Abstract
Background and Aims:
Elevated fecal calprotectin (FC) levels have been reported to correlate with histological activity in patients with ulcerative colitis (UC). However, the accuracy of FC for evaluating histological activity of UC remains to be determined. The aim of this study was to determine the accuracy of FC for evaluating histological activity of UC, based on updated definitions.
Methods:
Related studies were retrieved from the PubMed, Web of Science, Embase, and Cochrane databases. Adult participants diagnosed with UC were included when sufficient data could be extracted to calculate the accuracy of FC for evaluating histological activity. The primary outcome was histological response, and the secondary outcome was histological remission, defined according to a recently updated position paper of European Crohn’s and Colitis Organization. Statistics were pooled using bivariate mixed-effects models. The area under the curve was estimated by summary receiver-operating characteristic curves.
Results:
Nine studies were included, from which 1039 patients were included for the analysis of histological response and 591 patients for histological remission. For the evaluation of histological response, the pooled sensitivity, specificity, and the area under the curve were 0.69 [95% confidence interval (CI): 0.52–0.82], 0.77 (95% CI: 0.63–0.87), and 0.80 (95% CI: 0.76–0.83), respectively. For the evaluation of histological remission, the corresponding estimates were 0.76 (95% CI: 0.71–0.81), 0.71 (95% CI: 0.62–0.78), and 0.79 (95% CI: 0.75–0.82), respectively. FC had a higher accuracy in studies using Nancy Index. For histological response, the cut-off values of FC ranged from 50 to 172 µg/g, and the sensitivity was higher in studies with FC cut-off values >100 µg/g (0.77 versus 0.65).
Conclusion:
FC is a valuable biomarker for assessing histological activity in patients with UC. A cut-off value of 100–200 µg/g is more appropriate to spare patients from an unnecessary endoscopy and biopsy.
Keywords: calprotectin, histological remission, histological score, histology, inflammatory bowel disease, meta-analysis, ulcerative colitis
Introduction
Ulcerative colitis (UC) is a chronic, idiopathic inflammatory disease involving the colon, characterized by relapsing and remitting mucosal inflammation. The therapeutic target of UC was recommended as clinical and endoscopic remission.1 Recently, accumulating evidence suggests that histological remission more effectively reduces relapse, corticosteroid use, and hospitalization.2,3 Better long-term outcomes are associated with histological remission than with mucosal healing.2,3 Therefore, histological remission has also been proposed as a target for the treatment of UC.4
Biopsy is the gold standard for detecting and grading histological activity of UC. However, biopsy is invasive and may cause bleeding complications.5 Since the heterogenous distribution of inflammatory changes exists among different sample sites, the risk of sampling error creates additional challenges.1 Therefore, a non-invasive biomarker capable of surveillance of histological activity of UC would be helpful in clinical practice.
Calprotectin is a protein derived predominantly from neutrophils and accounts for approximately 60% of the cytosolic protein in neutrophils.6 Since neutrophil infiltration into the mucosa correlates with endoscopic severity and systemic inflammation,7 fecal calprotectin (FC) might be an appropriate marker to assess gastrointestinal inflammation.8 FC is expected to support or replace histological assessment as a non-invasive biomarker because of its proven strong correlation with histological activity.9,10 Recently, a systematic review attempted to summarize the relationship between FC and histological remission of UC.11 However, the definitions of histological remission are inconsistent in different studies and that review could not explore the threshold effect caused by various FC cut-off values. Moreover, that review could not summarize the quantitative effect of different patient characteristics, FC assays, histological activity criteria, and other factors. Since the European Crohn’s and Colitis Organization (ECCO) has recently updated the definitions of histological activity of UC, we applied the updated definitions to perform a meta-analysis to better assess the accuracy of FC for evaluating histological activity in UC.
Materials and methods
Search strategy
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.12 A review protocol was created before the search. We searched the PubMed, Web of Science, Embase, and Cochrane databases up to 31 December 2020. We used the following MeSH headings, keywords, and text words: “ulcerative colitis,” “UC,” “inflammatory bowel disease,” “IBD,” “fecal calprotectin,” “faecal calprotectin,” “calprotectin,” “FC,” “histologic activity,” “histologic score,” “histologic remission,” “histologic healing,” “Nancy index,” “Geboes score,” and “Robarts histopathologic score.” No language or publication date restrictions were applied. We also screened abstracts from the Digestive Disease Week and the ECCO from the past 5 years. Selected articles’ reference lists were checked for revision and screened for additional relevant publications.
Definition
The primary outcome was histological response, and the secondary outcome was histological remission. According to the position paper of ECCO,4 histological response is defined as continuous Geboes score (GS) ⩽12, GS < 3.0, Robarts histopathology index (RHI) ⩽9 (with subscores of 0 for neutrophils in the epithelium and without erosions or ulcers), Nancy Index (NI) ⩽1.13–15 Histological remission is defined as continuous GS ⩽ 6 GS, GS ⩽ 2.0, NI = 0, RHI ⩽ 3 (with subscores of 0 for lamina propria neutrophils and neutrophils in the epithelium and without ulcers or erosions).
Since both GS < 3.0 and GS < 3.1 indicate no neutrophils in the epithelium,13 studies with GS < 3.1 were also included in the analysis of histological response. Since both GS ⩽ 2.0 and GS < 2.0 indicate neither an increase in eosinophils nor neutrophils in the lamina propria,13 studies with GS < 2.0 were also included for the analysis of histological remission.
Selection criteria
The inclusion criteria were as follows: adult patients diagnosed with UC; detailed histological scores described; and the relationship between FC and histological activity of UC assessed. Studies were excluded for the following reasons: histological outcomes not matched with the definition of histological response or histological remission or those using a strict definition for a combined histological score and endoscopic score; lack of, or insufficient, data to calculate true-positive, false-positive, false-negative, or true-negative values; case reports, reviews, and editorials; and fewer than 20 samples. When patient data were reported more than once, only the most recent article with the most information was included.
Data extraction and quality assessment
The titles and abstracts were reviewed by two investigators (XY and SZ) independently. After the eligibility assessment, data were extracted, including the first author, publication year, study design, study region, patient sex, patient age, endoscopic activity, FC assay, median of FC, FC cut-off, interval between fecal sampling and endoscopy, and definition of histological remission or histological response. The absolute number of true-positive, false-positive, false-negative, and true-negative cases was collected or calculated to develop a 2 × 2 contingency table. When multiple cut-off values existed, we selected the pre-specified one or manufacturer’s reference first. If there is no pre-specified cut-off value or manufacturer’s reference, we select the optimal cut-off value with the highest Youden index.
Two authors (XY and SZ) independently assessed the study quality by using the Quality Assessment of Diagnostic Accuracy Studies 2 tool.16 In an case of discrepancies, we consulted with the senior reviewer (MC) to reach consensus.
Statistical analyses
A bivariate mixed-effects regression model was used to pool the diagnostic accuracy and calculate the sensitivity, specificity, likelihood ratio, diagnostic odds ratio, and confidence intervals (CIs). The summary receiver-operating characteristic (SROC) curve was plotted. An area under the SROC curve near 0.50 indicated low accuracy and that near 1.0 indicated perfect accuracy. Heterogeneity was measured by the I2 inconsistency test. Heterogeneity was ranked low (25%), moderate (50%), or high (75%).17 Two methods were used to identify the sources of heterogeneity: (1) the squared correlation coefficient of the proportion of heterogeneity due to threshold effects; and (2) univariate meta-regression and subgroup analyses (including study region, study design, endoscopic activity, definition of histological score, number of patients, FC assay, interval between fecal sampling and endoscopy and cut-off values). Sensitivity analysis was performed to assess the effect of individual studies on the summary estimates. Cook’s distance was used to identify outlier studies. As the number of included studies was below 10, publication bias was not explored.
Quality assessments were conducted using RevMan software (version 5.4, the Cochrane Collaboration, Oxford, UK). All statistical analyses were performed using Stata software (version 15, StataCorp, College Station, TX, USA) with the MIDAS module.18 A p value < 0.10 was considered to indicate statistical significance in meta-regression.
Results
Study selection and characteristics
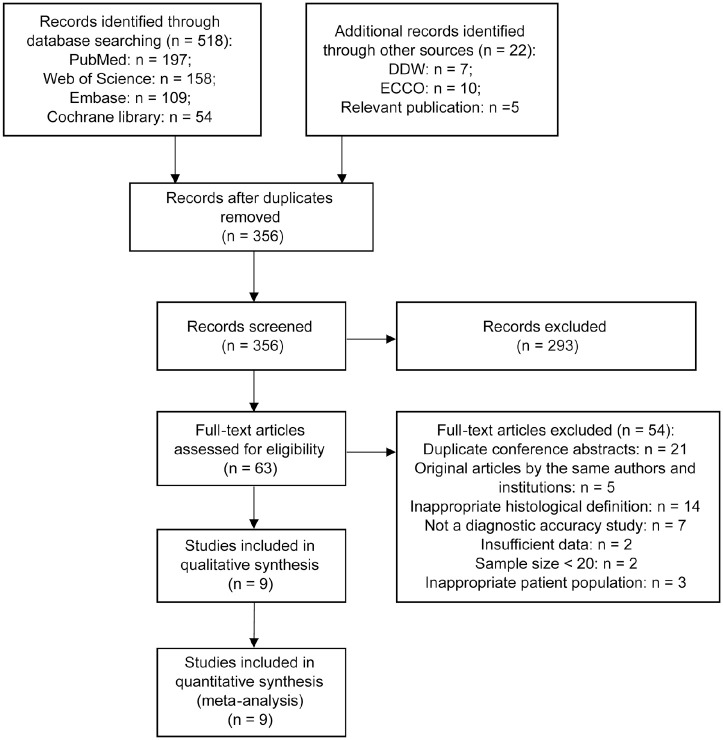
We searched databases and other sources and identified 517 and 22 records, respectively. After the titles and abstracts were screened, the full text of 63 articles were reviewed. Finally, nine studies were included for quantitative synthesis (Figure 1).
Figure 1.
Flow chart for the study selection procedure following the statement of PRISMA.
DDW, Digestive Disease Week; ECCO, European Crohn’s and Colitis Organization; PRISMA, preferred reporting items for systematic review and meta-analysis.
We included 1039 patients from six studies for the analysis of histological response and 591 patients from four studies for the analysis of histological remission (Tables 1 and 2). The studies were from various regions, with three from Asia,19–21 one from North America,22 and five from Europe.23–27 Eight studies were designed prospectively. The mean or median age of patients varied from 42 to 50 years. The FC assay differed between studies. The median FC levels were between 56.2 to 91 µg/g in patients with histological response or remission. The cut-off value of FC ranged from 50 to 237 µg/g. The interval between fecal sampling and endoscopy was within 14 days in all studies except one,23 which did not report the maximal interval (the FC sampling time in this study was before bowel preparation or at least 5 days after endoscopy to avoid false positive results, with unchanged therapy).
Table 1.
General characteristics of all included studies.
| Reference | Region | Publication year | Study design | Sex: male | Age | Endoscopic activity: MES ⩽ 1 |
|---|---|---|---|---|---|---|
| Cannatelli et al.23 | UK | 2020 | Prospective | 50% | 44 (mean) | 62% |
| Hart et al.22 | Canada | 2020 | Prospective | 53% | 48 (mean) | 86% |
| Kawashima et al.19 | Japan | 2020 | Prospective | 57% | 47 (median) | 100% |
| Langhorst et al.24 | Germany | 2019 | Post hoc | 40% | 45 (mean) | Not reported |
| Magro et al.25 | Portugal | 2017 | Prospective | 47% | 47 (median) | 96% |
| Magro et al.26 | Portugal | 2020 | Prospective | 47% | 45 (mean) | 78% |
| Sagami et al.21 | Japan | 2020 | Prospective | 74% | 42 (median) | 46% |
| Shi et al.20 | China | 2017 | Prospective | 48% | 50 (median) | 71% |
| Walsh et al.27 | UK | 2019 | Prospective | 50% | 46 (median) | UCEIS ⩽1: 40% |
MES, Mayo endoscopic score; UCEIS, ulcerative colitis endoscopic index of severity; UK, United Kingdom.
Table 2.
Summary of results of fecal calprotectin in included studies.
| Reference | FC assay | FC value (histological response /remission vs activity) | FC cut-off value (µg/g) | Intervals between FC and endoscopy | Definition | No. of patients | TP | FP | TN | FN | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Histological response | Histological remission | ||||||||||
| Cannatelli et al.23 | ELISA (Bulhmann) | 68.7 vs 810.9 (mean) | 172 | Before bowel preparation or at least 5 days after endoscopy | NI⩽1 | 76 | 27 | 13 | 35 | 1 | |
| Hart et al.22 | ELISA (Buhlmann) | 79.5 vs 148.5 (median) | 135 | 2 days | GS < 3.1 | 180 | 54 | 25 | 55 | 46 | |
| Kawashima et al.19 | Not reported | 56.2 vs 118.1 (median) | 82.7 | Within 3 days | GS < 2.0 | 74 | 29 | 12 | 22 | 11 | |
| Langhorst et al.24 | Not reported | Not reported | 50 | Within 7 days | NI ⩽ 1 | 228 | 68 | 62 | 82 | 16 | |
| Magro et al.25 | QB (Buhlmann) | 81.5 vs 231.0 (median) | 100 | Within 24 h | GS < 3.1 | 364 | 157 | 15 | 63 | 129 | |
| Magro et al.26 | QB (Buhlmann) | 73.5 vs 510.0 (median) | 237 | Within 24 h | RHI ⩽ 3a | 339 | 178 | 38 | 70 | 53 | |
| Sagami et al.21 | Colloidal gold aggregation (Alfresa) | 405 (overall median, IQR: 13 – 12517) | 100 | Within 10 days | GS ⩽ 2.0 | 39 | 9 | 4 | 21 | 5 | |
| Shi et al.20 | ELISA (Inova) | 40 (overall median, IQR: 6 – 242) | 50 | Within 3 days | NI ⩽ 1 | 139 | 64 | 11 | 40 | 24 | |
| 50 | GS < 2.0 | 139 | 60 | 15 | 48 | 16 | |||||
| Walsh et al.27 | Fcal test (Buhlmann) | 91 vs 985 (median) | 72 | Within 14 days | NI ⩽ 1 | 52 | 12 | 0 | 27 | 13 | |
ELISA, enzyme-linked immunosorbent assay; FC, fecal calprotectin; FN, false-negative; FP, false-positive; GS, Geboes score; IQR, interquartile range; NI, Nancy Index; QB, Quantum Blue; RHI, Robarts histopathology index; TN, true-negative; TP: true-positive.
With subscores of 0 for lamina propria neutrophils and neutrophils in the epithelium and without ulcers or erosions.
Quality assessment
The methodological quality assessment and risk of bias are summarized in Figure 2. All studies had low applicability concerns. In one study,24 the bias of patient selection was unclear owing to incomplete reporting on consecutive patient enrollment. The bias risk of the index test was high in four studies because they did not have a pre-specified FC cut-off and used the optimal cut-off value from the receiver operating characteristic curve.19,22,23,27 FC test was blinded to the histological result in one study,20 and the other studies did not report this blind method. In five studies,19–23 the risk of bias in the reference standard was unclear because of unclear blinding to the FC results. Although seven studies did not include all patients in the analysis of histological activity owing to the existence of other outcomes, the majority of overall patients (91%,21 97%,22 98%,25 80%,26 93%,28 99%,20 79%27) in these studies were still included in the analysis of the FC test and histological score.
Figure 2.
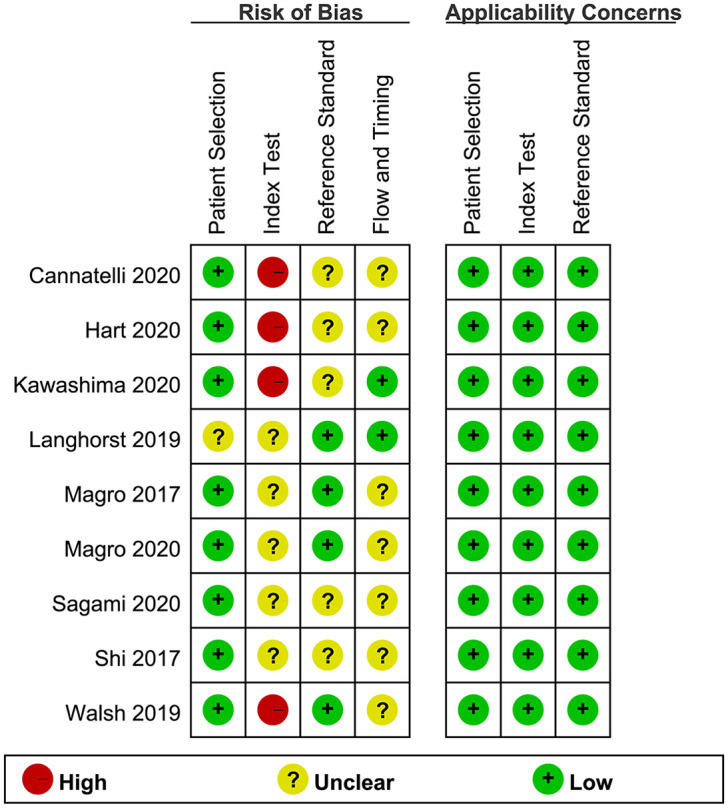
Summary of the methodological quality of the included studies. Assessed by the Quality Assessment of Diagnostic Accuracy Studies-2.
Quantitative synthesis
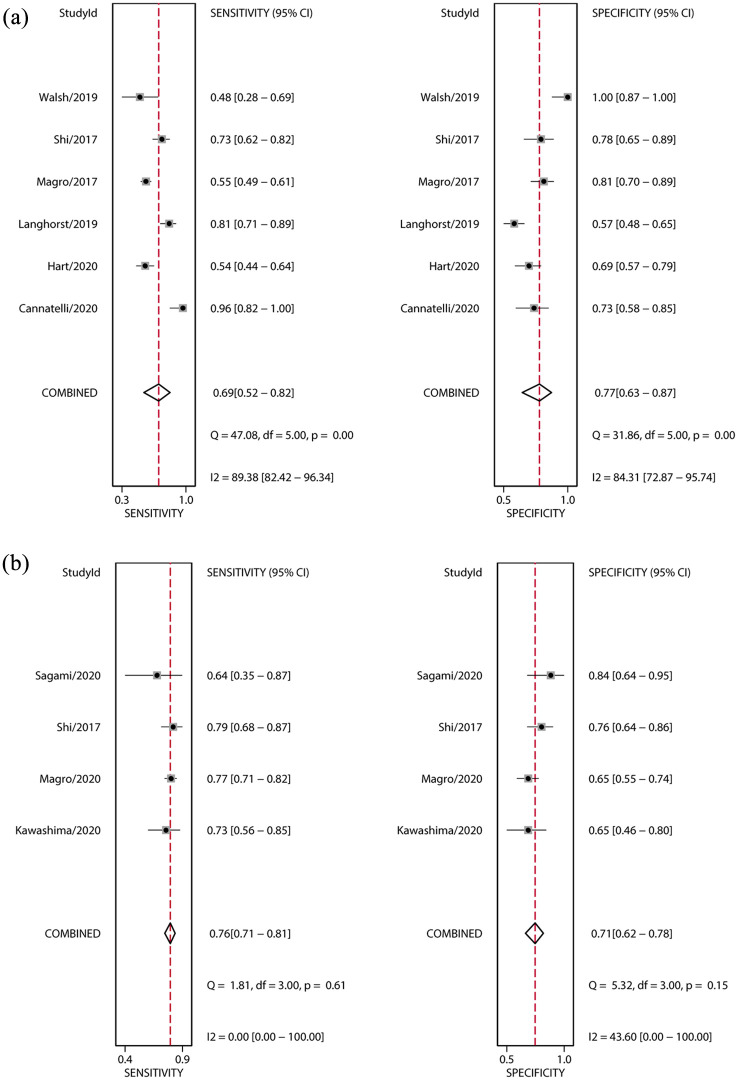
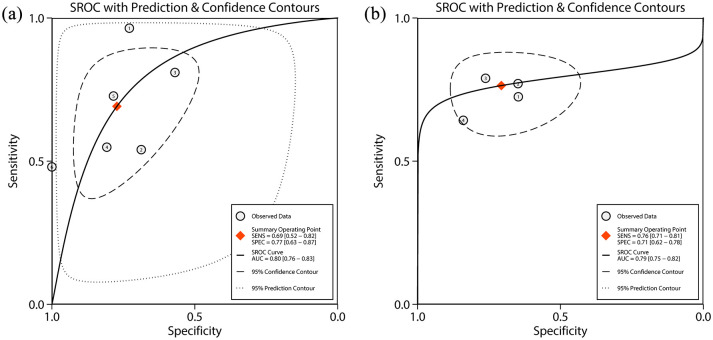
The primary outcome was histological response. The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratios were 0.69 (95% CI: 0.52–0.82), 0.77 (95% CI: 0.63–0.87), 3.0 (95% CI: 1.9–4.8), 0.40 (95% CI: 0.26–0.62), and 8 (95% CI: 4–16), respectively (Table 3, Figure 3a). The area under the SROC curve was 0.80 (95% CI: 0.76–0.83) (Table 3, Figure 4a). High heterogeneity was detected in these estimates (overall heterogeneity: I2 = 95%, sensitivity: I2 = 89%, specificity: I2 = 84%).
Table 3.
Meta-analysis of pooled diagnostic statistics.
| Parameter | Estimate | |
|---|---|---|
| Histological response (95% CI) | Histological remission (95% CI) | |
| Sensitivity | 0.69 (0.52–0.82) | 0.76 (0.71–0.81) |
| Specificity | 0.77 (0.63–0.87) | 0.71 (0.62–0.78) |
| Positive likelihood ratio | 3.0 (1.9–4.8) | 2.6 (2.0–3.4) |
| Negative likelihood ratio | 0.40 (0.26–0.62) | 0.33 (0.27–0.42) |
| Diagnostic odds ratio | 8 (4–16) | 8 (5–12) |
| Area under the SROC curve | 0.80 (0.76–0.83) | 0.79 (0.75–0.82) |
CI, confidence interval; SROC, summary receiver-operating characteristic.
Figure 3.
Forest plots of the sensitivity and specificity of FC for histological activity of UC. (a) Histological response; (b) histological remission. Bivariate mixed-effects models were applied. ■ point estimates; ◊ pooled estimates; error bars indicate 95% CI; data in parentheses are 95% CIs.
CI, confidence interval; FC, fecal calprotectin; UC, ulcerative colitis.
Figure 4.
SROC curve plots of FC for histological activity of UC. (a) Histological response; (b) histological remission. Numbered circles represent the respective individual studies. The square represents the point estimate of pooled sensitivity and specificity. The solid line indicates the SROC curve. The dashed line and dotted line represent 95% confidence contour and 95% prediction contour, respectively.
AUC: area under the curve; FC, fecal calprotectin; SENS: sensitivity; SPEC: specificity; SRAC, summary receiver-operating characteristic; UC, ulcerative colitis.
The secondary outcome was histological remission. The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio were 0.76 (95% CI: 0.71–0.81), 0.71 (95% CI: 0.62–0.78), 2.6 (95% CI: 2.0–3.4), 0.33 (95% CI: 0.27–0.42), and 8 (95% CI: 5–12), respectively (Table 3, Figure 3b). The area under the SROC curve was 0.79 (95% CI: 0.75–0.82) (Table 3, Figure 4b). Low heterogeneity was detected in these estimates (overall heterogeneity: I2 = 0%, sensitivity: I2 = 0%, specificity: I2 = 44%)
Exploration of source of heterogeneity
The squared correlation coefficient of the primary outcome indicated that 37% of heterogeneity came from the threshold effect. The study of Walsh et al. may have been an outlier because it selected a cut-off value to attain the highest specificity (100%) and lowest sensitivity (48%).27
The results of univariate meta-regression and subgroup analyses are summarized in Table 4. Two pre-specified subgroups could be combined into one subgroup because they had the identical division of studies (subgroups 6: interval between fecal sampling and endoscopy/number of patients). The sensitivity was significantly higher in studies using NI score (0.74 versus 0.56, p = 0.03). The specificity was significantly lower in studies with intervals within 7 days/number of patients ⩾ 100 (0.71 versus 0.90, p = 0.07). The sensitivity was higher in studies with FC cut-off values >100 µg/g (0.77 versus 0.65, p = 0.38), although the p value is not significant. The squared correlation coefficient of the secondary outcome indicated that 100% of heterogeneity came from the threshold effect.
Table 4.
Univariate meta-regression and subgroup analysis for the primary outcome (histological response).
| Covariate | κ | Sensitivity (95% CI) | p value | Specificity (95% CI) | p value |
|---|---|---|---|---|---|
| Study design characteristics | |||||
| Region | |||||
| Asian | 1 | 0.73 (0.41–1.00) | 0.87 | 0.79 (0.54–1.00) | 0.94 |
| Western | 5 | 0.68 (0.51–0.85) | 0.77 (0.64–0.90) | ||
| Study design | |||||
| Prospective | 5 | 0.66 (0.50–0.83) | 0.32 | 0.79 (0.71–0.87) | 0.48 |
| Post-hoc | 1 | 0.81 (0.58–1.00) | 0.57 (0.38–0.76) | ||
| Histological score | |||||
| NI | 4 | 0.74 (0.64–0.84) | 0.03* | 0.81 (0.64–0.98) | 0.61 |
| GS | 2 | 0.56 (0.40–0.72) | 0.75 (0.49–1.00) | ||
| MES ⩾ 1 | |||||
| ⩾70% | 3 | 0.61 (0.42–0.79) | 0.11 | 0.76 (0.57–0.96) | 0.59 |
| <70% | 3 | 0.77 (0.61–0.93) | 0.81 (0.61–1.00) | ||
| FC test characteristics | |||||
| FC assay | |||||
| ELISA | 3 | 0.75 (0.57–0.94) | 0.71 | 0.73 (0.56–0.90) | 0.26 |
| Not ELISA or unclear | 3 | 0.62 (0.41–0.84) | 0.81 (0.65–0.96) | ||
| Intervals between fecal sampling and endoscopy/number of patients | |||||
| Within 7 days/⩾100 | 4 | 0.67 (0.46–0.87) | 0.40 | 0.71 (0.56–0.87) | 0.07* |
| Over 7 days or unclear/<100 | 2 | 0.80 (0.56–1.00) | 0.90 (0.77–1.00) | ||
| FC cut-off value | |||||
| ⩽100 µg/g | 4 | 0.65 (0.47–0.84) | 0.38 | 0.80 (0.67–0.93) | 0.91 |
| >100 µg/g | 2 | 0.77 (0.53–1.00) | 0.71 (0.49–0.92) | ||
CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; FC, fecal calprotectin; GS, Geboes score; MES, Mayo endoscopic score; NI, Nancy Index.
p < 0.10.
Sensitivity analysis
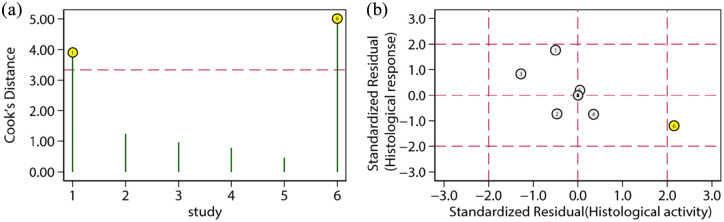
The sensitivity analysis is shown in Table 5. One article was removed each time to evaluate the impact of a single study on this meta-analysis. The outcome remained stable no matter which document was deleted. In the influence analysis (Figure 5), the study by Walsh et al. was an outlier.27 After removal of this study, the summary statistics were only slightly altered.
Table 5.
Sensitivity analysis for the primary outcome (histological response).
| Studies excluded | Sensitivity (95%CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) | Diagnostic odds ratio (95% CI) | Area under the curve (95% CI) |
|---|---|---|---|---|---|---|
| Cannatelli et al.23 | 0.62 (0.49– 0.74) | 0.78 (0.62–0.89) | 2.9 (1.7–5.0) | 0.48 (0.37–0.62) | 6 (3–12) | 0.75 (0.71–0.79) |
| Hart et al.22 | 0.71 (0.53– 0.85) | 0.79 (0.62–0.90) | 3.5 (2.0–6.2) | 0.36 (0.22–0.58) | 10 (5–20) | 0.82 (0.79– 0.85) |
| Langhorst et al.24 | 0.67 (0.47–0.83) | 0.80 (0.68–0.89) | 3.4 (2.0–5.7) | 0.41 (0.24–0.70) | 8 (3–20) | 0.82 (0.78–0.85) |
| Magro et al.25 | 0.73 (0.53–0.86) | 0.78 (0.58–0.90) | 3.3 (1.7–6.3) | 0.35 (0.20–0.62) | 9 (4–24) | 0.82 (0.78–0.85) |
| Shi et al.20 | 0.69 (0.48–0.84) | 0.78 (0.59–0.90) | 3.1 (1.7–5.8) | 0.40 (0.23–0.70) | 8 (3–20) | 0.80 (0.77–0.84) |
| Walsh et al.27 | 0.73 (0.56–0.86) | 0.71 (0.62–0.79) | 2.5 (1.9–3.4) | 0.38 (0.22–0.65) | 7 (3–14) | 0.77 (0.73–0.80) |
CI, confidence interval.
Figure 5.
Plots of Cook’s distance for histological response. (a) Spike plot: each study is presented as a vertical line, with its corresponding study number labeled along the x-axis. Studies above the dotted horizontal line are influential studies. (b) Scatter plot: Numbered circles represent the respective individual studies. Outlier studies are in yellow.
Discussion
To our knowledge, this is the first meta-analysis to assess the accuracy of FC for evaluating histological activity in patients with UC. Assessment of the histological activity may indicate the prognosis of UC and guide treatment decisions.29 Patients with UC in histological remission are more likely to be symptom-free and have a reduced risk of relapse, surgery, hospitalization, and colorectal cancer.30–32 Nevertheless, invasive endoscopy and biopsy are unpleasant for patients. FC, as a non-invasive marker, correlates well with histological activity and is expected to be a surrogate marker for histological evaluation.10 Therefore, we explored the accuracy of FC to evaluate the histological activity of UC. For histological response, the pooled sensitivity, specificity and the area under the SROC curve were 0.69, 0.77, and 0.80, respectively. For histological remission, the corresponding estimates were 0.76, 0.71, and 0.79, respectively. The area under the SROC curve indicated that FC had a good diagnostic accuracy. High heterogeneity was detected in the primary outcome, and low heterogeneity was detected in the secondary outcome. Then, the source of heterogeneity was explored for the primary outcome. A sensitivity analysis was further conducted to assess the robustness of the findings.
FC has been widely studied in inflammatory bowel disease for its ability to predict histological activity. It has a higher predictive value for histological remission in UC than in Crohn’s disease.33 FC also has a higher predictive value for histological remission than for endoscopic remission.34 FC can predict histological remission in adults and relates closely to histological activity in pediatric patients with UC.35 As for therapeutic monitoring, a low FC level showed good correlation to histological remission in patients treated with adalimumab for UC.36 Moreover, with technique improvement, FC could also be measured at home. The FC levels obtained through home-based measurement were in agreement with the result of enzyme-linked immunosorbent assay (ELISA).37 Therefore, FC is suitable and convenient for surveillance of histological activity in UC.
There are various histological scoring systems for UC. Neutrophil infiltration is a significant marker among these scores.38 According to the position paper of ECCO, histological activity is defined by neutrophil infiltration of the epithelium and/or lamina propria.4 Since FC is derived predominantly from neutrophils, it can reflect the neutrophilic granulocyte migration through the gut wall and reflect histological inflammation.39 Histological remission is defined by more specific values considered to indicate neutrophil infiltration than is histological response, which might partly explain the higher pooled sensitivity of histological remission in this study, in addition to the threshold effect. Histological response has also been reported to have a higher cut-off value for FC than does histological remission.40 However, a small portion of FC may also come from monocytes and macrophages rather than from neutrophils or from the proximal gastrointestinal tract due to neoplasia or infection, which can cause bias in the interpretation of FC related to histological activity of the colon.41
There are numerous histological scores for UC. Although NI, RHI, and GS showed a good concordance of identifying histological activity in patients with UC,42 different histological scores use different cut-off values of FC to identify histological activity.26 In the subgroup analysis of histological scores, studies using NI had a significantly higher sensitivity than did studies using GS (0.74 versus 0.56, p = 0.03). Moreover, studies using NI also had a higher specificity than did studies using GS, although the p value is not significant (0.81 versus 0.75, p = 0.61). NI is a fully validated score. It is the only histological score that is recommended by ECCO for both clinical trials and observational studies.4 Since FC correlated more closely with NI in this study, NI may be more suitable for evaluating histological activity when FC is used for disease surveillance.
The heterogeneity was also caused by the intervals between fecal sampling and endoscopy, and the number of patients. The specificity was significantly lower in studies with intervals within 7 days/number of patients ⩾100. There is no recommendation for the maximal interval between fecal sampling and endoscopy, but studies with longer or unclear intervals did not show a lower accuracy of FC than did studies with intervals within 7 days. Since studies with longer or unclear intervals also had a smaller number of patients, the difference in specificity should be carefully explained, considering both the effect of intervals and the number of patients.
We also assessed the heterogeneity of FC assays. The ELISA assay had a higher sensitivity and lower specificity than did other assays, which is not significant though. There are various FC assays from various manufacturers. While there was a qualitative correlation between assays from different manufacturers, quantitative agreement was reported to be suboptimal.41 In a previous study, different FC assays had obviously different sensitivity and specificity in distinguishing histological activity of UC when using the same cut-off values.42
The cut-off values of FC ranged from 50 to 172 µg/g in the analysis of histological response. Usually, FC levels less than 50 µg/g indicate normal or remission while FC levels over 200 µg/g indicate abnormality or inflammation. Uncertainty remains in patients who have FC levels of between 50 and 200 µg/g. Raising the cut-off value to 100 µg/g would have little effect (4%) on specificity but much more (14%) on sensitivity for non-inflammatory bowel disease.43 Therefore, we also explored whether raising the cut-off value to over 100 µg/g would get a better outcome. Studies with cut-off values >100 µg/g had a higher sensitivity than did studies with cut-off values ⩽100 µg/g (0.77 versus 0.65). Because the threshold effect also explained a small source (37%) of heterogeneity, the specificity decreases as the cut-off value increases. Studies with cut-off values >100 µg/g had a correspondingly lower, but acceptable, specificity (0.71 versus 0.80). Considering the range of FC cut-off values and the balance between sensitivity and specificity in this study, one should consider 100–200 µg/g for the cut-off value of FC. A higher sensitivity can identify more patients in histological response, which can spare these patients from unnecessary endoscopy and biopsy,25 but the exact cut-off value should be further decided according to the prevalence of histological activity and different FC assays.
The robustness of the results was assessed in the sensitivity analysis. The summary statistics changed only slightly no matter which study was removed, which suggested that the results were stable and reliable.
This study has several limitations. First, both histological scores and FC assays were inconsistent among studies, which might have increased the risk of bias of the index test and reference standard. Second, blinding is important for diagnostic accuracy, but most studies did not report whether blinding was adopted for the index test and reference standard, thereby increasing the risk of measurement bias. Third, individual patient characteristics (such as age and medication usage), sampling time, and laboratory storage conditions varied among studies. Heterogeneity could not be eliminated completely. Furthermore, the likelihood ratio was small, indicating a small change in the probability of the pretest rather than a large change.44 Thus, FC should be interpreted carefully and cannot completely replace endoscopy and biopsy. However, FC is still an acceptable and valuable biomarker for the continuous surveillance of histological activity.
Conclusion
FC has a good diagnostic accuracy for histological response and remission in patients with UC. A cut-off value of 100–200 µg/g is more appropriate to identify patients in histological response and spare patients from unnecessary endoscopy and biopsy. The exact cut-off value should be further decided according to the prevalence of histological activity and different FC assays. Further studies are required to confirm these findings.
Supplemental Material
Supplemental material, sj-doc-1-tag-10.1177_1756284821994741 for Can fecal calprotectin accurately identify histological activity of ulcerative colitis? A meta-analysis by Xiaoqi Ye, Ying Wang, Harry H. X. Wang, Rui Feng, Ziyin Ye, Jing Han, Li Li, Zhirong Zeng, Minhu Chen and Shenghong Zhang in Therapeutic Advances in Gastroenterology
Footnotes
Authors Contributions: Guarantor of the article: Shenghong Zhang.
Conceptualization: Shenghong Zhang, Minhu Chen; Analysis and interpretation of data: Xiaoqi Ye, Harry H.X. Wang, Ying Wang, Rui Feng, Ziyin Ye; Drafting of manuscript: Ying Wang, Li Li, Jing Han; Critical revision of manuscript for important intellectual content: Shenghong Zhang, Minhu Chen.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the National Natural Science Foundation of China (#82070538, #81870374), Guangzhou Science and Technology Department (#202002030041), and Guangdong Science and Technology Department (#2017A030306021).
ORCID iD: Shenghong Zhang  https://orcid.org/0000-0002-9088-8781
https://orcid.org/0000-0002-9088-8781
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Xiaoqi Ye, Department of Gastroenterology and Hepatology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong Province, China.
Ying Wang, Department of Gastroenterology and Hepatology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong Province, China.
Harry H. X. Wang, School of Public Health, Sun Yat-Sen University, Guangzhou, Guangdong Province, PR China General Practice and Primary Care, Institute of Health and Wellbeing, University of Glasgow, Scotland, UK.
Rui Feng, Department of Gastroenterology and Hepatology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong Province, China.
Ziyin Ye, Department of Gastroenterology and Hepatology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong Province, China.
Jing Han, Department of Gastroenterology and Hepatology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong Province, China.
Li Li, Department of Gastroenterology and Hepatology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong Province, China.
Zhirong Zeng, Department of Gastroenterology and Hepatology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong Province, China.
Minhu Chen, Department of Gastroenterology and Hepatology, The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong Province, China.
Shenghong Zhang, Department of Gastroenterology, The First Affiliated Hospital, Sun Yat-sen University, No. 58 Zhongshan 2nd Road, Guangzhou 510080, Guangdong Province, P.R. China.
References
- 1. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015; 110: 1324–1338. [DOI] [PubMed] [Google Scholar]
- 2. Hindryckx P, Baert F, Hart A, et al. Clinical trials in ulcerative colitis: a historical perspective. J Crohns Colitis 2015; 9: 580–588. [DOI] [PubMed] [Google Scholar]
- 3. Bryant RV, Burger DC, Delo J, et al. Beyond endoscopic mucosal healing in UC: histological remission better predicts corticosteroid use and hospitalisation over 6 years of follow-up. Gut 2016; 65: 408–414. [DOI] [PubMed] [Google Scholar]
- 4. Magro F, Doherty G, Peyrin-Biroulet L, et al. ECCO position paper: harmonisation of the approach to ulcerative colitis histopathology. J Crohns Colitis 2020; 14: 1503–1511. [DOI] [PubMed] [Google Scholar]
- 5. Yao MD, von Rosenvinge EC, Groden C, et al. Multiple endoscopic biopsies in research subjects: safety results from a National Institutes of Health series. Gastrointest Endosc 2009; 69: 906–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Røseth AG, Fagerhol MK, Aadland E, et al. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol 1992; 27: 793–798. [DOI] [PubMed] [Google Scholar]
- 7. Rosenberg L, Nanda KS, Zenlea T, et al. Histologic markers of inflammation in patients with ulcerative colitis in clinical remission. Clin Gastroenterol Hepatol 2013; 11: 991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walsham NE, Sherwood RA. Fecal calprotectin in inflammatory bowel disease. Clin Exp Gastroenterol 2016; 9: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muthas D, Reznichenko A, Balendran CA, et al. Neutrophils in ulcerative colitis: a review of selected biomarkers and their potential therapeutic implications. Scand J Gastroenterol 2017; 52: 125–135. [DOI] [PubMed] [Google Scholar]
- 10. Zittan E, Kelly OB, Kirsch R, et al. Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn’s disease. Inflam Bowel Dis 2016; 22: 623–630. [DOI] [PubMed] [Google Scholar]
- 11. D’Amico F, Bonovas S, Danese S, et al. Review article: faecal calprotectin and histologic remission in ulcerative colitis. Aliment Pharmacol Ther 2020; 51: 689–698. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009; 3: e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 13. Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000; 47: 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mosli MH, Feagan BG, Zou G, et al. Development and validation of a histological index for UC. Gut 2017; 66: 50–58. [DOI] [PubMed] [Google Scholar]
- 15. Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, et al. Development and validation of the Nancy histological index for UC. Gut 2017; 66: 43–49. [DOI] [PubMed] [Google Scholar]
- 16. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–536. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dwamena B. MIDAS: Stata module for meta-analytical integration of diagnostic test accuracy studies. Statistical Software Components 2007. Ann Arbor, Michigan: University of Michigan Medical School. [Google Scholar]
- 19. Kawashima K, Oshima N, Yuki T, et al. Low fecal calprotectin level predicts histological healing and prolonged clinical remission in ulcerative colitis patients with clinical remission and mucosal healing. Gastroenterology 2020; 158: S-461–S-462. [Google Scholar]
- 20. Shi HY, Chan FKL, Chan AWH, et al. Accuracy of faecal immunochemical test to predict endoscopic and histological healing in ulcerative colitis: a prospective study based on validated histological scores. J Crohns Colitis 2017; 11: 1071–1077. [DOI] [PubMed] [Google Scholar]
- 21. Sagami S, Kobayashi T, Aihara K, et al. Transperineal ultrasound predicts endoscopic and histological healing in ulcerative colitis. Aliment Pharmacol Ther 2020; 51: 1373–1383. [DOI] [PubMed] [Google Scholar]
- 22. Hart L, Chavannes M, Kherad O, et al. Faecal calprotectin predicts endoscopic and histological activity in clinically quiescent ulcerative colitis. J Crohns Colitis 2020; 14: 46–52. [DOI] [PubMed] [Google Scholar]
- 23. Cannatelli R, Bazarova A, Zardo D, et al. Fecal calprotectin thresholds to predict endoscopic remission using advanced optical enhancement techniques and histological remission in IBD patients. Inflamm Bowel Dis 2020; 22: 623–630. [DOI] [PubMed] [Google Scholar]
- 24. Langhorst J, Koch AK, Schroder D, et al. Diagnostic accuracy of fecal lactoferrin and calprotectin compared to the riley score and nancy index for the determination of histological inflammatory activity in patients with ulcerative colitis. Gastroenterology 2019; 156: S846–S846. [Google Scholar]
- 25. Magro F, Lopes S, Coelho R, et al. Accuracy of faecal calprotectin and neutrophil gelatinase B-associated Lipocalin in evaluating subclinical inflammation in UlceRaTIVE Colitis-the ACERTIVE study. J Crohns Colitis 2017; 11: 435–444. [DOI] [PubMed] [Google Scholar]
- 26. Magro F, Lopes J, Borralho P, et al. Comparing the continuous geboes score with the robarts histopathology index: definitions of histological remission and response and their relation to faecal calprotectin levels. J Crohns Colitis 2020; 14: 169–175. [DOI] [PubMed] [Google Scholar]
- 27. Walsh A, Kormilitzin A, Hinds C, et al. Defining faecal calprotectin thresholds as a surrogate for endoscopic and histological disease activity in ulcerative colitis-a prospective analysis. J Crohns Colitis 2019; 13: 424–430. [DOI] [PubMed] [Google Scholar]
- 28. Mak WY, Buisson A, Andersen MJ, Jr., et al. Fecal calprotectin in assessing endoscopic and histological remission in patients with ulcerative colitis. Digest Dis Sci 2018; 63: 1294–1301. [DOI] [PubMed] [Google Scholar]
- 29. Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet Gastroenterol Hepatol 2017; 389: 1756–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park S, Abdi T, Gentry M, et al. Histological disease activity as a predictor of clinical relapse among patients with ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol 2016; 111: 1692–1701. [DOI] [PubMed] [Google Scholar]
- 31. Zenlea T, Yee EU, Rosenberg L, et al. Histology grade is independently associated with relapse risk in patients with ulcerative colitis in clinical remission: a prospective study. Am J Gastroenterol 2016; 111: 685–690. [DOI] [PubMed] [Google Scholar]
- 32. Gupta RB, Harpaz N, Itzkowitz S, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology 2007; 133: 1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bojic D, Bojic B, Protic M, et al. Fecal calprotectin is reliable surrogate marker of endoscopic and histologic mucosal healing in Crohn’s disease and ulcerative colitis. J Crohns Colitis 2011; 5: S34. [Google Scholar]
- 34. Urushikubo J, Yanai S, Nakamura S, et al. Practical fecal calprotectin cut-off value for Japanese patients with ulcerative colitis. World J Gastroenterol 2018; 24: 4384–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bunn SK, Bisset WM, Main MJC, et al. Fecal calprotectin: validation as a noninvasive measure of bowel inflammation in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2001; 33: 14–22. [DOI] [PubMed] [Google Scholar]
- 36. Fernandez-Blanco I, Taxonera C, Cara C, et al. Endoscopic and histologic remission correlation with biomarkers in UC patients treated with adalimumab. Gastroenterology 2015; 148: S791–S791. [Google Scholar]
- 37. Heida A, Knol M, Kobold AM, et al. Agreement between home-based measurement of stool calprotectin and ELISA results for monitoring inflammatory bowel disease activity. Clin Gastroenterol Hepatol 2017; 15: 1742–1749.e1742. [DOI] [PubMed] [Google Scholar]
- 38. Mosli MH, Feagan BG, Sandborn WJ, et al. Histologic evaluation of ulcerative colitis: a systematic review of disease activity indices. Inflamm Bowel Dis 2014; 20: 564–575. [DOI] [PubMed] [Google Scholar]
- 39. Røseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol 1999; 34: 50–54. [DOI] [PubMed] [Google Scholar]
- 40. Magro F, Lopes J, Borralho P, et al. Comparison of the Nancy index with continuous Geboes score: histological remission and response in ulcerative colitis. J Crohn’s Colitis 2020; 14: 1021–1025. [DOI] [PubMed] [Google Scholar]
- 41. Ayling RM, Kok K. Fecal calprotectin. Adv Clin Chem 2018; 87: 161–190. [DOI] [PubMed] [Google Scholar]
- 42. Magro F, Lopes J, Borralho P, et al. Comparison of different histological indexes in the assessment of UC activity and their accuracy regarding endoscopic outcomes and faecal calprotectin levels. Gut 2019; 68: 594–603. [DOI] [PubMed] [Google Scholar]
- 43. Waugh N, Cummins E, Royle P, et al. Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess 2013; 17: xv–xix, 1–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oxman AD, Cook DJ, Guyatt GH. Users’ guides to the medical literature. VI. How to use an overview. Evidence-Based Medicine Working Group. JAMA 1994; 272: 1367–1371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-tag-10.1177_1756284821994741 for Can fecal calprotectin accurately identify histological activity of ulcerative colitis? A meta-analysis by Xiaoqi Ye, Ying Wang, Harry H. X. Wang, Rui Feng, Ziyin Ye, Jing Han, Li Li, Zhirong Zeng, Minhu Chen and Shenghong Zhang in Therapeutic Advances in Gastroenterology