Abstract
Aims:
We aimed to examine the frequency of polypharmacy in a large cohort of patients at the time of diagnosis of relapsing–remitting multiple sclerosis (RRMS) and to explore its effects on discontinuation of first disease-modifying treatment (DMT) using survival analysis.
Methods:
This was a cohort ambispective single-centre study. We enrolled RRMS patients starting their first DMT between 1st January 2013 and 31st December 2015. According to the number of medicines prescribed (except DMTs), we divided the patients into three groups: no-poly RRMS, minor-poly RRMS (from one to three medications), and major-poly RRMS (more than three medications).
Results:
A total of 392 RRMS patients were enrolled (mean age 41.1). The minor-poly RRMS group included 61 patients (15.6%) and the major-poly RRMS group included 112 (28.6%). Individuals in these groups were older and had higher median body mass index (BMI) than patients in the no-poly RRMS group (p < 0.05). Upon multinomial regression analysis, older age at onset was associated with minor and major polypharmacy (OR 1.050, CI 1.010–1.093, p = 0.015 and OR 1.063, CI 1.026–1.101, p = 0.001, respectively) and higher BMI was associated with major polypharmacy (OR 1.186, CI 1.18–1.29, p = 0.001). The rates of discontinuation of first DMT were similar among the three groups (50.7% for no-Poly RRMS, 50.8% for minor-Poly RRMS, and 53.3% for major-Poly RRMS, p = 0.264). At log-Rank test, there were no differences among the three groups (p = 0.834).
Conclusion:
Polypharmacy was more common in older RRMS patients with high BMI.
Keywords: discontinuation, disease-modifying therapy, multiple sclerosis, polypharmacy
Introduction
Multiple sclerosis (MS) is the most common immune-mediated neurological disease in young adults worldwide, and its most common course is relapsing–remitting MS (RRMS).1 MS management may require lifelong pharmacological and non-pharmacological interventions, and the choice of the first disease-modifying treatment (DMT) represents a crucial moment in MS management.2
When choosing the first DMT prescription, there is a need to take into account the presence of concomitant medical illnesses and the use of other medications (especially with the increasing age of the population) that can expose patients to adverse drug reactions, and drug–drug or drug–disease interactions.3–6
Several studies have shown the impact of comorbidities on the course of MS, such as an increased MS relapse rate (e.g. for patients with migraine and hyperlipidaemia), disability progression (e.g. for patients with cardiovascular comorbi-dities), and cognitive impairment.7–10 Because comorbidities may require adequate therapeutic management, MS patients may experience the administration of many drugs at the same time, that is, polypharmacy.7,11
However, the frequency and consequences of polypharmacy, usually defined as the intake of five or more medications,11–16 in actual cases of MS are largely unclear. Polypharmacy should be taken into account in MS therapeutic management because it should be included into the MS therapeutic algorithm.17–20
Studies on DMTs usually consider as outcomes relapse rates, new magnetic resonance imaging (MRI) and/or on safety concerns. However, no studies have assessed the impact of polypharmacy on discontinuing DMT nor on the reasons of discontinuation.
This study was established to examine the frequency of polypharmacy at the time of first DMT prescription in a large cohort of patients with RRMS. We also analysed the impact, if any, of polypharmacy on the discontinuation of first DMT during the entire follow-up of more than 36 months.
Methods
Setting and participants
This study was conducted at a tertiary MS centre in Catania, Italy. Data entry was performed using iMed© software (Merck Serono, Geneva, Switzerland), and the treating clinics used rigorous quality assurance procedures for the patient health records in coordination with the iMed© software data coordinators.21 Data were recorded retrospectively (up to 12 months) before the disease onset and start of the first DMT (the index date) and prospectively (until the last available follow-up visit) from the index date.
Key eligibility criteria were: (1) confirmed diagnosis of RRMS as per the 2010 McDonald criteria;22 (2) start of first DMT between 1st January 2013, and 31st December 2015; and (3) patient exposure to their first DMT for at least 6 months. Patients who participated in clinical trials or had fewer than two neurological visits during the follow-up were excluded.
The remaining patients were divided into three groups according to the number of administered medications (excluding DMT). For patients who changed the number of medications after the first 6 months of follow-up, we considered the first group to which they belonged:
- No-poly RRMS: patients taking no medications other than DMT.
- Minor-poly RRMS: patients taking between one and three medications.
- Major-poly RRMS: patients taking more than three medications.
Data collection
Clinicians entered all the clinical and radiological information available at every clinical visit (scheduled every 6 months or more frequently if necessary). The minimum information for all enrolled patients included demographic, clinical/radiological, and pharmacological data. Demographic data included sex, age, smoking status, and body mass index (BMI). Clinical data included comorbidities, type of disease onset, number of relapses in the year before diagnosis, level of disability measured with Expanded Disability Status Scale (EDSS), and radiological activity expressed as number of lesions in T2- and T1-gadolinium-weighted sequences on MRI. We also collected data on the different DMTs prescribed and then we grouped them together (based on Italian prescription rules) as first- and second-line DMTs.
Medication analysis
Medications were classified according to the therapeutic goal and the dosing schedule. In terms of the therapeutic goal, the classifications were DMT, specific symptomatic drugs for MS (for spasticity and pain), and medications for comorbidities.4 In terms of the dosing schedule, only long-term medications (taken daily or at regular intervals) for chronic diseases, taken for at least 6 months after inclusion, were considered for the analysis.
Outcomes
The primary outcome of this study was the frequency of polypharmacy in a large RRMS patient cohort. We analysed the demographic and clinical variables associated with polypharmacy.
Secondarily, we aimed to obtain evidence on the rate of discontinuation of first DMT among the three groups. We also assessed the time to discontinuation and the reasons for therapy withdrawal. Moreover, we explored the baseline demographic, clinical, and radiological factors associated with therapy discontinuation.
Discontinuation of DMTs was defined as a gap of treatment ⩾60 days. Time to discontinuation (in months) was measured as time between the index date and the end of supply of the DMT prescriptions dispensed.23
A relapse was defined as the occurrence of a new symptom or the exacerbation of an existing one persisting for at least 24 h in the absence of concurrent illness or fever, occurring at least 30 days after a previous relapse.
Ancillary, adverse events (AEs) were recorded, in accordance with the European Medication Agency definitions.24
Research ethics and patient consent
The study protocol was approved by the local ethics committee (Comitato Etico Catania 1, no. 87/2020/PO). Patients provided written informed consent to participate in the study. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and with the appropriate national regulations.
Data availability
Anonymised data will be shared upon request from any qualified investigator for the sole purpose of replicating the procedures and results presented in this report, provided that data transfer is in agreement with EU legislation on general data protection.
Statistical analysis
Univariate comparisons were performed using ANOVA, Kruskal–Wallis test, or chi-squared test.
According to the nature of variables, data were reported as mean ± standard deviation or as median [interquartile range (IQR)].
Multinomial regression analysis with forward selection was used to assess the relationship between polypharmacy class (expressed as an ordinal variable) and baseline demographic and clinical characteristics at baseline: sex, age, BMI, smoking (categorical), comorbidities (categorical), number of relapses (in the year prior to the index date; total number), baseline EDSS (median), and previous MRI activity (in the year prior to the index date; number of lesions on T2- and T1-gadolinium-weighted sequences).
DMT discontinuation was compared using chi-square test. Kaplan–Meier survival curve analysis and log-rank test were used to analyse the time to first DMT discontinuation. In our sensitivity analysis, we changed the permissible treatment gap period to 30 and 180 days. A Cox proportional hazards model adjusting for differences among the treatment cohorts was used to assess the risk of discontinuation. Covariates in the analysis included sex, age, BMI, smoking (categorical), hypertension (categorical), dyslipidaemia (categorical), number of relapses (in the year prior to the index date; total number), baseline EDSS (median), previous MRI activity (in the year prior to the index date); number of lesions on T2- and T1-gadolinium-weighted sequences, and DMT line.
The α-error was set at 0.05 and reported p-values are two-sided. Statistical analyses were conducted using SPSS Statistics 21 (IBM®).
Results
Study population
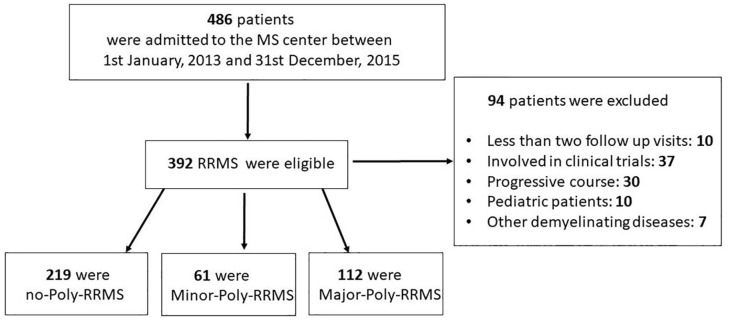
From a total of 486 patients entering the centre in the index window, 392 were enrolled (Figure 1). Their baseline characteristics are reported in Table 1. The mean age was 41.1 ± 11.7 years and 255 patients (65.1%) were women. The median EDSS score was 2.0 (IQR 1–2.5).
Figure 1.
Patient selection flow chart.
RRMS, relapsing–remitting multiple sclerosis.
Table 1.
Demographic and clinical characteristics at enrolment in the entire cohort and in the three groups.
| Variables* | Total cohort (n = 392) | No-poly RRMS (n = 219) | Minor-poly RRMS (n = 61) | Major-poly RRMS (n = 112) | p-value |
|---|---|---|---|---|---|
| Gender, n (%) | |||||
| Male | 137 (34.9) | 77 (35.2) | 24 (39.3) | 31 (27.7) | ns |
| Female | 255 (65.1) | 142 (64.8) | 37 (60.7) | 81 (72.3) | ns |
| Age | 41.1 ± 11.7 | 29.3 ± 9.9 | 35.7 ± 11.4 | 36.9 ± 11.7 | <0.05 |
| Smokers, n (%) | 119 (30.3) | 40 (18.3) | 27 (44.3) | 45 (40.2) | <0.05 |
| BMI (median, IQR) | 24 (21–27) | 22.7 (21–25.8) | 24.3 (21.8–26) | 26.7 (22.4–29) | <0.05 |
| EDSS (median, IQR) | 2.0 (1–2.5) | 2.0 (1–2.5) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | ns |
| N. of relapses one year before diagnosis | 1.8 ± 1 | 1.9 ± 0.9 | 1.6 ± 0.9 | 1.6 ± 1 | <0.05 |
| N. MRI T2 weighted brain lesions | 2.7 ± 1.2 | 2.7 ± 1.2 | 2.5 ± 1.3 | 2.7 ± 1.2 | ns |
| N. MRI T1 Gad+ weighted brain lesions | 0.8 ± 1.8 | 0.9 ± 1.9 | 0.9 ± 1.7 | 0.7 ± 1.7 | ns |
BMI, body mass index; EDSS, Expanded Disability Status Scale; Gad+, Gadolinium; IQR, interquartile range; MRI, magnetic resonance imaging; n, number.
Data are expressed as mean ± standard deviation unless otherwise specified.
Among the patients, 173 (44.1%) suffered from comorbidities, of which the most common was hypertension (n = 111), followed by dyslipidaemia (n = 107) (Table 2). According to our definition of polypharmacy, 219 patients (55.8%) were included in the no-poly RRMS group, 61 (15.6%) in the minor-poly RRMS group, and 112 patients (28.6%) were included in the major-poly RRMS group (Table 1).
Table 2.
Comorbidities in our RRMS cohort.
| Patients with comorbidities at enrolment, n (%) | Total cohort (n = 173/392) | No-poly RRMS (n = 24/219) | Minor-poly RRMS (n = 61/61) | Major-poly RRMS (n = 112/112) | p-value* |
|---|---|---|---|---|---|
| Hypertension | 111 (64.3) | – | 21 (34.4) | 90 (80.4) | <0.05 |
| Dyslipidaemia | 107 (61.8) | – | 20 (32.7) | 87 (77.7) | <0.05 |
| Diabetes | 97 (56.1) | – | 17 (27.9) | 80 (71.4) | <0.05 |
| Gastrointestinal comorbidities | 94 (54.3) | 7 (29.2) | 32 (52.4) | 62 (49.1) | ns |
| Osteoporosis | 82 (47.4) | – | 30 (49.2) | 52 (46.4) | ns |
| Depression/Anxiety | 44 (25.4) | – | 17 (27.8) | 27 (24.1) | ns |
| Hypothyroidism | 40 (23.1) | – | 15 (24.5) | 25 (22.3) | ns |
| Epilepsy | 35 (20.2) | – | 11 (18) | 24 (21.4) | ns |
| Headache | 30 (17.3) | 8 (33.3) | 8 (13.1) | 14 (12.5) | ns |
| Fatigue | 28 (16.2) | 5 (20.8) | 8 (13.1) | 15 (13.4) | ns |
| Asthma/allergy | 25 (14.5) | 4 (16.7) | 7 (11.5) | 14 (12.5) | ns |
| Urinary dysfunction | 23 (13.3) | – | 8 (13.1) | 15 (13.4) | ns |
via chi-square test, it is calculated between Minor-poly RRMS and Major-poly RRMS group.
RRMS, relapsing–remitting multiple sclerosis
The minor- and major-poly RRMS patients were, on average, older (35.7 ± 11.4 and 36.9 ± 11.7 versus 29.3 ± 9.9, p < 0.05) and had a higher median BMI (24.3 and 26.7 versus 22.7, p < 0.05) than those in the no-poly RRMS group. Furthermore, they included higher rates of smokers (44.3% and 40.2% versus 18.3%, both p < 0.05) (Table 1).
Table 2 shows the comorbidities observed in the entire cohort and separated among the three groups. The major-poly RRMS group had higher incidences of hypertension, dyslipidaemia, and diabetes than the minor-poly RRMS group (p < 0.05).
Medications prescribed
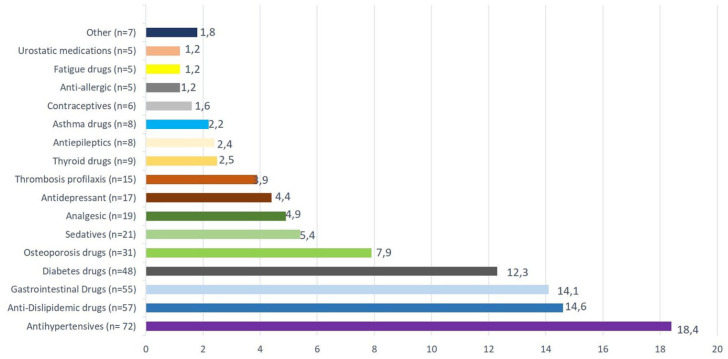
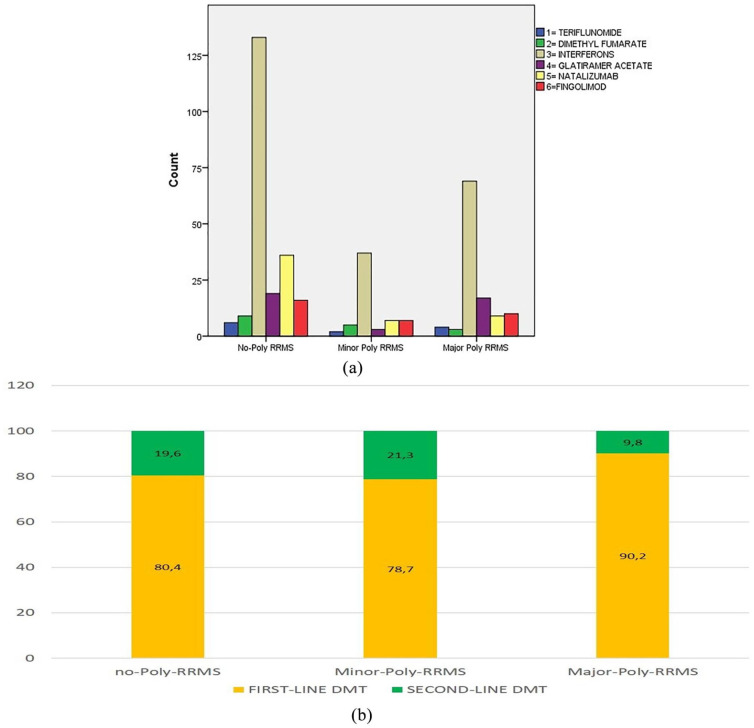
The most prescribed medications in the poly RRMS patients (minor and major) are shown in Figure 2. Figure 3a shows the first DMT prescribed in each group. The Figure 3b represents the DMT use according to the line of treatment. There were no differences in this regard among the three groups (Figure 3b).
Figure 2.
Proportion of categories of medications used by poly RRMS patients.
Groups were calculated according to the total number of drugs taken by poly RRMS patients.
Figure 3.
(a) DMTs’ distribution among the three groups; (b) DMTs’ line distribution among the three groups.
DMT, disease-modifying therapy; RRMS, relapsing–remitting multiple sclerosis.
Polypharmacy and associations with demographical/clinical parameters
Upon multinomial regression analysis, older age at onset was associated with minor and major polypharmacy [odds ratio (OR) 1.050, confidence interval (CI) 1.010–1.093, p = 0.015 and OR 1.063, CI 1.026–1.101, p = 0.001, respectively] and higher BMI was associated with major polypharmacy (OR 1.186, CI 1.18–1.29, p = 0.001).
First DMT discontinuation analysis
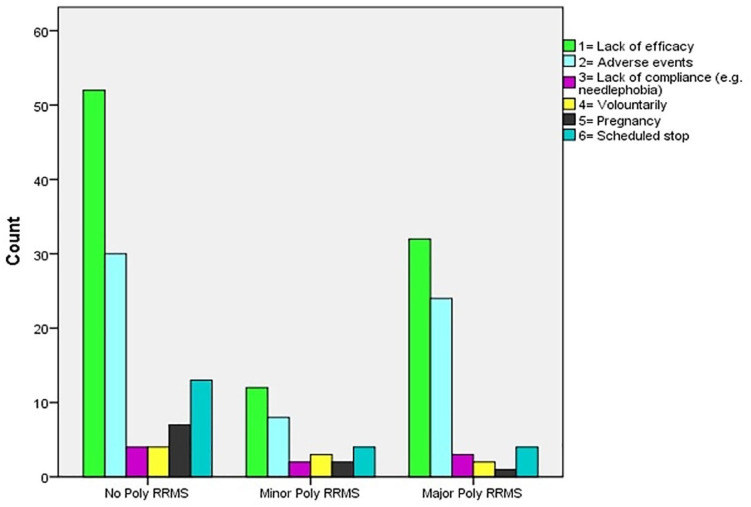
The rates of discontinuation of first DMT were similar among the three groups (50.7% for no-poly RRMS, 50.8% for minor-poly RRMS, and 53.3% for major-poly RRMS, p = 0.264). The most common reason for discontinuation was a lack of effectiveness (in terms of experiencing a relapse or newly enhanced MRI lesion) in 96 patients (52 for no-poly RRMS, 12 for minor-poly RRMS, and 32 major-poly RRMS with mean times of 35.5 ± 3.4, 41.9 ± 8.3, and 37.4 ± 4.9 months, not significant).
AEs led to discontinuation in 62 patients (30 for no-poly RRMS, eight for minor-poly RRMS, and 24 for major-poly RRMS with mean times of 36.3 ± 4.9, 46.6 ± 5.8, and 35.8 ± 6.6 months, not significant). The most frequent AEs that resulted in DMT discontinuation are shown in Table 3.
Table 3.
AEs leading to DMTs discontinuation among the three groups.
| Total (n = 62/202) | No-poly RRMS (n = 30/62) | Minor-poly RRMS (n = 8/62) | Major-poly RRMS (n = 24/62) | |
|---|---|---|---|---|
| Flu-like syndrome | 14 (22.6) | 9 (30) | 2 (25) | 3 (12.5) |
| Gastrointestinal side effects | 14 (22.6) | 7 (23.4) | 1 (12.5) | 6 (25) |
| Injection sites reactions | 4 (6.5) | 2 (6.6) | 1 (12.5) | 1 (4.2) |
| Transaminases elevation | 9 (14.5) | 3 (10) | 2 (25) | 4 (16.7) |
| Thrombocytopenia | 1 (1.6) | – | 1 (12.5) | – |
| Lymphocytopenia | 2 (3.2) | – | 1 (12.5) | 1 (4.2) |
| Dysthyroidism | 8 (12.9) | 5 (16.6) | – | 3 (12.5) |
| Arrhythmia | 1 (1.6) | – | – | 1 (4.2) |
| Depression | 9 (14.5) | 4 (13.4) | – | 5 (20.7) |
AE, adverse event; DMT, disease-modifying therapy; RRMS, relapsing–remitting multiple sclerosis.
The other reasons for discontinuing DMT are shown in Figure 4.
Figure 4.
Reasons of first DMT discontinuation among the three groups.
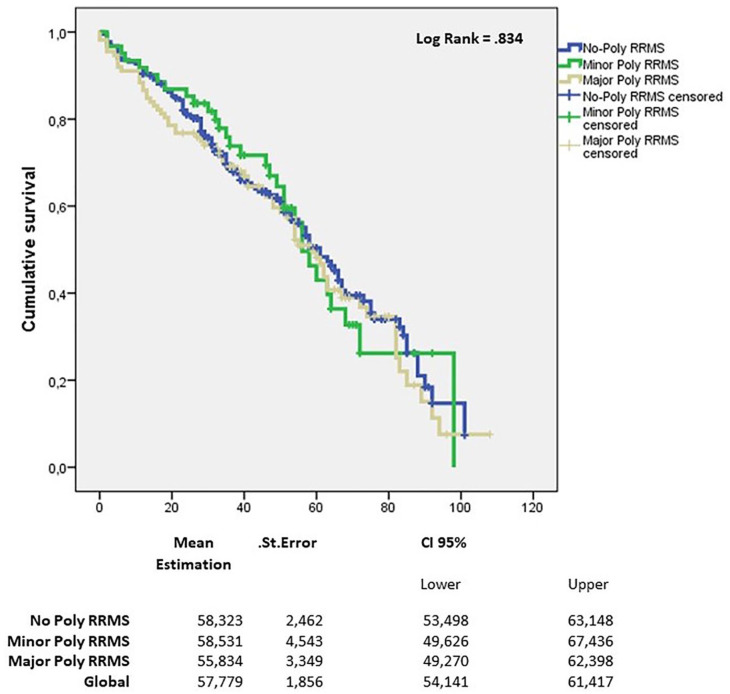
Kaplan–Meier estimates for the time to discontinuation of first DMT according to the polypharmacy group are shown in Figure 5. There were no differences among the three groups (p = 0.834, log-rank test). The analysis of predictors with a Cox regression model did not show differences among the three groups (p = 0.218). The number of relapses in the year before the diagnosis was an independent predictor of discontinuation of first DMT (Table 4).
Figure 5.
Kaplan–Meier survival analysis for the event time to first DMT discontinuation.
Table 4.
Variables in the Cox proportional hazard model for the time to first DMT discontinuation in the three groups.
| p-value | Exp(B) | CI 95.0% per exp(B) | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Gender | 0.624 | 1.093 | 0.765 | 1.562 |
| Age | 0.064 | 0.984 | 0.966 | 1.001 |
| Lag time (between onset and diagnosis) | 0.254 | 0.998 | 0.995 | 1.001 |
| Gender | 0.624 | 1.093 | 0.765 | 1.562 |
| Smokers | 0.614 | 1.015 | 0.712 | 1.075 |
| BMI | 0.627 | 1.015 | 0.954 | 1.080 |
| Lag time (between onset and diagnosis) | 0.330 | 1.003 | 0.997 | 1.008 |
| Hypertension | 0.374 | 1.566 | 0.583 | 4.210 |
| Dyslipidaemia | 0.941 | 1.029 | 0.485 | 2.184 |
| Number of relapses within the year before diagnosis | 0.029 | 2.340 | 1.090 | 5.022 |
| EDSS at the first DMT beginning | 0.346 | 1.099 | 0.903 | 1.336 |
| Number of T2-weighted lesions within the year before diagnosis | 0.327 | 1.059 | 0.839 | 1.336 |
| Number of T1 Gad+-weighted lesions within the year before diagnosis | 0.630 | 1.377 | 1.044 | 1.815 |
| Treatment line | 0.062 | 1.702 | 0.974 | 2.973 |
BMI, Body Mass Index; CI, confidence interval; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; Gad+, Gadolinium.
Discussion
In our cohort with newly diagnosed RRMS, polypharmacy was associated with an older age and higher BMI at the time of diagnosis. The rate of DMT discontinuation did not differ among the three groups, nor did the most common reasons for such discontinuation. Being older and having a higher BMI were associated with the administration of several medications in our RRMS cohort, but that did not seem to influence the first DMT discontinuation.
Polypharmacy represents an emerging challenge in therapeutic management globally, especially in the elderly.24 The World Health Organization (WHO) underlined that healthcare interventions are intended to benefit patients, but can actually also cause harm.25 According to WHO guidelines, polypharmacy in chronic diseases should be monitored carefully because it is linked in the general population to an increase of the drug burden, increases the risk of AEs from drug–drug interactions, reduces functional capacity and raises healthcare costs.20,25
The debate about structured evaluation of the use of medications is attracting increasing interest. A review of a patient’s medication should include engagement with the patient themselves. The patient’s perspective on managing and taking multiple medications should be assessed, as well as their goal from the care. A few studies have focused on the phenomenon of polypharmacy in a real-world MS setting, but they reported conflicting data regarding its frequency, with rates ranging from 14.9% to 59%.26,27 Recently, a single-centre study on MS patients with polypharmacy revealed that they were older and had higher disability levels than those without polypharmacy.28 However, in that study, only major polypharmacy was considered (⩾5 medications, including DMTs) and patients with a progressive course of MS with heterogeneous disease duration were included.28
Nonetheless, in our study, polypharmacy did not appear to influence the discontinuation of first DMT in RRMS and the reasons for DMT discontinuation did not differ among the three groups.
However, at the time of first DMT prescription, a complete medication review should be recommended, weighing the effective utility of each medication with the aim of optimising overall therapeutic management.29–31
Recently a multicentre Italian study on newly diagnosed RRMS patients investigated the prognostic factors for early switch after first therapy choice. Here, demographic, clinical, and MRI data were studied in terms of their potential associations with a switch in therapeutic strategy due to lack of efficacy or intolerance/poor safety, with stratification for lack of efficacy.32 In this cohort, comorbidities were associated with intolerance switch (HR = 1.28; p = 0.047). However, adjustment for the use of concomitant medications for different comorbidities was not performed.
Observational studies may significantly contribute to the identification of safety concerns or to the definition of the role of comorbidities/polypharmacy in discontinuation of DMTs. Clinical trials have several limits, mainly because of study duration, inclusion of patients without comorbidities or reduced statistical power.33–35 Real-world studies could provide a more realistic insight into the polypharmacy topic because they allow the inclusion of an unselected but highly representative population.36–40
Several limitations of this study need to be considered. The retrospective design of this study can be associated with recall bias (regarding the exact date of disease onset, time elapsed between onset and diagnosis, etc.). Moreover, the nature of the study limited the interpretation of data in terms of a direct risk analysis. The small sample size and data from a single centre also limited the study, underlining the need for further and larger studies on larger cohorts. Furthermore, the patients with RRMS in this study received different treatments, impeding our ability to understand the role of a specific therapy on clinical outcomes. As polypharmacy will continue to increase with the aging of MS cohorts, better characterisation of this phenomenon is required.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Research ethics and patient consent: The study protocol was approved by the local ethics committee (Comitato Etico Catania 1, no. 87/2020/PO). Patients provided written informed consent to participate in the study. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and with the appropriate national regulations.
ORCID iD: Emanuele D’Amico  https://orcid.org/0000-0001-7494-9057
https://orcid.org/0000-0001-7494-9057
Data availability: Anonymised data will be shared upon request from any qualified investigator for the sole purpose of replicating procedures and results presented in this report, provided that data transfer is in agreement with EU legislation on general data protection.
Contributor Information
Aurora Zanghì, Department “G.F. Ingrassia”; University of Catania, Catania, Italy.
Emanuele D’Amico, Department “G.F. Ingrassia”, Policlinico G. Rodolico, V. Santa Sofia 78, Catania, 95123, Italy; Department “G.F. Ingrassia”; University of Catania, Catania, Italy.
Salvatore Lo Fermo, Department “G.F. Ingrassia”; University of Catania, Catania, Italy.
Francesco Patti, Department “G.F. Ingrassia”; University of Catania, Catania, Italy.
References
- 1. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med 2018; 378: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zanghì A, D’Amico E, Patti F. Immunosuppression in relapsing remitting multiple sclerosis: moving towards personalized treatment. Expert Rev Neurother 2020; 20: 771–782. [DOI] [PubMed] [Google Scholar]
- 3. Ziemssen T, Thomas K. Treatment optimization in multiple sclerosis: how do we apply emerging evidence? Expert Rev Clin Immunol 2017; 13: 509–511. [DOI] [PubMed] [Google Scholar]
- 4. Marrie RA, Cohen J, Stuve O, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler 2015; 21: 263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang T, Tremlett H, Leung S, et al. Examining the effects of comorbidities on disease-modifying therapy use in multiple sclerosis. Neurology 2016; 86: 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D’Amico E, Patti F, Zanghì A, et al. Late-onset and young-onset relapsing-remitting multiple sclerosis: evidence from a retrospective long-term follow-up study. Eur J Neurol 2018; 25: 1425–1431. [DOI] [PubMed] [Google Scholar]
- 7. Moss BP, Rensel MR, Hersh CM. Wellness and the role of comorbidities in multiple sclerosis. Neurotherapeutics 2017; 14: 999–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petersen ER, Sondergaard HB, Laursen JH, et al. Smoking is associated with increased disease activity during natalizumab treatment in multiple sclerosis. Mult Scler 2019; 25: 1298–1305. [DOI] [PubMed] [Google Scholar]
- 9. Tettey P, Simpson S, Jr, Taylor BV, et al. The co-occurrence of multiple sclerosis and type 1 diabetes: shared aetiologic features and clinical implication for MS aetiology. J Neurol Sci 2015; 348: 126–131. [DOI] [PubMed] [Google Scholar]
- 10. Patti F, Leone C, D’Amico E, et al. Treatment options of cognitive impairment in multiple sclerosis. Neurol Sci 2010; 31(Suppl 2): S265–S269. [DOI] [PubMed] [Google Scholar]
- 11. Richardson K, Ananou A, Lafortune L, et al. Variation over time in the association between polypharmacy and mortality in the older population. Drugs Aging 2011; 28: 547–560. [DOI] [PubMed] [Google Scholar]
- 12. Fulton MM, Allen ER. Polypharmacy in the elderly: a literature review. J Am Acad Nurse Pract 2005; 17: 123–132. [DOI] [PubMed] [Google Scholar]
- 13. Haider SI, Johnell K, Thorslund M, et al. Analysis of the association between polypharmacy and socioeconomic position among elderly aged > or =77 years in Sweden. Clin Ther 2008; 30: 419–427. [DOI] [PubMed] [Google Scholar]
- 14. Jorgensen T, Johansson S, Kennerfalk A, et al. Prescription drug use, diagnoses, and healthcare utilization among the elderly. Ann Pharmacother 2001; 35: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 15. Linjakumpu T, Hartikainen S, Klaukka T, et al. Use of medications and polypharmacy are increasing among the elderly. J Clin Epidemiol 2002; 55: 809–817. [DOI] [PubMed] [Google Scholar]
- 16. Onder G, Liperoti R, Fialova D, et al. Polypharmacy in nursing home in Europe: results from the SHELTER study. J Gerontol A Biol Sci Med Sci 2012; 67: 698–704. [DOI] [PubMed] [Google Scholar]
- 17. Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health 2008; 11: 44–47. [DOI] [PubMed] [Google Scholar]
- 18. Rommer PS, Zettl UK. Managing the side effects of multiple sclerosis therapy: pharmacotherapy options for patients. Expert Opin Pharmacother 2018; 19: 483–498. [DOI] [PubMed] [Google Scholar]
- 19. D’Amico E, Patti F, Zanghì A, et al. A personalized approach in progressive multiple sclerosis: the current status of Disease Modifying Therapies (DMTs) and future perspectives. Int J Mol Sci 2016; 17: 1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frahm N, Hecker M, Zettl UK. Polypharmacy in patients with multiple sclerosis: a gender-specific analysis. Biol Sex Differ 2019; 10: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trojano M, Bergamaschi R, Amato MP, et al. The Italian multiple sclerosis register. Neurol Sci 2019; 40: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Havrdova E, Galetta S, Stefoski D, et al. Freedom from disease activity in multiple sclerosis. Neurology 2010; 74(Suppl. 3): S3–S7. [DOI] [PubMed] [Google Scholar]
- 24. European Medicines Agency. https://www.ema.europa.eu/en (accessed September 2020).
- 25. World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/325454/WHO-UHC-SDS-2019.11-eng.pdf?ua=1 (accessed September 2020).
- 26. Jelinek GA, Weiland TJ, Hadgkiss EJ, et al. Medication use in a large international sample of people with multiple sclerosis: associations with quality of life, relapse rate and disability. Neurol Res 2015; 37: 662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beiske GAG, Holmøy T, Beiske AG, et al. Antiepileptic and antidepressive, polypharmacy in patients with multiple sclerosis. Mult Scler Int 2015; 2015: 317859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Frahm N, Hecker M, Zettl UK. Multi-drug use among patients with multiple sclerosis: a cross-sectional study of associations to clinicodemographic factors. Sci Rep 2019; 9: 3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rotstein D, Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol 2019; 15: 287–300. [DOI] [PubMed] [Google Scholar]
- 30. Gourraud PA, Henry RG, Cree BA, et al. Precision medicine in chronic disease management: the multiple sclerosis BioScreen. Ann Neurol 2014; 76: 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalincik T, Manouchehrinia A, Sobisek L, et al. Towards personalized therapy for multiple sclerosis: prediction of individual treatment response. Brain 2017; 140: 2426–2443. [DOI] [PubMed] [Google Scholar]
- 32. Saccà F, Lanzillo R, Signori A, et al. Determinants of therapy switch in multiple sclerosis treatment-naïve patients: a real-life study. Mult Scler 2019; 25: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 33. Negrotto L, Farez MF, Correale J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA Neurol 2016; 73: 520–528. [DOI] [PubMed] [Google Scholar]
- 34. Pinhas-Hamiel O, Livne M, Harari G, et al. Prevalence of overweight, obesity and metabolic syndrome components in multiple sclerosis patients with significant disability. Eur J Neurol 2015; 22: 1275–1279. [DOI] [PubMed] [Google Scholar]
- 35. Slater N, White S, Venables R, et al. Factors associated with polypharmacy in primary care: a cross-sectional analysis of data from the English Longitudinal Study of Ageing (ELSA). BMJ Open 2018; 8: e020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blonde L, Khunti K, Harris SB, et al. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther 2018; 35: 1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lucchetta RC, Leonart LP, Becker J, et al. Safety outcomes of disease-modifying therapies for relapsing-remitting multiple sclerosis: a network meta-analysis. Mult Scler Relat Disord 2019; 35: 7–15. [DOI] [PubMed] [Google Scholar]
- 38. D’Amico E, Zanghì A, Sciandra M, et al. Discontinuation of teriflunomide and dimethyl fumarate in a large Italian multicentre population: a 24-month real-world experience. J Neurol 2019; 266: 411–416. [DOI] [PubMed] [Google Scholar]
- 39. D’Amico E, Zanghì A, Callari G, et al. Comparable efficacy and safety of dimethyl fumarate and teriflunomide treatment in Relapsing-Remitting Multiple Sclerosis: an Italian real-word multicenter experience. Ther Adv Neurol Disord 2018. doi:10.1177/1756286418796404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. D’Amico E, Zanghì A, Sciandra M, et al. Dimethyl fumarate vs Teriflunomide: an Italian time-to-event data analysis. J Neurol 2020; 267: 3008–3020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymised data will be shared upon request from any qualified investigator for the sole purpose of replicating the procedures and results presented in this report, provided that data transfer is in agreement with EU legislation on general data protection.