Abstract
Objective
To present an interesting case of unusual side effect of Mycobacterium W. in an adult COVID 19 positive male and discuss its assessment and management.
Methods
-
Design
Case Report.
Setting
Tertiary care hospital.
Patient
One.
Results
70 years male was admitted with complaints of fever, persistent dry cough since 10–12 days and progressive breathlessness since 3–4 days. Patient was found COVID-19 RTPCR positive and is known case of Type-II Diabetes with CAD (Post PTCA). Patient was managed conservatively with Oxygen support, I/V antibiotics, I/V Steroids, oral Favipiravir and other supportive treatment.
Patient was also given injection Mycobacterium W. in dose of 0.3 ml per day intradermally at 3 different sites (both deltoids) consecutively for three days. 7–8 days after administration, patient developed bright red pustules which later got converted into small punched out ulcerations on all nine local sites of administration, which were managed conservatively with oral analgesics and local steroids for 8–10 days which healed without any scar formation.
Conclusion
Injection Mycobacterium W. is used in COVID 19 patients as an immunomodulator agent and has been proved to be safe in most of the cases but we encountered this unusual side effect of bright red pustules formation at all nine local sites of injection in our case most likely because of being administered subcutaneously instead of intradermally, making this an interesting case which is being reported to scientific fraternity.
Keywords: Mycobacterium W, COVID-19, Intradermal, Adverse site reaction
1. Introduction
Coronaviruses are enveloped non segmented positive-sense RNA viruses belonging to the family coronaviridae and the order Nidovales and broadly distributed in humans and other mammals. Although most human coronavirus infections are mild, the epidemics of the two betacoronaviruses, severe acute respiratory syndrome coronavirus (SARS- CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), have caused more than 10,000 cumulative cases in the past two decades, with mortality rates of 10% for SARS-CoV and 37% for MERS-CoV. The coronaviruses already identified might only be the tip of the iceberg, with potentially more novel and severe zoonotic events to be revealed.
2. Case history
A 70 years male was admitted with complaints of fever, persistent dry cough since 10–12 days and progressive breathlessness since 3–4 days. Patient was found COVID-19 RTPCR positive and is known case of Type-II Diabetes with CAD (Post PTCA). On examination, vitals were stable, maintaining SpO2 94% on room air, on chest examination bilateral crepts present, rest systemic examination was within normal limits. Patient was managed conservatively with Oxygen support, I/V antibiotics, I/V Steroids, oral Favipiravir and other supportive treatment.
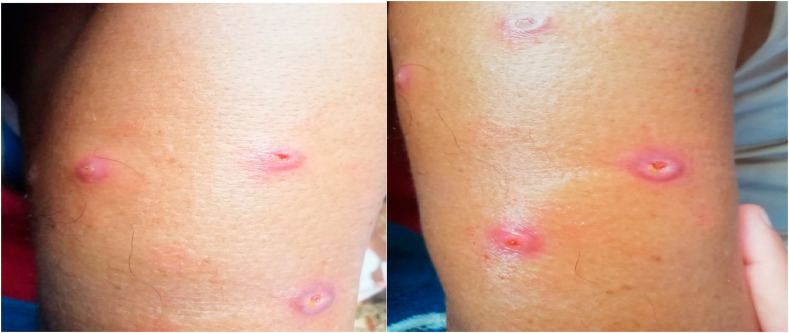
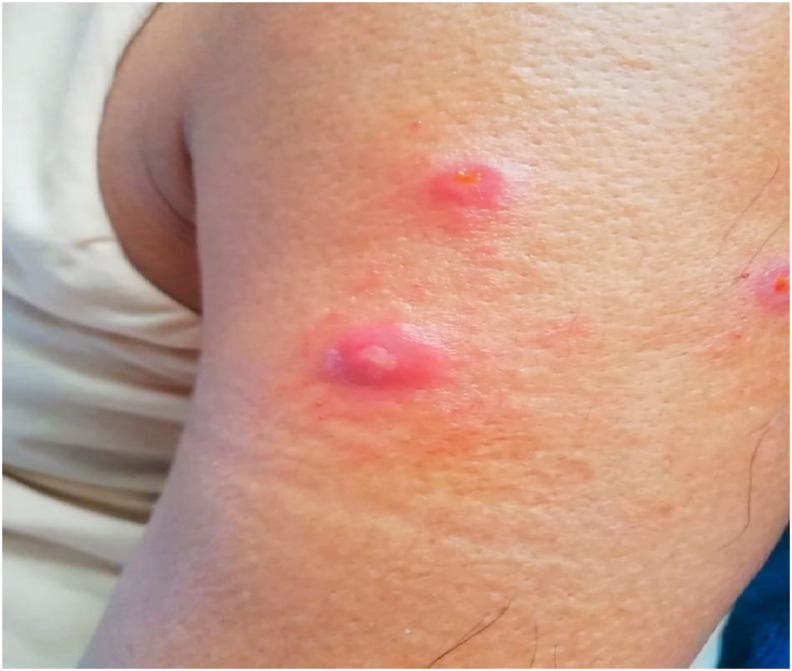
Patient was also given injection Mycobacterium W. 0.3 ml per day intradermally at 3 different sites (both deltoids) consecutively for three days. 7–8 days after administration, patient developed bright red pustules which later got converted into small punched out ulcerations on all nine local sites of administration (Fig. 1, Fig. 2 ). Lesions were painful, tender on touch, non-discharging in nature, local erythema present, with no signs of systemic inflammation, managed conservatively with oral analgesics and local steroids for 8–10 days which healed without any scar formation.
Fig. 1.
Bright red pustules formation at six local sites of administration (right deltoid).
Fig. 2.
Bright red pustules formation at three local sites of administration (left deltoid).
3. Discussion
Patients with COVID requiring intensive care unit (ICU) admission have higher
cytokine levels compared to those who do not need ICU care.1 Even among patients admitted to ICU, those discharged from hospital had lower cytokine levels compared to those who died.2 An immunomodulator may thus be of potential benefit in managing these critically ill COVID patients. The
Global Research Collaboration for Infectious Disease Preparedness (GLOPID-R) and the World Health Organization have identified adjuvant therapy as one of the key areas of research to save lives of patients infected with COVID-19.3 A heat-killed Mycobacterium w (Mw), originally developed as an
immunomodulator for leprosy, which acts through the toll-like receptors (TLRs) pathway and enhances the host-T cell responses.4 Sehgal IS et al showed the benefit of Mw in patients with severe sepsis.5 Use of Mw in COVID-19 patients was observed to be safe and well tolerated, without any major safety
concerns in patients with COVID-19.6
Herein our case report, we are discussing the issue arising due to use of Injection Mycobacterium W. in COVID 19 patients. Patient developed bright red pustules which later got converted into small punched out ulcerations on all nine local sites of administration which were painful, tender on touch, non-discharging in nature, local erythema present, with no signs of systemic inflammation, managed conservatively.
Local site reactions were observed at the site of injection of Mw in 85.47% of the patients. Out of which majority of the patients had mild reaction (54%). Patients developed erythema at the site of injection which was followed by development of induration and pustule formation. This was converted in to a small punched out ulceration which healed by a formation of healthy scar without the need of any specific treatment.6
Injections site immunological reaction to Mw is a known phenomenon.7 Sharma SK et al reported injection site reaction in 82.4% of the patients. 68% of the patients experienced mild intensity of the reaction whereas 12.91% had moderate to severe reaction at local site.8 d’Aleo F reported exaggerated response at the injection when BCG was administered into the subcutaneous space instead of intradermal inoculation which resulted in development of local abscess.9 Looking in to the skills required for the intradermal injection and paramedical staff working with PPE kits on, we believe there are chances of erroneous injection of Mw in to the subcutaneous space, instead of intradermal space, in some of the patients. We are reporting this case to make clinicians aware that this can also occur as adverse site reaction which resolved with oral anti-inflammatory drugs and local steroids for 8–10 days.
We used Injection Mycobacterium W. in COVID 19 patients as per protocol developed in our hospital, but patient developed this adverse site reaction most likely because of being administered subcutaneously instead of intradermally making this an interesting case, hence it is being reported to the scientific community.
4. Conclusion
Injection Mycobacterium W. is used in COVID 19 patients as an immunomodulator agent and proved to be safe in most of the cases, in our case patient had this unusual side effect most likely because of being administered subcutaneously instead of intradermally making this an interesting case to be reported to the scientific community and make clinicians aware of this possible unusual side effect of the forementioned injection.
Conflicts of interest
The authors have none to declare.
References
- 1.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [PMCID: PMC7159299] [PubMed: 31986264] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. doi:101007/s00134-020-05991-x. [PMCID: PMC7080116] [PubMed: 32125452] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID 19 public health emergency of international concern global research and innovation forum: towards a research roadmap. 2020. https://wwwwhoint/blueprint/priority-diseases/keyaction/Global_Research_Forum_FINAL_VERSION_for_web_14_feb_2020pdfua=1 [Last accessed on 2020 Mar 23]. Available from: [Google Scholar]
- 4.Desai N.M., Khamar B.M. Immunotherapy for tuberculous pericarditis. N Engl J Med. 2014;371:2533–2534. doi: 10.1056/NEJMc1413185. [PubMed: 25539119] [DOI] [PubMed] [Google Scholar]
- 5.Sehgal I.S., Agarwal R., Aggarwal A.N., Jindal S.K. A randomized trial of Mycobacterium w in severe sepsis. J Crit Care. 2015;30:85–89. doi: 10.1016/j.jcrc.2014.08.012. [PubMed: 25241089] [DOI] [PubMed] [Google Scholar]
- 6.Ingale A., Ingale F., Kunwar B., et al. Role of Mycobacterium w for the treatment of COVID-19: an observational study. Japi. 2021 Jan;69(2747444):1. [PubMed] [Google Scholar]
- 7.Walia R., Sarathchandra K.G., Pandey R.M., et al. Field trials on the use of Mycobacterium w vaccine in conjunction with multidrug therapy in leprosy patients for immunotherapeutic and immunoprophylactic purposes. Lepr Rev. 1993;64:302–311. doi: 10.5935/0305-7518.19930034. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S.K., Katoch K., Sarin R., et al. Efficacy and Safety of Mycobacterium indicus pranii as an adjunct therapy in Category II pulmonary tuberculosis in a randomized trial. Sci Rep. 2017;7:3354. doi: 10.1038/s41598-017-03514-1. PMID: 28611374; PMCID: PMC5469738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.d'Aleo F., Bonanno R., Constatino A.L.P., et al. A case of abscess after BCG vaccine in an immunocompetent child without other clinical signs. JMM Case Rep. 2015;2:1–3. doi: 10.1099/jmmcr.0.000103. [DOI] [Google Scholar]