Abstract
Objective
To determine the association between high flow supplementary oxygen and 30 day mortality in patients presenting with a suspected acute coronary syndrome (ACS).
Design
Pragmatic, cluster randomised, crossover trial.
Setting
Four geographical regions in New Zealand.
Participants
40 872 patients with suspected or confirmed ACS included in the All New Zealand Acute Coronary Syndrome Quality Improvement registry or ambulance ACS pathway during the study periods. 20 304 patients were managed using the high oxygen protocol and 20 568 were managed using the low oxygen protocol. Final diagnosis of ST elevation myocardial infarction (STEMI) and non-STEMI were determined from the registry and ICD-10 discharge codes.
Interventions
The four geographical regions were randomly allocated to each of two oxygen protocols in six month blocks over two years. The high oxygen protocol recommended oxygen at 6-8 L/min by face mask for ischaemic symptoms or electrocardiographic changes, irrespective of the transcapillary oxygen saturation (SpO2). The low oxygen protocol recommended oxygen only if SpO2 was less than 90%, with a target SpO2 of less than 95%.
Main outcome measure
30 day all cause mortality determined from linkage to administrative data.
Results
Personal and clinical characteristics of patients managed under both oxygen protocols were well matched. For patients with suspected ACS, 30 day mortality for the high and low oxygen groups was 613 (3.0%) and 642 (3.1%), respectively (odds ratio 0.97, 95% confidence interval 0.86 to 1.08). For 4159 (10%) patients with STEMI, 30 day mortality for the high and low oxygen groups was 8.8% (n=178) and 10.6% (n=225), respectively (0.81, 0.66 to 1.00) and for 10 218 (25%) patients with non-STEMI was 3.6% (n=187) and 3.5% (n=176), respectively (1.05, 0.85 to 1.29).
Conclusion
In a large patient cohort presenting with suspected ACS, high flow oxygen was not associated with an increase or decrease in 30 day mortality.
Trial registration
ANZ Clinical Trials ACTRN12616000461493.
Introduction
Oxygen has been given to patients with acute myocardial infarction for more than 50 years, despite limited evidence that this improves outcomes.1 Supplementary oxygen can correct or reduce hypoxaemia, which is common in patients with an acute coronary syndrome (ACS). Some evidence, however, suggests that arterial oxygen saturation levels above normal could be harmful by causing coronary vasoconstriction or increasing oxidative stress.1 2 The possibility of harm from supplementary oxygen was supported by a meta-analysis of clinical trials that compared liberal with conservative oxygen strategies in predominantly normoxaemic patients with a range of critical medical conditions.3 The only previous large, randomised trial in patients with suspected myocardial infarction, the DETermination of the role of Oxygen in suspected Acute Myocardial Infarction (DETO2X-AMI), reported no difference in one year mortality in patients given 12 hours of high flow oxygen compared with limited oxygen.4 Current clinical practice guidelines recommend that oxygen should not be given to non-hypoxaemic patients with ST elevation myocardial infarction (STEMI) or non-STEMI.5 6 7 8 9
The available evidence, however, has limitations. Firstly, patients included in randomised trials generally have a lower risk and might not be representative of all patients managed in usual care. Secondly, all previous trials, including DETO2X-AMI, had insufficient power to identify a small but clinically relevant 1-2% absolute difference in mortality with oxygen treatment. Thirdly, most patients included in previous trials had normal oxygen saturation levels. A benefit from oxygen might require the presence of hypoxaemia. The saturation threshold for starting oxygen and the target oxygen saturation level when oxygen is being administered, are uncertain. Fourthly, it is possible that the effects of oxygen depend on the diagnosis—for example, patients with an acute STEMI typically have prolonged and severe myocardial ischaemia and might show greater benefit than those with other conditions.
Recommendations for treating hypoxaemia would be better informed if evidence was clear on whether high flow oxygen is associated with harm, suggesting that high oxygen saturation (SpO2) needs to be avoided, or showed benefit from giving oxygen to correct modest reductions in SpO2. To determine whether and when oxygen might be indicated in patients with a suspected ACS we undertook a pragmatic, randomised comparison of high oxygen versus low oxygen delivery strategies as part of usual care in a large cohort of patients presenting to ambulances and acute cardiac care units thoughout New Zealand over two years.
Methods
The study population included all patients with a suspected ACS in two national registries during the defined study periods: the All New Zealand Acute Coronary Syndrome Quality Improvement (ANZACS-QI) registry, which includes about 98% of patients admitted to hospital in New Zealand with ACS who have coronary angiography with or without coronary revascularisation,10 11 and the ambulance ACS pathway, which includes all patients with suspected ACS managed by the St John Ambulance service, which covers about 90% of the New Zealand population. For patients with multiple ACS assessments, we included only the first presentation during the study.
Oxygen protocols
The high oxygen protocol recommended that patients with ischaemic chest pain or dyspnoea and ischaemic electrocardiographic changes should receive oxygen with a flow of 6-8 L/min by face mask or 4 L/min by nasal cannula, irrespective of SpO2. The oxygen flow rate could be increased if needed to achieve an oxygen saturation of greater than 95%. It was recommended to continue oxygen in the ambulance until hospital admission, and in hospital until clinical evidence that myocardial ischaemia had resolved. In the low oxygen protocol, oxygen was recommended only for patients with ischaemic chest pain or dyspnoea with electrocardiographic changes if SpO2 was less than 90%, with the flow rate adjusted to maintain SpO2 between 90% and 94% (see supplementary appendix for full protocols). SpO2 was routinely monitored using pulse oximeters.
The study aims and oxygen protocols were discussed with the doctors, nurses, and paramedics who care for patients with suspected or proven ACS across all geographical regions in New Zealand. Posters were displayed on walls, and reminder stickers on monitors in ambulances, emergency departments, acute cardiac care units, and cardiac catheterisation laboratories.
Cluster crossover design
This was a pragmatic, two arm, cluster randomised, crossover trial. At any one time the same oxygen protocol was used by all ambulances and hospitals across the pathway of care within each of the four New Zealand regions (northern, midlands, central, and southern). These geographical regions were chosen as the clusters because transfers between hospitals during an episode of ACS care occur within these regions. The independent study statistician used computer generated random numbers to allocate the four regions to the order of oxygen use, with two regions randomised to each oxygen protocol in each crossover period. The northern and southern regions started with the high oxygen protocol, and the midland and central regions started with the low oxygen protocol, for seven months from 11 July 2016 to 7 February 2017. The oxygen protocols were then switched for the next 12 months (8 February 2017 to 13 February 2018) and changed back to the original protocols for the last five months (14 February 2018 to 10 July 2018). The crossover dates were delayed from January to February to avoid the national summer holidays, which resulted in more patients being included in the first compared with the last study period. Thus each hospital and ambulance service used both the high and the low oxygen protocols for about one calendar year, and therefore during similar seasons. To minimise practical challenges related to changing protocols used as part of routine care, we limited the number of crossovers to two. In this way, two rather than one crossover better accounted for possible temporal trends not related to the oxygen protocols. To ensure familiarisation, we introduced protocols one to three months before the start of the study. Clinicians and paramedics were informed less than five days before each protocol change to reduce the risk of early switching. The analysis plan prespecified that patients admitted during the first two weeks after each protocol crossover were to be excluded, because protocol adherence might have been less during this time.
Clinical and personal characteristics of study population
New Zealand residents have a unique national health identifier number. For study participants, we linked the encrypted national health identifier to the matched encrypted national health identifier in New Zealand administrative data to determine all cause mortality up to 2 August 2019 with no known missing data. Demographic data and all hospital admissions with discharge diagnoses based on ICD-10 codes (international classification of diseases, 10th revision) obtained from the National Minimum Dataset included age, sex, ethnicity (New Zealand European, Māori, Pacific Islander, Asian, other), and socioeconomic status from the New Zealand deprivation index 2013.12 Previous myocardial infarction, previous heart failure, and Charlson comorbidity index score,13 excluding myocardial infarction and heart failure, were also determined from ICD-10 codes for the past five years. Current smoking status, diabetes, and Killip class for heart failure on admission were only available for patients in the ANZACS-QI registry.
Clinical staff recorded the discharge diagnosis for patients in the ANZACS-QI registry.10 11 For patients managed using the ambulance ACS pathway, the incidence date was used to identify the relevant hospital admission in the national hospital admission records, and discharge diagnosis from ICD-10 codes used to identify the type of ACS. Patients with an unknown diagnosis did not have an ICD-10 discharge code. This group included patients discharged from hospital within three hours and those transferred to healthcare centres or hospitals that do not routinely care for patients with ACS.
Study end points
The primary outcome was all cause mortality 30 days after first presentation with suspected ACS. The prespecified subgroup analyses were 30 day mortality in patients with a final diagnosis of STEMI and non-STEMI. Secondary outcomes were one year all cause mortality, and length of hospital stay for patients discharged alive at the index admission. The 30 day cause specific mortality was assessed for those patients admitted by November 2017, because of data availability at the end of the study.
Audit of protocol adherence
A research nurse completed the audit form for 779 of 870 (89.5%) patients in the ANZACS-QI registry with ACS admitted on 28 randomly allocated days throughout the study (about one day each month). This form recorded whether oxygen was used, the presence of ischaemic symptoms or dynamic electrocardiographic changes, SpO2 before starting and when receiving oxygen, and contraindications to oxygen use in each of four locations: ambulance, emergency department, cardiac cathetisation laboratory, and acute cardiac care unit. The duration of oxygen use was not recorded.
For most participants from the ambulance ACS pathway, oxygen usage, the first SpO2 measured after arrival by ambulance, and the last SpO2 recorded in the ambulance were documented.
Statistical analysis
We chose a two year trial for pragmatic reasons. Based on historical registry records, we estimated that a study with nearly 30 000 patients would have 80% power at a 5% level of significance (two sided) to detect a 20% relative and 0.6% absolute difference in all cause 30 day mortality between the two oxygen protocols, assuming a 30 day mortality of 3% for patients with suspected ACS. Historical data suggested that the intracluster correlation coefficient was small (<0.0001). With only four regional networks serving as the clusters, the power calculation did not account for the cluster effect, which was considered as a fixed effect in final analysis. We estimated the potential cluster effect using random effects mixed model, and the estimated intracluster correlation coefficient was consistent with the original trial design.
An intention-to-treat analysis was performed for all eligible patients identified from the two trial registries, irrespective of oxygen received. Baseline characteristics and clinical outcomes of patients were first summarised descriptively for each treatment period. We compared the primary outcome (30 day all cause mortality) between the two oxygen protocols using a generalised linear regression model with a binomial distribution and logit link. Both unadjusted and adjusted analyses were considered to evaluate potential confounding effects of individual baseline characteristics associated with mortality. The regression model adjusted for the several prespecified baseline variables: region (northern, midland, central, southern), ACS type (STEMI, non-STEMI, unstable angina, not ACS, diagnosis not classified), age at admission (in years), sex, ethnicity (Māori, Pacific Islander, Asian, New Zealand European, or other), socioeconomic status (New Zealand deprivation 10th), and season. For the primary analysis, complete data were available for vital status and baseline variables, except for socioeconomic status, which was not known in 286 (0.7%) patients as part of the national data collections. No imputation for missing data was performed.
Unadjusted and adjusted odds ratios are reported with corresponding 95% confidence intervals and associated P values. An odds ratio of less than 1 indicates a lower risk with the high oxygen protocol compared with the low oxygen protocol. We also estimated relative risks using Poisson regression models with robust error estimates. Cardiovascular disease as the cause of death was only available to the end of 2017 and is reported on 30 days after first presentation for patients admitted to hospital by November 2017.
We used the same regression model on one year mortality. The Kaplan-Meier plot is presented for all cause mortality to illustrate deaths by duration of follow-up, together with the unadjusted and adjusted hazard ratios and 95% confidence intervals using Cox proportional hazard models.
Prespecified subgroup analysis explored the consistency of treatment effects in patients with a final diagnosis of STEMI and non-STEMI, and in post hoc analyses for patients with other ACS disgnoses, using the same method as for the primary outcome measure. We tested the interaction effect between oxygen protocol and ACS diagnosis in the main model by adding an interaction term between treatment group and ACS type.
The ambulance ACS pathway was implemented just before starting the trial, so data from this source were not available at the time of the trial design. Post hoc subgroup analyses were conducted for patients managed in the ambulance ACS pathway only with first SpO2 recorded, including their baseline SpO2 level in the adjusted models to explore its impact on mortality outcomes and potential interaction with the treatment effect.
For patients discharged alive from hospital, we reported length of hospital stay as mean (standard deviation) and median (interquartile range) days in hospital. The mean difference in length of stay and 95% confidence interval for patients managed under the high and low oxygen protocols are also reported.
Statistical tests are two sided at a 5% significance level. Statistical analysis was conducted using SAS version 9.4 (SAS Institute, Cary, NC). No interim efficacy analysis was conducted during the trial.
Patient and public involvement
The study was considered by research review committees and Māori health advisory committees at district health boards throughout New Zealand, and for the two national ambulance services, each of which included community representation.
Results
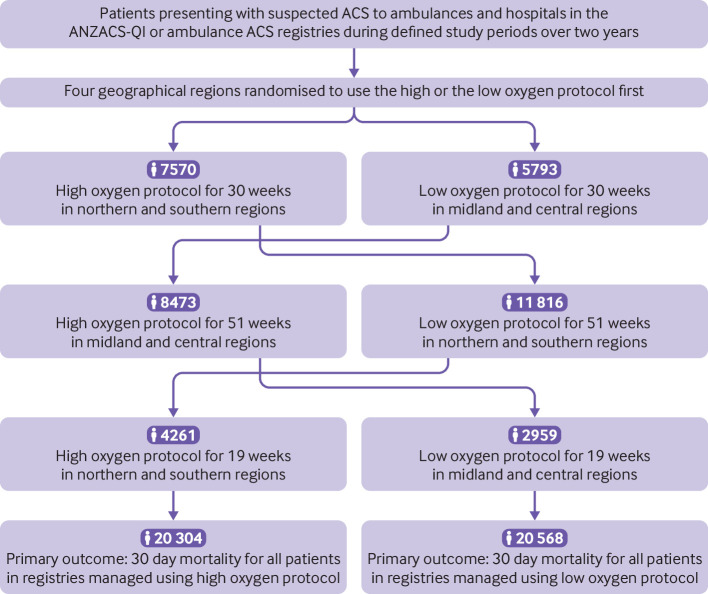
A total of 40 872 patients with suspected or confirmed ACS were enrolled: 20 304 were managed using the high oxygen protocol and 20 568 were managed using the low oxygen protocol (fig 1). The study cohort included patients in the ANZACS-QI registry (high oxygen: n=9726; low oxygen: n=9840) and patients on the ambulance ACS pathway who were not in the ANZACS-QI registry (high oxygen: n=10 578; low oxygen: n=10 728). Personal and clinical characteristics of patients managed using the two protocols were well matched (table 1).
Fig 1.
Study flow diagram. ACS=acute coronary syndrome; ANZACS-QI=All New Zealand Acute Coronary Syndrome Quality Improvement registry
Table 1.
Baseline characteristics of study population with a suspected acute coronary syndrome according to oxygen protocol. Values are numbers (percentages) unless stated otherwise
| Characteristics | High oxygen (n=20 304) | Low oxygen (n=20 568) |
|---|---|---|
| Personal characteristics | ||
| Mean (SD) age (years) | 66.5 (14.4) | 66.4 (14.6) |
| Men | 11 777 (58.0) | 11 943 (58.1) |
| Ethnic group: | ||
| Māori | 2298 (11.3) | 2370 (11.5) |
| Pacific Islander | 919 (4.5) | 958 (4.7) |
| Asian | 1404 (6.9) | 1495 (7.3) |
| New Zealand European* | 15 683 (77.2) | 15 745 (76.6) |
| Clinical characteristics | ||
| Previous myocardial infarction | 2343 (11.5) | 2349 (11.4) |
| Previous heart failure | 1723 (8.5) | 1741 (8.5) |
| Comorbidity index score: | ||
| 0 | 15 373 (75.7) | 15 648 (76.1) |
| 1 or 2 | 3812 (18.8) | 3854 (18.7) |
| 3† | 1119 (5.5) | 1066 (5.2) |
| Current smoker‡ | 1661 (17.1) | 1725 (17.5) |
| Diabetes‡ | 2283 (23.5) | 2323 (23.6) |
| Killip class‡§: | ||
| 1 | 8632 (88.7) | 8787 (89.3) |
| 2 | 770 (7.9) | 724 (7.4) |
| 3 or 4 | 324 (3.3) | 328 (3.3) |
| Socioeconomic fifth†: | ||
| 1 (least deprived) | 3234 (15.9) | 3203 (15.6) |
| 2 | 3512 (17.3) | 3554 (17.3) |
| 3 | 3953 (19.5) | 4034 (19.6) |
| 4 | 4717 (23.2) | 4770 (23.2) |
| 5 (most deprived) | 4753 (23.4) | 4856 (23.6) |
| Not recorded | 135 (0.7) | 151 (0.7) |
| Geographical region: | ||
| Northern | 6913 (34.1) | 7109 (34.6) |
| Midland | 4815 (23.7) | 4916 (23.9) |
| Central | 3658 (18.0) | 3836 (18.7) |
| Southern | 4918 (24.2) | 4707 (22.9) |
| Final diagnosis: | ||
| STEMI | 2031 (10.0) | 2128 (10.4) |
| Non-STEMI | 5152 (25.4) | 5066 (24.6) |
| Unstable angina | 1606 (7.9) | 1678 (8.2) |
| Non-ACS condition | 9681 (47.7) | 9838 (47.8) |
| Not classified | 1834 (9.0) | 1858 (9.0) |
STEMI=ST elevation myocardial infarction; NSTEMI=non-ST elevation myocardial infarction; ACS=acute coronary syndrome.
Other ethnic groups were <2% of the study population and are included in the New Zealand European group.
Socioeconomic status determined from New Zealand deprivation index 2013 census data in fifths of the New Zealand general population (data were missing for 286 (0.7%) patients).
Data on hospital admission were recorded only for patients in All New Zealand Acute Coronary Syndrome Quality Improvement registry (high oxygen protocol: n=9726; low oxygen protocol: n=9840).
Killip class refers to clinical evidence of heart failure, 1=no heart failure, 2=rales or raised jugular venous pressure, 3=pulmonary oedema, 4=cardiogenic shock.
Outcomes for patients with suspected ACS
For patients with suspected ACS, 30 day mortality associated with the high oxygen protocol was 3.0% (n=613) and for the low oxygen protocol was 3.1% (n=642). The unadjusted odds ratio was 0.97 (95% confidence interval 0.86 to 1.08) and the adjusted odds ratio was 0.96 (0.86 to 1.08; table 2). Because the mortality rate was low, the results were similar when expressed as a relative risk. The unadjusted relative risk was 0.97 (95% confidence interval 0.87 to 1.08) and the adjusted relative risk was 0.96 (0.87 to 1.07).
Table 2.
All cause mortality by oxygen protocol. Values are numbers (percentages) unless stated otherwise
| High oxygen | Low oxygen | Unadjused odds ratio (95% CI) | P value | Adjusted odds ratio (95% CI)* | P value | |
|---|---|---|---|---|---|---|
| All cause mortality†: | n=20 304 | n=20 568 | ||||
| 30 days | 613 (3.0) | 642 (3.1) | 0.97 (0.86 to 1.08) | 0.55 | 0.96 (0.86 to 1.08) | 0.50 |
| 1 year | 1726 (8.5) | 1682 (8.2) | 1.04 (0.97 to 1.12) | 0.24 | 1.05 (0.97 to 1.13) | 0.21 |
| STEMI‡: | n=2031 | n=2128 | ||||
| 30 days | 178 (8.8) | 225 (10.6) | 0.81 (0.66 to 1.00) | 0.049 | 0.78 (0.63 to 0.97) | 0.027 |
| 1 year | 272 (13.4) | 321 (15.1) | 0.87 (0.73 to 1.04) | 0.12 | 0.84 (0.70 to 1.01) | 0.063 |
| Non-STEMI‡: | n=5152 | n=5066 | ||||
| 30 days | 187 (3.6) | 176 (3.5) | 1.05 (0.85 to 1.29) | 0.67 | 1.02 (0.83 to 1.27) | 0.84 |
| 1 year | 555 (10.8) | 512 (10.1) | 1.07 (0.95 to 1.22) | 0.27 | 1.07 (0.94 to 1.23) | 0.31 |
| Unstable angina: | n=1606 | n=1678 | ||||
| 30 days | 13 (0.8) | 22 (1.3) | 0.61 (0.31 to 1.22) | 0.17 | 0.59 (0.29 to 1.18) | 0.14 |
| 1 year | 65 (4.0) | 76 (4.5) | 0.89 (0.63 to 1.25) | 0.50 | 0.85 (0.60 to 1.21) | 0.36 |
| Non-ACS: | n=9681 | n=9838 | ||||
| 30 days | 174 (1.8) | 175 (1.8) | 1.01 (0.82 to 1.25) | 0.92 | 1.04 (0.84 to 1.29) | 0.71 |
| 1 year | 702 (7.3) | 648 (6.6) | 1.11 (0.99 to 1.24) | 0.067 | 1.16 (1.03 to 1.30) | 0.013 |
| Non-classified diagnosis: | n=1834 | n=1858 | ||||
| 30 days | 61 (3.3) | 44 (2.4) | 1.42 (0.95 to 2.10) | 0.081 | 1.45 (0.97 to 2.18) | 0.072 |
| 1 year | 132 (7.2) | 125 (6.7) | 1.08 (0.83 to 1.39) | 0.58 | 1.09 (0.83 to 1.43) | 0.54 |
| Patients with first SpO2§: | n=10 137 | n=10 293 | ||||
| 30 days | 293 (2.9) | 315 (3.1) | 0.94 (0.80 to 1.11) | 0.47 | 0.91 (0.76 to 1.09) | 0.31 |
| 1 year | 945 (9.3) | 923 (9.0) | 1.04 (0.95 to 1.15) | 0.38 | 1.03 (0.92 to 1.14) | 0.64 |
ACS=acute coronary syndrome; NSTEMI=non ST elevation myocardial infarction; STEMI=ST elevation myocardial infarction; SpO2=transcapillary oxygen saturation level.
Adjusted for age, sex, region, and diagnosis of acute coronary syndrome. Age is stratified at median age for study population.
Primary outcome.
Prespecified secondary analyses.
First oxygen saturation level measured on ambulance arrival. Data are for 20 430 patients from the ambulance ACS pathway only.
Of the 29 695 patients admitted to hospital by November 2017 with available cause specific mortality data, 942 died within 30 days—658 due to cardiovascular disease (high oxygen: n=303/14 127; low oxygen: n=355/14 910). The unadjusted and adjusted odds ratios for 30 day mortality due to cardiovascular disease were 0.90 (0.77 to 1.05) and 0.86 (0.73 to 1.01), respectively.
One year mortality for patients managed using the high oxygen protocol was 8.5% (n=1726) and for patients managed using the low oxygen protocol was 8.2% (n=1682). The unadjusted odds ratio was 1.04 (0.97 to 1.12) and the adjusted odds ratio was 1.05 (0.97 to 1.13). Figure 2 shows the Kaplan-Meier survival plot for all cause mortality. Unadjusted and adjusted hazard ratios comparing the high with low oxygen protocols were 1.03 (95% confidence interval 0.98 to 1.09) and 1.05 (0.99 to 1.11), respectively.
Fig 2.
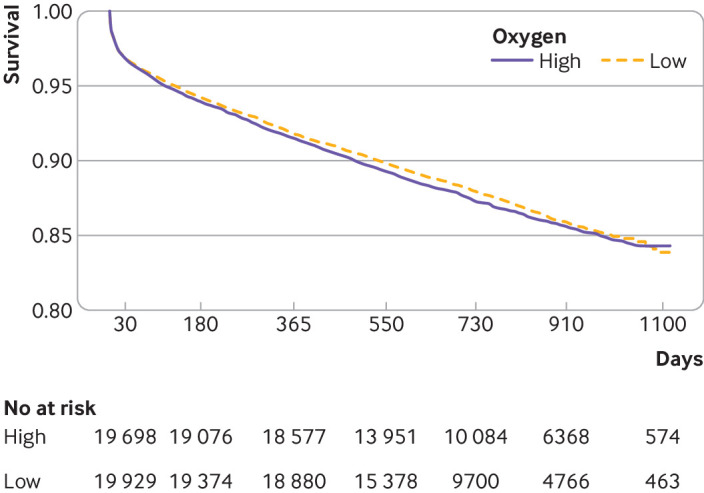
Kaplan-Meier plot showing all cause mortality for New Zealand patients with suspected acute coronary syndrome (ACS) managed on high and low oxygen protocols. All cause mortality was determined from national administrative data assessed on 12 August 2019 for all patients. The length of follow-up after one year varied according to the date of first assessment of ACS during the study
No significant differences were found in either 30 day or one year mortality by region. Results were similar for patients in the ANZACS-QI registry and those in the ambulance ACS pathway not in the ANZACS-QI registry (see supplementary table 1).
Length of hospital stay was compared between the two oxygen protocols in 36 342 patients admitted to hospital and discharged alive. The mean length of hospital stay for patients managed using the high oxygen protocol was 3.89 (SD 8.81) days and for patients managed using the low oxygen protocol was 3.86 (SD 8.23) days. The median (interquartile range) for hospital stay was 2 (1-5) days in both groups. The adjusted mean difference in length of stay was 0.012 days (95% confidence interval −0.16 to 0.18).
Outcomes in patients with different ACS diagnoses
For the 4159 patients with STEMI (10% of the total), 30 day mortality for those managed using the high oxygen protocol was 8.8% (n=178) and for those managed using the low oxygen protocol was 10.6% (n=225). The unadjusted odds ratio was 0.81 (0.66 to 1.00) and the adjusted odds ratio was 0.78 (0.63 to 0.97). One year mortality for those managed using the high oxygen protocol was 13.4% (n=272) and for those managed using the low oxygen protocol was 15.1% (n=321). The unadjusted odds ratio was 0.87 (0.73 to 1.04) and the adjusted odds ratio was 0.84 (0.70 to 1.01).
For the 10 218 (25%) patients with non-STEMI, 30 day mortality was 3.6% (n=187) in those managed using the high oxygen protocol and 3.5% (n=176) in those managed using the low oxygen protocol. The unadjusted odds ratio was 1.05 (0.85 to 1.29) and the adjusted odds ratio was 1.02 (0.83 to 1.27). One year mortality for the high oxygen group was 10.8% (n=555) and for the low oxygen group was 10.1% (n=512). The unadjusted odds ratio was 1.07 (0.95 to 1.22) and the adjusted odds ratio was 1.07 (0.94 to 1.23).
For patients with unstable angina or those without a final diagnosis of ACS, no significant differences were found in 30 day or one year mortality for either of the oxygen protocols (table 2). For the patients without a diagnosis of ACS, one year mortality was significantly higher in those managed using the high oxygen protocol (odds ratio 1.16, 1.03 to 1.30).
The differences in treatment effect between patients with different ACS diagnoses were explored using an interaction term between oxygen protocol and ACS type in the adjusted analysis. For 30 day all cause mortality, no statistically significant interaction effect was found between patients with STEMI and those with non-STEMI (0.75, 95% confidence interval 0.55 to 1.02, P=0.065), patients with unstable angina (1.30, 0.63 to 2.68, P=0.48), or patients without ACS (0.75, 0.55 to 1.01, P=0.062). For one year mortality, a significant interaction effect was found between patients with and without STEMI (0.77, 0.61 to 0.97, P=0.025), and patients with STEMI and without ACS (0.72, 0.58 to 0.89, P=0.003).
Outcomes by SpO2 level on ambulance arrival
First SpO2 levels recorded on the ambulance ACS pathway in 20 430 patients were similar between the high oxygen and low oxygen protocols (96.9% (SD 3.6%) v 96.9% (SD 3.4%)). The first SpO2 recorded in the ambulance was lower in patients who died within 30 days of presentation compared with those who survived (SpO2 mean 91.7% (SD 9.5%) v 97.1% (SD 3.0%)) but was similar for both oxygen protocol groups. The unadjusted odds ratio for 30 day mortality by oxygen protocol was 0.94 (0.80 to 1.11) and the adjusted odds ratio was 0.91 (0.76 to 1.09; table 2). Results were the same after additional adjustment for baseline SpO2.
Protocol adherence
About half of the patients with suspected ACS had no indication for oxygen under either protocol, mostly because they did not have clinical evidence of ongoing myocardial ischaemia. Forty two per cent of patients were given oxygen at least once across the ACS pathway under the high oxygen protocol compared with 22% under the low oxygen protocol. For patients given oxygen, SpO2 was higher for those who received the high oxygen protocol compared with those who received the low oxygen protocol before starting oxygen (high oxygen: median 96% (interquartile range 92-98%) v low oxygen: 90% (86-95%)) and when receiving oxygen (high oxygen: 98% (96-100%) v low oxygen: 96% (94-98%)).
Audit of medical records for protocol adherence was completed in 779 of 812 patients from the ANZACS-QI registry admitted on randomly selected days (table 3). Reasons for non-completion of the audit were not documented. Personal and clinical characteristics for audited patients were similar to those of the overall cohort (supplementary table 2). Eight per cent of patients on the high oxygen protocol did not receive oxygen despite having ischaemic chest pain or electrocardiographic changes during medical assessment. Fifteen per cent of patients were considered non-adherent to the low oxygen protocol because they were given oxygen without documentation of SpO2 less than 90% at least once during care (table 3).
Table 3.
Audit of protocol adherence and oxygen use in a random sample of patients from the ANZACS-QI registry. Values are numbers (percentages) unless stated otherwise
| High oxygen | Low oxygen | |
|---|---|---|
| No of patients audited | 385 | 394 |
| Received oxygen | 162 (42) | 88 (22) |
| SpO2 before first oxygen use: | ||
| ≥90% | 138 (36) | 60 (15)* |
| <90% | 24 (6) | 22 (6) |
| Oxygen not given: ischaemic symptoms | 35 (8)† | 118 (30) |
| No ischaemic symptoms | 182 (47) | 188 (48) |
| Contraindication for oxygen | 6 (2) | 6 (2) |
| Median (interquartile range) SpO2 in oxygen recipients: | ||
| Before first oxygen use | 96 (92-98) | 90 (86-95) |
| After first oxygen use | 98 (96-100) | 96 (94-98) |
ANZACS-QI=All New Zealand Acute Coronary Syndrome Quality Improvement.
SpO2=transcapillary oxygen saturation level when measured before and after first use of oxygen across the care pathway.
Oxygen use was audited from review of medical records on 28 random days during the study.
Clinical characteristics of audited patients were similar to those of non-audited patients in the ANZACS-QI registry cohort (see supplementary table 2).
Patients given oxygen but with no documented SpO2 <90% were non-adherent for the low oxygen protocol.
Patients with ischaemic symptoms who were not given oxygen protocol were non-adherent for the high oxygen protocol.
Discussion
This study found neither benefit nor harm from a protocol that recommended high flow oxygen for ischaemic symptoms or electrocardiographic changes as part of routine care in a broad general population of patients presenting with suspected ACS. This finding is consistent with previous studies, suggesting that high flow oxygen is unlikely to benefit most patients with presumed ischaemic chest pain and a normal oxygen saturation level.4 14 15 Because oxygen is widely used, it is important to determine that it is not associated with harm. In the current study, as in real world use, oxygen was given to patients with suspected ACS before the diagnosis was confirmed, with the knowledge that many patients will not have a final diagnosis of ACS.16 In this trial almost two thirds of the study population were subsequently found to have neither STEMI nor non-STEMI.
Patients with STEMI
STEMI is identified from a 12 lead electrocardiogram usually taken at presentation in patients with a suspected ACS. It is possible that patients with STEMI could have more to gain from supplementary oxygen because they usually present with severe prolonged myocardial ischaemia. Also, STEMI can be diagnosed early after first medical contact from the electrocardiogram, allowing targeted oxygen use if appropriate. This trial included a larger cohort of patients with STEMI than in previous trials,4 even though such patients comprised only 10% of the whole study population. The pragmatic cluster randomised trial design, without individual participant consent, meant that all patients in the registries were included in the analysis. This included acutely unwell individuals who would be unable to provide consent, and therefore not included in a traditional study protocol. The inclusion of high risk participants is shown by the 30 day mortality of about 10% for patients with STEMI compared with about 2% of patients in the DETO2X-AMI trial.4 However, despite a nominally lower 30 day mortality for patients with STEMI under the high oxygen protocol, it is difficult to draw definite conclusions on benefit for this subgroup given the neutral result for the primary outcome of the study.
Importance of oxygen saturation level
Most previous oxygen strategy trials3 14 were designed to evaluate effects of maintaining high SpO2 in normoxaemic patients and excluded people with an oxygen saturation level of less than 94%15 or 90%.4 Current clinical practice guidelines recommend giving oxygen to patients with STEMI or non-STEMI when the SpO2 is less than 94% or less than 90%,5 6 7 8 9 despite little evidence from randomised trials. In this trial the low oxygen protocol included a lower threshold for starting oxygen (SpO2 <90%) and lower oxygen flow rates to avoid high SpO2 levels. The low oxygen protocol was therefore associated with less effectiveness than the high oxygen protocol for preventing mild hypoxaemia. In a sample of the study population with oxygen saturation levels recorded on first ambulance attendance, SpO2 was lower in patients who died. No change was, however, found in the odds ratios for 30 day or one year mortality for the group receiving high oxygen compared with low oxygen when analysis also included adjustment for first SpO2. A secondary analysis from DETO2X-AMI also reported a much higher mortality for patients with an SpO2 of less than 95%, but no difference in mortality, reinfarction, or hospital admission for heart failure over the next 1-4 years between liberal and conservative oxygen protocols.17
Stastistical power
Low statistical power for modest but clinically important effects of oxygen is a limitation of the current and all previous studies. In a meta-analysis of the eight studies14 that compared liberal with conservative oxygen use in a total of 7732 patients, in-hospital mortality was only 1.7% (133 deaths), and a 30% relative mortality difference with high flow oxygen could not be excluded. One year mortality was a secondary outcome in the current study, because it was considered less likely than 30 day mortality to be influenced by early oxygen use.
Strengths and limitations of this study
The pragmatic cluster randomised design had both strengths and limitations. Strengths include the ability to compare outcomes with two protocols used as part of usual care, in a representative population and at multiple locations across the ACS pathway. This made it possible to evaluate oxygen use in a much larger population than would be possible in a conventional trial with individual consent and randomisation. The pragmatic design allowed the trial to be undertaken with a low administrative burden and at modest cost. Sicker, higher risk patients, who are generally less likely to be enrolled in clinical trials were included. A risk of the cluster design is imbalance between study groups. The crossover allowed the two oxygen protocols to be compared within regions accounting for possible hospital, temporal, and seasonal effects. Baseline characteristics of patients managed under two protocols differed by less than1%, suggesting that randomisation resulted in equivalent groups. The risk of bias from patient selection or evaluation of outcomes was minimised by using electronic linkage of all patients in the two registries admitted on the specified study dates to independently collected administrative data for diagnosis and outcomes, with no or minimal missing data.
A disadvantage of the study design was that the study population included all patients in the two registries, whether a patient had an indication for oxygen or not, and irrespective of how likely they were to have a final diagnosis of ACS. Thus many patients included in the analysis did not have ischaemic symptoms when seen and they had no indication for supplementary oxygen under either protocol. Most of these patients had a low risk of 30 day mortality. An additional limitation is that information on oxygen use and protocol adherence was not available for most participants, and it was missing for 10% of audited patients. We do not know the specific reasons for protocol non-adherence. They are likely to include not documenting oxygen use, an active decision by clinical staff or the patient not to follow the recommended oxygen protocol based on personal judgment, or failure to remember to use the recommended protocol. Overall, we believe the level of non-adherence is consistent with the expected level in a pragmatic clinical trial undertaken in real world settings. Protocol non-adherence would bias results towards no difference and decrease the statistical power of the trial to detect any true difference in outcomes related to the oxygen protocol. The study protocols did not specify the duration of oxygen use, which would vary between patients according to clinical circumstances, consistent with usual clinical practice. These oxygen protocols contrast with most previous oxygen strategy trials where oxygen (or air) was usually administered for a defined time irrespective of clinical status.3 14 It is possible oxygen only benefits patients who are hypoxaemic, but the study design did not allow a definite answer to this possibility.
Conclusions
This study found no benefit from high flow oxygen in most patients presenting with a suspected ACS. The result supports current clinical practice guidelines,5 6 8 9 which recommend that oxygen is not given to patients with suspected ACS who have a normal SpO2. However, the study neither confirmed nor excluded the possibility of a small benefit from supplementary oxygen in patients presenting with STEMI.
What is already known on this topic
Previous trials have reported no benefit and possible harm from supplementary oxygen given to normoxaemic patients presenting with a suspected or confirmed acute coronary syndrome (ACS)
Previous trials were too small to reliably evaluate modest favourable or unfavourable effects of oxygen on early mortality, or specific circumstances when oxygen might be beneficial
What this study adds
Supplementary high flow oxygen given for ischaemic symptoms to patients with suspected ACS was not associated with a change in 30 day mortality
The study did not confirm or exclude a small mortality benefit from supplementary oxygen in selected circumstances—for example, hypoxaemic patients with ST elevation myocardial infarction
Acknowledgments
We thank the study investigators (see supplementary appendix), the St John Ambulance and Wellington Free Ambulance servicee, the National Cardiac and Emergency Medicine Clinical Networks, the hospitals that participate in the All New Zealand Acute Coronary Syndromes Quality Improvement (ANZACS-QI) registry, the data monitoring committee (see supplementary appendix), contributors to ANZACS-QI and Vascular Informatics using Epidemiology and the Web (VIEW) programme, and physicians, nurses, and paramedics who used the study oxygen protocols. We also thank the study participants.
The study was approved by the National Health and Disability ethics committee and Māori research review committee without requiring individual patient consent. It was agreed that obtaining consent might not be appropriate during a medical emergency for a simple low risk intervention. Study conduct and progress were reviewed by the data monitoring committee of the Health Research Council of New Zealand (see supplementary appendix). The trial protocol was registered on the Australia New Zealand Clinical Trials Website (ACTRN12616000461493).
Web extra.
Extra material supplied by authors
Supplementary information: appendices
Contributors: RAHS was study chair, conceived the study, and drafted the manuscript. YJ coordinated data linkage and undertook all statistical analyses. All authors contributed to critical discussion of trial design, and conduct, review of trial data, and revisions of the manuscript. The following authors contributed to study conduct as leaders of relevant national or regional clinical networks or services: RAH chair of National Cardiac Clinical Network; PJ chair of Emergency Medicine Network; GD clinical leader of Midlands Clinical Network; BD clinical leader of St John Ambulance; JE clinical leader of Southern Network; AK clinical leader of the All New Zealand Acute Coronary Syndrome Quality Improvement (ANZACS-QI); AR clinical leader of Central Regional Network; TS clinical leader of Northern Regional Network; TSm medical director of St John Ambulance; DS clinical leader of Southern Regional Network (Christchurch); AS medical director of Wellington Free Ambulance; and MT clinical leader of Emergency Medicine Network Southern region. MW and HDW were expert steering committee members. RAHS and YJ are the guarantors. The corresponding author attests that all listed authors had full access to all of the data (including statistical reports and tables), takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: This work was supported by the National Heart Foundation of New Zealand (grant No 1649) and by the Health Research Council of New Zealand (HRC) clinical practitioner fellowships to RAHS and MT. The trial was undertaken as part of the All New Zealand Acute Coronary Syndrome Quality Improvement (ANZACS-QI), which is funded by the Ministry of Health (ANZACS-QI 41). Data linkage for mortality was obtained through the VIEW programme funded by the HRC. The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the National Heart Foundation of New Zealand (grant No 1649) and from the Health Research Council of New Zealand clinical practitioner fellowships to RAHS and MT; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years ; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by the National Health and Disability ethics committee (15/NTA/117).
Data sharing: Requests for study data can be made to the corresponding author.
The lead author (RAHS) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Patient consent: The National Ethics Committees approved the study without patient consent because they considered there was clinical equipoise, that study conduct did not place demands on patients or reduce their autonomy, and obtaining informed consent might not be appropriate during a medical emergency. Information on the trial was openly available for patients and doctors, who could decide whether to follow the study oxygen protocols.
Dissemination to participants and related patient and public communities: Results of the study have been reported to relevant healthcare providers through the national cardiac clinical networks and ambulance services. Results have been made available to the public through the National Heart Foundation of New Zealand (the study sponsor) and through the media.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Beasley R, Aldington S, Weatherall M, Robinson G, McHaffie D. Oxygen therapy in myocardial infarction: an historical perspective. J R Soc Med 2007;100:130-3. 10.1177/014107680710000311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McNulty PH, King N, Scott S, et al. Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. Am J Physiol Heart Circ Physiol 2005;288:H1057-62. 10.1152/ajpheart.00625.2004 [DOI] [PubMed] [Google Scholar]
- 3. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet 2018;391:1693-705. 10.1016/S0140-6736(18)30479-3 [DOI] [PubMed] [Google Scholar]
- 4. Hofmann R, James SK, Jernberg T, et al. DETO2X–SWEDEHEART Investigators . Oxygen Therapy in Suspected Acute Myocardial Infarction. N Engl J Med 2017;377:1240-9. 10.1056/NEJMoa1706222 [DOI] [PubMed] [Google Scholar]
- 5. Amsterdam EA, Wenger NK, Brindis RG, et al. ACC/AHA Task Force Members. Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons . 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. [correction in: Circulation 2014;130:e431-2.] Circulation 2014;130:2354-94. 10.1161/CIR.0000000000000133 [DOI] [PubMed] [Google Scholar]
- 6. Ibanez B, James S, Agewall S, et al. ESC Scientific Document Group . 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 7. O’Driscoll BR, Howard LS, Earis J, Mak V, British Thoracic Society Emergency Oxygen Guideline Group. BTS Emergency Oxygen Guideline Development Group . BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax 2017;72(Suppl 1):ii1-90. 10.1136/thoraxjnl-2016-209729 [DOI] [PubMed] [Google Scholar]
- 8. O’Gara PT, Kushner FG, Ascheim DD, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362-425. 10.1161/CIR.0b013e3182742c84 [DOI] [PubMed] [Google Scholar]
- 9. Roffi M, Patrono C, Collet JP, et al. ESC Scientific Document Group . 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267-315. 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 10. Kerr A, Williams MJ, White H, et al. The All New Zealand Acute Coronary Syndrome Quality Improvement Programme: Implementation, Methodology and Cohorts (ANZACS-QI 9). N Z Med J 2016;129:23-36. [PubMed] [Google Scholar]
- 11. Kerr AJ, Lee M, Jiang Y, et al. High level of capture of coronary intervention and associated acute coronary syndromes in the all New Zealand acute coronary syndrome quality improvement cardiac registry and excellent agreement with national administrative datasets (ANZACS-QI 25). N Z Med J 2019;132:19-29. [PubMed] [Google Scholar]
- 12. Atkinson JSC, Crampton P. NZDep2013 Index of Deprivation. Department of Public Health, University of Otago, 2014. [Google Scholar]
- 13. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004;57:1288-94. 10.1016/j.jclinepi.2004.03.012 [DOI] [PubMed] [Google Scholar]
- 14. Sepehrvand N, James SK, Stub D, Khoshnood A, Ezekowitz JA, Hofmann R. Effects of supplemental oxygen therapy in patients with suspected acute myocardial infarction: a meta-analysis of randomised clinical trials. Heart 2018;104:1691-8. 10.1136/heartjnl-2018-313089 [DOI] [PubMed] [Google Scholar]
- 15. Stub D, Smith K, Bernard S, et al. AVOID Investigators . Air Versus Oxygen in ST-Segment-Elevation Myocardial Infarction. Circulation 2015;131:2143-50. 10.1161/CIRCULATIONAHA.114.014494 [DOI] [PubMed] [Google Scholar]
- 16. Kohn MA, Kwan E, Gupta M, Tabas JA. Prevalence of acute myocardial infarction and other serious diagnoses in patients presenting to an urban emergency department with chest pain. J Emerg Med 2005;29:383-90. 10.1016/j.jemermed.2005.04.010 [DOI] [PubMed] [Google Scholar]
- 17. James SK, Erlinge D, Herlitz J, et al. DETO2X-SWEDEHEART Investigators . Effect of Oxygen Therapy on Cardiovascular Outcomes in Relation to Baseline Oxygen Saturation. JACC Cardiovasc Interv 2020;13:502-13. 10.1016/j.jcin.2019.09.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: appendices