Abstract
Background:
Long non-coding RNA bladder cancer associated transcript 1 (BLACAT1) is oncogenic in several types of cancers. However, little is known concerning its expression and function in prostate cancer.
Methods:
Paired prostate cancer samples were collected, and the expression levels of BLACAT1, miR-29a-3p and disheveled segment polarity protein 3 (DVL3) were examined by quantitative real-time polymerase chain reaction (qRT-PCR); BLACAT1 shRNAs were transfected into PC-3 and LNCaP cell lines, and proliferative ability was detected by cell counting kit-8 (CCK-8) assay; qRT-PCR and Western blot were used to analyze the changes of miR-29a-3p and DVL3; dual-luciferase reporter gene assay was used to determine the regulatory relationships between miR-29a-3p and BLACAT1, and miR-29a-3p and DVL3.
Results:
BLACAT1 expression was significantly up-regulated in cancerous tissues of prostate cancer samples and positively correlated with the expression of DVL3, while negatively associated with miR-29a-3p. After the transfection of BLACAT1 shRNAs into prostate cancer cells, the proliferative ability and metastatic ability of cancer cells were significantly inhibited; BLACAT1 shRNAs could reduce the expression of DVL3 on both mRNA and protein expressions levels, the luciferase activity of BLACAT1 reporter was inhibited by miR-29a-3p, and DVL3 was validated as a target gene of miR-29a-3p.
Conclusion:
BLACAT1 expression is abnormally up-regulated in prostate cancer tissues. BLACAT1 can modulate the proliferative and metastatic ability of prostate cancer cells and have the potential to be the “ceRNA” to regulate the expression of DVL3 by sponging miR-29a-3p.
Keywords: prostate cancer, lncRNA BLACAT1, miR-29a-3p, DVL3
Introduction
Prostate cancer (PCa) is one of the most common malignant tumors in male genitourinary system.1-2 Because of the lack of specific symptoms of early PCa, it is easy to be confused with benign prostatic hyperplasia. When obvious symptoms appear, the tumors are often getting progressed to the middle and late stages, even accompanied by metastasis, and thus the patients are deprived of the best treatment opportunity. Searching for reliable biomarkers is conducive to the early diagnosis of PCa and it is also of great significance to prolong the survival time and improve the quality of life of the patients. Meanwhile, it can provide new ideas and research directions for gene therapy of PCa.
Long non-coding RNA (lncRNA) is a type of non-coding RNA, named for its length of more than 200 nucleotides and the lack of protein coding ability.3 Accumulating studies authenticate that lncRNAs figure prominently in epigenetic regulation, cell cycle regulation, cell differentiation and protein metabolism.3 Besides, some studies confirm that there are differences in the expression profile of lncRNA between normal cells and tumor cells.4 Bladder cancer associated transcript 1 (BLACAT1) is located on human chromosome 1q32.1. It is identified for the first time that in bladder cancer, it is overexpressed in many cancers and can promote the proliferation, migration and invasion of cancer cells.5
MicroRNA (miRNA) is a kind of non-coding RNA with a length of about 22 nucleotides. It is widely involved in multiple biological processes.6 A large number of studies unearth that the abnormal expression of miRNAs is closely related to the tumorigenesis and progression of tumors, including PCa.7 For example, the down-regulation of miR-564 expression is involved in the progression of PCa by modulating the proto-oncogene MLLT3.8 The expression and role of miR-29a-3p in gastric cancer, thyroid cancer and other cancers have been studied.9-11 However, the regulating mechanism of miR-29a-3p in PCa has not been fully elucidated.
Wnt signaling pathway is a key pathway to maintain stem cell-like characteristics such as stem cell growth and self-renewal. Abnormal activation or mutation of Wnt signaling pathway is closely related to tumorigenesis.12,13 As important proteins widely existing in the cytoplasm, disheveled (DVL) family members also participate in the regulation of Wnt signaling pathway. It is revealed that the high expression of DVL3, which is a member of DVL family, is associated with the high risk of recurrence in PCa patients.14 However, the molecular mechanism of DVL3 dysregulation in PCa needs further study.
This study aimed to explore the expression changes of BLACAT1 and miR-29a-3p in PCa, and also tried to delve into their roles in PCa. We demonstrated the oncogenic function of BLACAT1/miR-29a-3p/DVL3 axis in the progression of PCa, which provided potential biomarkers and therapy targets for clinical diagnosis and treatment of this disease.
Materials and Methods
Tissue Specimens
We selected 42 patients with PCa who underwent PCa surgery in the Affiliated Hospital of North Sichuan Medical College from January 2017 to January 2018 to get the cancerous and adjacent normal tissue samples. The tissue samples were immediately preserved in liquid nitrogen at -196 °C after the surgical excision.
Cell Culture
Human PCa cell lines DU145, LNCap, PC-3 and normal prostatic epithelial cell line RWPE-1 were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). RWPE-1 cells were cultured in prostate epithelial cell medium (ScienCell, Carlsbad, CA, USA). All the cancer cells were cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) at 37 °C in 5% CO2. The medium was changed at the intervals of every 3 d.
Cell Transfection
PCa cells in logarithmic phase were seeded into 6-well plates with 2.5 × 105 cells per well. Cell transfection was carried out according to the instruction of LipofectamineTM 2000 (Thermo Fisher Scientific, Rockford, IL, USA). shRNAs targeting BLACAT1 (BLACAT1_shRNA1 and BLACAT1_shRNA2), BLACAT1 over-expression plasmid (pcDNA-BLACAT1), miR-29a-3p mimics, miR-29a-3p inhibitor and their negative controls (shRNA_control, blank vector, mimics control and inhibitor control) were transfected into the corresponding cells, respectively. The vectors and shRNAs mentioned above were designed and constructed by GenePharma (Shanghai, China). MiR-29a-3p mimics and inhibitors were purchased from RiboBio (Guangzhou, China).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted and purified from tissues and cell lines using TRIzol reagent (Invitrogen, Carlsbad, USA), and then reversely transcribed to cDNA with SuperScript First Strand cDNA System (Invitrogen, Grand Island, NY, USA). qRT-PCR was then conducted on ABI 7300 instrument (Applied Biosystems, Foster City, CA, USA) with SYBR Green PCR Master mix (Thermo Fisher Scientific, Rockford, IL, USA). GAPDH and U6 were used to normalize the expressions, and the relative expressions of BLACAT1, miR-29a-3p and DVL3 were calculated by 2-ΔΔCt method. Primers used in this study are listed in Table 1.
Table 1.
Primer Sequences Used in this Study.
| Name | Primer sequences(5’-3’) |
|---|---|
| BLACAT1 | Forward: CAAGAGGAGCCGGCTTAGCATCTA |
| Reverse: ACGGTTCCAGTCCTCAGTCAG | |
| miR-29a-3p | Forward: AGCACCAUCUGAAAUCGGUUA |
| universal primer: GTGCAGGGTCCGAGGT | |
| DVL3 | Forward: CCATCACCAGCTCCATCC |
| Reverse: CTGCTGCGTGTAGTGTGG | |
| GAPDH | Forward: TGATGGGTGTGAACCACGAG |
| Reverse: CTGATGGGTGTGAACCACGAG | |
| U6 | Forward: CTCGCTTCGGCAGCACA |
| Reverse: AACGCTTCACGAATTTGCGT |
Cell Counting Kit-8 (CCK-8) Assay
HCT-116 and SW480 cells (3000 cells per well) were incubated in 96 well plates, and cultured for 1, 2, 3 and 4 d, respectively. On each day, 10 µL CCK-8 kit (Beyotime, Beijing, China) was added, with which the cells were incubated for 1 h. Then the absorbance (OD value) of each well was measured at 450 nm using a microplate reader. The average of OD values from 3 wells in each group was calculated and the proliferation curve was drawn.
Transwell Experiment
In invasion assay, 1 day before the inoculation, the chambers were coated with Matrigel (BD, San Jose, CA, USA) on the basal membrane, while the Matrigel was not needed in migration assay. In the lower chamber, RPMI-1640 medium with 10% FBS was added, and in the upper chamber, cell suspension without FBS was added. After the incubation for 24 h, the cells that failed to migrate or invade were removed from the upper chamber. The migrated or invaded cells were fixed with 4% polyformaldehyde for 10 min and then stained with 0.5% crystal violet. After that, the cells were observed under an inverted microscope. The cells were counted in 6 random visual fields, and the average value was obtained.
Flow Cytometry Analysis
Flow cytometry was used to detect cellular apoptosis in this study. After 24 h of culture, the cells were washed twice with PBS buffer, fixed with 70% ethanol, and stored overnight at 4 °C. After dyeing with 10 µL Annexin V staining solution and 5 µL PI staining solution (Yeasen Biotech Co., Ltd., Shanghai, China), the cells were fully mixed and incubated in the dark at room temperature for 15 min. Following the culture, binding buffer was then added and the apoptosis rate of cells in each group was detected.
Dual-Luciferase Reporter Assay
Luciferase reporter assay system (Promega, Madison, WI, USA) was used to validate the bind sites. The target fragments of wild type (wt) BLACAT1 and mutated (mut) BLACAT1 were constructed and integrated into psiCHECK-2 plasmids (Promega, Madison, WI, USA), respectively. BLACAT1-wt plasmid or BLACAT1-mut plasmid was co-transfected with miR-29a-3p mimics or negative controls into HEK293 T cells. After 48 h of transfection, luciferase activity was determined according to the manufacturer’s instruction. Similar method was used to construct DVL3-wt and DVL3-mut, and detect the binding relationship between miR-29a-3p and DVL3.
Western Blot
Cells were lysed with RIPA buffer containing PMSF (Beyotime, Shanghai, China). After high-speed centrifugation, the supernatant was collected and heated in a water bath at 100°C for 10 min to denature the protein. Protein samples were then separated by SDS-PAGE and transferred to nitrocellulose membrane (Millipore, Bedford, MA, USA). Blocked by 5% fat-free milk, the membranes were then incubated with anti-DVL3 (diluted 1:1000, Cell Signaling Technology) and anti-GAPDH (diluted 1:2000, Santa Cruz) antibodies, respectively. Next, the membranes were washed by TBST 3 times and then incubated with the secondary antibody (1:2000, Santa Cruz Biotechnology) conjugated with horseradish peroxidase (HRP) for 1 h at room temperature. ECL chemiluminescence substrates A and B (Millipore, Bedford, MA, USA) were mixed in equal volumes and added to the membranes, and an automatic developer (ChemiDoc XRS imaging system) was used to detect the signal.
Statistical Analysis
SPSS18.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for data analysis. All data were expressed as mean ± SD. Student’s t-test was used for statistical analysis. P < 0.05 was considered to have statistical significance.
Result
Expressions of BLACAT1 and miR-29a-3p in PCa
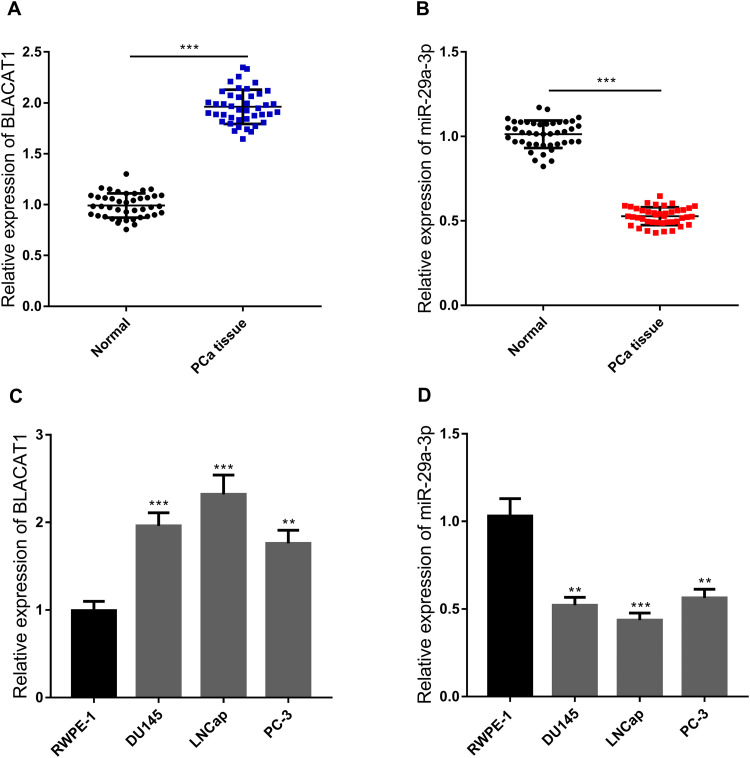
We conducted qRT-PCR to detect the expressions of BLACAT1 and miR-29a-3p in 42 pairs of PCa tissues and adjacent tissues. The results showed that the expression of BLACAT1 in cancer tissues was significantly higher than that in adjacent normal tissues (Figure 1A), while the expression of miR-29a-3p was markedly lower than that in the adjacent normal tissues (Figure 1B). Further detection in cell lines showed that the expression of BLACAT1 in PCa cell lines (DU145, LNCap and PC-3) was significantly higher than that in normal prostate epithelial cells RWPE-1, whereas the expression of miR-29a-3p was observably lower than that in RWPE-1 group (Figure 1C and D).
Figure 1.
Expressions of BLACAT1 and miR-29a-3p in PCa. (A-B) qRT-PCR was used to detect the expressions of BLACAT1 and miR-29a-3p in 42 pairs of cancer tissues. n = 42, ** P < 0.01 and *** P < 0.001. (C-D) qRT-PCR was used to detect the expressions of BLACAT1(C) and miR-29a-3p(D) in normal prostate epithelial cell line and PCa cell lines. ** P < 0.01 and *** P < 0.001. All the experiments were replicated 3 times.
BLACAT1 Promoted the Proliferation, Invasion and Migration, and Inhibited the Apoptosis of PCa Cells
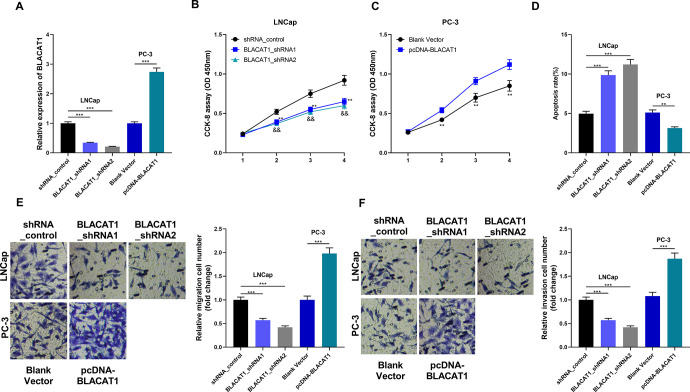
To verify the effects of BLACAT1 in PCa, we knocked down and up-regulated BLACAT1 expression in LNCap and PC3 cell lines, respectively (Figure 2A). CCK-8 assay was used to detect the proliferation of PC3 cells. We found that knockdown of BLACAT1 significantly slowed down the proliferation of LNCap cells. Conversely, overexpression of BLACAT1 could promote the proliferation of PC3 cells (Figure 2B and C). Flow cytometry was used to detect cellular apoptosis, the results of which showed that apoptosis of PC3 cells was decreased after the overexpression of BLACAT1, while the apoptosis of LNCap cells was increased after the knockdown of BLACAT1 (Figure 2D). Transwell assay was used to detect the effect of BLACAT1 on the migration and invasion of PCa cells. The results showed that overexpression of BLACAT1 significantly increased the migration and invasion ability of PC3 cells, while after knocking down BLACAT1, the migration and invasion of LNCap cells were decreased markedly (Figure 2E and F).
Figure 2.
The function of BLACAT1 in PCa. (A) qRT-PCR was used to detect the expression of BLACAT1 after transfection. (B-C) CCK-8 assay was used to examine cell proliferation. (D) Flow cytometry was used to detect cell apoptosis. (E-F) Transwell method was used to detect cell invasion and migration. * P < 0.05, ** P < 0.01 and *** P < 0.001. In B, ** P < 0.01 shRNA_control group vs BLACAT1_shRNA1 group; && P < 0.01 shRNA_control group vs BLACAT1_shRNA2 group. All the experiments were replicated 3 times.
BLACAT1 Targeted miR-29a-3p
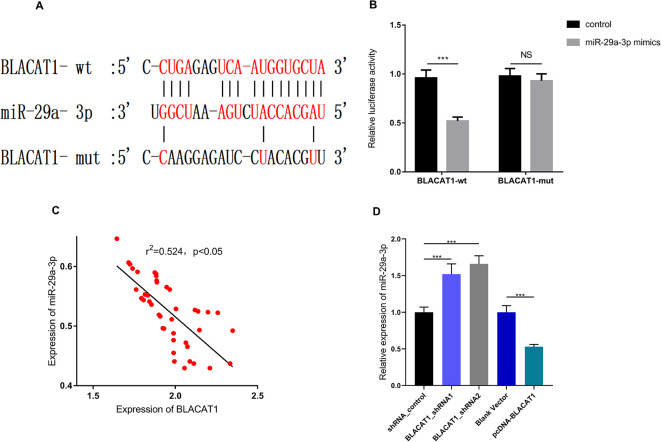
To further pinpoint the downstream mechanism of BLACAT1, we searched the LncBase Predicted v.2 database and found that BLACAT1 had a potential binding site with miR-29a-3p (Figure 3A). In order to verify whether BLACAT1 can directly adsorb miR-29a-3p, dual-luciferase reporter assay was performed, and we demonstrated that miR-29a-3p markedly reduced the luciferase activity of wild-type BLACAT1 reporter, but had no significant effect on the mutated BLACAT1 reporter (Figure 3B). In addition, we found that BLACAT1 was negatively correlated with the expression of miR-29a-3p in PCa samples (Figure 3C, r2 = 0.524, P < 0.05). Moreover, the expression of miR-29a-3p was significantly down-regulated after the overexpression of BLACAT1, while up-regulated by knocking down BLACAT1 in PCa cells (Figure 3D). Collectively, these data indicated that BLACAT1 could be a sponge of miR-29a-3p and reduce its expression in PCa.
Figure 3.
BLACAT1 targeted on miR-29a-3p. Base pairing relationship between BLACAT1 and miR-29a-3p as predicted by LncBase Predicted v.2 database. Luciferase gene reporter assay was used to verify the targeting binding relationship between BLACAT1 and iR-29a-3p. The correlation between the expression of BLACAT1 and miR-29a-3p in PCa tissues (n = 42). Detection of the expression of miR-29a-3p after BLACAT1 knockdown or overexpression of BLACAT1 by qRT-PCR in PCa cell lines. ** P < 0.01, *** P < 0.001 and NS P > 0.05. All the experiments were replicated 3 times.
MiR-29a-3p Inhibited the Proliferation, Invasion and Migration, and Promoted the Apoptosis of PCa Cells
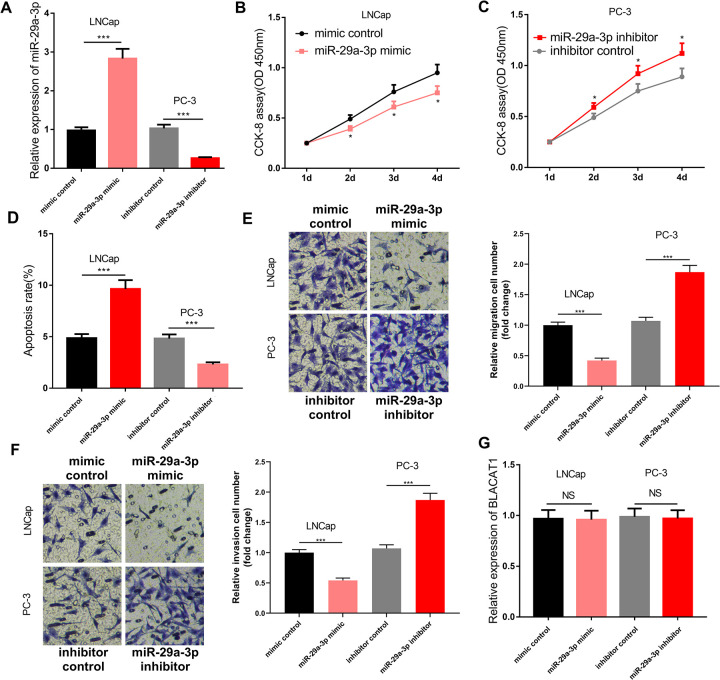
To investigate the role of miR-29a-3p in PCa, we up-regulated or inhibited miR-29a-3p expression in LNCap and PC3 cell lines, respectively (Figure 4A). We found that the transfection of miR-29a-3p mimics could inhibit the proliferation of LNCap cells; conversely, transfection of miR-29a-3p inhibitors could promote the proliferation of PC3 cells (Figure 4B and C). Transwell assay showed that the transfection of miR-29a-3p mimics significantly reduced the migration and invasion of PCa cells, while the transfection of miR-29a-3p inhibitors markedly promoted these processes (Figure 4D and E). Flow cytometry analysis showed that the transfection of miR-29a-3p mimics promoted apoptosis of LNCap cells, and the transfection of miR-29a-3p inhibitors reduced apoptosis of PC3 cells (Figure 4F). These data implied that miR-29a-3p was a tumor suppressor in PCa. Additionally, we also found that miR-29a-3p could not change the expression of BLACAT1, which proved that miR-29a-3p was a downstream molecule, rather than an upstream molecule of BLACAT1 (Figure 4G).
Figure 4.
Function of miR-29a-3p in PCa. qRT-PCR was used to detect the expression of miR-29a-3p after transfection. (B-C) CCK-8 assay was used to examine the cell proliferation. (D-E) Transwell method was used to detect cell invasion and migration. Flow cytometry was used to detect cell apoptosis. qRT-PCR was used to detect the expression of BLACAT1 after the transfection of miR-29a-3p mimics or inhibitors in PCa cells. * P < 0.05, ** P < 0.01 and *** P < 0.001. All the experiments were replicated 3 times.
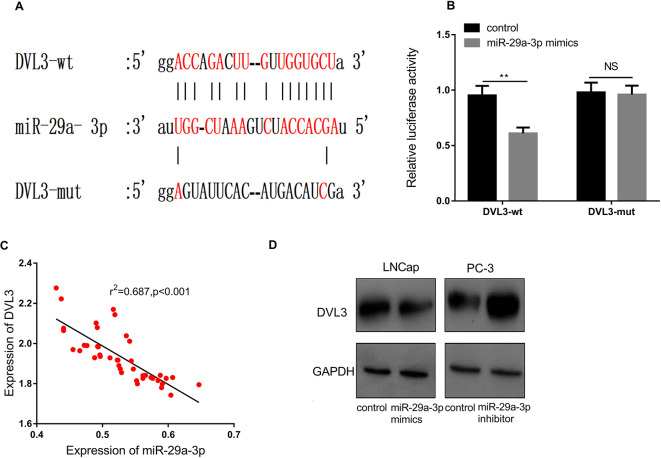
MiR-29a-3p Targeted and Down-Regulated DVL3 Expression in PCa
By querying TargetScan database, we noticed that 3’UTR of DVL3 contained a potential binding site with miR-29a-3p (Figure 5A). Subsequently, the luciferase report assay confirmed that miR-29a-3p could bind to the 3’UTR of DVL3 (Figure 5B). To further delve into the relationship between the expression of DVL3 and miR-29a-3p expression in PCa, we used qRT-PCR to detect the expression of DVL3 in PCa tissue. We found that the expression of DVL3 was negatively correlated with the expression of miR-29a-3p (Figure 5C, r2 = 0.687, P < 0.05). Afterward, with Western blot, we found that the expression of DVL3 was decreased significantly after the transfection of miR-29a-3p mimics, whereas increased observably after the transfection of miR-29a-3p inhibitors (Figure 5D). Collectively, these data suggested that DVL3 was a target of miR-29a-3p.
Figure 5.
MiR-29a-3p targeted on DVL3. Base pairing relationship between the 3’UTR of DVL3 and miR-29a-3p as predicted by TargetScan database. Luciferase reporter assay was used to detect the targeting binding relationship between DVL3 and miR-29a-3p. The correlation between DVL3 expression and miR-29a-3p expression in PCa tissues (n = 42). Detection of DVL3 expression after knockdown or overexpression of miR-29a-3p by Western blot. ** P < 0.01 and NS P > 0.05. All the experiments were replicated 3 times.
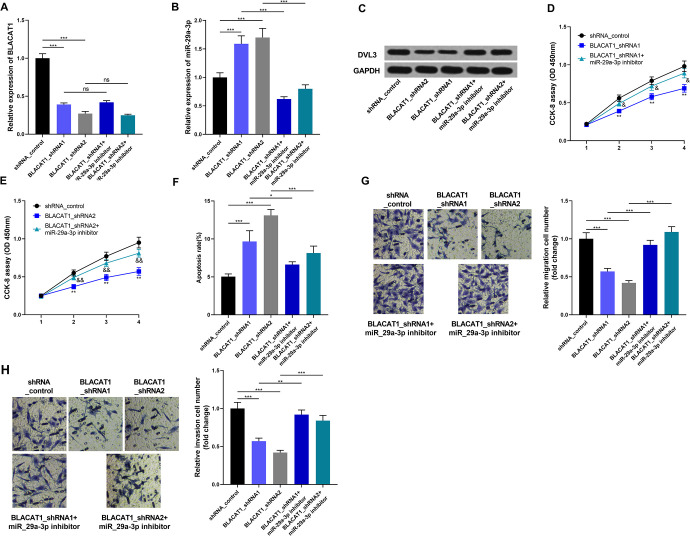
BLACAT1 Knockdown Repressed PCa cell Viability, Migration, and Invasion but Induced Cell Apoptosis via Regulating miR-29a-3p and DVL3
In order to verify the role of BLACAT1/miR-29a-3p/DVL3 axis in PCa progression, BLACAT1 shRNA and miR-29a-3p inhibitor were co-transfected into LNCap cells. The expressions of BLACAT1 and miR-29a-3p in different groups of cells were detected by qRT-PCR (Figure 6A and B). The results of Western blot indicated that knockdown of BLACAT1 inhibited the expression of DVL3, while co-transfection of miR-29a-3p partly reversed it (Figure 6). Consistently, the functional assays showed that knockdown of BLACAT1 weakened the malignant phenotypes of LNCap cells, while co-transfection of miR-29a-3p inhibitors abolished them (Figure 6D-H). These results implied that BLACAT1 modulated the progression of PCa dependent on miR-29a-3p and DVL3.
Figure 6.
BLACAT1 promoted the expression of DVL3 and progression of PCa by targeting miR-29a-3p. (A-B) qRT-PCR was used to detect the expressions of BLACAT1 and miR-29a-3p in LNCap cells, respectively, after the transfection; (C) Western blot was used to detect the expression of DVL3 in LNCap cells after transfection. (D-E) CCK-8 assay was used to examine the cell proliferation of LNCap cells. (F) Flow cytometry was used to detect LNCap cell apoptosis. (G-H) Transwell method was used to detect invasion and migration of LNCap cells. * P < 0.05, ** P < 0.01 and *** P < 0.001. All the experiments were replicated 3 times.
Discussion
LncRNA is proved to be involved in the development of a variety of tumors and is also widely studied in PCa. For example, the high expression of lncRNA HULC in PCa promotes the proliferation and metastasis of cancer cells, and is positively correlated with the unfavorable clinicopathological features and poor overall survival of patients15; lncRNA FOXP4-AS1 is involved in the growth of prostate cancer by regulating miR-3184-5p and FOXP4 expressions.16 BLACAT1 is a widely studied lncRNA with potential as a molecular marker and therapy target. Previous studies unveil that the high expression of BLACAT1 is related to the poor prognosis of cancers such as endometrial cancer and non-small cell lung cancer.17,18 In osteosarcoma, overexpression of BLACAT1 can promote the proliferation and invasion of cancer cells via activating STAT3.19 The expression of BLACAT1 in cervical cancer is also significantly up-regulated and BLACAT1 promotes cancer progression by activating Wnt/β-catenin signaling pathway.20 In this study, we found that the expression of BLACAT1 was significantly up-regulated in PCa tissues. In vitro studies showed that overexpression of BLACAT1 could promote the proliferation, invasion and migration of PCa cells, while opposite effects could be observed by knocking down it. Our data indicated that BLACAT1 was an oncogenic lncRNA, which is consistent with previous reports.
The dysregulation of miRNAs is also involved in the tumorigenesis and progression of PCa.21,22 It is reported that miR-29a-3p suppresses the progression of several types of cancers. For example, in hepatocellular carcinoma, miR-29a-3p targets insulin-like growth factor-1 receptor and participates in the immune regulatory response in tumor microenvironment23; in gastric cancer, the expression of miR-29a-3p is significantly down-regulated and its low expression contributes to the proliferation, migration and invasion of cancer cells.9 In this study, we demonstrated that the expression of miR-29a-3p was reduced in PCa and it could repress the malignant phenotypes of PCa cells.
Mounting studies demonstrate that lncRNAs are able to sponge miRNAs to influence the progress of cancer. For examples, in papillary thyroid cancer, lncRNA TUG1 promotes the proliferation and migration of cancer cells by targeting miR-145, and participates in the process of epithelial-mesenchymal transition24; lncRNA ZEB2-AS1 is highly expressed in bladder cancer, and can promote bladder cancer by regulating the expression of miR-27b.25 In this study, we identified the binding site between BLACAT1 and miR-29a-3p, and demonstrated that BLACAT1 could negatively regulate miR-29a-3p expression. Meanwhile, through rescue experiments, we confirmed that the oncogenic role of BLACAT1 was partly dependent on regulating miR-29a-3p. Our data not only clarified the downstream mechanism of BLACAT1, but also decoded the mechanism of miR-29a-3p dysregulation in cancers including PCa.
In previous studies, several modulators of Wnt signaling pathway, including DVL3, FZD3, FZD5, FRAT2 and CK2A2, are identified as direct targets of miR-29 family members.26 Previous studies confirm that DVL3 is highly expressed in PCa, which can regulate cell proliferation, differentiation and stem cell renewal by activating classical Wnt/β-catenin signaling pathway, and inactivation of Wnt signaling can inhibit the self-renewal of PCa stem cells.14,27 Herein, we elucidated that miR-29a-3p could directly target DVL3, and DVL3 was indirectly modulated by BLACAT1 with a “competing endogenous RNA (ceRNA)” mechanism. Our results gave the evidence that the aberrant expression of DVL3 in PCa tissues might be related to the disorder of BLACAT1 and miR-29a-3p. Based on these results, we can make a hypothesis that BLACAT1 may be a crucial lncRNA in regulating Wnt signaling, which needs to be further investigated and validated.
There are some limitations to this study. The relationship between BLACAT1 and the clinicopathologic characteristics of PCa patients remains unclear, and its relationship with patients’ survival also requires investigation. Additionally, in vivo data are needed to validate our conclusion in the future. Besides, although the lncRNA-miRNA-protein interaction is the most widely studied mechanism by which lncRNA participates in cancer biology, some other mechanisms are reported. For example, in prostate cancer, the risk-associated SNP rs11672691 mediates lncRNA PCAT19 expression, and PCAT19 interacts with HNRNPAB to promote the aggressive phenotypes of prostate cancer cells.27-29 It remains unclear whether BLACTA1 can mediate the progression of PCa via other mechanisms. Despite these drawbacks, we highlight that BLACAT1 promotes the expression of DVL3 through sponging miR-29a-3p, thus facilitating the progression of PCa. This study provides a new theoretical basis for developing potential therapeutic strategies for PCa.
Footnotes
Authors’ Note: The data used to support the findings of this study are available from the corresponding author upon request. Our study was approved by the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College (201607KS014). All patients provided written informed consent prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by City and College Strategic Cooperation Project of Nanchong (18SXHZ0576 18SXHZ0429) and “Thirteenth Five-Year Plan” for Social Science Research in Nanchong City (NC2018B035, NC2018B044).
ORCID iD: Sha Ke  https://orcid.org/0000-0002-7501-2773
https://orcid.org/0000-0002-7501-2773
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016,66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 3. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–874. [DOI] [PubMed] [Google Scholar]
- 4. Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophy Act. 2014;1839(11):1097–1109. [DOI] [PubMed] [Google Scholar]
- 5. Lu H, Liu H, Yang X, et al. LncRNA BLACAT1 may serve as a prognostic predictor in cancer: evidence from a meta-analysis. Biomed Res Int. 2019;2019:1275491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vishnoi A, Rani S.MiRNA biogenesis and regulation of diseases: an overview. Methods Mol Biol. 2017;1509:1–10. [DOI] [PubMed] [Google Scholar]
- 7. Acunzo M, Romano G, Wernicke D, Croce CM. MicroRNA and cancer—a brief overview. Adv Biol Regul. 2015;57:1–9. [DOI] [PubMed] [Google Scholar]
- 8. Meng FJ, Meng FM, Wu HX, Cao XF. miR-564 inhibited metastasis and proliferation of prostate cancer by targeting MLLT3. Eur Rev Med Pharmacol Sci. 2017;21(21):4828–4834. [PubMed] [Google Scholar]
- 9. Zhao Z, Wang L, Song W, et al. Reduced miR-29a-3p expression is linked to the cell proliferation and cell migration in gastric cancer. World J Surg Oncol. 2015;13:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma Y, Sun Y. miR-29a-3p inhibits growth, proliferation, and invasion of papillary thyroid carcinoma by suppressing NF-κB signaling via direct targeting of OTUB2. Cancer Manag Res. 2018;11:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. He H, Wang N, Yi X, Tang C Wang D. Long non-coding RNA H19 regulates E2F1 expression by competitively sponging endogenous miR-29a-3p in clear cell renal cell carcinoma. Cell Biosci. 2017;7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pai VC, Hsu CC, Chan TS, et al. ASPM promotes prostate cancer stemness and progression by augmenting Wnt-Dvl-3-β-catenin signaling. Oncogene. 2019;38(8):1340–1353. [DOI] [PubMed] [Google Scholar]
- 13. Zou YF, Xie CW, Yang SX, Xiong JP. AMPK activators suppress breast cancer cell growth by inhibiting DVL3-facilitated Wnt/β-catenin signaling pathway activity. Mol Med Rep. 2017;15(2):899–907. [DOI] [PubMed] [Google Scholar]
- 14. Kim PJ, Park JY, Kim HG, Cho YM, Go H. Dishevelled segment polarity protein 3 (DVL3): a novel and easily applicable recurrence predictor in localised prostate adenocarcinoma. BJU Int. 2017;120(3):343–350. [DOI] [PubMed] [Google Scholar]
- 15. Zheng P, Li H, Xu P, et al. High lncRNA HULC expression is associated with poor prognosis and promotes tumor progression by regulating epithelial-mesenchymal transition in prostate cancer. Arch Med Sci. 2018;14(3):679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu X, Xiao Y, Zhou Y, Zhou Z, Yan W. LncRNA FOXP4-AS1 is activated by PAX5 and promotes the growth of prostate cancer by sequestering miR-3184-5p to upregulate FOXP4. Cell Death Dis. 2019;10(7):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen X, Dai M, Zhu H, et al. Evaluation on the diagnostic and prognostic values of long non-coding RNA BLACAT1 in common types of human cancer. Mol Cancer. 2017;16(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen W, Hang Y, Xu W, et al. BLACAT1 predicts poor prognosis and serves as oncogenic lncRNA in small-cell lung cancer. J Cell Biochem. 2018. doi:10.1002/jcb.27548 [DOI] [PubMed] [Google Scholar]
- 19. Dong Z, Wang Y. LncRNA BLACAT1 accelerates the proliferation and migration of osteosarcoma cells through regulating STAT3. Pathol Res Pract. 2019;215(3):571–579. [DOI] [PubMed] [Google Scholar]
- 20. Wang CH, Li YH, Tian HL, Wang ZM. Long non-coding RNA BLACAT1 promotes cell proliferation, migration and invasion in cervical cancer through activation of Wnt/β-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2018; 22(10):3002–3009. [DOI] [PubMed] [Google Scholar]
- 21. Sekhon K, Bucay N, Majid S, Dahiya R, Saini S. MicroRNAs and epithelial-mesenchymal transition in prostate cancer. Oncotarget. 2016;7(41):67597–67611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang S, Wa Q, Pan J, et al. Downregulation of miR-141-3p promotes bone metastasis via activating NF-κB signaling in prostate cancer. J Exp Clin Cancer Res. 2017;36(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang X, Liu S, Cao L, et al. miR-29a-3p suppresses cell proliferation and migration by downregulating IGF1 R in hepatocellular carcinoma. Oncotarget. 2017;8(49):86592–86603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lei H, Gao Y, Xu X. LncRNA TUG1 influences papillary thyroid cancer cell proliferation, migration and EMT formation through targeting miR-145. Acta Biochim Biophys Sin (Shanghai). 2017;49(7):588–597. [DOI] [PubMed] [Google Scholar]
- 25. Wu X, Yan T, Wang Z, Cao G, Wu X, Zhang C. LncRNA ZEB2-AS1 promotes bladder cancer cell proliferation and inhibits apoptosis by regulating miR-27b. Biomed Pharmacother. 2017:96:299–304. [DOI] [PubMed] [Google Scholar]
- 26. Le LT, Swingler TE, Crowe N, et al. The microRNA-29 family in cartilage homeostasis and osteoarthritis. J Mol Med (Berl). 2016;94(5):583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kharaishvili G, Simkova D, Makharoblidze E, Trtkova K, Kolar Z, Bouchal J. Wnt signaling in prostate development and carcinogenesis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155(1):11–18. [DOI] [PubMed] [Google Scholar]
- 28. Hua JT, Ahmed M, Guo H, et al. Risk SNP-mediated promoter-enhancer switching drives prostate cancer through lncRNA PCAT19. Cell. 2018;174(3):564–575. [DOI] [PubMed] [Google Scholar]
- 29. Gao P, Xia JH, Sipeky C, et al. Biology and clinical implications of the 19q13 aggressive prostate cancer susceptibility locus. Cell. 2018;174(3):576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]