Abstract
OBJECTIVES:
To identify whether delirium biomarkers aligned with the National Institute on Aging-Alzheimer’s Association (NIA-AA) research framework, a conceptual model which describes the use of diagnostic biomarkers for Alzheimer’s disease and other related dementias (ADRD).
DESIGN:
Systematic review following PRISMA guidelines
SETTING:
Acute care and outpatient settings
PARTICIPANTS:
Adults diagnosed with delirium
METHODS AND MEASUREMENTS:
MEDLINE, PsycInfo, Embase, and the Cochrane Library were searched for English‐language studies published from January 2010 to February 2020. Studies included adults older than 18 years, identified delirium with a standardized assessment tool, and measured an ADRD biomarker. Independent reviewers determined whether an association between delirium and ADRD biomarker was found, the quality of biomarker data based on the REMARK checklist, and the study bias based on the Newcastle Ottawa Scale.
RESULTS:
A total of 61,256 citations were identified; 113 studies were included. Most studies did not examine amyloid, tau, or neurodegeneration biomarkers. Delirium may be associated with neurodegeneration biomarkers, but few to no studies found an association with amyloid and tau biomarkers. Delirium was not consistently associated with inflammatory biomarkers. The quality of biomarker data was moderate, and the risk of bias was moderate to high. Studies often did not collect pre- and post-hospital cognitive data.
CONCLUSION:
Most delirium diagnostic biomarker studies did not measure amyloid, tau, and/or neurodegenerative biomarkers, making characterization of the relationship between delirium and ADRD difficult. Future delirium biomarker diagnostic studies could improve the understanding of pathophysiologic links between delirium with other conditions affecting cognition.
Keywords: delirium, Alzheimer’s disease, mild cognitive impairment, preclinical Alzheimer’s disease, biomarkers, inflammation, neuroimaging
INTRODUCTION
The National Institute on Aging-Alzheimer’s Association (NIA-AA) research framework describes a novel concept of diagnosing Alzheimer’s disease (AD) by using disease state biomarkers, which are classified into the categories amyloid (A), tau (T), and neurodegeneration (N).1 This research framework represents a paradigm shift from the traditional diagnostic approach of AD based on clinical symptoms towards a biologically driven approach designed to detect AD pathophysiology in its earliest stages before symptoms arise. One possible application of this framework is the early detection of neurodegenerative processes in those diagnosed with neuropsychiatric disorders which are associated with a higher risk of Alzheimer’s disease and other related dementias (ADRD). One such disorder may be delirium. Delirium is defined as an acute change in attention and consciousness with a fluctuating course, and affects 14–56% of all hospitalized older adults and costs the US $152 billion each year.2–3 Patients who experience an episode of delirium are at a higher risk of developing subsequent ADRD.4–6
Better characterization of this interrelationship could advance our understanding of whether the pathophysiology of delirium overlaps with ADRD. To more deeply understand how well integrated the literature for delirium disease state biomarkers is with the NIA-AA framework, we conducted a systematic review on disease state biomarkers of delirium and examined their alignment with the NIA-AA framework.
METHODS
Eligibility and Search Strategy
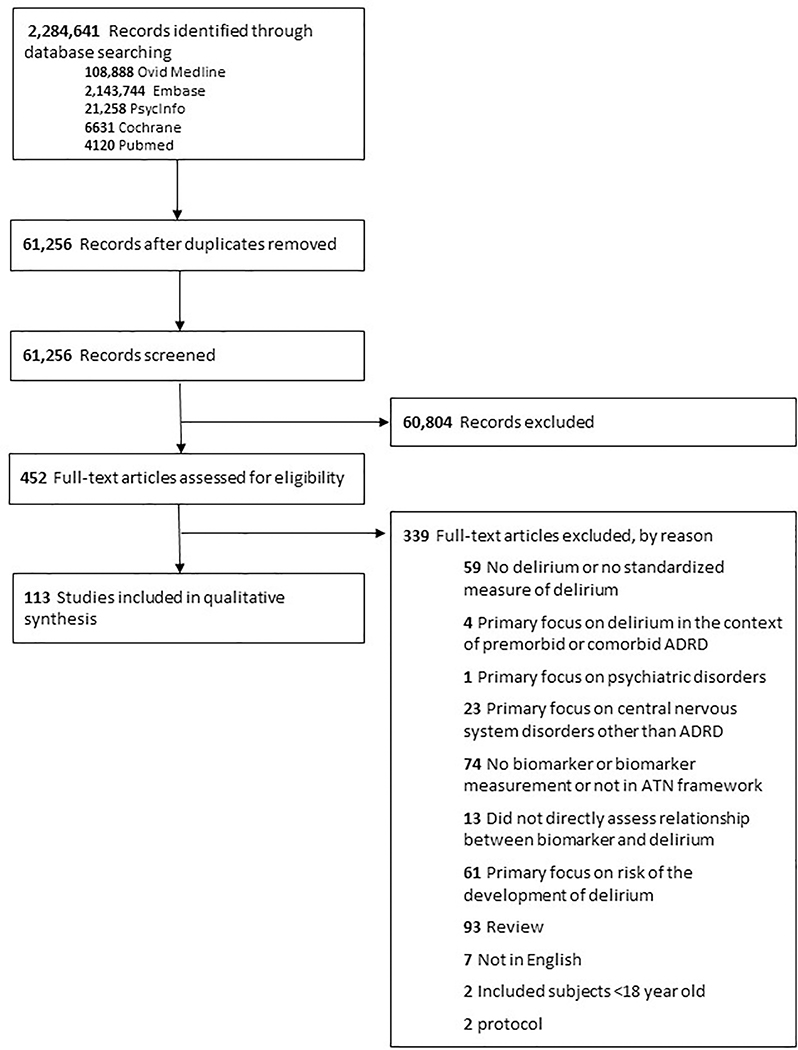
PRISMA guidelines were followed to design and conduct this systematic review.7–8 Ovid MEDLINE, PsycInfo, Embase, and the Cochrane Library were searched from January 1, 2000, through February 20, 2020, using a combination of controlled vocabulary and keyword terms developed in collaboration with a medical librarian and content experts in ADRD and delirium. The overall search strategy was designed to ensure citations included both the concept of delirium and AT(N)-X (X = other) biomarkers. The complete list of search strategies and a flow diagram are provided in Supplementary Table S1 and Figure 1.
Figure 1:
PRISMA Flow Search Strategy
Study inclusion criteria were: 1) age ≥ 18 years old, 2) used standardized delirium screening tools or diagnostic assessments, and 3) biomarker measurements listed in NIA-AA framework (Supplementary Table S1). Study exclusion criteria were: 1) delirium not included or measured using standardized screening tools or diagnostic assessments, 2) Age < 18 years old, 3) biomarker measurements not aligned with AT(N)-X categories (Supplementary Table S1), 4) primary focus on delirium in the following contexts: 4a) premorbid or comorbid ADRD (i.e. delirium superimposed on dementia); 4b) psychiatric disorders (e.g. alcohol dependence, alcohol withdrawal, major depressive disorder, bipolar disorder, and psychotic disorders) 4c) central nervous system disorders other than ADRD; 4d) terminal illness (palliative care or hospice services); 4e) persistent delirium (etiology may be different); 4f) long-term care settings; 4g) risk of delirium; 5) non-human studies; and 6) non-English articles.
Study Selection
The study selection procedures were conducted with independent, blinded reviewers and discrepancies were evaluated by consensus panels. Following exclusion of citations based on title and abstract review, the full-text was independently assessed for the primary outcomes (associations between delirium and biomarkers) and secondary outcomes (quality of the studies and risk of bias). Included and excluded studies are illustrated per PRISMA in Figure 1.
Primary Outcome
Primary outcome was defined as the association between delirium and the AT(N)-X biomarkers. For the determination of the association between delirium and the biomarker, each biomarker was categorized by the biomarker group (A, T, N, X), the method of measurement, and the presence or absence of a statistically significant association (p ≤ 0.05). Subcategories of X included inflammation, vascular, synucleinopathy, frontotemporal dementia, endothelial cell-cell adhesion molecule (EC-CAM) and neuronal function.
Secondary Outcomes
The secondary outcomes were: 1) the quality of studies and risk of bias, 2) description of delirium assessment, and 3) description of pre- and post-hospital cognitive assessments. Study quality was determined using two measurements, the REMARK (REporting recommendations for tumour MARKer prognostic studies) checklist and the Newcastle Ottawa Scale (NOS). Two quality scales were chosen because the modified REMARK checklist is focused on the quality of methodology, reporting, and interpretation of biomarker studies, whereas the NOS focused on cohort study design and risk of bias.9–11 Modifications were made to the REMARK checklist to account for differing biomarker measurements, including neuroimaging and electroencephalography. A 25-point scale to measure REMARK study quality was used (Supplementary Table S2). The quality of a study per REMARK checklist score was categorized as follows: 1) low < 12.5 points, 2) moderate 12.5–18.75 points, and 3) high > 18.75 points. Information about study design, including selection of subjects, comparability of findings, and determination of exposure to delirium, were extracted per the NOS guidelines for cohort and case control studies. A 9-point scale was used to determine the study quality and categorized as follows: 1) low < 5 points, 2) moderate 5–7 points, and 3) high > 7 points.12
The mean, standard deviation (SD), and range for the REMARK checklist and NOS scores were calculated for all 113 studies and for each of the AT(N)-X biomarker. SPSS 26.0 did the analysis.
The following data were extracted to describe delirium assessment: tool(s) used, frequency of assessment, who conducted the assessment, and characterization (i.e. subtypes, severity, and duration) and its relationship with biomarker(s). The following data were extracted to describe pre-hospital cognitive assessment: if completed (yes/no), approach used (e.g. chart review, structured tool and/or neuropsychological testing), and whether consensus was completed. Post-hospital cognitive assessment (yes/no) was extracted.
RESULTS
Study Selection
One hundred thirteen full text articles were selected for inclusion. The initial search resulted in 61,256 titles, with 452 identified for full-text review as detailed in Figure 1.
Study Characteristics
The study designs included 7 clinical trials (2 randomized controlled trial (RCT), 5 secondary analysis of an RCT), 89 prospective cohort studies, 4 case-control studies, 4 retrospective chart reviews, and 9 other types (Supplementary Table S3). These studies included 13,809 participants, of which 9,174 (66.4%) had delirium. Studies were conducted mostly in Europe (N = 57, 50.4%) and North America (N = 31, 27.4%), and in urban areas (N = 87, 77.0%) at academic hospitals (n = 97, 85.8%) (Supplementary Table S3). Studies were divided between intensive care unit (ICU) (n = 55, 48.7%) and non-ICU settings (N = 58, 51.3%)). Of the included studies, 75.2% (N = 85) reported medical comorbidities, 65.4% (N = 74) reported a severity of illness measurement, 19.4% (N = 22) reported education levels, and 6.2% (N = 7) studies reported on race/ethnicity with mostly Caucasian subjects (no delirium = 80–98%, delirium = 51–100%). Types of biomarker measurements included fluid biomarkers (blood-based and CSF, N = 80, 70.8%), neuroimaging (N = 11, 9.7%), post-mortem examination (N = 3, 2.7%), electroencephalography (N = 19, 16.8%), and one study combined fluid biomarkers with EEG.13
Association between Delirium and Biomarkers
Table 1 summarizes the presence or absence of significant associations between ATN(X) biomarkers with delirium. Associations between biomarkers and delirium for individual studies and quality of studies are listed Supplementary Tables S4–S7. Twenty-three studies (20.4%) investigated at least one biomarker within the amyloid14–21 (A, N = 8), tau17–21 (T, N = 4), and neurodegeneration14,16,17,21–35 (N, N = 18) framework. Three out of the eight studies reported a significant association between delirium and amyloid biomarkers. No studies reported a significant association between delirium and tau biomarkers. Thirteen out of 18 studies (72.2%) reported a relationship between delirium and various neurodegeneration biomarkers.
Table 1.
Association between ATN-X Biomarkers and Delirium
| Likely Association with Delirium | Equivocal | No Likely Association with Delirium |
|---|---|---|
| Neurodegeneration IL-6 IL-8 C-reactive protein Synaptic function (EEG and fMRI) |
Amyloid S-100B Vascular |
Tau IL-1 beta IL-10 Tumor necrosis factor-alpha |
FTD, EC-CAM, synucleinopathy, and actigraphy studies were not included because there were less than 4 studies.
Only inflammatory biomarkers with 15 or more articles are included in this table.
Likely association with delirium was defined as > 50% of studies finding an association between the biomarker or category of biomarkers and the presence of delirium.
Equivocal was defined as 25–50% of studies finding an association between the biomarker or category of biomarkers and the presence of delirium.
No likely association with delirium was defined < 25% of studies finding an association between the biomarker or category of biomarkers and the presence of delirium.
The majority of included studies examined “X” biomarkers, which were divided into the following subcategories: inflammation13–16,18,22–25,27–30,32 (N = 76), vascular19–21,34 (N = 6), synucleinopathy19,20 (N = 2), frontotemporal dementia (FTD) (N = 1), endothelial cell-cell adhesion molecule (EC-CAM) (N = 1), neuronal function (N = 22), and sleep (N = 4). (Details about X biomarkers are listed in Supplementary Table S5–8.)
Only six inflammatory biomarkers had 15 or more studies: interleukin (IL)-1β14,16,18,32, IL-613–16,18,28,32, IL-814,32, tumor necrosis factor (TNF)-α14,16, IL-1014,16, CRP14,15,18,25, and S100B14,22–25,28,30. Three inflammatory biomarkers showed minimal associations with delirium (<25% reported positive relationship): 1) IL-1β (3/19, 15.8%); 2) tumor necrosis factor (TNF)-α (5/21, 23.8%); and 3) IL-10 (3/15, 20%). S-100B was equivocal (9/19, 47.3%). Three inflammatory biomarkers showed a moderate trend (>50% reported a positive relationship) towards an association with delirium: 1) IL-6 (18/31, 58.1%); 2) IL-8 (9/16, 56.3%), and; 3) C-reactive protein (CRP) (22/37, 59.5%). Three out of six studies found a relationship between delirium and vascular biomarkers. Other less frequently occurring biomarkers are described in the supplementary material (Supplementary Table 6). There were no associations between delirium and markers of synucleinopathy or EC-CAM. Nineteen out of twenty-two studies examining synaptic function found an association between delirium and EEG (17/19, 89%) or fMRI (2/3, 67%) biomarkers. Two out of four actigraphy studies found a relationship between delirium and measures of sleep. (Details about X biomarkers are listed in Supplementary Table S5–8.)
Quality of Studies and Assessment of Risk Bias
The studies were of moderate quality, as indicated by mean scores (SD) of 15.68 (3.76) for the REMARK checklist and 6.68 (1.48) for NOS indicating a potential for moderate to high risk of bias. REMARK checklist items most often not reported include sample size calculations, statistical methods including the handling of missing data, cut-off points for biomarkers, and effect sizes of findings (Supplementary Figure S1).
Moderate study quality was indicated in most AT(N)-X categories (mean (SD) REMARK and NOS): Amyloid 17.25 (2.24) and 7.50 (0.93); Tau 18.00 (1.78) and 7.25 (0.96); Neurodegeneration,16.69 (3.27) and 6.57 (1.29). And for the X category: Inflammation (15.39 (3.71) and 6.77 (1.48)), neuronal function (15.20 (3.60) and 6.36 (1.50), and sleep (14.75 (6.59) and 5.75 (2.87)) categories. High study quality was indicated for vascular (19.67 (1.33) and 6.83 (0.41)), synucleinopathy (19.25 (1.77) and 6.50 (0.71)), FTD (18.50 (2.83) and 7.50 (0.71)), and EC-CAM (17.50 and 7.00).
Table 2 shows the numbers of studies with significant associations aligned with the AT(N)-X framework, categorized by REMARK and NOS scores for studies. Supplementary Tables S4–S7 show the REMARK and NOS individual scores for studies.
Table 2.
Association and Study Quality Based on the REMARK Checklist and Newcastle Ottawa Scale (NOS) for Selected ATN-(X) Biomarkers
| Study Quality | ||||||
|---|---|---|---|---|---|---|
| REMARK | NOS | |||||
| Biomarker | Low | Medium | High | Low | Medium | High |
| Amyloid (A) biomarkers | ||||||
| Fluid-based amyloid | 0/0 | 2/3 | 1/1 | 0/0 | 0/0 | 3/4 |
| Amyloid Plaques | 0/1 | 0/2 | 0/1 | 0/1 | 0/3 | 0/0 |
| Tau (T) biomarkers | ||||||
| Phosphorylated tau | 0/0 | 0/1 | 0/0 | 0/0 | 0/0 | 0/1 |
| Neurofibrillary tangles | 0/1 | 0/1 | 0/1 | 0/1 | 0/2 | 0/0 |
| Neurodegenerative (N) biomarkers | ||||||
| Neuron Specific Enolase (NSE) | 2/3 | 2/5 | 1/1 | 1/2 | 4/5 | 0/2 |
| Neurofilament Light (NFL) | 0/0 | 1/1 | 1/1 | 0/0 | 1/1 | 1/1 |
| Total Tau | 0/0 | 2/2 | 0/1 | 0/0 | 0/1 | 2/2 |
| Atrophy or hypometabolism | 0/0 | 2/2 | 2/2 | 0/0 | 4/4 | 0/0 |
| Inflammatory | ||||||
| IL-1β | 1/5 | 1/11 | 1/2 | 1/1 | 1/13 | 1/5 |
| IL-6 | 5/8 | 11/19 | 2/4 | 4/4 | 7/17 | 7/10 |
| IL-8 | 1/9 | 11/25 | 3/3 | 0/2 | 9/23 | 6/12 |
| IL-10 | 0/4 | 2/9 | 1/2 | 0/1 | 2/9 | 1/5 |
| CRP | 4/10 | 12/20 | 6/7 | 0/4 | 10/19 | 11/14 |
| S100B | 1/5 | 5/9 | 3/5 | 1/5 | 3/6 | 5/8 |
| TNFα | 0/5 | 2/13 | 1/1 | 3/19 | 0/0 | 0/0 |
| Vascular | ||||||
| Post-mortem lesions | 0/0 | 0/1 | 0/1 | 0/0 | 0/2 | 0/0 |
| MRI | 0/0 | 1/1 | 2/3 | 0/0 | 3/4 | 0/0 |
| Synucleinopathy | 0/0 | 0/1 | 0/1 | 0/0 | 0/2 | 0/0 |
| EC-CAM | 0/0 | 0/1 | 0/0 | 0/0 | 0/1 | 0/0 |
| Synaptic Function | ||||||
| EEG | 4/4 | 6/7 | 3/3 | 2/2 | 4/5 | 7/7 |
| Bispectral EEG | 2/2 | 2/3 | 0/0 | 0/0 | 4/5 | 0/0 |
| fMRI | 0/0 | 2/3 | 0/0 | 2/2 | 0/1 | 0/0 |
EEG = electroencephalogram; fMRI = functional magnetic resonance imaging
The numerator represents number of studies that found an association between biomarker and delirium. The denominator represents the number of studies in that category of study quality.
The REMARK (Reporting Recommendations for Tumor Marker Prognostic Studies) checklist is scored on a range of 0–25 checklist score, and categorized as L = low, < 12.5 points; M = moderate, 12.5–18.75 points; H = high > 18.75 points. The Newcastle Ottawa Scale (NOS) score has a range of 1–9 and was categorized as L = low, < 5 points; M = moderate, 5–7 points; H = high, > 7 points.
Delirium Assessment
The Confusion Assessment Method (CAM, N = 46) or one of its variants (CAM-ICU (N = 46) and 3D-CAM (N = 2)) were the most commonly used delirium assessments (83.2%). Delirium administration in general care was approximately once a day, whereas for the ICU setting it was 1–2 times per day. The median duration for delirium assessments for both general and ICU settings was 6 days. 46.0% of studies (N = 52) reported the duration of delirium assessment and 66.4% of studies (N = 75) reported who conducted the delirium assessment. Geriatricians were more frequently reported conducting assessments in general care compared to ICU (16.7% vs 0.0%), and bedside clinicians were more frequently reported to do assessments in ICU compared to non-ICU (28.2% vs 5.6%). Only 32.7% (N = 37) of studies collected additional information about delirium subtypes (N = 16, 14.2%), or examined the association between delirium severity (N = 21, 18.6%) or duration (N = 12, 10.6%) and biomarkers. Supplementary Tables S9–S11 describe further information on types of validated delirium tools used, frequency of delirium assessment, and personnel conducting assessments.
Cognitive Assessment
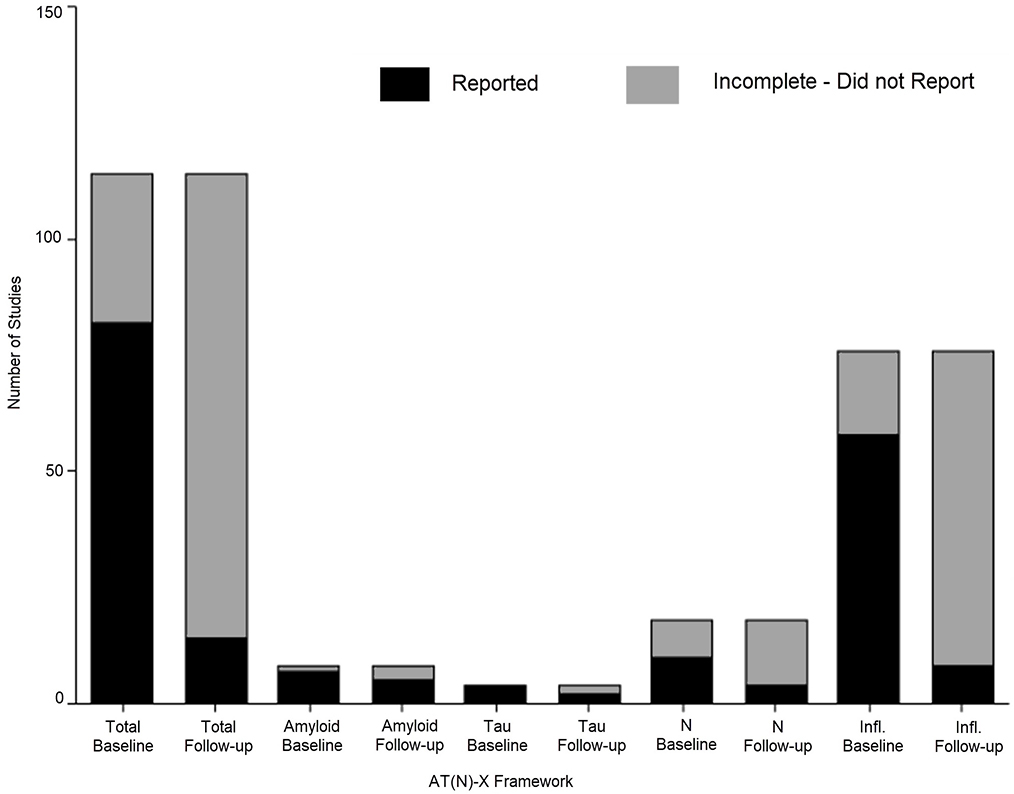
Supplementary Table S12 provides detailed information about pre- and post-hospital cognitive assessments. Pre-existing cognitive status was assessed in 62.8% (N = 71). These studies used at least one of the following approaches: a validated tool (N = 45), informant report (n = 31), or medical history review (N = 16). The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (N = 27) and Mini-Mental State Examination (MMSE) (N = 25) were most frequently used assessment tools for pre-hospital cognition. Post-hospital cognition was assessed in 12.4% (N = 14) studies. Figure 2 shows completion for pre-hospital and post-hospital cognitive assessments for the AT(N) and inflammatory biomarker categories.
Figure 2:
Number of Completed Pre-Hospital and Post-Hospital Cognitive Assessments
DISCUSSION
The NIA-AA framework was designed to provide a deeper understanding of the pathophysiology of Alzheimer’s disease. While epidemiologic studies suggest Alzheimer’s disease and other related dementias are connected to other disorders such as delirium and depression, understanding these relationships through the lens of the NIA-AA framework is in the early stages. This systematic review identified 113 delirium biomarker studies that aligned with the NIA-AA framework. However, only 20% of these studies measured amyloid, tau, and/or neurodegeneration biomarkers. Most studies examined “X” category biomarkers, most notably inflammatory cytokines. Moderate study quality and risk of bias were identified largely due to incomplete methodology and insufficient reporting of study conduct. This review identified important limitations to current delirium biomarker literature. More thorough characterization of delirium in the context of the AT(N) framework and standardized methods for biomarker data collection and for pre-and post-hospital cognitive assessment would enrich the understanding of the biological relationship between delirium and ADRD.
Delirium and NIA-AA Framework
The included studies suggest a potential relationship between delirium and neurodegeneration processes associated with ADRD. There is minimal support for a relationship between amyloid and delirium and no support for tau, as there were few studies that evaluated the relationship between delirium and these two categories of the NIA-AA framework. No studies examined biomarkers simultaneously in all of the AT(N) categories, precluding a deeper understanding of how the NIA-AA framework fits into the pathophysiology of delirium. Furthermore, the post-hospital course is incomplete since there were few longitudinal studies. Another methodological challenge is the navigation and balance of rigorous delirium assessment and thorough AD biomarker measurement. For example, while post-mortem studies are generally considered to be the gold standard biomarker for AD research, in these studies, researchers relied on patient and caregiver reports and medical chart documentation to diagnose delirium.19,20 This may lead to an underestimation of delirium prevalence and disproportionate representation of hyperactive delirium. Conversely, Simons et al. performed the CAM-ICU multiple times a day, which has higher validity for delirium assessment than chart review and informant reports and obtained a temporal course of biomarkers.16 However, the biomarkers were blood-based. Although the sensitivity of amyloid blood-based biomarkers is significantly improving, the test characteristics remain lower than brain-based biomarkers and post-mortem studies. Additionally, the timing of delirium assessments and biomarker collection were unclear in most studies. As the key features of delirium include an acute onset and fluctuating course, understanding the temporal relationship between the onset and resolution of symptoms and biomarkers is important. Overcoming these challenges will be critical to elucidating relationships between delirium and AT(N) biomarkers.
Delirium Etiologic Hypotheses
Neuroinflammation is one of the central hypotheses of delirium, which posits that an acute peripheral event, such as infection or surgery, triggers a systemic inflammatory response. The acute peripheral response leads the activation of microglia in the central nervous system resulting in neuroinflammation and symptoms of delirium.18 Three biomarkers show a moderate positive associative trend with delirium; IL-6, IL-8, and C-Reactive Protein (CRP). Each of these biomarkers has been tied to the neuroinflammatory pathway and neurodegeneration, not only in delirium, but also in ADRD and other neurodegenerative disorders.19–23 While these studies demonstrate a plausible pathway from delirium to continued neurodegeneration, it is important to consider the limitations. The included studies are limited by moderate study quality and risk of bias, are blood-based, and provide moderate support for involvement of one or more inflammatory pathways in the neuroinflammatory hypothesis of delirium. Few studies examined cerebrospinal fluid biomarkers and obtaining these data will be crucial to better understanding neuroinflammatory pathways and involvement. Further, future studies should consider measuring delirium severity and duration and exploring different subgroups and phenotypes of delirium. Both of these metrics provide the opportunity to investigate dose-dependent relationships between biomarker levels and the intensity of the delirious episode, as shown by recent studies.24–25
Electrophysiological studies investigating the underlying connectivity hypotheses of delirium have proliferated in recent years, with ten studies published in 2019. While a number of these studies’ central aim is to identify signature cortical patterns to identify or diagnose delirium, findings also reveal important insights into the neurobiological responses that are elemental to delirium.36 One of these insights is that cortical slow wave activity is a hallmark of delirium.37 A critical step in moving delirium etiology work forward will be the multimodal integration of biomarker measurement to begin to understand the interconnectedness of connectivity and neuroinflammation. A recent published study focused on the Cognitive Disintegration Theory of delirium demonstrates this approach, reporting that changes in connectivity significantly correlated with delirium severity, IL-10, and monocyte chemoattractant protein.38–39 NIA-AA framework may be a useful theoretical construct to methodically characterize current and novel biological pathways linking delirium and neurodegenerative processes in future research.
Implications for Future Study Design and Reporting
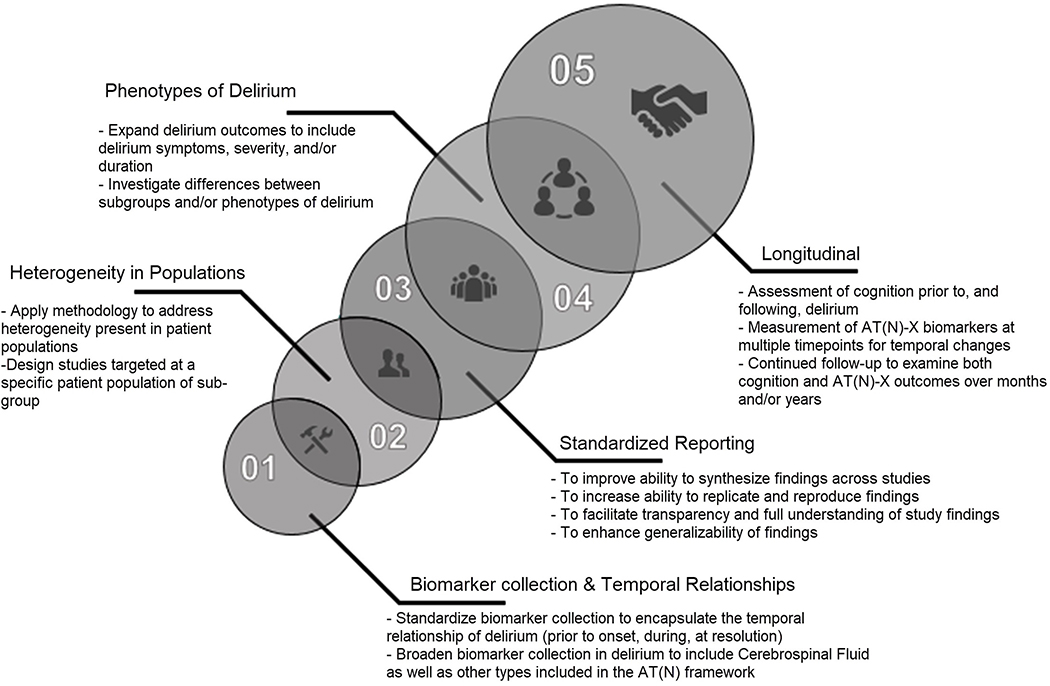
Figure 3 outlines the future directions for study design and reporting to better align delirium biomarkers with the NIA-AA framework. Longitudinal studies that incorporate rigorous measurement of pre- and post-cognition, delirium (incidence, severity, duration, and subtypes), and multimodal biomarker measurement are needed to move the science connecting delirium with ADRD forward. This systematic review identified four major areas that will need to be addressed to advance this research including: 1) methodology of biomarker collection and analysis including temporal relationships and reporting of results; 2) methodology to address heterogeneity of studied populations; 3) need for refinement of clinical presentations and measurements of delirium in relation to biomarkers; and 4) concurrent, longitudinal characterization of cognitive symptoms and biomarkers.
Figure 3:
Future Directions for Study Design and Reporting to Better Align Delirium Biomarkers with the NIA-AA Research Framework
Methodology of biomarker collection, analysis, and reporting needs to be standardized for delirium biomarker studies. This review was not able to synthesize findings because of the inconsistencies in reporting and methodology in delirium biomarker studies. These findings are supported by a recent systematic review and a subsequent study outlining best practice methods for delirium biomarker studies through a three-round Delphi survey.40–41 Future studies also need to consider the temporal relationship between biomarker measurement, delirium onset and resolution.
Methodology to address heterogeneity of studied populations is needed, because it remains unclear whether different precipitants of delirium (infection versus surgery) result in differing pathophysiology, e.g. postoperative delirium in a fairly healthy older adult versus a younger ICU patient with a complicated medical course. Future studies should consider focusing on a specific patient population, using statistical methods to control for differences, or using matched case-control design in a limited resource environment.42
The included studies used validated tools to identify delirium. Future studies need to continue this practice and incorporate refined measures of delirium. Few studies have examined the relationship between delirium subtypes, phenotypes, severity, duration, coma and biomarkers.16,23,32,60,80,123 The few that did investigate subgroups highlight important underlying relationships. Simons et al. found an association between Tau/Aβ1–42 biomarker for the hypoactive delirium subtype, but not other delirium subtypes.16 Regarding cognitive subgroups, Idland et al. found differences in CSF measurements of Aβ1–42 and Aβ42/AβX-40 for premorbid non-demented subjects who had delirium but not those with premorbid dementia.17 These subgroup differences highlight the importance of delirium subgroup or phenotype characterization along with premorbid cognitive status when examining biomarker relationships.
Finally, longitudinal studies which characterize pre- and post-hospital cognition and concurrent ADRD biomarker measurements will provide invaluable insight about the context in which delirium occurs. Longitudinal characterization of pre-hospital biomarker and cognitive status may clarify whether the observed differences represent true disease-state biomarkers of delirium versus pre-existing differences and if delirium is “priming” the brain for future neurodegeneration.
Strengths and Limitations
Strengths of this study are the inclusion of studies that used standardized assessments of delirium, application of the PRISMA guidelines, and rigorous evaluation of study quality and risk of bias using two standardized reporting guidelines. Nevertheless, there were some limitations. Although we tried to develop a comprehensive list of AT(N)-X biomarkers, the framework itself is still evolving, with new biomarkers being constantly added. Likewise, despite creating an exhaustive list of X biomarkers for delirium, we cannot exclude the possibility of addition of new X biomarkers to the field of delirium biomarkers since our search term list was developed. The modification of the REMARK biomarker checklist for delirium studies may have also influenced the results. Despite us making only minor changes to the REMARK checklist, with the anticipated growth of the number of delirium biomarker studies, a standardized REMARK biomarker checklist developed by a Delphi method of consensus would ensure consistent grading for future systematic reviews. This systematic review was also limited by selection bias as non-English studies were excluded, and the majority of the studies were conducted in the U.S. and Europe at an academic medical center.
Only few studies have examined the role of AD biomarkers in other disorders such as depression and delirium. This study is consistent with a previous systematic review of CSF-based measurements in delirium, which did not find strong evidence for AD-based biomarkers as either a risk factor or disease state biomarker for delirium, and a systematic review of neuroimaging delirium studies found an associated with white matter hyperintensities (WMH), lower brain volume, and atrophy.43–44 Our comprehensive approach builds on this previous work by identify cross-cutting themes across various modalities and biomarker categories. This allows a more complete picture to emerge that highlights the complexity and current gaps in the biological characterization of delirium in relation to ADRD.
CONCLUSION
In summary, this systematic review identified 113 studies with ADRD biomarkers, which mostly measured inflammatory biomarkers in category “X.” This review identified several limitations to current delirium biomarker literature including the need to more thoroughly characterize delirium in the context of the AT(N) framework and the lack of standardized methodology for biomarker data collection and for pre-and post-hospital cognitive assessment. These limitations will need to be addressed by future delirium biomarker studies in order to work towards delineating the biological relationship between delirium and ADRD.
Supplementary Material
ACKNOWLEDGEMENTS
Grant support: SW is supported by NIA 2P30AG010133 and K23AG062555-01. HL is supported by NHLBI 5T32HL091816-07. AJS is supported by NIA P30 AG010133, R01 AG019771, and R01 CA129769. BK is supported by NHLBI R01HL131730, and NIA R01AG055391.
Footnotes
Conflicts of interest: There are no conflicts of interest.
Sponsor’s role: No sponsor involvement.
Supplemental files include search strategy, modified REMARK checklists, REMARK and NOS scores for individual studies, individual references, and details about study characteristics.
REFERENCES
- 1.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol 2009;5(4):210–220. doi: 10.1038/nrneurol.2009.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leslie DL, Inouye SK. The importance of delirium: economic and societal costs. J Am Geriatr Soc 2011;59 Suppl 2(Suppl 2):S241–S243. doi: 10.1111/j.1532-5415.2011.03671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults [published correction appears in Lancet Neurol. 2015 Aug;14(8):788]. Lancet Neurol 2015;14(8):823–832. doi: 10.1016/S1474-4422(15)00101-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg TE, Chen C, Wang Y, et al. Association of Delirium With Long-term Cognitive Decline: A Meta-analysis [published online ahead of print, 2020 Jul 13]. JAMA Neurol 2020;e202273. doi: 10.1001/jamaneurol.2020.2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. Published 2009 Jul 21. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation [published correction appears in BMJ. 2016 Jul 21;354:i4086]. BMJ 2015;350:g7647. Published 2015 Jan 2. doi: 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 9.Iafolla MAJ, Picardo S, Aung K, Hansen AR. Systematic review and REMARK scoring of renal cell carcinoma prognostic circulating biomarker manuscripts. PLoS One. 2019;14(10):e0222359. Published 2019 Oct 22. doi: 10.1371/journal.pone.0222359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauerbrei W, Taube SE, McShane LM, Cavenagh MM, Altman DG. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): An Abridged Explanation and Elaboration. J Natl Cancer Inst 2018;110(8):803–811. doi: 10.1093/jnci/djy088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P: The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 12.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol 2014;14:45. Published 2014 Apr 1. doi: 10.1186/1471-2288-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plaschke K, Fichtenkamm P, Schramm C, et al. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med 2010;36(12):2081–2089. doi: 10.1007/s00134-010-2004-4 [DOI] [PubMed] [Google Scholar]
- 14.van den Boogaard M, Kox M, Quinn KL, et al. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care. 2011;15(6):R297. doi: 10.1186/cc10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun L, Jia P, Zhang J, et al. Production of inflammatory cytokines, cortisol, and Aβ1–40 in elderly oral cancer patients with postoperative delirium. Neuropsychiatr Dis Treat. 2016;12:2789–2795. Published 2016 Oct 27. doi: 10.2147/NDT.S113077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simons KS, van den Boogaard M, Hendriksen E, et al. Temporal biomarker profiles and their association with ICU acquired delirium: a cohort study. Crit Care. 2018;22(1):137. Published 2018 May 25. doi: 10.1186/s13054-018-2054-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idland AV, Wyller TB, Støen R, et al. Preclinical Amyloid-β and Axonal Degeneration Pathology in Delirium. J Alzheimers Dis 2017;55(1):371–379. doi: 10.3233/JAD-160461 [DOI] [PubMed] [Google Scholar]
- 18.van Munster BC, Aronica E, Zwinderman AH, Eikelenboom P, Cunningham C, Rooij SE. Neuroinflammation in delirium: a postmortem case-control study. Rejuvenation Res 2011;14(6):615–622. doi: 10.1089/rej.2011.1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis DH, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012;135(Pt 9):2809–2816. doi: 10.1093/brain/aws190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis DH, Muniz-Terrera G, Keage HA, et al. Association of Delirium With Cognitive Decline in Late Life: A Neuropathologic Study of 3 Population-Based Cohort Studies. JAMA Psychiatry. 2017;74(3):244–251. doi: 10.1001/jamapsychiatry.2016.3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolandi E, Cavedo E, Pievani M, et al. Association of postoperative delirium with markers of neurodegeneration and brain amyloidosis: a pilot study. Neurobiol Aging. 2018;61:93–101. doi: 10.1016/j.neurobiolaging.2017.09.020 [DOI] [PubMed] [Google Scholar]
- 22.Herrmann M, Ebert AD, Galazky I, Wunderlich MT, Kunz WS, Huth C. Neurobehavioral outcome prediction after cardiac surgery: role of neurobiochemical markers of damage to neuronal and glial brain tissue. Stroke. 2000;31(3):645–650. doi: 10.1161/01.str.31.3.645 [DOI] [PubMed] [Google Scholar]
- 23.van Munster BC, Korse CM, de Rooij SE, Bonfrer JM, Zwinderman AH, Korevaar JC. Markers of cerebral damage during delirium in elderly patients with hip fracture. BMC Neurol 2009;9:21. Published 2009 May 27. doi: 10.1186/1471-2377-9-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caplan GA, Kvelde T, Lai C, Yap SL, Lin C, Hill MA. Cerebrospinal fluid in long-lasting delirium compared with Alzheimer’s dementia. J Gerontol A Biol Sci Med Sci 2010;65(10):1130–1136. doi: 10.1093/gerona/glq090 [DOI] [PubMed] [Google Scholar]
- 25.Schramm P, Klein KU, Falkenberg L, et al. Impaired cerebrovascular autoregulation in patients with severe sepsis and sepsis-associated delirium. Crit Care. 2012;16(5):R181. Published 2012 Oct 4. doi: 10.1186/cc11665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson BJ, Reilly JP, Shashaty MGS, et al. Admission plasma levels of the neuronal injury marker neuron-specific enolase are associated with mortality and delirium in sepsis. J Crit Care. 2016;36:18–23. doi: 10.1016/j.jcrc.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson BJ, Chesley CF, Theodore M, et al. Incidence, risk factors, and clinical implications of postoperative delirium in lung transplant recipients. J Heart Lung Transplant. 2018;37(6):755–762. doi: 10.1016/j.healun.2018.01.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erikson K, Ala-Kokko TI, Koskenkari J, et al. Elevated serum S-100β in patients with septic shock is associated with delirium. Acta Anaesthesiol Scand 2019;63(1):69–73. doi: 10.1111/aas.13228 [DOI] [PubMed] [Google Scholar]
- 29.Gailiušas M, Andrejaitienė J, Širvinskas E, Krasauskas D, Švagždienė M, Kumpaitienė B. Association between serum biomarkers and postoperative delirium after cardiac surgery. Acta Med Litu 2019;26(1):8–10. doi: 10.6001/actamedica.v26i1.3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Yu ZX, Ji MS, et al. A Pilot Study of the Use of Dexmedetomidine for the Control of Delirium by Reducing the Serum Concentrations of Brain-Derived Neurotrophic Factor, Neuron-Specific Enolase, and S100B in Polytrauma Patients. J Intensive Care Med 2019;34(8):674–681. doi: 10.1177/0885066617710643 [DOI] [PubMed] [Google Scholar]
- 31.Halaas NB, Blennow K, Idland AV, et al. Neurofilament Light in Serum and Cerebrospinal Fluid of Hip Fracture Patients with Delirium. Dement Geriatr Cogn Disord 2018;46(5–6):346–357. doi: 10.1159/000494754 [DOI] [PubMed] [Google Scholar]
- 32.Casey CP, Lindroth H, Mohanty R, et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2020;143(1):47–54. doi: 10.1093/brain/awz354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunther ML, Morandi A, Krauskopf E, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study*. Crit Care Med 2012;40(7):2022–2032. doi: 10.1097/CCM.0b013e318250acc0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown CH 4th, Faigle R, Klinker L, et al. The Association of Brain MRI Characteristics and Postoperative Delirium in Cardiac Surgery Patients. Clin Ther 2015;37(12):2686–2699.e9. doi: 10.1016/j.clinthera.2015.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haggstrom LR, Nelson JA, Wegner EA, Caplan GA. 2–18F-fluoro-2-deoxyglucose positron emission tomography in delirium. J Cereb Blood Flow Metab 2017;37(11):3556–3567. doi: 10.1177/0271678X17701764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cerejeira J, Firmino H, Vaz-Serra A, Mukaetova-Ladinska EB. The neuroinflammatory hypothesis of delirium. Acta Neuropathol 2010;119(6):737–754. doi: 10.1007/s00401-010-0674-1 [DOI] [PubMed] [Google Scholar]
- 37.Sleigh J, Sanders RW. Slow waves, cognitive disintegration, and delirium. Br J Anaesth 2019;122(1):9–11. doi: 10.1016/j.bja.2018.10.024 [DOI] [PubMed] [Google Scholar]
- 38.Tanabe S, Mohanty R, Lindroth H, et al. Cohort study into the neural correlates of postoperative delirium: the role of connectivity and slow-wave activity. Br J Anaesth 2020;125(1):55–66. doi: 10.1016/j.bja.2020.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanders RD. Hypothesis for the pathophysiology of delirium: role of baseline brain network connectivity and changes in inhibitory tone. Med Hypotheses. 2011;77(1):140–143. doi: 10.1016/j.mehy.2011.03.048 [DOI] [PubMed] [Google Scholar]
- 40.Amgarth-Duff I, Hosie A, Caplan G, Agar M. A systematic review of the overlap of fluid biomarkers in delirium and advanced cancer-related syndromes. BMC Psychiatry. 2020;20(1):182. Published 2020 Apr 22. doi: 10.1186/s12888-020-02584-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amgarth-Duff I, Hosie A, Caplan G, Agar M. Toward best practice methods for delirium biomarker studies: An international modified Delphi study. Int J Geriatr Psychiatry. 2020;35(7):737–748. doi: 10.1002/gps.5292 [DOI] [PubMed] [Google Scholar]
- 42.Fong TG, Vasunilashorn SM, Libermann T, Marcantonio ER, Inouye SK. Delirium and Alzheimer disease: A proposed model for shared pathophysiology. Int J Geriatr Psychiatry. 2019;34(6):781–789. doi: 10.1002/gps.5088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall RJ, Watne LO, Cunningham E, et al. CSF biomarkers in delirium: a systematic review. Int J Geriatr Psychiatry. 2018;33(11):1479–1500. doi: 10.1002/gps.4720 [DOI] [PubMed] [Google Scholar]
- 44.Nitchingham A, Kumar V, Shenkin S, Ferguson KJ, Caplan GA. A systematic review of neuroimaging in delirium: predictors, correlates and consequences. Int J Geriatr Psychiatry. 2018;33(11):1458–1478. doi: 10.1002/gps.4724 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.