Abstract
Background
Surgical therapy still offers the only chance of long-term survival for patients with perihilar cholangiocarcinoma (pCCA). The aim of this narrative review is to summarize the current standards and challenges in the surgical treatment of pCCA.
Summary
After imaging and defining resectability, the first step towards optimal surgical treatment is optimizing biliary drainage and preventing cholangitis, followed by securing adequate future liver remnant volume and/or function. The main goal of resection for pCCA is achieving radical resection and ultimately long-term survival. In order to achieve radical resection, several points will be addressed (e.g., vascular resection and reconstruction, intraoperative frozen sections, right versus left hemihepatectomy, and the usefulness of preoperative [chemo]therapy).
Key Messages
In order to optimize long-term outcomes for patients with pCCA, collaboration between leading centers should be increased. In addition, this collaboration is necessary to design large prospective randomized controlled trials, as the incidence of pCCA is low and the number of resectable patients is even lower. Currently, most results are based on small retrospective cohort studies resulting in low evidence. In order to properly investigate how to improve long-term survival, we need to set up trials to confirm the results of small series suggesting the positive effect of preoperative chemotherapy and extended lymph node resection.
Keywords: Perihilar cholangiocarcinoma, Surgical therapy, Radical resection, Survival, Vascular reconstruction
Introduction
Perihilar cholangiocarcinoma (pCCA) is a rare tumor with a poor prognosis. Resection offers the only chance of long-term survival, with 5-year overall survival (OS) rates up to 44%. Tumor margin-negative (R0) resection increases the OS up to 67% [1, 2, 3]. Unfortunately, only one-fifth of patients is eligible for resection because most patients have locally advanced or metastatic disease at presentation [1, 4]. Due to the complex anatomy and the central location of the tumor in the hilum of the liver, no validated and objective resection criteria for pCCA are available. Therefore, resectability is mainly based on expert opinion in multidisciplinary team meetings. The aim of this narrative review is to summarize the current standards and challenges in the surgical treatment of pCCA.
Optimal surgical treatment starts during workup and consists of different parts such as biliary drainage as well as securing adequate future liver remnant (FLR) volume and/or function. The first step in the treatment of pCCA consists of biliary drainage and prevention of cholangitis, depending on the volume of the FLR [5]. The optimal strategy is still under debate, and choices made in the crucial time early after diagnosis have consequences throughout the surgical treatment of these complex patients. Based on our experience in the DRAINAGE trial we prefer to selectively drain the FLR endoscopically [6]. Percutaneous drainage is reserved for cases unsuitable for the endoscopic approach or when endoscopic drainage fails. Adequate biliary drainage prior to surgery is mandatory in patients with obstructive cholangitis to lower the risk of postoperative mortality. Postoperative mortality rates up to 18% are described in patients suffering from cholangitis prior to surgery [5]. For patients without cholangitis, biliary drainage should be performed with caution because of the risk of inducing cholangitis and is not recommended for patients with a FLR >50% [1, 5]. Besides cholangitis a high preoperative bilirubin level >2.9 mg/dL is an independent predictor for post-hepatectomy liver failure [7]. This suggests that patients with bilirubin levels >2.9 mL/dL have benefit from preoperative drainage. However, a uniform standpoint has not yet been adopted. A recently published systematic review showed that patients with low FLR volume and bilirubin levels >15.0 mg/dL benefit from preoperative drainage in terms of lower mortality rates [8].
The next step in the workup towards resection is securing adequate liver volume or function. Since most patients will have compromised livers due to cholestasis even after adequate biliary drainage, the preoperative FLR volume should be >40% in most patients [1]. A quantitative liver function test, such as hepatobiliary scintigraphy of the FLR, is a helpful tool to adequately secure the function of the FLR in order to prevent post-hepatectomy liver failure. The generally accepted value for the function of the FLR is 2.7%/min/m2. Olthof et al. [9] investigated hepatobiliary scintigraphy in 116 patients undergoing resection for pCCA. They found a cutoff value of >8.5%/min (note: this is uncorrected for body surface area) with a positive predictive value (PPV) of 41% and a negative predictive value (NPV) of 94% versus a PPV of 38% and a NPV of 82% for 2.7%/min/m2. The cutoff is still being discussed, but a higher value seems to be safer for this complex group of patients. Portal vein embolization (PVE) is necessary in case of insufficient function or volume of the FLR, a common situation when adhering to a function threshold of 8.5%/min [5, 10]. After PVE, volume and function discrepancy may be even greater, increasing the clinical value of hepatobiliary scintigraphy [9]. The kinetic growth rate after PVE seem as important as the actual FLR function [11]. In case PVE does not result in a sufficient increase in FLR volume/function, only few alternatives remain. First, it should be noted that such a patient is probably not a candidate for major resection since the FLR did not, and probably after resection will not, regenerate adequately. Alternatively, associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) has been advocated in cases where PVE was unsuccessful. In liver resections for colorectal metastasis this option, and especially the modified versions such as the mini-ALPPS, have led to safe resections in patients unresectable after PVE only [12]. In pCCA, however, ALPPS is associated with dramatic infectious complications and 90-day mortality rates up to 48% [13, 14]. As a result, the prevalence of hilar biliary tumors in the international ALPPS registry decreased from 24 to 9%. The high complication rate after ALPPS is mainly caused by the typically infected bile in patients with pCCA, increasing the risk of bile-originated infection and impairing the regenerative capacity of the liver. However, technical refinements (minimally invasive first stage including biliary drainage of both the FLR and deportalized liver followed by biliary reconstruction at the second stage) have the potential to overcome these limitations of the ALPPS procedure and to reduce morbidity after the operation. Thus in selected patients, the indication for ALPPS procedure may be extended for pCCA with preoperative jaundice.
An emerging alternative is double vein embolization. During double vein embolization not only the contralateral portal vein of the FLR is embolized, but also the efferent hepatic vein. Hypertrophy appears to be faster and more pronounced. Since it does not involve parenchymal dissection or surgery, infectious complications are minimal, making it an interesting concept for patients with pCCA [15]. This concept is currently under investigation for patients suffering from colorectal liver metastasis in the international DRAGON trial (NCT04272931).
Resectability Criteria
The Memorial Sloan Kettering Cancer Center staging system is a preoperative staging system based on imaging data that contains radial tumor growth, portal venous involvement, and hepatic lobar atrophy [16]. This so called “Blumgart staging system” effectively predicted resectability and metastatic disease [17]. Although updated in 2001, this system is now less applicable in view of the more extended resections devised in the last decade. Another staging system designed by Chaiteerakij et al. [18] is based on nonoperative information of pCCA to classify patients into four prognostic stages. Kuriyama et al. [19] have recently proposed a new anatomical resectability classification system in which they make a subdivision between locally advanced, borderline resectable, and resectable patients with pCCA. However, none of these staging systems are widely implemented, resulting in major differences in daily practice of resectability across centers. Nevertheless, uniform pCCA resectability criteria are mandatory to set up clinical trials and to compare their results.
When determining resectability or “candidates for exploration,” patients with distant metastasis, poor prognosis due to high age, or poor performance status will be excluded as exploration for resection is clearly not recommended [20]. In case of suspicious N2 (AJCC 7th edition) positive lymph nodes, endoscopic ultrasound-guided fine needle aspiration to confirm this suspicion is recommended as patients with N2 node positivity are excluded from exploration in most centers [21]. For use in preoperative therapy studies, we can pragmatically consider three patient categories: clearly resectable, borderline resectable, and clearly unresectable. Patients with clearly resectable disease have no vascular involvement of the FLR. In addition, radical resection of the tumor and involved biliary ducts appears to be possible on imaging. Patients with clearly unresectable disease have circumferential and unreconstructable vascular (portal venous or arterial) involvement of the FLR, insufficient FLR for potential radical resection despite PVE, and/or no possibility for radical resection of the bile ducts especially in relation to (branches of) the FLR artery. Patients with borderline resectable disease are placed between these two aforementioned groups and form a group of great debate providing an interesting field for research. In addition, patients with borderline resectable disease are therefore not always treated in the best way.
Nevertheless, in order to define these three patient categories, the vascular and biliary involvement of the pCCA need to be assessed. Predicting vascular involvement of the hepatic artery and portal vein on CT and MRI scans remains difficult. In a study performed by Ruys et al. [22], the pooled sensitivity for portal vein and hepatic artery involvement on contrast-enhanced CT scans was 89 and 84%, respectively. This is in contrast to our recent clinical experience. A study performed at Amsterdam UMC showed acceptable PPVs for occluded, irregular, or narrowed portal veins and stenotic or occluded hepatic arteries on CT imaging, but for any other suspicion of involvement of hepatic arteries the PPV was low (unpublished results). The fact that imaging unreliably reflects the true intraoperative situation results in 30–50% futile laparotomies for patients with borderline resectable pCCA, ending up without resection [3, 4]. This suggests that new or improved radiological modalities are warranted.
Surgery
Surgery for pCCA should only be performed in specialized, high-volume centers [23]. Complete resection of pCCA generally involves resection of the extrahepatic bile ducts in combination with (extended) hemihepatectomy and segment I, including lymphadenectomy of the hepatoduodenal ligament and biliary reconstruction [1]. Due to the extent and complexity of the disease, resection is unfortunately associated with substantial morbidity (50%) [3] and mortality (14%) [5]. In highly selected cases, especially young patients and patients with primary sclerosing cholangitis, liver transplantation may be an option as well [1, 24]. Minimally invasive surgery for pCCA is mainly due to the complexity of the disease, still in its infancy. A systematic review performed by Franken et al. [25] stated that robotic and/or laparoscopic (hemi)hepatectomy is technically feasible, but that besides the inherent benefits (e.g., less blood loss, faster functional recovery, and less postoperative complications), the major outcomes (mortality, liver failure, and bile leak) of minimally invasive surgery for pCCA need further investigation.
Staging Laparoscopy
Staging laparoscopy should be considered prior to explorative laparotomy to exclude peritoneal metastasis or positive N2 lymph nodes. Coelen et al. [26, 27] described an advantage of staging laparoscopy for 25% of patients with pCCA, especially for detecting peritoneal metastasis, and developed a preoperative risk score to predict unresectable pCCA at staging laparoscopy.
Radical Resection
The main goal of resection for pCCA is achieving R0 resection and, ultimately, long-term survival. The secondary benefits of resection for pCCA may lie in optimizing biliary drainage and palliation. R0 resection is defined as tumor-free margins of ≥1 mm of the following planes: distal ductal margin (common bile duct), proximal margin (hepatic duct), portal vein resection (PVR) plane, hepatic artery resection plane, liver parenchyma resection plane, and periductal dissection plane [28]. Therefore, to properly assess the microscopic radicality of the resection, all resection and dissection planes need to be examined and described in the pathology reports. Roos et al. [28] investigated a total of 146 reports in one institution, where one or more planes were missing in 64% of the reports. This resulted in a reclassified residual disease of 15% (22 patients were reclassified from R0 to R1), underscoring the importance of dedicated pathologists examining the specimen and widely implemented pathological assessment strategies. In the current literature, R0 resection rates range from 50 to 90% [2, 3], which is associated with a 5-year OS up to 67%, while R1 resection is associated with a 5-year OS of <10% while long-term survivors have also been reported [1]. There are several points that can be addressed to increase the chance of achieving a radical resection.
Neoadjuvant/Induction Chemotherapy. A recent systematic review published by our group stated that induction chemotherapy including, e.g., gemcitabine(-based) chemotherapy or 5-FU(-based) chemotherapy followed by resection in adequately selected patients improves median OS (pooled HR 0.31, 95% CI 0.19–0.50; p < 0.0001) [29]. Other reviews show comparable conclusions [30, 31]. However, these reviews found only studies with small sample sizes with different chemotherapy regimens. Therefore, in order to further investigate this effect, prospective randomized controlled trials are needed.
(Extended) Left or Right Hemihepatectomy. The choice between (extended) left or right hemihepatectomy depends on the tumor predominantly infiltrating into the left or right hemiliver, the presence of lobar atrophy, as well as vascular and biliary involvement and is regularly decided before exploration. In addition, the side of resection is determined by the embolized lobe when PVE needs to be performed. Due to the central anatomical position of pCCA, curative resection (R0) of the tumor is difficult. Because of frequent anterior and posterior ductal infiltration, resection of segment IV and complete excision of the caudate lobe are strongly advised to achieve free resection margins [32]. Therefore, for patients with Bismuth-Corlette type III or IV pCCA, an extended hemihepatectomy is usually indicated, which contributes to a higher rate of R0 resections and improved survival. Based on segmental anatomy, extended right hemihepatectomy is the most commonly performed procedure for pCCA, with the highest percentage of R0 resections [33]. However, two recent publications showed no difference between short- and long-term outcomes between patients undergoing left or right hemihepatectomy with or without caudate lobectomy [34, 35]. In addition, a recent systematic review by Pinotti et al. [36] stated that caudate lobectomy may improve the R0 resection rate and OS in patients with pCCA and thus should be considered. In conclusion, although overall no survival benefit was found after either right or left resection, (extended) right hemihepatectomy with caudate lobectomy may lead to a slightly higher rate of radical resections, but is also hampered by a higher percentage of post-hepatectomy liver failure [34, 35].
Standard or Selective PVR. PVR and reconstruction is usually undertaken when the portal vein bifurcation is infiltrated by tumor. PVR is performed in approximately 17–36% of cases, leading to a slightly increased 5-year OS compared to no PVR [2, 3, 37, 38]. In few centers, PVR with (extended) right hemihepatectomy is the standard of care as part of a “no-touch” technique. The procedure termed “hilar en bloc resection” may be oncologically superior to conventional major hepatectomy, providing a chance of long-term survival even in advanced tumors [39]. However, from our own data combined with data of a specialized center performing standard PVR, we conclude that standard PVR was not associated with increased severe morbidity or mortality compared to selective PVR. In addition, survival was comparable in both selective and standard PVR groups, which suggests no superiority of one or the other method (unpublished results).
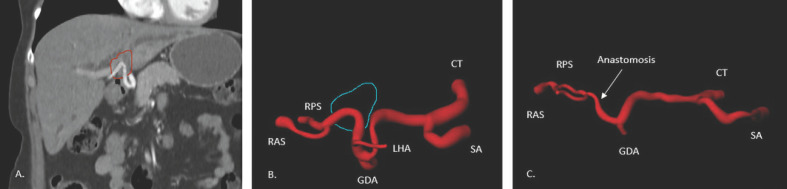
Arterial Resection and Reconstruction. Most cases of concomitant resection of (branches of) the hepatic artery and reconstruction to the FLR are being performed in East Asian countries. There are some Asian case series available with a hepatic artery resection rate of >10% [2, 37, 40]. In Western case series, hepatic artery resection and reconstruction are being performed in <7% of patients (Table 1A) [41]. A meta-analysis performed by Abbas and Sandroussi [42] showed increased morbidity (OR 1.61, 95% CI 0.80–3.25; p = 0.19) and mortality (OR 4.48, 95% CI 1.97–10.16; p = 0.0003) when hepatic artery resection and reconstruction was performed. The increased risk of hepatic artery resection suggests that additional arterial resection and reconstruction should only be performed in highly selected patients. We believe that preoperative chemotherapy should be considered in all cases with a high probability of arterial reconstruction. Preoperative chemotherapy may serve as an induction therapy, but more importantly, it provides a test of time to select patients who may benefit most from these high-risk procedures. The crucial point to consider when discussing arterial involvement and reconstruction is that its complexity varies from case to case. Essentially, if it is possible to separate the FLR artery at the point of biliary transection, reconstruction is possible. On the practical level, however, there are two types of involvement of the FLR artery. First, if an upstream part of the bile duct is involved at a point past the second division of the proper hepatic artery, a safe reconstruction is very unlikely due to the small caliber and intraparenchymal location of the anastomosis. On the other hand, there is a second type as illustrated in Figure 1; here the involvement is extrahepatic, and due to the contraction of the tumor (typical for cholangiocarcinoma), a large surplus of the artery exists. The large diameter of this artery and the possibility to anastomose end to end without interposition or transposition makes this an ideal candidate for arterial reconstruction. If a direct reconstruction is impossible due to insufficient length, a transposition of the gastroduodenal or left hepatic artery (since it is often not involved in the tumor) can be considered. Again, when these vessels are of sufficient caliber, a safe reconstruction can be made [37]. As a precaution we do not mobilize the FLR retroperitoneally (e.g., triangular ligament) to preserve any arterial collaterals that may exist. This can serve as a backup when the arterial reconstruction fails. In general, postoperative occlusion of a reconstructed artery is a dramatic complication associated with a very high mortality rate [42].
Table 1.
Overview of publications describing hepatic artery resection and IFS
A Overview of publications describing hepatic artery resection
| Reference (first author) | Asian/Western countries | Resected patients | Number of hepatic artery resections | Morbidity (Clavien-Dindo >3a) | Mortality (90 days) | 5-year OS |
|---|---|---|---|---|---|---|
| Mizuno, 2020 [37] | Asian | 1,055 | 146 (13.8%) | 74 (50.7%) | 6 (4.0%) | 29.5% |
| Matsuyama, 2016 [40] | Asian | 172 | 44 (25.5%) | 29 (65.9%) | 4 (9.0%) | 22.3% |
| Schimizzi, 2018 [41] | Western | 201 | 12 (6.0%) | 8 (66.7%) | 0a | NAb |
| Nuzzo, 2012 [59] | Western | 376 | 5 (1.3%) | NA | 8c | 22.8%c |
B Small overview of publications describing IFS
| Reference (first author) | IFS | Positive IFS | Proximal/distal | Additional resection | R0 resection | 5-year OS |
|---|---|---|---|---|---|---|
| Otsuka, 2019 [44] | 558 | 74 (13.3%) | distal | 53 (71.6%) | 30 (56.6%) | 31% EBDX, 67% PPPD |
| Zhang, 2018 [45] | 215 | 80 (37.2%) | proximal/distal | 58 (72.5%) | 29 (50%) | 44.3% |
| Mantel, 2016 [43] | 67 | 17 (25.3%) | proximal | 10 (58.8%) | 3 (30%) | 39% |
EBDX, external bile duct resection; IFS, intraoperative frozen section; NA, not applicable; OS, overall survival; PPPD, pylorus-preserving pancreaticoduodenectomy.
Thirty-day mortality.
Median survival 33 months.
Postoperative mortality/5-year OS for total group of vascular resections (n = 42).
Fig. 1.
A Coronal CT imaging (red: tumor). B Hepatic artery upfront to surgery (blue: tumor). C Hepatic artery after resection and reconstruction. Imaging by a specialized CT technician with the use of syngo.via (Siemens Medical Systems). CT, celiac trunk; GDA, gastroduodenal artery; LHA, left hepatic artery; RAS, artery right anterior segments; RPS, artery right posterior segments; SA, splenic artery.
Intraoperative Frozen Sections. In patients undergoing resection for pCCA, intraoperative frozen sections are often routinely performed to assess the completeness of the proximal and distal bile duct resection planes. In case of a positive proximal margin, additional resection of the bile duct stump is usually undertaken to achieve R0 resection. In case of a positive distal margin, an additional pancreatoduodenectomy is considered. However, in case of a positive proximal margin, extending the resection can be technically difficult because of available proximal bile duct remnant and is often accompanied by increased morbidity [43]. In the current literature, there are several studies that investigated the value of intraoperative frozen sections in relation to survival (Table 1B) [43, 44, 45, 46, 47, 48]. These studies describe a similar OS after additional resection of the proximal and distal resection planes as after initial R0 resections. If additional pylorus-preserving pancreaticoduodenectomy needs to be performed the OS is similar, but the perioperative mortality rate increases towards 17% [44, 49]. In addition, an intraoperative switch to additional pylorus-preserving pancreaticoduodenectomy is associated with a worse recurrence-free survival [50]. A recent systematic review with meta-analysis performed by Ke et al. [51] showed no difference in 5-year OS between initial R0 and R0 after additional resection of the proximal or distal resection plane. However, the authors found a significant difference in 5-year OS between R0 after additional resection and initial R1 in favor of R0 after additional resection (OR 3.54, 95% CI 1.67–7.50; p = 0.0010), suggesting that frozen section and additional resection should routinely be performed.
Lymph Node Sampling
The prognosis of patients resected for pCCA is significantly determined by the presence of lymph node metastasis. At resection about 35% of patients are shown to have positive lymph nodes, which is associated with a 5-year OS of <20% versus 55% in case of negative lymph nodes [1, 2, 52]. Also the number of lymph nodes (<4) retrieved during resection has been reported to negatively influence survival due to possible understaging [53]. Lymph node metastases have been reported to be a major determinant of OS and poor recurrence-free survival [54, 55]. Buettner et al. [21] stated that the actual benefit of resection in patients with positive lymph nodes is <7 months and should be weighed against considerable postoperative morbidity and mortality. Ma et al. [56] reported that extended lymphadenectomy, including the right celiac, superior mesenteric, and para-aortic lymph nodes, may lead to improved survival prediction and prevents understaging. They found a median OS in radically resected patients of 33 versus 21 months (p = 0.044) for extended and regional lymphadenectomy, respectively. In addition, a recent narrative review performed by Li et al. [57] stated that a standardized extended lymphadenectomy, including the para-aortic lymph node, might help to more accurately stage pCCA, but that further studies are required to further investigate the added value of extended lymphadenectomy in patients with negative para-aortic, celiac, or superior mesenteric lymph nodes. We advocate routine intraoperative sampling and performing frozen sections to be able to weigh the number of positive nodes and to determine the extent and risk of the resection (including age, preoperative cholangitis, and other risk factors). We use this information to decide whether to proceed with the resection or not.
Discussion and Future Perspectives
The low incidence and thus the low number of patients being resected for pCCA hampers research, and therefore improving outcomes on a wider scale is difficult. A meta-analysis by Franken et al. [58], including 4,659 patients among 79 centers worldwide, showed a median annual volume of four hepatectomies performed per center. In addition, only half of the centers performed over four procedures per year, while only 11 centers performed more than eight resections a year. Low-volume centers performing less than four resections showed higher mortality (11%) than high-volume centers (7%) worldwide. This corroborates an earlier systematic review in which specialized care in high-volume centers proved to result in better outcomes [23].
Over the years, more complex surgeries including vascular reconstructions are being undertaken. Selection of patients should therefore be done judiciously, aiming at improving postoperative outcomes. Preoperative biliary decompression should be decided according to the volume of FLR and control of cholangitis. PVE should be liberally applied when the volume of the FLR is insufficient. In addition, if there is any doubt about the resectability and oncological outcome of a patient, preoperative chemotherapy should be considered. Besides, a possible tumor response or progression could provide a “test of time” determining further therapy.
In order to achieve better selection and minimize postresectional mortality and morbidity, it is clear that we should focus on collaboration between leading centers. Currently, most research is based on small, retrospective cohort studies due to the minimal number of available randomized controlled trials. In order to properly investigate how to improve long-term survival, we need to set up large multi-institutional, randomized controlled trials towards the effects of minimally invasive surgery, the proposed preoperative chemotherapy, and extended lymph node resection. In addition, resectability criteria and pathological assessments need to be uniform, clear, and widely implemented to adequately compare results derived from different studies.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There was no funding to support this study.
Author Contributions
Conception and design of the work: L.E. Nooijen, T.M. van Gulik, J.I. Erdmann. All authors participated in the critical revision of the manuscript for important intellectual content. All authors finally approved the manuscript.
Acknowledgment
The authors acknowledge the contribution of Klaas Jan Franssen (specialized CT technician) for his help with Figure 1.
References
- 1.Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, et al. Surgery for cholangiocarcinoma. Liver Int. 2019 May;39((S1 Suppl 1)):143–55. doi: 10.1111/liv.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagino M, Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013 Jul;258((1)):129–40. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]
- 3.Rassam F, Roos E, van Lienden KP, van Hooft JE, Klümpen HJ, van Tienhoven G, et al. Modern work-up and extended resection in perihilar cholangiocarcinoma: the AMC experience. Langenbecks Arch Surg. 2018 May;403((3)):289–307. doi: 10.1007/s00423-018-1649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaspersz MP, Buettner S, Roos E, van Vugt JL, Coelen RJ, Vugts J, et al. A preoperative prognostic model to predict surgical success in patients with perihilar cholangiocarcinoma. J Surg Oncol. 2018 Sep;118((3)):469–76. doi: 10.1002/jso.25174. [DOI] [PubMed] [Google Scholar]
- 5.Wiggers JK, Groot Koerkamp B, Cieslak KP, Doussot A, van Klaveren D, Allen PJ, et al. Postoperative Mortality after Liver Resection for Perihilar Cholangiocarcinoma: Development of a Risk Score and Importance of Biliary Drainage of the Future Liver Remnant. J Am Coll Surg. 2016 Aug;223((2)):321–331.e1. doi: 10.1016/j.jamcollsurg.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coelen RJ, Roos E, Wiggers JK, Besselink MG, Buis CI, Busch OR, et al. Endoscopic versus percutaneous biliary drainage in patients with resectable perihilar cholangiocarcinoma: a multicentre, randomised controlled trial. Lancet Gastroenterol Hepatol. 2018 Oct;3((10)):681–90. doi: 10.1016/S2468-1253(18)30234-6. [DOI] [PubMed] [Google Scholar]
- 7.Olthof PB, Wiggers JK, Groot Koerkamp B, Coelen RJ, Allen PJ, Besselink MG, et al. Postoperative Liver Failure Risk Score: Identifying Patients with Resectable Perihilar Cholangiocarcinoma Who Can Benefit from Portal Vein Embolization. J Am Coll Surg. 2017 Sep;225((3)):387–94. doi: 10.1016/j.jamcollsurg.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Teng F, Tang YY, Dai JL, Li Y, Chen ZY. The effect and safety of preoperative biliary drainage in patients with hilar cholangiocarcinoma: an updated meta-analysis. World J Surg Oncol. 2020 Jul;18((1)):174. doi: 10.1186/s12957-020-01904-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olthof PB, Coelen RJ, Bennink RJ, Heger M, Lam MF, Besselink MG, et al. 99mTc-mebrofenin hepatobiliary scintigraphy predicts liver failure following major liver resection for perihilar cholangiocarcinoma. HPB (Oxford) 2017 Oct;19((10)):850–8. doi: 10.1016/j.hpb.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 10.van Lienden KP, van den Esschert JW, de Graaf W, Bipat S, Lameris JS, van Gulik TM, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol. 2013 Feb;36((1)):25–34. doi: 10.1007/s00270-012-0440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Zhu S. Present status and future perspectives of preoperative portal vein embolization. Am J Surg. 2009 May;197((5)):686–90. doi: 10.1016/j.amjsurg.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Rassam F, Olthof PB, van Lienden KP, Bennink RJ, Erdmann JI, Swijnenburg RJ, et al. Comparison of functional and volumetric increase of the future remnant liver and postoperative outcomes after portal vein embolization and complete or partial associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) Ann Transl Med. 2020 Apr;8((7)):436. doi: 10.21037/atm.2020.03.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balci D, Sakamoto Y, Li J, Di Benedetto F, Kirimker EO, Petrowsky H. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure for cholangiocarcinoma. Int J Surg. 2020 Oct;82S:97–102. doi: 10.1016/j.ijsu.2020.06.045. [DOI] [PubMed] [Google Scholar]
- 14.Olthof PB, Coelen RJ, Wiggers JK, Groot Koerkamp B, Malago M, Hernandez-Alejandro R, et al. High mortality after ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford) 2017 May;19((5)):381–7. doi: 10.1016/j.hpb.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esposito F, Lim C, Lahat E, Shwaartz C, Eshkenazy R, Salloum C, et al. Combined hepatic and portal vein embolization as preparation for major hepatectomy: a systematic review. HPB (Oxford) 2019 Sep;21((9)):1099–106. doi: 10.1016/j.hpb.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 16.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BS J, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001 Oct;234((4)):507–17. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuo K, Rocha FG, Ito K, D'Angelica MI, Allen PJ, Fong Y, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg. 2012 Sep;215((3)):343–55. doi: 10.1016/j.jamcollsurg.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 18.Chaiteerakij R, Harmsen WS, Marrero CR, Aboelsoud MM, Ndzengue A, Kaiya J, et al. A new clinically based staging system for perihilar cholangiocarcinoma. Am J Gastroenterol. 2014 Dec;109((12)):1881–90. doi: 10.1038/ajg.2014.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuriyama N, Usui M, Gyoten K, Hayasaki A, Fujii T, Iizawa Y, et al. Neoadjuvant chemotherapy followed by curative-intent surgery for perihilar cholangiocarcinoma based on its anatomical resectability classification and lymph node status. BMC Cancer. 2020 May;20((1)):405. doi: 10.1186/s12885-020-06895-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Z, Yang Y, Zhao Z, Wei K, Meng W, Li X. The clinicopathological factors associated with prognosis of patients with resectable perihilar cholangiocarcinoma: A systematic review and meta-analysis. Medicine (Baltimore) 2018 Aug;97((34)):e11999. doi: 10.1097/MD.0000000000011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buettner S, van Vugt JL, Gaspersz MP, Coelen RJ, Roos E, Labeur TA, et al. Survival after resection of perihilar cholangiocarcinoma in patients with lymph node metastases. HPB (Oxford) 2017 Aug;19((8)):735–40. doi: 10.1016/j.hpb.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruys AT, van Beem BE, Engelbrecht MR, Bipat S, Stoker J, Van Gulik TM. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. Br J Radiol. 2012 Sep;85((1017)):1255–62. doi: 10.1259/bjr/88405305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson AJ, Pang TC, Johnston E, Hollands MJ, Lam VW, Pleass HC. The volume effect in liver surgery—a systematic review and meta-analysis. J Gastrointest Surg. 2013 Nov;17((11)):1984–96. doi: 10.1007/s11605-013-2314-2. [DOI] [PubMed] [Google Scholar]
- 24.Vugts JJ, Gaspersz MP, Roos E, Franken LF, Olthof PB, Coelen RJ, et al. Eligibility for Liver Transplantation in Patients with Perihilar Cholangiocarcinoma. Ann Surg Oncol. 2020 Sep; doi: 10.1245/s10434-020-09001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franken LC, van der Poel MJ, Latenstein AE, Zwart MJ, Roos E, Busch OR, et al. Minimally invasive surgery for perihilar cholangiocarcinoma: a systematic review. J Robot Surg. 2019 Dec;13((6)):717–27. doi: 10.1007/s11701-019-00964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coelen RJ, Ruys AT, Besselink MG, Busch OR, van Gulik TM. Diagnostic accuracy of staging laparoscopy for detecting metastasized or locally advanced perihilar cholangiocarcinoma: a systematic review and meta-analysis. Surg Endosc. 2016 Oct;30((10)):4163–73. doi: 10.1007/s00464-016-4788-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coelen RJ, Ruys AT, Wiggers JK, Nio CY, Verheij J, Gouma DJ, et al. Development of a Risk Score to Predict Detection of Metastasized or Locally Advanced Perihilar Cholangiocarcinoma at Staging Laparoscopy. Ann Surg Oncol. 2016 Dec;23((S5 Suppl 5)):904–10. doi: 10.1245/s10434-016-5531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roos E, Franken LC, Soer EC, van Hooft JE, Takkenberg RB, Klumpen HJ, et al. Lost in translation: confusion on resection and dissection planes hampers the interpretation of pathology reports for perihilar cholangiocarcinoma. Virchows Archiv : an international journal of pathology. 2019;475((4)):435–43. doi: 10.1007/s00428-019-02621-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belkouz A, Nooijen LE, Riady H, Franken LC, van Oijen MG, Punt CJ, et al. Efficacy and safety of systemic induction therapy in initially unresectable locally advanced intrahepatic and perihilar cholangiocarcinoma: A systematic review. Cancer Treat Rev. 2020 Dec;91:102110. doi: 10.1016/j.ctrv.2020.102110. [DOI] [PubMed] [Google Scholar]
- 30.Frosio F, Mocchegiani F, Conte G, Bona ED, Vecchi A, Nicolini D, et al. Neoadjuvant therapy in the treatment of hilar cholangiocarcinoma: review of the literature. World J Gastrointest Surg. 2019 Jun;11((6)):279–86. doi: 10.4240/wjgs.v11.i6.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grendar J, Grendarova P, Sinha R, Dixon E. Neoadjuvant therapy for downstaging of locally advanced hilar cholangiocarcinoma: a systematic review. HPB (Oxford) 2014 Apr;16((4)):297–303. doi: 10.1111/hpb.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinant S, Gerhards MF, Busch OR, Obertop H, Gouma DJ, Van Gulik TM. The importance of complete excision of the caudate lobe in resection of hilar cholangiocarcinoma. HPB (Oxford) 2005;7((4)):263–7. doi: 10.1080/13651820500372376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999 Dec;230((6)):808–18. doi: 10.1097/00000658-199912000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franken LC, Olthof PB, Erdmann JI, van Delden OM, Verheij J, Besselink MG, et al. Short- and long-term outcomes after hemihepatectomy for perihilar cholangiocarcinoma: does left or right side matter? Hepatobiliary Surg Nutr. 2020 doi: 10.21037/hbsn-19-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bednarsch J, Czigany Z, Lurje I, Tacke F, Strnad P, Ulmer TF, et al. Left- versus right-sided hepatectomy with hilar en-bloc resection in perihilar cholangiocarcinoma. HPB (Oxford) 2020 Mar;22((3)):437–44. doi: 10.1016/j.hpb.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Pinotti E, Sandini M, Famularo S, Tamini N, Romano F, Gianotti L. Resection of the caudate lobe for the treatment of hilar cholangiocarcinoma. Minerva Chir. 2019 Aug;74((4)):348–58. doi: 10.23736/S0026-4733.18.07498-9. [DOI] [PubMed] [Google Scholar]
- 37.Mizuno T, Ebata T, Yokoyama Y, Igami T, Yamaguchi J, Onoe S, et al. Combined Vascular Resection for Locally Advanced Perihilar Cholangiocarcinoma. Ann Surg. 2020 doi: 10.1097/SLA.0000000000004322. doi:10.1097/SLA.0000000000004322 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.de Jong MC, Marques H, Clary BM, Bauer TW, Marsh JW, Ribero D, et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi-institutional analysis of 305 cases. Cancer. 2012 Oct;118((19)):4737–47. doi: 10.1002/cncr.27492. [DOI] [PubMed] [Google Scholar]
- 39.Neuhaus P, Thelen A, Jonas S, Puhl G, Denecke T, Veltzke-Schlieker W, et al. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann Surg Oncol. 2012 May;19((5)):1602–8. doi: 10.1245/s10434-011-2077-5. [DOI] [PubMed] [Google Scholar]
- 40.Matsuyama R, Mori R, Ota Y, Homma Y, Kumamoto T, Takeda K, et al. Significance of Vascular Resection and Reconstruction in Surgery for Hilar Cholangiocarcinoma: With Special Reference to Hepatic Arterial Resection and Reconstruction. Ann Surg Oncol. 2016 Aug;23((S4 Suppl 4)):475–84. doi: 10.1245/s10434-016-5381-2. [DOI] [PubMed] [Google Scholar]
- 41.Schimizzi GV, Jin LX, Davidson JT, 4th, Krasnick BA, Ethun CG, Pawlik TM, et al. Outcomes after vascular resection during curative-intent resection for hilar cholangiocarcinoma: a multi-institution study from the US extrahepatic biliary malignancy consortium. HPB (Oxford) 2018 Apr;20((4)):332–9. doi: 10.1016/j.hpb.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abbas S, Sandroussi C. Systematic review and meta-analysis of the role of vascular resection in the treatment of hilar cholangiocarcinoma. HPB (Oxford) 2013 Jul;15((7)):492–503. doi: 10.1111/j.1477-2574.2012.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mantel HT, Westerkamp AC, Sieders E, Peeters PM, de Jong KP, Boer MT, et al. Intraoperative frozen section analysis of the proximal bile ducts in hilar cholangiocarcinoma is of limited value. Cancer Med. 2016 Jul;5((7)):1373–80. doi: 10.1002/cam4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otsuka S, Ebata T, Yokoyama Y, Mizuno T, Tsukahara T, Shimoyama Y, et al. Clinical value of additional resection of a margin-positive distal bile duct in perihilar cholangiocarcinoma. Br J Surg. 2019 May;106((6)):774–82. doi: 10.1002/bjs.11125. [DOI] [PubMed] [Google Scholar]
- 45.Zhang XF, Squires MH, 3rd, Bagante F, Ethun CG, Salem A, Weber SM, et al. The Impact of Intraoperative Re-Resection of a Positive Bile Duct Margin on Clinical Outcomes for Hilar Cholangiocarcinoma. Ann Surg Oncol. 2018 May;25((5)):1140–9. doi: 10.1245/s10434-018-6382-0. [DOI] [PubMed] [Google Scholar]
- 46.Endo I, House MG, Klimstra DS, Gönen M, D'Angelica M, Dematteo RP, et al. Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann Surg Oncol. 2008 Aug;15((8)):2104–12. doi: 10.1245/s10434-008-0003-2. [DOI] [PubMed] [Google Scholar]
- 47.Ribero D, Amisano M, Lo Tesoriere R, Rosso S, Ferrero A, Capussotti L. Additional resection of an intraoperative margin-positive proximal bile duct improves survival in patients with hilar cholangiocarcinoma. Ann Surg. 2011 Nov;254((5)):776–81. doi: 10.1097/SLA.0b013e3182368f85. [DOI] [PubMed] [Google Scholar]
- 48.Shingu Y, Ebata T, Nishio H, Igami T, Shimoyama Y, Nagino M. Clinical value of additional resection of a margin-positive proximal bile duct in hilar cholangiocarcinoma. Surgery. 2010 Jan;147((1)):49–56. doi: 10.1016/j.surg.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 49.D'Souza MA, Valdimarsson VT, Campagnaro T, Cauchy F, Chatzizacharias NA, D'Hondt M, et al. E-AHPBA scientific and research committee Hepatopancreatoduodenectomy -a controversial treatment for bile duct and gallbladder cancer from a European perspective. HPB (Oxford) 2020 Sep;22((9)):1339–48. doi: 10.1016/j.hpb.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Aoki T, Sakamoto Y, Kohno Y, Akamatsu N, Kaneko J, Sugawara Y, et al. Hepatopancreaticoduodenectomy for Biliary Cancer: Strategies for Near-zero Operative Mortality and Acceptable Long-term Outcome. Ann Surg. 2018 Feb;267((2)):332–7. doi: 10.1097/SLA.0000000000002059. [DOI] [PubMed] [Google Scholar]
- 51.Ke Q, Chen Y, Huang Q, Lin N, Wang L, Liu J. Does additional resection of a positive microscopic ductal margin benefit patients with perihilar cholangiocarcinoma: A systematic review and meta-analysis. PLoS One. 2020 May;15((5)):e0232590. doi: 10.1371/journal.pone.0232590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawasaki S, Imamura H, Kobayashi A, Noike T, Miwa S, Miyagawa S. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003 Jul;238((1)):84–92. doi: 10.1097/01.SLA.0000074984.83031.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groot Koerkamp B, Wiggers JK, Gonen M, Doussot A, Allen PJ, Besselink MG, et al. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann Oncol. 2015 Sep;26((9)):1930–5. doi: 10.1093/annonc/mdv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Groot Koerkamp B, Wiggers JK, Allen PJ, Besselink MG, Blumgart LH, Busch OR, et al. Recurrence Rate and Pattern of Perihilar Cholangiocarcinoma after Curative Intent Resection. J Am Coll Surg. 2015 Dec;221((6)):1041–9. doi: 10.1016/j.jamcollsurg.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bird NT, McKenna A, Dodd J, Poston G, Jones R, Malik H. Meta-analysis of prognostic factors for overall survival in patients with resected hilar cholangiocarcinoma. Br J Surg. 2018 Oct;105((11)):1408–16. doi: 10.1002/bjs.10921. [DOI] [PubMed] [Google Scholar]
- 56.Ma WJ, Wu ZR, Hu HJ, Wang JK, Yin CH, Shi YJ, et al. Extended Lymphadenectomy Versus Regional Lymphadenectomy in Resectable Hilar Cholangiocarcinoma. J Gastrointest Surg. 2020 Jul;24((7)):1619–29. doi: 10.1007/s11605-019-04244-7. [DOI] [PubMed] [Google Scholar]
- 57.Li J, Zhou MH, Ma WJ, Li FY, Deng YL. Extended lymphadenectomy in hilar cholangiocarcinoma: what it will bring? World J Gastroenterol. 2020 Jun;26((24)):3318–25. doi: 10.3748/wjg.v26.i24.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franken LC, Schreuder AM, Roos E, van Dieren S, Busch OR, Besselink MG, et al. Morbidity and mortality after major liver resection in patients with perihilar cholangiocarcinoma: A systematic review and meta-analysis. Surgery. 2019 May;165((5)):918–28. doi: 10.1016/j.surg.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 59.Nuzzo G, Giuliante F, Ardito F, Giovannini I, Aldrighetti L, Belli G, et al. Italian Chapter of the International Hepato-Pancreato-Biliary Association Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012 Jan;147((1)):26–34. doi: 10.1001/archsurg.2011.771. [DOI] [PubMed] [Google Scholar]