783–790. doi: 10.1128/AAC.48.3.783-790.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhutta Z.A., Black R.E., Brown K.H., Gardner J.M., Gore S., Hidayat A. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. Zinc Investigators' Collaborative Group. J Pediatr. 1999;135:689–697. doi: 10.1016/s0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- 24.Prasad A.S., Fitzgerald J.T., Bao B., Beck F.W., Chandrasekar P.H. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2000;133:245–252. doi: 10.7326/0003-4819-133-4-200008150-00006. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari E., Wright-Minogue J., Fang J.W., Baroudy B.M., Lau J.Y., Hong Z. Characterization of soluble hepatitis C virus RNA-dependent RNA polymerase expressed in Escherichia coli. J Virol. 1999;73:1649–1654. doi: 10.1128/jvi.73.2.1649-1654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumura H., Nirei K., Nakamura H., Arakawa Y., Higuchi T., Hayashi J. Zinc supplementation therapy improves the outcome of patients with chronic hepatitis C. J Clin Biochem Nutr. 2012;51:178–184. doi: 10.3164/jcbn.12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuoka S., Matsumura H., Nakamura H., Oshiro S., Arakawa Y., Hayashi J. Zinc supplementation improves the outcome of chronic hepatitis C and liver cirrhosis. J Clin Biochem Nutr. 2009;45:292–303. doi: 10.3164/jcbn.08-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murakami Y., Koyabu T., Kawashima A., Kakibuchi N., Kawakami T., Takaguchi K. Zinc supplementation prevents the increase of transaminase in chronic hepatitis C patients during combination therapy with pegylated interferon alpha-2b and ribavirin. J Nutr Sci Vitaminol (Tokyo) 2007;53:213–218. doi: 10.3177/jnsv.53.213. [DOI] [PubMed] [Google Scholar]
- 29.Cakman I., Kirchner H., Rink L. Zinc supplementation reconstitutes the production of interferon-alpha by leukocytes from elderly persons. J Interferon Cytokine Res. 1997;17:469–472. doi: 10.1089/jir.1997.17.469. [DOI] [PubMed] [Google Scholar]
- 30.Salas M., Kirchner H. Induction of interferon-gamma in human leukocyte cultures stimulated by Zn2+ Clin Immunol Immunopathol. 1987;45:139–142. doi: 10.1016/0090-1229(87)90120-6. [DOI] [PubMed] [Google Scholar]
- 31.Takagi H., Nagamine T., Abe T., Takayama H., Sato K., Otsuka T. Zinc supplementation enhances the response to interferon therapy in patients with chronic hepatitis C. J Viral Hepatitis. 2001;8:367–371. doi: 10.1046/j.1365-2893.2001.00311.x. [DOI] [PubMed] [Google Scholar]
- 32.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenkranz E., Metz C.H., Maywald M., Hilgers R.D., Wessels I., Senff T. Zinc supplementation induces regulatory T cells by inhibition of Sirt-1 deacetylase in mixed lymphocyte cultures. Mol Nutr Food Res. 2016;60:661–671. doi: 10.1002/mnfr.201500524. [DOI] [PubMed] [Google Scholar]
- 35.Lee M.-C., Chen Y.-K., Hsu Y.-J., Lin B.-R. Zinc supplement augments the suppressive effects of repurposed drugs of NF-kappa B inhibitor on ACE2 expression in human lung cell lines in vitro. bioRxiv. 2021 doi: 10.1016/j.lfs.2021.119752. 2021.2001.2027.428372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Sena L.W.P., Mello A., Ferreira M.V.D., de Ataide M.A., Dias R.M., Vieira J.L.F. Doses of chloroquine in the treatment of malaria by Plasmodium vivax in patients between 2 and 14 years of age from the Brazilian Amazon basin. Malar J. 2019;18:439. doi: 10.1186/s12936-019-3072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centor R.M., Kim A.H., Sparks J.A. Annals on call - COVID-19: is chloroquine the answer? Ann Intern Med. 2020;172 doi: 10.7326/A20-0003. [DOI] [PubMed] [Google Scholar]
- 38.Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;105938 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferner R.E., Aronson J.K. Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020;369 doi: 10.1136/bmj.m1432. [DOI] [PubMed] [Google Scholar]
- 40.Huang M., Tang T., Pang P., Li M., Ma R., Lu J. Treating COVID-19 with chloroquine. J Mol Cell Biol. 2020 doi: 10.1093/jmcb/mjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinkeler F.S., Berger F.A., Muntinga H.J., Jansen M. The risk of QTc-interval prolongation in COVID-19 patients treated with chloroquine. Neth Heart J. 2020;28:418–423. doi: 10.1007/s12471-020-01462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Offerhaus J.A., Wilde A.A.M., Remme C.A. Prophylactic (hydroxy)chloroquine in COVID-19: potential relevance for cardiac arrhythmia risk. Heart Rhythm. 2020;17:1480–1486. doi: 10.1016/j.hrthm.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Javelot H., El-Hage W., Meyer G., Becker G., Michel B., Hingray C. COVID-19 and (hydroxy)chloroquine-azithromycin combination: should we take the risk for our patients? Br J Clin Pharmacol. 2020;86:1176–1177. doi: 10.1111/bcp.14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grewal S., Jankelson L., van den Broek M.P.H., Cour M., Bachmann G., Kostis J.B. QTc prolongation risk evaluation in female COVID-19 patients undergoing chloroquine and hydroxychloroquine with/without azithromycin treatment. Front Cardiovasc Med. 2020;7:152. doi: 10.3389/fcvm.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Decloedt E.H., Reuter H., Allwood B., Parker A., Koegelenberg C.F.N., Blockman M. Benefit v. risk when using chloroquine in patients with severe COVID-19 disease. S Afr Med J. 2020;110:12903. doi: 10.7196/SAMJ.2020.v110i5.14761. [DOI] [PubMed] [Google Scholar]
- 46.Xue J., Moyer A., Peng B., Wu J., Hannafon B.N., Ding W.Q. Chloroquine is a zinc ionophore. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlucci P, Ahuja T, Petrilli CM, Rajagopalan H, Jones S, Rahimian J, Hydroxychloroquine and azithromycin plus zinc vs hydroxychloroquine and azithromycin alone: outcomes in hospitalized COVID-19 patients, medRxiv, (2020) 2020.2005.2002.20080036.
- 48.Pal A., Pawar A., Goswami K., Sharma P., Prasad R. Hydroxychloroquine and Covid-19: a cellular and molecular biology based update. Indian J Clin Biochem. 2020;35:274–284. doi: 10.1007/s12291-020-00900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Megarbane B. Chloroquine and hydroxychloroquine to treat COVID-19: between hope and caution. Clin Toxicol (Phila) 2021;59:70–71. doi: 10.1080/15563650.2020.1748194. [DOI] [PubMed] [Google Scholar]
- 50.Abdulrahman A., AlSayed I., AlMadhi M., AlArayed J., Mohammed S.J., Sharif A.K. The efficacy and safety of hydroxychloroquine in patients with COVID-19: a multicenter national retrospective cohort. Infect Dis Ther. 2021 doi: 10.1007/s40121-021-00397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee Z., Rayner C.R., Forrest J.I., Nachega J.B., Senchaudhuri E., Mills E.J. The rise and fall of hydroxychloroquine for the treatment and prevention of COVID-19. Am J Trop Med Hyg. 2021;104:35–38. doi: 10.4269/ajtmh.20-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez J., Roustit M., Lepelley M., Revol B., Cracowski J.L., Khouri C. Reported adverse drug reactions associated with the use of hydroxychloroquine and chloroquine during the COVID-19 pandemic. Ann Intern Med. 2021 doi: 10.7326/M20-7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang CW, Lee MC, Lin BR, Lu YP, Hsu YJ, Chuang CY, Huang TT, Chen YK, Azithromycin Plus Zinc Sulfate Rapidly and Synergistically Suppresses IκBα-Mediated In Vitro Human Airway Cell ACE2 Expression for SARS-CoV-2 Entry, bioRxiv, (2021) 2021.2001.2019.427206.
- 54.Razzaque M.S. Exacerbation of antimicrobial resistance: another casualty of the COVID-19 pandemic? Expert Rev Anti Infect Ther. 2020 doi: 10.1080/14787210.2021.1865802. [DOI] [PubMed] [Google Scholar]
- 55.Razzaque M.S. Commentary: microbial resistance movements: an overview of global public health threats posed by antimicrobial resistance, and how best to counter. Front Public Health. 2020;8:629120. doi: 10.3389/fpubh.2020.629120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Razzaque M.S. Implementation of antimicrobial stewardship to reduce antimicrobial drug resistance. Expert Rev Anti Infect Ther. 2020 doi: 10.1080/14787210.2021.1840977. [DOI] [PubMed] [Google Scholar]
- 57.Kodama H., Tanaka M., Naito Y., Katayama K., Moriyama M. Japan’s practical guidelines for zinc deficiency with a particular focus on taste disorders, inflammatory bowel disease, and liver cirrhosis. Int J Mol Sci. 2020;21:2941. doi: 10.3390/ijms21082941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roohani N., Hurrell R., Kelishadi R., Schulin R. Zinc and its importance for human health: an integrative review. J Res Med Sci. 2013;18:144–157. [PMC free article] [PubMed] [Google Scholar]
- 59.Hess SY, Peerson JM, King JC, Brown KH, Use of Serum Zinc Concentration as an Indicator of Population Zinc Status, Food and Nutrition Bulletin, 28 (2007) S403-S429. [DOI] [PubMed]
- 60.Maares M., Haase H. Zinc and immunity: an essential interrelation. Arch Biochem Biophys. 2016;611:58–65. doi: 10.1016/j.abb.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 61.Field C.J., Johnson I.R., Schley P.D. Nutrients and their role in host resistance to infection. J Leukoc Biol. 2002;71:16–32. [PubMed] [Google Scholar]
- 62.Overbeck S., Uciechowski P., Ackland M.L., Ford D., Rink L. Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J Leukoc Biol. 2008;83:368–380. doi: 10.1189/jlb.0307148. [DOI] [PubMed] [Google Scholar]
- 63.Fraker PJ, King LE, Laakko T, Vollmer TL, The dynamic link between the integrity of the immune system and zinc status, J Nutr, 130 (2000) 1399S-1406S. [DOI] [PubMed]
- 64.Prasad A.S. Zinc: mechanisms of host defense. J Nutr. 2007;137:1345–1349. doi: 10.1093/jn/137.5.1345. [DOI] [PubMed] [Google Scholar]
- 65.Telford W.G., Fraker P.J. Preferential induction of apoptosis in mouse CD4+CD8+ alpha beta TCRloCD3 epsilon lo thymocytes by zinc. J Cell Physiol. 1995;164:259–270. doi: 10.1002/jcp.1041640206. [DOI] [PubMed] [Google Scholar]
- 66.Fraker P.J., Telford W.G. A reappraisal of the role of zinc in life and death decisions of cells. Proc Soc Exp Biol Med. 1997;215:229–236. doi: 10.3181/00379727-215-44132. [DOI] [PubMed] [Google Scholar]
- 67.Sazawal S., Jalla S., Mazumder S., Sinha A., Black R.E., Bhan M.K. Effect of zinc supplementation on cell-mediated immunity and lymphocyte subsets in preschool children. Indian Pediatr. 1997;34:589–597. [PubMed] [Google Scholar]
- 68.Shankar AH, Prasad AS, Zinc and immune function: the biological basis of altered resistance to infection, Am J Clin Nutr, 68 (1998) 447S-463S. [DOI] [PubMed]
- 69.DePasquale-Jardieu P., Fraker P.J. Interference in the development of a secondary immune response in mice by zinc deprivation: persistence of effects. J Nutr. 1984;114:1762–1769. doi: 10.1093/jn/114.10.1762. [DOI] [PubMed] [Google Scholar]
- 70.Fraker P.J., Caruso R., Kierszenbaum F. Alteration of the immune and nutritional status of mice by synergy between zinc deficiency and infection with Trypanosoma cruzi. J Nutr. 1982;112:1224–1229. doi: 10.1093/jn/112.6.1224. [DOI] [PubMed] [Google Scholar]
- 71.Fraker P.J., Hildebrandt K., Luecke R.W. Alteration of antibody-mediated responses of suckling mice to T-cell-dependent and independent antigens by maternal marginal zinc deficiency: restoration of responsivity by nutritional repletion. J Nutr. 1984;114:170–179. doi: 10.1093/jn/114.1.170. [DOI] [PubMed] [Google Scholar]
- 72.Lee G.R. The balance of Th17 versus treg cells in autoimmunity. Int J Mol Sci. 2018;19:730. doi: 10.3390/ijms19030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kulik L., Maywald M., Kloubert V., Wessels I., Rink L. Zinc deficiency drives Th17 polarization and promotes loss of Treg cell function. J Nutr Biochem. 2019;63:11–18. doi: 10.1016/j.jnutbio.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 74.Kitabayashi C., Fukada T., Kanamoto M., Ohashi W., Hojyo S., Atsumi T. Zinc suppresses Th17 development via inhibition of STAT3 activation. Int Immunol. 2010;22:375–386. doi: 10.1093/intimm/dxq017. [DOI] [PubMed] [Google Scholar]
- 75.Kido T., Ishiwata K., Suka M., Yanagisawa H. Inflammatory response under zinc deficiency is exacerbated by dysfunction of the T helper type 2 lymphocyte-M2 macrophage pathway. Immunology. 2019;156:356–372. doi: 10.1111/imm.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hojyo S, Fukada T, Roles of Zinc Signaling in the Immune System, J Immunol Res, 2016 (2016) 6762343-6762343. [DOI] [PMC free article] [PubMed]
- 77.Liu J., Li S., Liu J., Liang B., Wang X., Wang H. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen X, Ling J, Mo P, Zhang Y, Jiang Q, Ma Z, et al., Restoration of leukomonocyte counts is associated with viral clearance in COVID-19 hospitalized patients, medRxiv, (2020) 2020.2003.2003.20030437.
- 79.Briefel RR, Bialostosky K, Kennedy-Stephenson J, McDowell MA, Ervin RB, Wright JD, Zinc intake of the U.S. population: findings from the third National Health and Nutrition Examination Survey, 1988-1994, J Nutr, 130 (2000) 1367S-1373S. [DOI] [PubMed]
- 80.Ervin R.B., Kennedy-Stephenson J. Mineral intakes of elderly adult supplement and non-supplement users in the third national health and nutrition examination survey. J Nutr. 2002;132:3422–3427. doi: 10.1093/jn/132.11.3422. [DOI] [PubMed] [Google Scholar]
- 81.Haase H., Hebel S., Engelhardt G., Rink L. Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells. Anal Biochem. 2006;352:222–230. doi: 10.1016/j.ab.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 82.Mocchegiani E., Costarelli L., Giacconi R., Cipriano C., Muti E., Tesei S. Nutrient-gene interaction in ageing and successful ageing. A single nutrient (zinc) and some target genes related to inflammatory/immune response. Mech Ageing Dev. 2006;127:517–525. doi: 10.1016/j.mad.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 83.Mocchegiani E., Rink L., Blasco M. Zinc and ageing (ZINCAGE Project) Biogerontology. 2006;7:305–306. doi: 10.1007/s10522-006-9044-8. [DOI] [PubMed] [Google Scholar]
- 84.Gorodetsky R., Sheskin J., Weinreb A. Iron, copper, and zinc concentrations in normal skin and in various nonmalignant and malignant lesions. Int J Dermatol. 1986;25:440–445. doi: 10.1111/j.1365-4362.1986.tb03449.x. [DOI] [PubMed] [Google Scholar]
- 85.Oon B.B., Khong K.Y., Greaves M.W., Plummer V.M. Trophic skin ulceration of leprosy: skin and serum zinc concentrations. Br Med J. 1974;2:531–533. doi: 10.1136/bmj.2.5918.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ej D., Vm P., Mw G. Venous leg ulceration: skin and serum zinc concentrations. Acta Dermato-venereologica. 1975;55:497. [PubMed] [Google Scholar]
- 87.Braun L.A., Rosenfeldt F. Pharmaco-nutrient interactions - a systematic review of zinc and antihypertensive therapy. Int J Clin Pract. 2013;67:717–725. doi: 10.1111/ijcp.12040. [DOI] [PubMed] [Google Scholar]
- 88.Prasad A.S. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14:353–357. doi: 10.2119/2008-00033.Prasad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prasad A.S., Beck F.W., Bao B., Fitzgerald J.T., Snell D.C., Steinberg J.D. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85:837–844. doi: 10.1093/ajcn/85.3.837. [DOI] [PubMed] [Google Scholar]
- 90.Armitage R., Nellums L.B. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5:e256. doi: 10.1016/S2468-2667(20)30061-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kunz R., Minder M. COVID-19 pandemic: palliative care for elderly and frail patients at home and in residential and nursing homes. Swiss Med Wkly. 2020;150 doi: 10.4414/smw.2020.20235. [DOI] [PubMed] [Google Scholar]
- 92.Anderson R.A., Roussel A.M., Zouari N., Mahjoub S., Matheau J.M., Kerkeni A. Potential antioxidant effects of zinc and chromium supplementation in people with type 2 diabetes mellitus. J Am Coll Nutr. 2001;20:212–218. doi: 10.1080/07315724.2001.10719034. [DOI] [PubMed] [Google Scholar]
- 93.Acevedo-Murillo J.A., Garcia Leon M.L., Firo-Reyes V., Santiago-Cordova J.L., Gonzalez-Rodriguez A.P., Wong-Chew R.M. Zinc supplementation promotes a Th1 response and improves clinical symptoms in fewer hours in children with pneumonia younger than 5 years old. A randomized controlled clinical trial. Front Pediatr. 2019;7:431. doi: 10.3389/fped.2019.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heller R.A., Sun Q., Hackler J., Seelig J., Seibert L., Cherkezov A. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021;38 doi: 10.1016/j.redox.2020.101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: a report on four patients. Int J Infect Dis. 2020;99:307–309. doi: 10.1016/j.ijid.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jothimani D., Kailasam E., Danielraj S., Nallathambi B., Ramachandran H., Sekar P. COVID-19: poor outcomes in patients with zinc deficiency. Int J Infect Dis. 2020;100:343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elalfy H., Besheer T., El-Mesery A., El-Gilany A.H., Abd Elazez M.S., Alhawarey A. Effect of a combination of Nitazoxanide, Ribavirin and Ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. J Med Virol. 2021 doi: 10.1002/jmv.26880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frontera J.A., Rahimian J.O., Yaghi S., Liu M., Lewis A., de Havenon A. Treatment with zinc is associated with reduced in-hospital mortality among COVID-19 patients: a multi-center cohort study. Res Square. 2020 doi: 10.21203/rs.3.rs-94509/v1. [DOI] [Google Scholar]
- 99.Thomas S, Patel D, Bittel B, Wolski K, Wang Q, Kumar A et al., Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction Among Ambulatory Patients With SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial, JAMA Network Open, 4 (2021) e210369-e210369. [DOI] [PMC free article] [PubMed]
- 100.Yao J.S., Paguio J.A., Dee E.C., Tan H.C., Moulick A., Milazzo C. The minimal effect of zinc on the survival of hospitalized patients with COVID-19: an observational study. Chest. 2020;159:108–111. doi: 10.1016/j.chest.2020.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fosmire G.J. Zinc toxicity. Am J Clin Nutr. 1990;51:225–227. doi: 10.1093/ajcn/51.2.225. [DOI] [PubMed] [Google Scholar]
- 102.Richards C.D., Burke R. Local and systemic effects of targeted zinc redistribution in Drosophila neuronal and gastrointestinal tissues. Biometals. 2015;28:967–974. doi: 10.1007/s10534-015-9881-5. [DOI] [PubMed] [Google Scholar]
- 103.Razzaque M. COVID-19 pandemic: can boosting immune responses by maintaining adequate nutritional balance reduce viral insults? Adv Hum Biol. 2020;10:99–102. doi: 10.4103/AIHB.AIHB_75_20. [DOI] [Google Scholar]
- 104.Quiles J.L., Rivas-García L., Varela-López A., Llopis J., Battino M., Sánchez-González C. Do nutrients and other bioactive molecules from foods have anything to say in the treatment against COVID-19? Environ Res. 2020;191 doi: 10.1016/j.envres.2020.110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abukabda A.B., Razzaque M.S. COVID-19 pandemic: impacts of social lockdown on nutritional health and beyond. Adv Hum Biol. 2021;11:3–7. doi: 10.4103/aihb.aihb_130_20. [DOI] [Google Scholar]
- 106.Zabetakis I., Lordan R., Norton C., Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12:1466. doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Galmés S., Serra F., Palou A. Current state of evidence: influence of nutritional and nutrigenetic factors on immunity in the COVID-19 pandemic framework. Nutrients. 2020;12:2738. doi: 10.3390/nu12092738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Calder P.C., Carr A.C., Gombart A.F., Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12:1181. doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stachowska E., Folwarski M., Jamioł-Milc D., Maciejewska D., Skonieczna-Żydecka K. Nutritional support in coronavirus 2019 disease. Medicina (Kaunas) 2020;56:289. doi: 10.3390/medicina56060289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hakeem R., Sheikh M.A. Beyond transmission: dire need for integration of nutrition interventions in COVID-19 pandemic-response strategies in Developing Countries like Pakistan. Pak J Med Sci. 2020;36:S85–s89. doi: 10.12669/pjms.36.COVID19-S4.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I. Zinc and respiratory tract infections: perspectives for COVID-19 (Review) Int J Mol Med. 2020;46:17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rahman M.T., Idid S.Z. Can Zn be a critical element in COVID-19 treatment? Biol Trace Elem Res. 2021;199:550–558. doi: 10.1007/s12011-020-02194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mossink J.P. Zinc as nutritional intervention and prevention measure for COVID-19 disease. BMJ Nutr Prev Health. 2020;3:111–117. doi: 10.1136/bmjnph-2020-000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McPherson S.W., Keunen J.E., Bird A.C., Chew E.Y., van Kuijk F.J. Investigate oral zinc as a prophylactic treatment for those at risk for COVID-19. Am J Ophthalmol. 2020;216:A5–a6. doi: 10.1016/j.ajo.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Razzaque M.S. COVID-19 pandemic: can maintaining optimal zinc balance enhance host resistance? Tohoku J Exp Med. 2020;251:175–181. doi: 10.1620/tjem.251.175. [DOI] [PubMed] [Google Scholar]
- 116.Arentz S., Hunter J., Yang G., Goldenberg J., Beardsley J., Myers S.P. Zinc for the prevention and treatment of SARS-CoV-2 and other acute viral respiratory infections: a rapid review. Adv Integr Med. 2020;7:252–260. doi: 10.1016/j.aimed.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee Y.H., Bang E.S., Lee J.H., Lee J.D., Kang D.R., Hong J. Serum concentrations of trace elements zinc, copper, selenium, and manganese in critically Ill patients. Biol Trace Elem Res. 2019;188:316–325. doi: 10.1007/s12011-018-1429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pal A., Squitti R., Picozza M., Pawar A., Rongioletti M., Dutta A.K. Zinc and COVID-19: basis of current clinical trials. Biol Trace Elem Res. 2020:1–11. doi: 10.1007/s12011-020-02437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dabbagh-Bazarbachi H., Clergeaud G., Quesada I.M., Ortiz M., O'Sullivan C.K., Fernández-Larrea J.B. Zinc ionophore activity of quercetin and epigallocatechin-gallate: from Hepa 1–6 cells to a liposome model. J Agric Food Chem. 2014;62:8085–8093. doi: 10.1021/jf5014633. [DOI] [PubMed] [Google Scholar]
- 120.Pawar A., Pal A. Molecular and functional resemblance of dexamethasone and quercetin: a paradigm worth exploring in dexamethasone-nonresponsive COVID-19 patients. Phytother Res. 2020;34:3085–3088. doi: 10.1002/ptr.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bonaventura P., Benedetti G., Albarède F., Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015;14:277–285. doi: 10.1016/j.autrev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 122.Beyersmann D. Homeostasis and cellular functions of zinc. Materialwiss Werkstofftech. 2002;33:764–769. [Google Scholar]