Abstract
Background:
Laryngeal cancer is one of the most common malignant tumors among head and neck cancers. Accumulating studies have indicated that long noncoding RNAs (lncRNAs) play an important role in laryngeal cancer occurrence and progression, however, the functional roles and relative regulatory mechanisms of lncRNA growth arrest-specific transcript 5 (GAS5) in laryngeal cancer progression remain unclear.
Methods:
The expression of lncRNA GAS5 in both laryngeal cancer tissues and cell lines was evaluated using quantitative reverse transcription-polymerase chain reaction (RT-qPCR) assay. The relationships between lncRNA GAS5 expression and clinical parameters were also analyzed. To determine the biological function of lncRNA GAS5, a lncRNA GAS5-specific plasmid was first transfected into laryngeal cancer cells using lentiviral technology. Cell counting kit-8 assay, flow cytometry, and Transwell assays were used to detect in vitro cell proliferation, apoptosis, cycle distribution, and metastasis abilities, respectively. Furthermore, in vivo cell growth experiments were also performed using nude mice. Additionally, western blotting was performed to identify the underlying regulatory mechanism.
Results:
In the current study, lncRNA GAS5 was downregulated in laryngeal cancer tissues and its low expression was closely associated with poor tumor differentiation, advanced TNM stage, lymph node metastasis, and shorter overall survival time. In addition, lncRNA GAS5 upregulation significantly inhibited laryngeal cancer cell proliferation both in vitro and in vivo. Moreover, in response to lncRNA GAS5 overexpression, more laryngeal cancer cells were arrested at the G2/M stage, accompanied by increased cell apoptosis rates and suppressed migration and invasion capacities. Mechanistically, our data showed that the overexpression of lncRNA GAS5 significantly regulated the PI3K/AKT/mTOR signaling pathway.
Conclusion:
LncRNA GAS5 might act as a suppressor gene during laryngeal cancer development, as it suppressed cell proliferation and metastasis by regulating the PI3K/AKT/mTOR signaling pathway; thus, lncRNA GAS5 is a promising therapeutic biomarker for the treatment of laryngeal cancer.
Keywords: long noncoding RNA_GAS5, laryngeal neoplasms, cell proliferation, cell metastasis, phosphatidylinositol 3-Kinase (PI3 K)
Introduction
Laryngeal cancer is one of the most common malignant tumors among head and neck cancers.1,2 Radical surgical resection accompanied by postoperative adjuvant radiotherapy or chemotherapy is the standard treatment for patients with laryngeal cancer. However, laryngeal cancer is a complex disease, mainly caused by various genetic and environmental factors.3 Consequently, there are few effective biomarkers to identify the early-stage patients with laryngeal cancer, since its underlying pathogenesis remains unclear. Most patients are diagnosed with advanced laryngeal cancer and have relatively high morbidity and mortality rates.4 Moreover, advanced laryngeal cancer seriously affects patients’ quality of life.5 Therefore, it is of great importance to elucidate the potential molecular pathogenesis mechanism involved in the progression of laryngeal cancer and to identify potential novel effective therapeutic strategies.
In recent years, with the rapid development of sequencing technology, numerous types of non-coding RNAs have been identified, including circular RNAs (circRNAs), long non-coding RNAs (lncRNAs), and microRNAs (miRNAs).5 Notably, lncRNAs are a group of noncoding RNAs with more than 200 nucleotides in length6 that play crucial roles in diverse biological processes by regulating target genes at the transcription or translation levels.7 Wang reported that lncRNA RHPN1-AS1 might function as an oncogene in the development of ovarian cancer.8 In addition, another study showed that lncRNA_SNHG1 could suppress the cell metastasis ability of esophageal squamous cell cancer cells by regulating the miR-204/HOXC8 axis.9 However, whether some specifically expressed lncRNAs might also act as predictors and treatment targets in laryngeal cancer remains unknown. Furthermore, the biological function of lncRNAs in laryngeal cancer progression has not been fully elucidated.
Among lncRNAs, lncRNA GAS5 (growth arrest-specific transcript 5) is a member of the 5’ terminal oligo-pyrimidine class of genes. Numerous studies have identified lncRNA GAS5 as a tumor-suppressor gene in multiple cancers, including hepatocellular carcinoma,10 non-small lung cancer,11 pancreatic cancer12 and cervical cancer.13 Furthermore, Lyu et al showed that lncRNA GAS5 inhibited cell proliferation and increased the cell apoptosis rate in laryngeal squamous cell carcinoma by regulating the miR-21/BAX/CDK6 axis.14 However, Lyu’s study had some limitations.14 First, the function and relative regulatory mechanism of lncRNA GAS5 on laryngeal cancer cell migration and invasion ability in vitro have not been elucidated. Second, in vivo animal experiments have not yet been performed. Therefore, further studies should be performed to confirm the biological role of lncRNA GAS5 in laryngeal cancer progression.
In the current study, we first determined the expression levels of lncRNA GAS5 in laryngeal cancer tissues and adjacent normal tissues and then evaluated the relationships between its expression pattern and clinicopathological characteristics of laryngeal cancer patients. Subsequently, we constructed lncRNA GAS5-overexpressing laryngeal cancer cell models using lentiviral-based plasmid transfection technology. Furthermore, both in vitro and in vivo experiments were performed to investigate the biological role of lncRNA GAS5 in laryngeal cancer cell proliferation, cycle, apoptosis, migration, and invasion progression and its relative regulatory mechanism. Our data consistently indicated the therapeutic implications of lncRNA GAS5 for the treatment of laryngeal cancer.
Methods
Tissue Samples
In the current study, a total of 40 paired laryngeal cancer tissues and adjacent normal tissues were obtained from patients who had undergone radical surgeries in the Department of Otorhinolaryngology of Guangdong Provincial People’s Hospital. All tissue samples were histologically confirmed by 3 experienced pathologists and stored at -80°C. In addition, the use of patient tissue samples and animal experiments were approved by both Ethical Committees of our hospital. Furthermore, all patients enrolled in this study voluntarily provided signed informed consent.
Cell Culture and RNA Transfection
Human laryngeal cancer cell lines (HEP-2, TU212, and TU686) and normal oral epithelial cell (HOK2) lines were obtained from the Cell Bank of the Chinese Academy of Sciences. Additionally, Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) was used for cell incubation. All cell lines were cultured in an incubator at 37°C and 5% CO2 in a humid environment. For RNA transfection, lentiviruses (multiplicity of infection = 100) and 5 μg/mL polybrene were used for the construction of lncRNA GAS5-overexpressing TU212 and TU686 cell models. The cell models were screened by puromycin and were further evaluated by quantitative reverse transcription-polymerase chain reaction (RT-qPCR).
RT-qPCR
Following the manufacturer’s instructions, Trizol reagent (Invitrogen) was used to extract total RNA from the tissue samples and cell lines. Subsequently, complementary DNA (cDNA) was synthesized using PrimeScript RT Master Mix (TaKaRa, Dalian, China). Finally, the relative expression level of lncRNA GAS5 was detected with SYBR Premix Ex Taq II Kit (TaKaRa) on a QuantStudio 5 Real-time PCR system (Thermo Fisher Scientific) and analyzed using the 2−ΔΔCt method. The following primer sequences were used: lncRNA GAS 5′ Forward-5′-TCT AGC TTG GGT GAG GCA-3′ and Reverse-5′-TGG AGA GTC GGC TTG ACT A-3′; GAPDH Forward-5′-ACG GAT TTG GTC GTA TTG G-3′ and Reverse-5′-TCC CGT TCT CAG CCT TG-3′.
In Vitro Cell Proliferation Assay
The cell counting kit-8 (CCK-8) assay was performed to evaluate the proliferation ability of the TU212 and TU686 cell lines. Briefly, the transfected cells were seeded in 96-well plates. Cell viability was then determined with the CCK-8 assay (Donjindo, Kumamoto, Japan) at the indicated time points.
Cell Apoptosis and Cell Cycle Assays
Forty-eight hours after the transfection, transfected cells were collected and treated with the Annexin V-FITC/PI apoptosis detection kit (Keygen) and the cell cycle detection kit (Keygen, Nanjing, China), respectively, following the manufacturer’s instructions. Finally, the cell apoptosis rate and cell cycle distribution were analyzed using the FACSCanto II flow cytometer (BD Biosciences).
Transwell Assay
To further evaluate the cell migration and invasion abilities, Transwell chambers (0.8. μm; Corning, NY, USA) with or without Matrigel coating were used. Briefly, 48 h after the transfection, the transfected cells were collected and added into the upper chamber (Corning, NY, USA), while DMEM containing 15% FBS was added to the lower chamber. Finally, the cells captured in the basement membrane were fixed and stained for imaging.
In Vivo Proliferation Study
To elucidate the function of lncRNA GAS5 in vivo, we collected transfected cells and injected them subcutaneously into nude mice. Twenty-one days later, the volumes of the subsequent tumors were measured and calculated. Finally, subcutaneous tumor tissues were collected and weighed after euthanasia.
Western Blotting
Un-transfected and transfected cells were collected and suspended in RIPA lysis buffer (Beyotime, China) for total protein extraction. Subsequently, 50 µg of proteins was loaded and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by transfer onto polyvinylidene fluoride membranes. After blocking with 10% bovine serum albumin, the membranes were incubated with primary and secondary antibodies. Finally, the protein bands were analyzed with a super-sensitive enhanced chemiluminescence reagent (Meilunbio, Dalian, China).
Statistics
In this study, all experiments were performed at least 3 times separately, and all data are presented as means ± SD. Statistical analysis and graph construction were performed using GraphPad Prism 7.0. Significant differences between groups were determined using Student’s t-tests, chi-square tests, or 1-way analysis of variance (ANOVA). The statistical significance criterion was P ≤ 0.05.
Results
Low lncRNA GAS5 Expression Predicts Poor Prognosis in Laryngeal Cancer
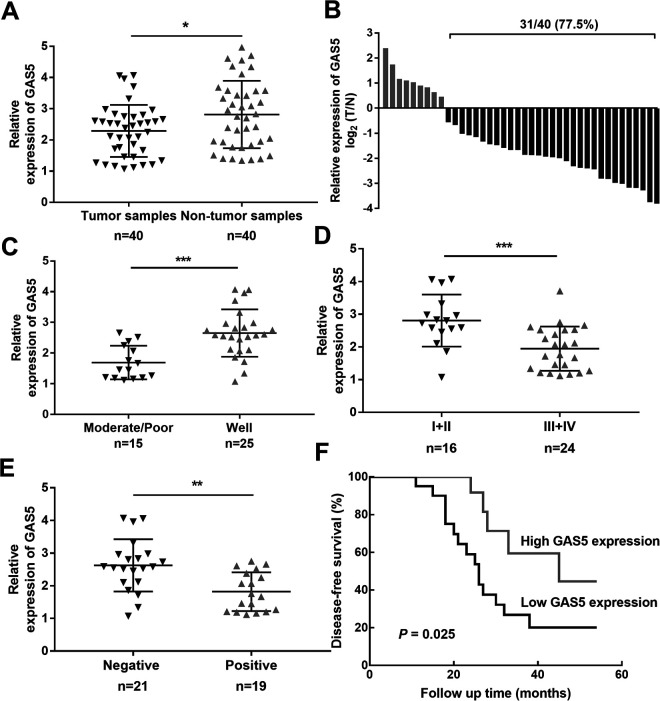
To confirm the biological role of lncRNA GAS5 in the development of laryngeal cancer, RT-qPCR assays were performed. As shown in Figure 1A, the expression level of lncRNA GAS5 in laryngeal cancer tissues was lower than that in adjacent normal specimens. In addition, low lncRNA GAS5 expression was detected in 77.5% (31/40) of laryngeal cancer samples (Figure 1B). Subsequently, 40 laryngeal cancer patients were divided into lncRNA GAS5 high-expression and low-expression groups based on the median lncRNA GAS5 expression value. The results of our statistical analysis showed that lncRNA GAS5 expression was closely related to tumor differentiation (P = 0.022), TMN stage (P = 0.001), and lymph node metastasis (P = 0.027) (Table 1). Consistent with these results, laryngeal cancer patients with low lncRNA GAS5 expression were inclined to have poor tumor differentiation (Figure 1C, P = 0.0001), advanced TNM stage (Figure 1D, P = 0.0007), lymph node metastasis (Figure 1E, P = 0.0012), and shorter overall survival time (Figure 1F, P = 0.025). Therefore, lncRNA GAS5 might be considered a tumor-suppressor factor in the development of laryngeal cancer.
Figure 1.
Expression patterns and roles of lncRNA GAS5 in patients with laryngeal cancer. (A) lncRNA GAS5 expression in laryngeal cancer tissues and adjacent normal tissues. (B) Significant downregulation of lncRNA GAS5 (77.5%, 31/40) in patients with laryngeal cancer. (C) Relationship between lncRNA GAS5 expression and tumor differentiation. (D) Relationship between lncRNA GAS5 expression and TMN stage. (E) Relationship between lncRNA GAS5 expression and lymph node metastasis. (F) Kaplan–Meier curves of the overall survival of laryngeal cancer patients with high and low lncRNA GAS5 expression. *P < 0.05, **P < 0.01, ***P < 0.001.
Table 1.
Correlation Between GAS5 Expression and Clinicopathological Features in Patients With Laryngeal Squamous Cell Carcinoma.
| Clinicopathological features | All cases | GAS5 expression | χ2 value | P value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Sex | 0.690 | 0.490 | |||
| Male | 28 | 13 | 15 | ||
| Female | 12 | 7 | 5 | ||
| Age (year) | 0.921 | 0.337 | |||
| ≤ 60 | 23 | 10 | 13 | ||
| > 60 | 17 | 10 | 7 | ||
| Tumor differentiation | 5.227 | 0.022 | |||
| Well | 15 | 4 | 11 | ||
| Moderate/Poor | 25 | 16 | 9 | ||
| TNM stage | 10.420 | 0.001 | |||
| I+II | 16 | 3 | 13 | ||
| III+IV | 24 | 17 | 7 | ||
| Lymph node metastasis | 4.912 | 0.027 | |||
| Negative | 21 | 7 | 14 | ||
| Positive | 19 | 13 | 6 | ||
| Anatomical region | 0.417 | 0.519 | |||
| Glottis | 24 | 11 | 13 | ||
| Supraglottis | 16 | 9 | 7 | ||
LncRNA GAS5 Upregulation Suppresses Laryngeal Cancer Cell Growth
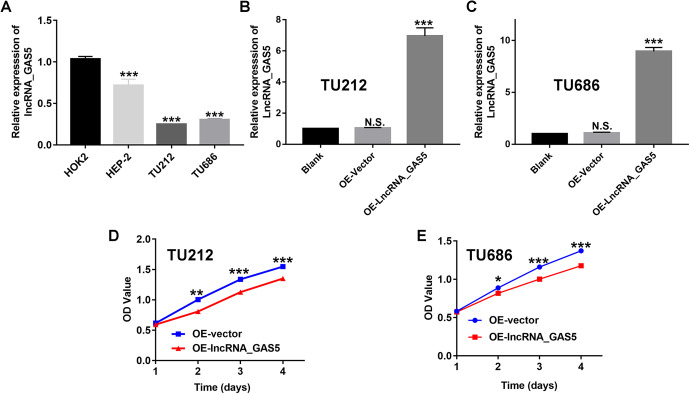
To further elucidate the biological function of lncRNA GAS5 in laryngeal cancer progression, we also determined the expression levels of lncRNA GAS5 in laryngeal cancer cells and normal oral epithelial cells (HOK2). The results of RT-qPCR revealed dramatic downregulation of lncRNA GAS5 in laryngeal cancer cell lines, including HEP-2, TU212, and TU686, compared to that in the normal oral epithelial cells (HOK2). Accordingly, the lncRNA GAS5 expression level in both TU212 and TU686 cell lines was lower than that in HEP-2 cell lines (Figure 2A). Therefore, in the current study, we selected TU212 and TU686 cells as research models for the in vitro and in vivo experiments. Subsequently, we constructed lncRNA GAS5-overexpressing TU212 and TU686 cells through lentivirus-mediated plasmid transfection. As shown in Figure 2B and C, we observed no significant difference between the OE-vector group (transfected with negative control plasmids) and the blank group (control). However, after transfection with pcDNA3.1-lncRNA GAS5 (OE-lncRNA_GAS), the expression levels of lncRNA GAS5 were significantly increased in both TU212 and TU686 cells, indicating the successful construction of the lncRNA GAS5 over-expressing laryngeal cancer cell lines. Subsequently, these lncRNA GAS5-overexpressing laryngeal cancer cells were used for in vitro cell growth assays. The results of the CCK-8 assay showed a much lower OD value in the OE-LncRNA_GAS group than in the OE-vector group at the indicated time points (Figure 2D and E), demonstrating that the upregulation of lncRNA GAS5 significantly inhibited the proliferation abilities of laryngeal cells.
Figure 2.
Overexpression of lncRNA GAS5 inhibits the proliferation of laryngeal cancer cells in vitro. (A) Expression levels of lncRNA GAS5 in laryngeal cancer cells and normal oral epithelial cells (HOK2). (B&C) lncRNA GAS5-overexpressing TU212 and TU686 laryngeal cancer cell lines were successfully constructed. (D&E) The cell proliferation abilities of the lncRNA GAS5-overexpressing TU212 and TU686 laryngeal cancer cell lines as assessed by the CCK-8 assay. *P < 0.05, **P < 0.01, ***P < 0.001.
LncRNA GAS5 Upregulation Induces Cell Apoptosis and Arrests More Cells at the G2/M Stage
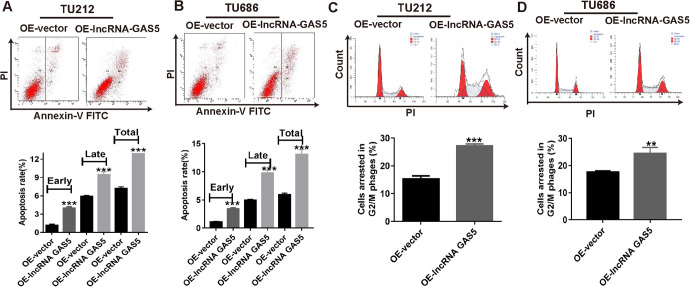
In the current study, we identified that lncRNA GAS5 overexpression significantly inhibited laryngeal cancer cell growth in vitro. However, it was not clear whether cell apoptosis contributed to the proliferation inhibition caused by lncRNA GAS5 overexpression. Consequently, we performed flow cytometry experiments to evaluate the cell apoptosis rates in TU212 and TU686 cells. As shown in Figure 3A and B, lncRNA GAS5 overexpression resulted in dramatic increases in early, late, and total apoptosis rates compared to those in the OE-vector groups.
Figure 3.
Cell apoptosis rate and cell cycle distribution of laryngeal cancer cells after lncRNA GAS5 overexpression. (A & B) Cell apoptosis images (upper) and flow cytometry statistics analysis (lower) of TU212 and TU686 cells after lncRNA GAS5 overexpression. (C&D) Cell cycle images (upper) and flow cytometry statistics analysis (lower) of TU212 and TU686 cells after lncRNA GAS5 overexpression. **P < 0.01, ***P < 0.001.
The cell cycle is also closely associated with cancer cell growth capacity; thus, we performed another flow cytometry experiment to observe the cell cycle distribution. The flow cytometry data also showed that lncRNA GAS5 overexpression arrested more TU212 cells (27.2%) at the G2/M stage than the OE-vector group (15.3%) (Figure 3C). Similarly, lncRNA GAS5 up-regulation also arrested more TU686 cells (24.5%) at the G2/M stage compared to the OE-vector group (17.7%) (Figure 3D). Therefore, we concluded that lncRNA GAS5 upregulation inhibited cell proliferation, induced cell apoptosis, and arrested more cells at the G2/M stage.
LncRNA GAS5 Upregulation Inhibits Cell Migration and Invasion of Laryngeal Cancer Cells
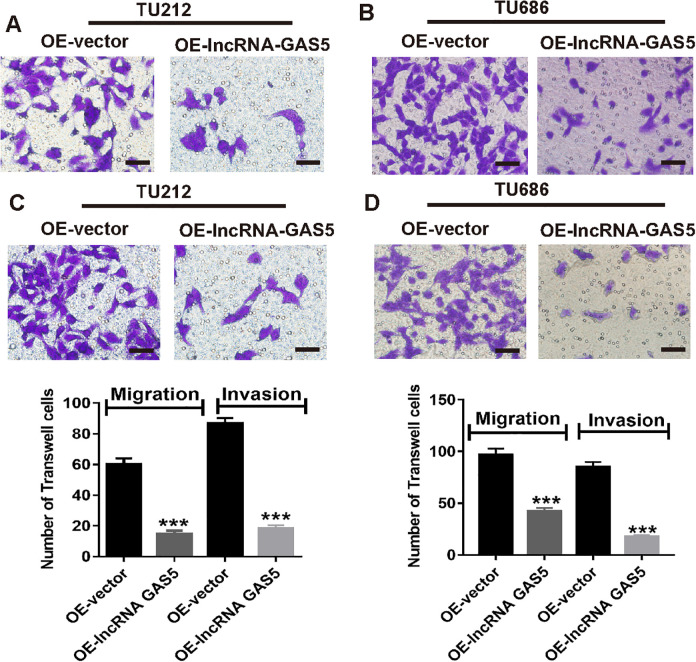
The clinical parameter analysis showed that laryngeal cancer patients with low lncRNA GAS5 expression were more likely to have lymph node metastasis. Therefore, we assumed that lncRNA GAS5 might also play a crucial role in cell migration and invasion of laryngeal cancer cells. To validate this, we next performed a Transwell assay. As shown in Figure 4A and C, lncRNA GAS5 overexpression in TU212 cells resulted in less migration and invasion. Similarly, Figure 4B and D also demonstrated that lncRNA GAS5 overexpression in TU686 cells resulted in less migration and invasion than that in the cells in the OE-vector group. Thus, lncRNA GAS5 upregulation significantly suppressed the cell migration and invasion ability of laryngeal cancer cells.
Figure 4.
Inhibition of cell migration and laryngeal cancer invasion after lncRNA GAS5 overexpression. (A & C) Cell migration and invasion images (upper), and statistical analysis (lower) of TU212 cells after lncRNA GAS5 overexpression. (B & D) Cell migration and invasion images (upper), and statistical analysis (lower) of TU686 cells after lncRNA GAS5 overexpression. ***P < 0.001.
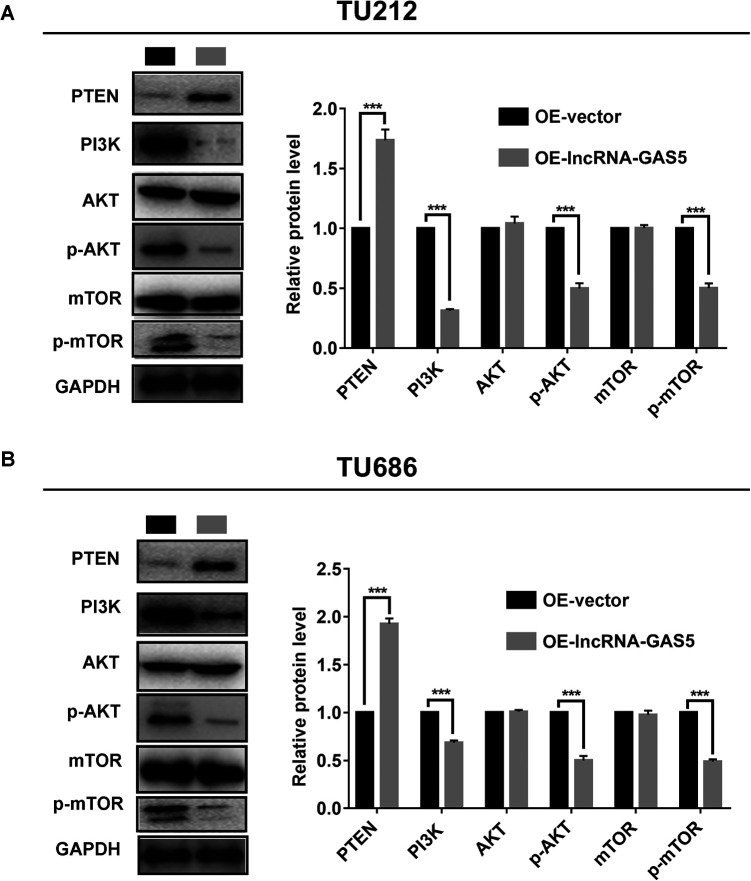
LncRNA GAS5 Demonstrates Antitumor Effects by Regulating the Phosphatidylinositol 3-Kinase/Protein Kinase B/Mechanistic Target of the Rapamycin (PI3K/AKT/mTOR) Signaling Pathway
The PI3K/AKT/mTOR signaling pathway is the pivotal regulatory mechanism for regulating cell proliferation and metastasis. The correlation between GAS5 and the PI3K/AKT/mTOR signaling pathway is clearly shown in the StarBase database. Therefore, western blotting assays were performed to determine whether lncRNA GAS5 also exerted its anti-tumor effects through the PI3K/AKT/mTOR signaling pathway in laryngeal cancer. As shown in Figure 5A and 5B, compared to the OE-vector groups, lncRNA GAS5 upregulation led to increased phosphatase and tensin homolog (PTEN) expression, while the expression levels of PI3 K, phosphorylated AKT (p-AKT), and phosphorylated mTOR (p-mTOR) were consistently decreased. Together, these results consistently demonstrated that lncRNA GAS5 inhibited cell proliferation and metastasis in laryngeal cancer through the PI3K/AKT/mTOR signaling pathway.
Figure 5.
lncRNA GAS5 overexpression significantly inhibits the PI3K/AKT/mTOR signaling pathway. (A) Protein expression of PTEN, PI3 K, total AKT, mTOR, phosphorylated AKT, and phosphorylated mTOR expression in TU212 cells with lncRNA GAS5 overexpression (left), and the corresponding quantification of the gray value for each protein (right). (B) Protein expression of PTEN, PI3 K, total AKT, mTOR, phosphorylated AKT, and phosphorylated mTOR expression in TU686 cells with lncRNA GAS5 overexpression (left), and the corresponding quantification of the gray value for each protein (right). ***P < 0.001.
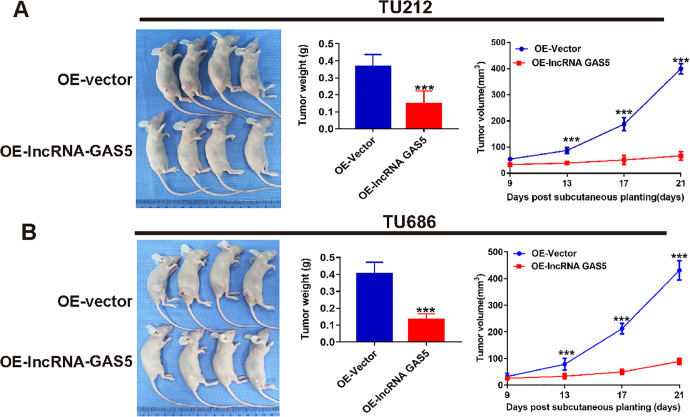
LncRNA GAS5 Upregulation Suppresses Laryngeal Cancer Tumor Growth In Vivo
Given the inhibitory role of lncRNA GAS5 in cell proliferation in vitro, we then constructed tumor-bearing xenograft models by subcutaneous injection of normal and lncRNA GAS5-overexpressing TU212 and TU686 cells into nude mice. As shown in Figure 6A and B, the tumor weights in the OE-lncRNA GAS5 groups were significantly lower than those in OE-vector groups, which were almost 40% of those in the OE-vector groups. In addition, the tumor volume curves also indicated much slower tumor growth in the lncRNA GAS5-overexpressing groups. Therefore, consistent with the in vitro experiments, we found that lncRNA GAS5 overexpression also suppressed tumor growth in vivo.
Figure 6.
Overexpression of lncRNA GAS5 suppresses laryngeal cancer tumor growth in vivo. (A) Growth rate of a xenograft model tumor comprising lncRNA GAS5-overexpressing TU212 cells. (B) Growth rate of a xenograft model tumor comprising lncRNA GAS5-overexpressing TU686 cells. ***P < 0.001.
Discussion
Laryngeal cancer is one of the most common lethal tumors among head and neck cancers worldwide.15 While molecular targeted therapy and immunotherapy have made significant advances in recent years,16,17 the total survival time of patients with advanced laryngeal cancer has not improved significantly. Therefore, it is of great importance to identify an effective biological marker and clarify its regulatory mechanism.
A recent study demonstrated that lncRNA GAS5 might act as a tumor inhibitor in laryngeal cancer progression, and its overexpression could significantly inhibit cell proliferation and induce cell apoptosis; however, its effect on cell metastasis ability remains unclear.14 Similarly, in the current study, we also observed lncRNA GAS5 downregulation in laryngeal cancer tissues relative to the adjacent normal tissues. After statistical analysis, our data also showed that patients with reduced lncRNA GAS5 expression were more likely to have poor tumor differentiation, advanced TNM stage, lymph node metastasis, and shorter overall survival time. Together, these findings indicate that lncRNA GAS5 plays a significant role in the development of laryngeal cancer.
The lncRNA GAS5-overexpressed laryngeal cancer cell models were constructed to explore its biological role in laryngeal cancer progression. In the context of cellular function, lncRNA GAS5 upregulation suppressed cell proliferation and tumor growth both in vitro and in vivo and also increased the cell apoptosis rate. Indeed, increasing numbers of studies have confirmed that cell cycle distribution is closely associated with cell proliferation ability, as successful G2/M transformation is the key to cell division.18,19 In other words, the cell proliferation ability was suppressed when more cells were arrested at the G2/M stage. In this study, the results of flow cytometric analysis showed that, compared to the control group, lncRNA GAS5 upregulation arrested more cells at the G2/M stage, suggesting that lncRNA GAS5 could inhibit laryngeal cancer cell proliferation by arresting more cells at the G2/M stage.
As lncRNA GAS5 expression is closely related to lymph node metastasis, we further investigated the function of lncRNA GAS5 in laryngeal cancer cell metastasis. A previous study showed that lncRNA GAS5 overexpression suppressed cell metastasis by regulating the miR-221/SOCS3 axis.12 Another study reported that lncRNA GAS5 acted as a suppressor in gastric cancer metastasis.20 Similarly, our data also showed significantly suppressed cell migration and invasion ability with increased lncRNA GAS5 expression. Taken together, these data consistently suggested that lncRNA GAS5 could serve as a tumor suppressor gene in the development of laryngeal cancer; however, the specific regulatory mechanism is still not clear.
Accumulating evidence has shown that lncRNAs can function as the target genes of tumorigenesis and malignant progression by activating or inactivating various signaling pathways.21,22 Emerging evidence has also indicated that PI3 K is activated by various oncogenes and growth factor receptors, followed by phosphorylation of downstream regulatory proteins, including AKT and mTOR.23,24 In addition, numerous studies have demonstrated that the PI3K/AKT/mTOR signaling pathway plays a significant role in a broad spectrum of physiological processes such as cell proliferation, apoptosis, and metastasis.25-27 For example, Jiang et al reported that lncRNA HOXB-AS3 promoted cell proliferation and metastasis of lung cancer by regulating the PI3K/AKT pathway.28 Another study showed that lncRNA TCL6 directly regulated miR-106a-5p expression, suppressed the PI3K/AKT signaling pathway, and exerted anti-tumor effects in hepatocellular carcinoma.29 Therefore, the present study performed western blot assays to confirm whether PI3K/AKT/mTOR signaling pathway was involved in anti-tumor effects induced by lncRNA GAS5 overexpression in laryngeal cancer. Our data showed that lncRNA GAS5 upregulation downregulated PI3 K expression and suppressed p-AKT and p-mTOR expression, with no change in total AKT and mTOR expression. Furthermore, the expression level of the PTEN protein, which served as a tumor suppressor phosphatase, was also increased upon lncRNA GAS5 overexpression. In brief, these results verified that the PI3K/AKT/mTOR signaling pathway might be involved in lncRNA GAS5-overexpressed mediated tumor suppression of laryngeal cancer.
In summary, we identified a novel mechanism by which lncRNA GAS5 could act as a tumor suppressor gene by interfering with the PI3K/AKT/mTOR signaling pathway. However, this study has some limitations; among these is the lack of data on how lncRNA GAS5 regulates the PI3K/AKT/mTOR pathway in laryngeal cancer; thus, further research is needed.
Conclusion
Our data showed that lncRNA GAS5 was significantly downregulated in laryngeal cancer tissues and that its low expression contributed to the poor prognosis of patients with laryngeal cancer. Moreover, the results of in vitro and in vivo experiments revealed that lncRNA GAS5 overexpression suppressed cell proliferation and cell metastasis, induced cell apoptosis, and arrested more cells at the G2/M stage by regulating the PI3K/AKT/mTOR signaling pathway. The limitation of this study was that the downstream target genes, such as miRNAs, and the corresponding regulatory mechanisms have not yet been determined. Overall, lncRNA GAS5 could serve as a tumor suppressor gene in laryngeal cancer, which would be a promising therapeutic biomarker for laryngeal cancer treatment.
Abbreviations
- ANOVA
analysis of variance
- CCK-8
cell counting kit-8
- cDNA
complementary DNA
- circRNAs
circular RNA
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- GAS5
growth arrest-specific transcript 5
- lncRNAs
long noncoding RNAs
- miRNAs
microRNAs
- mTOR
mechanistic target of rapamycin
- PI3 K
phosphatidylinositol 3-kinase
- PTEN
phosphatase and tensin homolog
- RT-qPCR
quantitative reverse transcription-polymerase chain reaction
Footnotes
Authors’ Note: Wenlin Liu and Jiandong Zhan contributed equally to this work. The datasets used during the present study are available from the corresponding author upon reasonable request. Our study was approved by The Ethical Committees of Guangdong Provincial People’s Hospital (approval no. LAEC-202006-001). All patients provided written informed consent prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Siyi Zhang  https://orcid.org/0000-0002-7730-9546
https://orcid.org/0000-0002-7730-9546
References
- 1. He Y, Liang D, Li D, et al. Incidence and mortality of laryngeal cancer in China, 2015. Chin J Cancer Res. 2020;32(1):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steuer CE, El-Deiry M, Parks JR, Higgins KA, Saba NF. An update on larynx cancer. CA Cancer J Clin. 2017;67(1):31–50. [DOI] [PubMed] [Google Scholar]
- 3. Turati F, Negri E, La Vecchia C. Family history and the risk of cancer: genetic factors influencing multiple cancer sites. Expert Rev Anticancer Ther. 2014;14(1):1–4. [DOI] [PubMed] [Google Scholar]
- 4. Li H, Wang Y, Zhu C, Wang X, Du L. Incidence and mortality of laryngeal cancer in Zhejiang cancer registry, 2000-2011. J Cancer Res Ther. 2015;11(suppl 2):C155–160. [DOI] [PubMed] [Google Scholar]
- 5. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12(12):861–874. [DOI] [PubMed] [Google Scholar]
- 6. Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17(1):47–62. [DOI] [PubMed] [Google Scholar]
- 7. Chan JJ, Tay Y. Noncoding RNA: RNA regulatory networks in cancer. Int J Mol Sci. 2018;19(5):1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang J, Ding W, Xu Y, et al. Long non-coding RNA RHPN1-AS1 promotes tumorigenesis and metastasis of ovarian cancer by acting as a ceRNA against miR-596 and upregulating LETM1. Aging (Albany NY). 2020;12(5):4558–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li HM, Yu YK, Liu Q, et al. LncRNA SNHG1 regulates the progression of esophageal squamous cell cancer by the miR-204/HOXC8 axis. Onco Targets Ther. 2020;13:757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang F, Yang C, Xing Z, et al. LncRNA GAS5-mediated miR-1323 promotes tumor progression by targeting TP53INP1 in hepatocellular carcinoma. Onco Targets Ther. 2019;12:4013–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xue Y, Ni T, Jiang Y, Li Y. Long noncoding RNA GAS5 inhibits tumorigenesis and enhances radiosensitivity by suppressing miR-135b expression in non-small cell lung cancer. Oncol Res. 2017;25(8):1305–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu B, Wu S, Ma J, et al. lncRNA GAS5 reverses EMT and tumor stem cell-mediated gemcitabine resistance and metastasis by targeting miR-221/SOCS3 in pancreatic cancer. Mol Ther Nucleic Acids. 2018;13:472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang W, Hong L, Xu X, Wang Q, Huang J, Jiang L. LncRNA GAS5 suppresses the tumorigenesis of cervical cancer by downregulating miR-196a and miR-205. Tumour Biol. 2017;39(7):1010428317711315. [DOI] [PubMed] [Google Scholar]
- 14. Lyu K, Xu Y, Yue H, et al. Long noncoding RNA GAS5 Acts as a tumor suppressor in laryngeal squamous cell carcinoma via miR-21. Cancer Manag Res. 2019;11:8487–8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGuire S. World Cancer Report 2014. World Health Organization, International Agency for Research on Cancer, WHO Press; 2015. Adv Nutr. 2016;7(2):418–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karpathiou G, Casteillo F, Giroult J-B, et al. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: Immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget. 2017;8(12):19310–19322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kimura T, Suzuki K, Motai H, et al. Final report of a randomized controlled study with streptococcal preparation OK-432 as a supplementary immunopotentiator for laryngeal cancer. Acta Otolaryngol Suppl. 1996;525:135–141. [PubMed] [Google Scholar]
- 18. Xue L, Wu Z, Liu J, Luo J. FPHPB inhibits gastric tumor cell proliferation by inducing G2-M cell cycle arrest. Biomed Pharmacother. 2018;98:694–700. [DOI] [PubMed] [Google Scholar]
- 19. Li Q, Chen Z-G, Xia Q, et al. Mefloquine inhibits chondrocytic proliferation by arresting cell cycle in G2/M phase. Int J Clin Exp Pathol. 2015;8(10):12583–12588. [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Yin L, Chen C, Zhang X, Wang S. Long non-coding RNA GAS5 inhibits migration and invasion in gastric cancer via interacting with p53 protein. Dig Liver Dis. 2020;52(3):331–338. [DOI] [PubMed] [Google Scholar]
- 21. Liu F, Hu L, Pei Y, et al. Long non-coding RNA AFAP1-AS1 accelerates the progression of melanoma by targeting miR-653-5p/RAI14 axis. BMC Cancer. 2020;20(1):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin J, Jia Z-H, Luo X-H, Zhai H-F. Long non-coding RNA HOXA11-AS accelerates the progression of keloid formation via miR-124-3p/TGFβR1 axis. Cell Cycle. 2020;19(2):218–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3 K pathway in human disease. Cell. 2017;170(4):605–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayer IA, Arteaga CL. The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med. 2016;67:11–28. [DOI] [PubMed] [Google Scholar]
- 25. Bertacchini J, Heidari N, Mediani L, et al. Targeting PI3K/AKT/mTOR network for treatment of leukemia. Cell Mol Life Sci. 2015;72(12):2337–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guerrero-Zotano A, Mayer IA, Arteaga CL. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016;35(4):515–524. [DOI] [PubMed] [Google Scholar]
- 27. Delaloge S, DeForceville L. Targeting PI3K/AKT pathway in triple-negative breast cancer. Lancet Oncol. 2017;18(10):1293–1294. [DOI] [PubMed] [Google Scholar]
- 28. Jiang W, Kai J, Li D, Wei Z, Wang Y, Wang W. lncRNA HOXB-AS3 exacerbates proliferation, migration, and invasion of lung cancer via activating the PI3K-AKT pathway. J Cell Physiol. 2020;235(10):7194–7203. [DOI] [PubMed] [Google Scholar]
- 29. Luo LH, Jin M, Wang L-Q, et al. Long noncoding RNA TCL6 binds to miR-106a-5p to regulate hepatocellular carcinoma cells through PI3K/AKT signaling pathway. J Cell Physiol. 2020;235(9):6154–6166. [DOI] [PubMed] [Google Scholar]