Abstract
We propose the use of the 1-minute sit-to-stand test (1STST) to evaluate the physical capacity and exertional desaturation one month after discharge in a sample of patients who survived COVID-19 pneumonia. This was a cross-sectional study that collected routine data from consecutive patients admitted to the outpatient program in a public hospital in Chile. Patients were asked to complete a 1STST. Data were analyzed according to those with and without a prolonged hospital stay of >10 days. Eighty-three percent of the patients were able to complete the test (N = 50). The median age was 62.7 ± 12.5 years. The average number of repetitions in the 1STST was 20.9 ± 4.8. Thirty-two percent of patients had a decrease in pulse oxygen saturation (SpO2) ≥ 4 points. The prolonged hospital stay subgroup had a significant increase in exertional desaturation (mean difference = 2.6; 95% CI = 1.2 to 3.9; p = 0.001) and dyspnea (mean difference = 1.1; 95% CI = 0.4 to 2.1; p = 0.042) compared to the group of length of stay ≤10 days. In-hospital survivors of COVID-19, the 1STST showed a decrease in physical capacity at one month in those 90% who were able to complete it. The 1STST was able to discriminate between those with and without a prolonged hospital stay and was able to detect exertional desaturation in some patients.
Keywords: SARS-CoV-2, coronavirus disease 2019, functional capacity, rehabilitation
Introduction
Coronavirus Disease 2019 (COVID-19) was first found in Wuhan city, China,1 and evolved into a pandemic in a few weeks.2 It is estimated that up to 20% of patients develop severe respiratory illness,3 and some cases must be admitted to intensive care units.4 Together with the pulmonary impairments related to disease,5 prolonged hospital stays can seriously affect the physical condition of patients due to the negative effects of bed rest and immobility.6
The assessment of functional capacity in patients post COVID-19 after hospitalization has become an important issue for estimating functional consequences, disability and exertional desaturation.7–9 To date, the 6-minute walk test (6WMT) and 1-minute sit-to-stand test (1STST) have been the most common tests used,10 the physical capacity assessment at discharge and follow-up show a low-percentage of subjects performing both tests.7,11 In this context, it is a challenge for clinicians to objectify changes in physical capacity for future decision-making.
The 6MWT requires certain technical execution conditions that are not easy to meet, such as a 30-meter corridor, especially in this time of a pandemic when precautionary measures are even greater. On the other hand, patients after a prolonged hospitalization may present balance problems that may affect the correct execution of the test.
In some situations, not all the technical requirements necessary to perform the 6MWT can be fulfilled. If the distance available is less than 30 meters, it is recommended to choose tests that can be performed in minor distances, as the incremental or the endurance shuttle walking (ISWT or ESWT).12 In other cases, the clinical condition of the patient does not allow a demanding effort, in which case we can use modifications of the 6MWT as the two minute walking test (2MWT).13 Particularly, other exercise tests, the 1STST have emerged because can be used in a reduced space and that has shown a good correlation with the result of the 6MWT in other respiratory diseases. In patients with interstitial lung diseases (ILD), similar to pulmonary impairment produced by COVID-19,14 the 1STST showed good consistency and correlated strongly with 6MWT to measure exercise-induced desaturation.15 Moreover, 1STST is a reliable and valid tool to assess the physical capacity in older adults16 and chronic obstructive pulmonary disease (COPD) patients.17 Furthermore, the 1STST has shown a significant correlation with clinical outcomes in COPD subjects, such as 6MWT, 4-m gait speed test, and physical activity in daily life.18 A recent systematic review19 concluded that 1STST could be a practical, reliable, valid, and responsive alternative to measure physical capacity, and that performance is correlated with 6MWT, making it an ideal alternative for use in office settings and during routine visits.
Knowing the space and isolation limitations of patients post COVID-19, we propose the use of 1STST to evaluate the physical capacity and exertional desaturation one month after discharge in a sample of patients who survived COVID-19 pneumonia and the success rate in the execution of this test. Our hypothesis was that patients with COVID-19 have a limitation in physical capacity, which is higher in patients with a long hospital stay (>10 days). Furthermore, we consider that 1STST may be a good test to detect exertional desaturation.
Methods
Participants and design
This was a cross-sectional study that collected routine data from consecutive patients with COVID-19 admitted to the follow-up program in the Day Hospital of the Hospital Clínico La Florida (Santiago, Chile) between August 4, 2020 and September 11, 2020 (6 weeks). The inclusion criteria were as follows: a) over 18 years of age; b) outpatients discharged from the hospital four weeks prior to evaluation; c) positive PCR assay findings for nasal and pharyngeal swab specimens20 or diagnosis according to chest computed tomography scan evidence.14 We excluded from the 1STST: a) Inability to perform either the test (limited mobility or any joint/mobility pain); b) hemodynamic instability (systolic blood pressure >180 mmHg or diastolic blood pressure >100 mmHg). This study was approved by the ethics committee of our institution, and all patients gave written informed consent. The investigation was performed in conformity to the ethical principles of the Declaration of Helsinki. This study was performed in accordance with the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) Guidelines.
Data collection
At the moment of the admission, demographic characteristics, medical history, exposure history and underlying comorbidities were collected. The main outcome measures were physical capacity, assessed through the number of times that subjects were able to sit-and-stand during the 1STST. All tests were conducted in the same room, in the Day Hospital of our institution with only the presence of the researcher and the patient to avoid distractions. The test was performed with a chair of standard height (46 cm) without armrests positioned against a wall;21 the same chair was used for all participants. Participants were not allowed to use their hands/arms to push the seat of the chair or their body. Participants were instructed to complete as many sit-and-stand cycles as possible in 60 seconds at self-paced speed. We used the reference values based on the healthy adult population previously reported by Strassmann et al.22 The 1STST has good test-retest reliability (ICC = 0.80).16,19
The modified Borg scale (0–10) was used to measure dyspnea and fatigue immediately before and after the 1STST. A finger oximeter was used to record pulse oxygen saturation (SpO2) and heart rate (HR). A desaturation level of ≥4% was considered clinically significant.15 Patients were evaluated one month after discharge, and all measurements were taken by a single evaluator. The evaluator had previous experience in this test. We divided our sample into two subgroups according to the length of stay (≤10 or >10 days) to determine the effects of prolonged hospital stay in physical capacity and exercise-induced oxygen desaturation. This cut-off value has been considered in previous studies to indicate long-term hospital stay in patients with COVID-1923 and in patients with pneumonia.24 Furthermore, this was defined considering the median hospital stay reported in previous studies, which was 10 days in patients discharged for COVID-1925,26 and considering that patients’ condition worsens on the 10th day after illness onset.20,27
Statistical analyses
The data normality was verified through a Shapiro–Wilk test. The mean and standard deviations were calculated for the quantitative variables and the percentages for the categorical variables. T-Test was used to compare continuous variables between groups, and Chi-square was used to compare the categorical variables. Statistical significance was established in p < 0.05. All statistical analyses were performed in SPSS version 22.0 (IBM Corporation, Armonk, NY). Post hoc power was calculated using the software G*Power 3.1, with a significance level of 0.05 and an effect size of 0.98.
Results
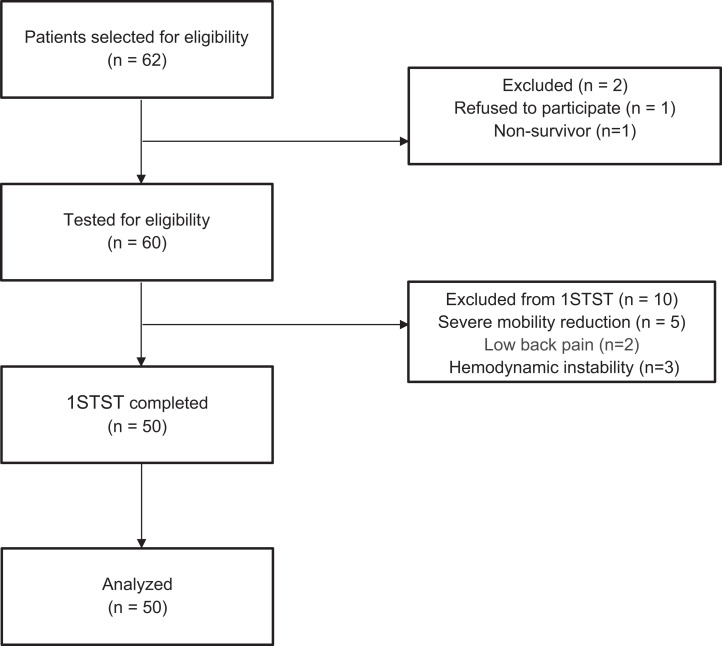
Sixty patients were tested for eligibility. Ten patients did not meet the criteria for the 1STST, seven due to mobility limitations and three due to hemodynamic instability (Figure 1). The median age was 62.7 ± 12.5 years, and 27 (54%) patients were male. The baseline patient characteristics are presented in Table 1. Eighty-three percent of the patients were able to complete the test (N = 50). The average number of repetitions in the 1STST was 20.9 ± 4.8, 42% were under the 2.5th percentile of predicted value, and 90% were under the 25th percentile, in relation to the reference values.22 For a multivariate analysis with ANOVA, there was no interaction (p > 0.05) when comparing the number of repetitions in the 1STST between both groups with the subject’s individual factors: sex, body mass index (BMI) and age. Thirty-two percent of patients had a decrease in Sp02 ≥ 4 points. The prolonged hospital stay subgroup had a significant increase in exertional desaturation (mean difference =2.6; 95% CI = 1.2 to 3.9; p = 0.001) and dyspnea (mean difference =1.1; 95% CI = 0.4 to 2.1; p = 0.042) compared to the group of length of stay ≤10 days. The power to detect exertional desaturation was 92%. There was no association between the number of repetitions of the 1STST with the degree of desaturation or dyspnea after the test (R = −0.12; p > 0.05). Table 2 shows the results of sit-to-stand and cardiorespiratory parameters. There were no significant differences in the variables studied when comparing patients with and without invasive mechanical ventilation (Supplementary material).
Figure 1.
Patient flow diagram.
Table 1.
Baseline patient characteristics.
| Characteristics | Total group (n = 50) | Hospital stay ≤10 days (n = 14) | Hospital stay >10 days (n = 36) | p value |
|---|---|---|---|---|
| Age (mean ± SD) | 62.7 ± 12.5 | 58.6 ± 13.8 | 64.3 ± 11.9 | 0.157 |
| Sex, male, n (%) | 27 (54) | 5 (35.7) | 22 (61.1) | 0.097 |
| BMI (mean ± SD) | 30.3 ± 5.3 | 32.4 ± 5.8 | 29.4 ± 4.9 | 0.075 |
| Days of discharge (mean ± SD) | 35.7 ± 8.6 | 32.7 ± 5.6 | 36.8 ± 9.3 | 0.125 |
| ICU stay, n (%) | 12 (24) | 0 (0) | 12 (33.3) | 0.010 |
| Invasive ventilation, n (%) | 12 (24) | 0 (0) | 12 (33.3) | 0.010 |
| Hospitalization days (mean ± SD) | 18.0 ± 12.3 | 6.7 ± 1.5 | 22.4 ± 11.9 | <0.001 |
| Comorbidities, n (%) | ||||
| Obesity | 25 (50) | 9 (64.3) | 16 (44.4) | 0.173 |
| Hypertension | 29 (58) | 9 (64.3) | 20 (55.6) | 0.407 |
| Diabetes | 19 (38) | 7 (50) | 12 (33.3) | 0.221 |
| Cardiovascular disease | 7 (14) | 2 (14.3) | 5 (13.9) | 0.643 |
| Stroke | 2 (4.1) | 0 (0) | 2 (5.7) | 0.506 |
| COPD | 1 (2) | 1 (7.1) | 0 (0) | 0.280 |
| Chronic kidney diseases | 3 (6) | 0 (0) | 3 (8.3) | 0.364 |
| Malignancy | 3 (6) | 1 (7.1) | 2 (5.6) | 0.556 |
| Previous smoker | 5 (10) | 2 (14.3) | 3 (8.3) | 0.433 |
| CT Patterns, n (%) | ||||
| Ground-glass opacities | 19 (38) | 7 (50) | 12 (33.3) | 0.221 |
| Consolidation | 1 (2) | 1 (7.1) | 0 (0) | 0.280 |
| Crazy-paving pattern | 1 (2) | 0 (0) | 1 (2.8) | 0.720 |
| Mixed | 28 (56) | 5 (35.7) | 23 (63.9) | 0.069 |
| Without alteration | 1 (2) | 1 (7.1) | 0 (0) | 0.280 |
| CT Findings distribution, n (%) | ||||
| Unilateral | 2 (4) | 1 (7.1) | 1 (2.8) | 0.061 |
| Bilateral | 47 (94) | 12 (85.7) | 35 (97.2) | 0.186 |
| Without alteration | 1 (2) | 1 (7.1) | 0 (0) | 0.280 |
BMI = body mass index; ICU = intensive care units; COPD = chronic obstructive pulmonary disease; 1STST = 1 minute sit-to-stand test; CT = computed tomography; SD = standard deviation.
Table 2.
Results of sit-to-stand and cardiorespiratory parameters.
| Characteristics | Total group (n = 50) | Hospital stay ≤10 days (n = 14) | Hospital stay >10 days (n = 36) | p value |
|---|---|---|---|---|
| 1STST (repetitions) | 20.9 ± 4.8 | 21.0 ± 5.1 | 20.8 ± 4.7 | 0.916 |
| Repetitions <2.5th percentile, n (%) | 21 (42) | 3 (21.4) | 18 (50) | 0.062 |
| Repetitions <25th percentile, n (%) | 45 (90) | 12 (85.7) | 33 (91.7) | 0.433 |
| SpO2 Baseline (%) | 96.3 ± 1.4 | 96.3 ± 1.4 | 96.3 ± 1.4 | 0.986 |
| SpO2 Post (%) | 93.6 ± 3.5 | 95.4 ± 1.4 | 92.8 ± 3.8 | 0.01* |
| Change in SpO2 (points) | −2.7 ± 3.2 | −0.8 ± 1.5 | −3.4 ± 3.4 | 0.001** |
| Change in SpO2 ≥ 4, n (%) | 16 (32) | 1 (7.1) | 15 (41.7) | 0.019* |
| SpO2 <90% post test, n (%) | 8 (16) | 0 (0) | 8 (22.2) | 0.056 |
| Heart rate Baseline (beats/min) | 86.2 ± 11.1 | 89.7 ± 8.5 | 84.9 ± 11.6 | 0.112 |
| Heart rate Post (beats/min) | 106.1 ± 15.4 | 110.1 ± 16.5 | 104.6 ± 14.9 | 0.286 |
| Change in Heart rate (points) | 19.9 ± 11.5 | 20.4 ± 12.2 | 19.7 ± 11.4 | 0.842 |
| Heart rate reserve (%) | 28.9 ± 17.3 | 30.3 ± 20.6 | 28.3 ± 16.1 | 0.712 |
| Dyspnea Baseline, Borg Index (0–10) | 1.6 ± 1.9 | 1.6 ± 1.8 | 1.6 ± 1.9 | 0.984 |
| Dyspnea Post, Borg Index (0–10) | 3.9 ± 2.6 | 3.2 ± 2.2 | 4.1 ± 2.8 | 0.220 |
| Change in Dyspnea (points) | 2.4 ± 1.9 | 1.6 ± 1.4 | 2.7 ± 1.9 | 0.042* |
| Fatigue Baseline, Borg Index (0–10) | 1.3 ± 2.1 | 1.4 ± 2.3 | 1.3 ± 2.0 | 0.802 |
| Fatigue Post, Borg Index (0–10) | 2.5 ± 2.8 | 2.1 ± 2.8 | 2.7 ± 2.7 | 0.539 |
| Change in Fatigue (points) | 2.3 1.8 | 0.7 ± 0.8 | 1.5 ± 2.1 | 0.059 |
1STST = 1 minute sit-to-stand test; SpO2 = pulse oxygen saturation. Data are presented as mean ± SD unless otherwise indicated. *Statistically significant difference (p < 0.05); **Statistically significant difference (p < 0.01).
Discussion
Our results are consistent with the evidence showing that patients post COVID-19 have a decrease in physical capacity one month after discharge. Additionally, patients who were hospitalized for more than ten days had more significant exertional desaturation and dyspnea.
Our results coincide with those of Belli et al.7 in finding a decrease in physical capacity in patients post COVID-19. However, Belli et al.’s values are even lower.7 One possible explanation is the age of the patients. In their series, the mean age was 74 years versus 66 in our population. Obviously, the 8 years of difference determine a significant decrease not only given by the disease but also by the age of the patients.
Another essential aspect that we highlight is that the performed tests rate (i.e. % of patients who complete the 1STST) of this test in our patients is over 80%. This percentage is almost the double that reported by Belli and Simonelli.7,8 The reports of both have ages of over 70 in common, so perhaps, to evaluate physical capacity in the older population, it makes sense to apply other tests that are commonly used to evaluate older adults, such as the Short Physical Performance Battery (SPPB) or the Timed Up and Go test (TUG), which in both authors showed success rates greater than 90%.7,8
Thirty-two percent of our patients showed a drop of more than 4 points in SpO2 and 16% desaturated, even below 90%, in line with the increase in dyspnea. These results are lower than those shown by Fuglebjerg et al.,28 who reported a desaturation of less than 90% in half of the subjects to whom she applied the 6MWT at discharge, which would explain our better results. Another difference in our results is that we did not stop the test when it dropped below 90%, since the objective of these tests is to detect not only desaturation but also its magnitude.29 The use of the 1STST showed in our results to detect the exertional desaturation, making it a good alternative tool for the 6MWT, similar to shown in other pathologies as ILD.15
On the other hand, the 6MWT needs specific technical requirements for its execution as a 30-meter corridor.12 These are not easy to achieve within a hospital and much less within an ICU, especially in this time of a pandemic when rehabilitation services have had to be reconverted.30 If we add to that the impairment of the balance of patients who have had prolonged hospitalizations,31 and considering that the balance influences the result of the 6MWT, the 1STST appears as a valid alternative, especially because it allows to maintain safe conditions when using a chair and has proven to be an excellent follow-up tool in face-to-face or remote rehabilitation programs.10,32
We used the reference values recommended by our national guidelines for post COVID-19 rehabilitation guide.33 Although these equations are for the Swiss population,22 have some advantages of using them as cover a wide age range from 20 to 79 years and establishes the normality values in percentiles. On the other hand, using the Strassman values allows comparisons with previous articles that have investigated the 1STST since they have used the same values.7,34
Limitations
Finally, our study has some limitations, such as the non-comparison with the 6MWT, mainly due to the lack of technical and safety conditions in the hospital to carry it out. In addition, the results were compared with international reference values since they are not available for our country. Furthermore, it is difficult to determine or state whether the 1STST is a test of physical capacity with no comparison to the gold standard (i.e. cardiopulmonary exercise test) and future research should delve into this aspect. Another limitation of the study was the small sample included. Due to the nature of convenience sampling, all patients who survived hospitalization and were referred to the follow-up program were included, so we cannot rule out a selection bias. To overcome this limitation, we calculated the power of the study, which was high (92%).
Conclusions
In hospital survivors of COVID-19, the 1STST showed a decrease in physical capacity at one month in those 90% who were able to complete it. The 1STST was able to discriminate between those with and without a prolonged hospital stay and was able to detect exertional desaturation in some patients.
Supplemental material
Supplemental Material, sj-pdf-1-crd-10.1177_1479973121999205 for Use of sit-to-stand test to assess the physical capacity and exertional desaturation in patients post COVID-19 by Rodrigo Núñez-Cortés, Gonzalo Rivera-Lillo, Marisol Arias-Campoverde, Dario Soto-García, Roberto García-Palomera and Rodrigo Torres-Castro in Chronic Respiratory Disease
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Rodrigo Núñez-Cortés  https://orcid.org/0000-0002-4068-9338
https://orcid.org/0000-0002-4068-9338
Rodrigo Torres-Castro  https://orcid.org/0000-0001-7974-4333
https://orcid.org/0000-0001-7974-4333
Supplemental material: Supplemental material for this article is available online.
References
- 1. Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA 2020; 323: 709–710. [DOI] [PubMed] [Google Scholar]
- 2. Adhikari SP, Meng S, Wu Y-J, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty 2020; 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi 2020; 41: 145–151. [DOI] [PubMed] [Google Scholar]
- 4. Murthy S, Gomersall CD, Fowler RA. Care for critically Ill patients With COVID-19. JAMA 2020; 323: 1499–1500. [DOI] [PubMed] [Google Scholar]
- 5. Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. Epub ahead of print 25 November 2020. DOI: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Candan SA, Elibol N, Abdullahi A. Consideration of prevention and management of long-term consequences of post-acute respiratory distress syndrome in patients with COVID-19. Physiother Theory Pract 2020; 36: 663–668. [DOI] [PubMed] [Google Scholar]
- 7. Belli S, Balbi B, Prince I, et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur Respir J 2020; 56(4):2002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simonelli C, Paneroni M, Fokom AG, et al. How the COVID-19 infection tsunami revolutionized the work of respiratory physiotherapists: an experience from Northern Italy. Monaldi Arch Chest Dis 2020; 90: 1085. [DOI] [PubMed] [Google Scholar]
- 9. Bai C, Chotirmall SH, Rello J, et al. Updated guidance on the management of COVID-19: from an American Thoracic Society/European Respiratory Society coordinated International Task Force (29 July 2020). Eur Respir Rev 2020; 29(157): 200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holland AE, Malaguti C, Hoffman M, et al. Home-based or remote exercise testing in chronic respiratory disease, during the COVID-19 pandemic and beyond: a rapid review. Chronic Respir Dis 2020; 17: 147997312095241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Curci C, Pisano F, Bonacci E, et al. Early rehabilitation in post-acute COVID-19 patients: data from an Italian COVID-19 rehabilitation unit and proposal of a treatment protocol. a cross-sectional study. Eur J Phys Rehabil Med 2020; 56(5):633–641. [DOI] [PubMed] [Google Scholar]
- 12. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44: 1428–1446. [DOI] [PubMed] [Google Scholar]
- 13. Fumagalli A, Misuraca C, Bianchi A, et al. Pulmonary function in patients surviving to COVID-19 pneumonia. Infection 1–5. Epub ahead of print 28 July 2020. DOI: 10.1007/s15010-020-01474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zu ZY, Jiang MD, Xu PP, et al. Coronavirus Disease 2019 (COVID-19): a perspective from China. Radiology 2020; 296: E15–E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Briand J, Behal H, Chenivesse C, et al. The 1-minute sit-to-stand test to detect exercise-induced oxygen desaturation in patients with interstitial lung disease. Ther Adv Respir Dis 2018; 12: 1753466618793028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ritchie C, Trost SG, Brown W, et al. Reliability and validity of physical fitness field tests for adults aged 55 to 70 years. J Sci Med Sport 2005; 8: 61–70. [DOI] [PubMed] [Google Scholar]
- 17. Crook S, Büsching G, Schultz K, et al. A multicentre validation of the 1-min sit-to-stand test in patients with COPD. Eur Respir J 2017; 49(3):1601871. [DOI] [PubMed] [Google Scholar]
- 18. Morita AA, Bisca GW, Machado FVC, et al. Best protocol for the sit-to-stand test in subjects with COPD. Respir Care 2018; 63: 1040–1049. [DOI] [PubMed] [Google Scholar]
- 19. Bohannon RW, Crouch R. 1-Minute sit-to-stand test: systematic review of procedures, performance, and clinimetric properties. J Cardiopulm Rehabil Prev 2019; 39: 2–8. [DOI] [PubMed] [Google Scholar]
- 20. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ozalevli S, Ozden A, Itil O, et al. Comparison of the sit-to-stand test with 6 min walk test in patients with chronic obstructive pulmonary disease. Respir Med 2007; 101: 286–293. [DOI] [PubMed] [Google Scholar]
- 22. Strassmann A, Steurer-Stey C, Lana KD, et al. Population-based reference values for the 1-min sit-to-stand test. Int J Public Health 2013; 58: 949–953. [DOI] [PubMed] [Google Scholar]
- 23. Yue H, Yu Q, Liu C, et al. Machine learning-based CT radiomics method for predicting hospital stay in patients with pneumonia associated with SARS-CoV-2 infection: a multicenter study. Ann Transl Med 2020; 8: 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iroezindu MO, Isiguzo GC, Chima EI, et al. Predictors of in-hospital mortality and length of stay in community-acquired pneumonia: a 5-year multi-centre case control study of adults in a developing country. Trans R Soc Trop Med Hyg 2016; 110: 445–455. [DOI] [PubMed] [Google Scholar]
- 25. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qiu C, Deng Z, Xiao Q, et al. Transmission and clinical characteristics of coronavirus disease 2019 in 104 outside-Wuhan patients, China. J Med Virol 2020; 92: 2027–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu X-W, Wu X-X, Jiang X-G, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020; m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fuglebjerg NJU, Jensen TO, Hoyer N, et al. Silent hypoxia in patients with SARS CoV-2 infection before hospital discharge. Int J Infect Dis 2020; 99: 100–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Du Plessis JP, Fernandes S, Jamal R, et al. Exertional hypoxemia is more severe in fibrotic interstitial lung disease than in COPD. Respirology 2018; 23: 392–398. [DOI] [PubMed] [Google Scholar]
- 30. Rivera-Lillo G, Torres-Castro R, Fregonezi G, et al. Challenge for rehabilitation after hospitalization for COVID-19. Arch Phys Med Rehab 2020; 101: 1470–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Denehy L, Nordon-Craft A, Edbrooke L, et al. Outcome measures report different aspects of patient function three months following critical care. Intensive Care Med 2014; 40: 1862–1869. [DOI] [PubMed] [Google Scholar]
- 32. Torres-Castro R, Solis-Navarro L, Sitjà-Rabert M, et al. Functional limitations post-COVID-19: a comprehensive assessment strategy. Arch Bronconeumol 2021; 57(Suppl 1): 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodríguez-Núñez I, Torres-Castro R, Vera R, et al. Consenso de rehabilitación respiratoria en pacientes con COVID-19. Technical Report. August 2020. DOI: 10.13140/RG.2.2.16594.17607/1. [Google Scholar]
- 34. D’Cruz RF, Waller MD, Perrin F, et al. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res 2020; 7(1): 00655–02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-crd-10.1177_1479973121999205 for Use of sit-to-stand test to assess the physical capacity and exertional desaturation in patients post COVID-19 by Rodrigo Núñez-Cortés, Gonzalo Rivera-Lillo, Marisol Arias-Campoverde, Dario Soto-García, Roberto García-Palomera and Rodrigo Torres-Castro in Chronic Respiratory Disease