Abstract
Background:
The handgrip strength is a practical, valid, reliable, low-cost tool that presents strong correlations with several health conditions. However, handgrip strength may be inaccurate to prospectively predict the variability of muscular function since the decrease in muscular strength over the years varies according to a muscular group or between upper and lower limbs. Our hypothesis is that the handgrip strength cannot explain the variance of muscle function prospectively.
Purpose:
The aim of this study was to evaluate the cross-sectional and prospective association between handgrip strength and isokinetic muscle function of the knee and elbow in 780 asymptomatic adults.
Methods:
In a sample of 780 adults, we obtained handgrip strength and elbow and knee muscle function (for both flexion and extension at 60°/s and 300°/s) using, respectively, a hydraulic dynamometer and an isokinetic dynamometer. In a cross-sectional analysis, we analyzed the data obtained from baseline assessment. Then, we calculated the absolute change as a result of the variation data between the baseline and the 1-year follow-up assessment of each participant. The correlations were analyzed using Pearson or Spearman coefficients. We used multivariate models to investigate the association between handgrip strength and isokinetic muscle function.
Results and Discussion:
The cross-sectional correlations were significantly moderate-to-strong (r = 0.41–0.71, p < 0.01), but became weak-to-moderate (r = 0.26–0.34, p < 0.01) prospectively. In the cross-sectional analysis, the handgrip strength was selected as a strong predictor for isokinetic variables (∆R2 = 0.171–0.583, p < 0.05) as expected. Although handgrip strength was also selected as a significant predictor in prospective analysis, it explained only a little variance in isokinetic muscle function of the knee (∆R2 = 0.7–0.117, p < 0.05). Regarding the predictive models for the elbow, handgrip strength was not selected prospectively.
Conclusion:
The 1-year absolute change of the handgrip strength cannot explain the variance of the isokinetic muscle function. Thus, specific measures are required for assessing muscle function in epidemiological studies.
Keywords: Hand strength, muscle strength dynamometer, cohort analysis, muscle function
Introduction
The measurement of handgrip strength (HGS) provides an indicative of overall strength, nutritional status, muscle mass, physical fitness and general health condition, besides being a predictor of time spent on hospitalization and functional exercise capacity, mainly for middle-aged and elderly subjects.1 Nevertheless, the HGS is much more practical and less costly when compared to the isokinetic dynamometer (the gold standard for muscle strength assessment), which favors its application.
Regarding the association between HGS and muscle strength from other segments, such as knee extensor muscles, cross-sectional and cohort studies showed moderate-to-strong correlations in elderly.2,3 Although the relationship between HGS and global strength (sum of the peak torque (PT) of muscle groups of the trunk and dominant lower limbs) and/or isolated muscle function of upper and lower limbs has been widely addressed,4 previous studies suggest that the HGS must be used with caution to represent the strength of other body segments5,6 questioning the utilization of this index to estimate the overall strength.
In addition, little is known about the prospective correlation between the HGS and the muscle strength from other body segments, as well as its ability to predict these aforementioned values. Xue et al.,7 in a cohort study, reported a weak correlation between the decline of HGS and the muscle strength from hip and knee in older women. Although the muscle strength decline arises from the fourth decade of life, its progression varies depending on the muscles and also between upper and lower limbs.8 Thus, evaluations that can predict global strength decline in middle-aged and elderly asymptomatic subjects may be necessary to identify early alterations and to develop preventive strategies directly oriented to this population.
Considering the divergent data about this theme and the fact that we are unaware about studies that investigated the association between HGS and isokinetic muscle function, especially in upper limbs, we hypothesized that the HGS might not be suitable to predict the global muscle strength prospectively. Therefore, we aimed to evaluate the association between HGS and isokinetic muscle function for both knee and elbow joints in a cross-sectional design as well as over 1-year follow-up in asymptomatic adults.
Methods
Experimental approach to the problem
To evaluate the association between HGS and isokinetic muscle function of both knee and elbow, we used data collected from a cohort design of clinical assessment over 1-year follow-up with asymptomatic adults. These variables were chosen due to their recognized relationship to muscle function.
Participants
This study is part of the Epidemiology and Human Movement Study (EPIMOV study). The EPIMOV study, funded by São Paulo Research Foundation (Grant No. 2011/07282-6), is a cohort study that was kicked off in late 2013. We conducted the study in the metropolitan area of the city of Santos, São Paulo, Brazil.
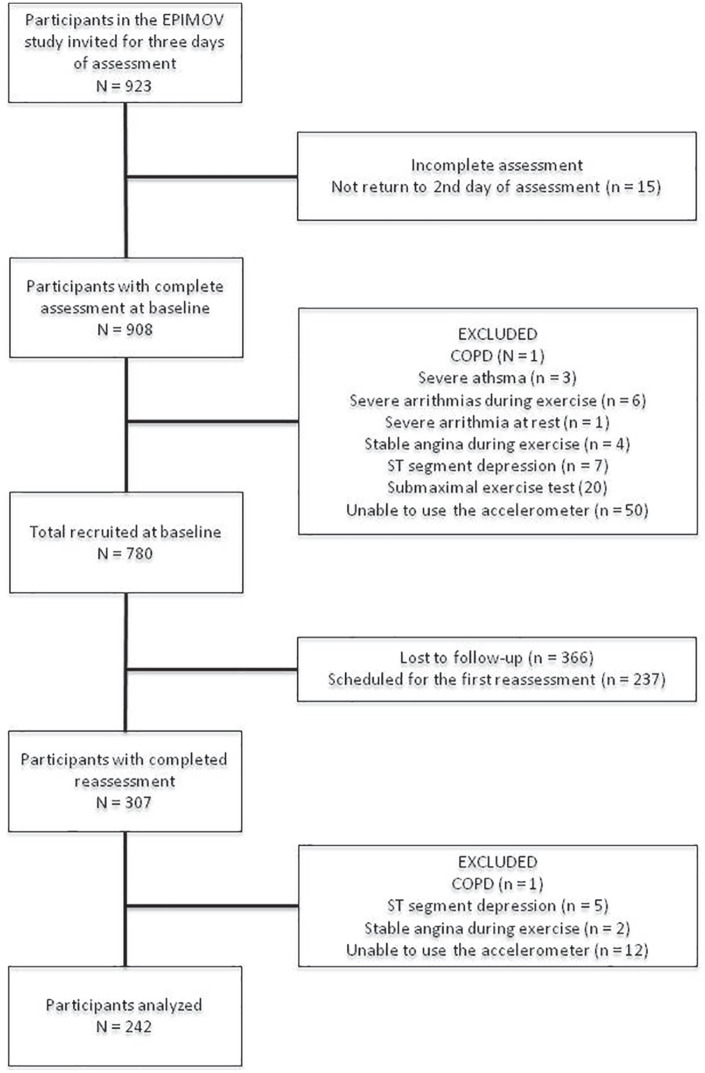
Our study includes EPIMOV participants evaluated prospectively from 2013 to 2016, over the first year of follow-up (n = 923). The EPIMOV study includes adults (18–80 years of age), which at baseline are free of cardiopulmonary and locomotive diseases. Participants diagnosed with electrocardiogram disturbance, indicating that the individual would not be able to perform physical exercises safely, and spirometric abnormalities, suggesting obstructive lung disease, are excluded. Thus, 780 individuals are part of our sample (Figure 1). The volunteers are recruited by announcements in social media, in regional universities, magazines and local journals. After the selection, the subjects were informed about the procedures should be performed and then all participants provided written informed consent.
Figure 1.
Flow diagram of the study participants.
After 14 ± 3 months have passed since the first evaluation, all participants are invited by telephone to repeat the entire research protocol. The loss to follow-up and the excluded participants in the second evaluation can be seen in the flowchart (Figure 1).
The Research Ethics Committee of human beings approved the EPIMOV study (CEP: 186.796), which was conducted according to 466/12 resolution from the National Health Council.
Data collection
Assessments from the EPIMOV study were carried out over 2 days, 1 week apart. In the first visit, participants underwent general health screening, anthropometrics, spirometry and cardiopulmonary exercise testing. At the end of the first assessment, participants were informed about the use of the triaxial accelerometer for the subsequent 7 days. In the second visit, they returned the accelerometer, and then underwent assessments of heart rate variability, body composition (bioelectrical impedance), HGS and muscle function of the knee and the elbow directly using an isokinetic dynamometer.
General health screening
Before tests evaluation, we questioned the volunteers about past health problems, regular use of medication and risk factors for cardiovascular diseases, such as age, family history, hypertension, dyslipidemia, diabetes mellitus, physical inactivity and obesity. Moreover, we tracked possible contraindications to performing the physical exercise using the Physical Activity Readiness Questionnaire (Supplemental material).9,10
Anthropometry
We measured height (m) and body mass (kg) using a digital balance with a stadiometer (Toledo®, São Paulo, Brazil). The body mass index (BMI) was calculated through weight divided by height squared.
Accelerometer-based physical activity
We used a triaxial accelerometer previously validated11,12 (ActiGraph GT3x, MTI, Pensacola, FL) and attached to the waist by an elastic band to assess the physical activity level of the participants. Carefully, we instructed all volunteers about the use of the devices above. The assessments were performed during a week after the first-day test in this study. Each day of evaluation has, at least, 12 h of awakening hours of monitoring. Thus, the assessment provides a pattern representative from a physical activity of the week of the participants. We obtained the intensity of activity and energy expenditure by the average of two valid days. The minimum level of physical activity was considered as 30 min of moderate-to-vigorous physical activity (MVPA) for, at least, 5 days a week as a minimal recommendation.13 Subjects that did not achieve this level were considered physically inactive.
Body composition
We determine the body composition using bioelectrical impedance (310e BIODYNAMICS, Detroit, EUA) at ambient temperature. The impedance and the reactance were collected from the subject in the supine position with arms and legs in 30° and 45° of abduction, as the following procedures described.14,15 After cleaning the skin, two electrodes were positioned on hand and foot at the same side of the body. Using the equation developed for healthy subjects,16 we calculated lean body mass (LBM) and fat body mass (FBM). Before the test, we carefully instructed volunteers not to ingest any liquid or food for the previous 4 h and not to practice exercise for 12 h before the test.
Isokinetic muscle function
We assessed muscle function of elbow and knee through an isokinetic dynamometer (Biodex System 3 PRO, Lumex Inc., Ronkonkoma, NY, EUA) as recommendations previously described.17,18 In seated position, the dominant upper body and the dominant lower limb were fully fixed by strips. We aligned the mechanical axis of rotation of the device to the rotational axis of the assessed joint. For elbow evaluation, we kept the position described and instructed the volunteer to perform the movement with the forearm in a neutral position. For both assessments, we adjusted the angles and positions of the chair and the dynamometer as recommended by the manufacturer. Equipment software has properly corrected the influence of gravity and the weight of the evaluated limb during all tests. To warm up and get familiar with the trials, the participants performed three to five submaximal repetitions. The PT of knee extension and elbow flexion, in Nm, was achieved by two tests with five movements each at 60°/s. After a rest period of 2 min, the subjects executed 30 repetitions at 300°/s to collect the total work (TW) of flexion and extension of elbow and knee, in kJ. Finally, the peak isometric torque of the elbow flexion and the knee extension, registered in Nm, was obtained by performing three tests against a fixed resistance at 60° of range of motion, for 5 s of a maximal isometric contraction, followed by 30 s of recovery between each repetition. We selected the greater absolute value for further analysis in all the tests above mentioned. In addition, we calculated the absolute change of isokinetic muscle function of elbow and knee joints to prospective analysis.
HGS
We also obtained the muscle function by performing a HGS test. The HGS from a dominant hand was evaluated using a hydraulic dynamometer (SH5001 SAEHAN®) as suggested.19 We defined the dominant hand as the priority hand to realize the daily living activities. We positioned the subjects in seated position with an adducted arm, elbow flexed at 90° and forearm in a neutral position. Wrist hyperextension of up to 30° and ulnar deviation of up to 15° was allowed during the tests. Each volunteer executed three tests with an interval of 30 s between them. We submitted the greater absolute value obtained for further analysis for both baseline and follow-up. In addition, we calculated the absolute change of HGS to prospective analysis.
Statistical analysis
The statistical analysis was performed using SPSS, version 23 (SPSS Inc., Chicago, IL, EUA), and the level of statistical significance was set at p < 0.05. Initially, the data were analyzed descriptively for baseline assessment and 1-year follow-up. We present the continuous variables as means ± SD. In turn, we present the categorical variables as frequency and percentage. For cross-sectional analysis, we used the values obtained on the first evaluation. Regarding the prospective analysis, we calculated the absolute change as a result of the variation data between the baseline and the 1-year follow-up assessment of each participant. We performed a paired t-test to compare the baseline and 1-year follow-up data. Then, we evaluated the correlations between continuous variables by Pearson’s or Spearman’s coefficients according to data distribution. At last, we developed several multiple stepwise linear regression models to identify the main predictors for PT, TW and peak isometric torque and their respective absolute changes. First, we verify the multicollinearity among the main variables of interest to enter the model. Among the anthropometric and body composition variables, an univariate analysis was performed to verify which one was more relevant to be added to the model. We also verified the univariate association of variables related to cardiovascular risk, to know which variables really influence the outcome and should be included in the model. We estimated the sample size based on the number of independent variables of interest for inclusion in multiple regression models. Thus, we reached a total of 12 predictors for inclusion in the model, that is, HGS, height, age, sex, body mass, lean mass, LBM, MVPA, diabetes, dyslipidemia, obesity and hypertension. Therefore, considering 15 observations for each predictor, we fixed at least 150 individuals for inclusion in the baseline analyses and 180 individuals for follow-up analysis. Nevertheless, even if all variables were included in the model, the sample size would be enough to perform this analysis. In the follow-up analyses, we also adjusted the multiple regression models for the 1-year absolute change in MVPA obtained in the first assessment.
Results
We selected 780 (297 men and 493 women) subjects from the EPIMOV study from 2013 to 2016. After 1 year, all the volunteers were invited by phone. Among the total sample of our study, 242 (62 men and 180 women) subjects returned to our laboratory and completed the second evaluation (Figure 1).
The sample of our study was composed of middle-aged and overweight subjects, mainly by women. Also, our sample presents a significant prevalence of hypertension, dyslipidemia and obesity (Table 1).
Table 1.
Baseline characteristics of the studied sample.
| Total sample (n = 780) | Follow-up sample (n = 242) | |
|---|---|---|
| Age (years) | 44 ± 14 | 46 ± 14 |
| Males/females | 297/493 | 158/84 |
| Weight (kg) | 76 ± 17 | 76 ± 17 |
| Height (m) | 1.63 ± 0.09 | 1.63 ± 0.09 |
| Body mass index (kg/m2) | 28 ± 6 | 28 ± 6 |
| Moderate-to-vigorous physical activity (h) | 5.06 ± 2.56 | 5.19 ± 2.72 |
| Lean body mass (kg) | 52 ± 10 | 52 ± 10 |
| Lean body mass (% of total) | 68 ± 8 | 68 ± 9 |
| Fat body mass (kg) | 24 ± 10 | 24 ± 10 |
| Fat body mass (% of total) | 31 ± 8 | 31 ± 9 |
| Risk factors for cardiovascular disease, n (%) | ||
| Arterial hypertension | 139 (17.8) | 49 (20.2) |
| Diabetes | 83 (10.6) | 24 (9.9) |
| Dyslipidemia | 212 (27.2) | 75 (30.9) |
| Obesity | 278 (35.6) | 90 (37.1) |
Data are reported as means ± SD or frequency (%).
When compared to the baseline with the 1-year follow-up, we found significant differences for HGS, peak isometric torque of knee flexion and elbow extension, and TW of elbow extension. The 1-year absolute changes in peak isometric torque of the knee extension and the elbow flexion were negative, as well as TW of elbow flexion, which indicate a decrease in 2.6%, 14.2% and 10% in muscle strength from the first to the second evaluation, respectively. However, we did not observe the same decrease for the others isokinetic indexes. The HGS also decreased after 1-year follow-up (Table 2), reducing, on average, 2.2% in muscle strength.
Table 2.
Muscle function assessment variables of the studied sample at baseline, 1-year follow-up and the 1-year absolute change values.
| Muscle function assessment variables | Baseline total sample (n = 780) | 1-year follow-up (n = 242) | p value | 1-year absolute change (n = 242) |
|---|---|---|---|---|
| Hand grip strength (kgF) | 34.05 ± 9.73 | 34.99 ± 10.62* | 0.040 | −0.76 ± 5.51 |
| Peak torque of knee extension 60°/s (N m) | 133.41 ± 53.53 | 134.94 ± 56.60 | 0.236 | 2.62 ± 32.17 |
| Total work of knee extension 300°/s (kJ) | 1528.43 ± 622.34 | 1555.11 ± 664.19 | 0.674 | 11.27 ± 389.44 |
| Peak torque of knee flexion 60°/s (N m) | 64.73 ± 28.91 | 62.10 ± 31.10* | 0.000 | 4.75 ± 17.30 |
| Total work of knee flexion 300°/s (kJ) | 833.62 ± 451.66 | 819.10 ± 519.56 | 0.112 | 34.10 ± 309.63 |
| Peak torque of elbow extension 60°/s (N m) | 42.30 ± 20.66 | 38.46 ± 18.73* | 0.023 | 4.59 ± 17.73 |
| Total work of elbow extension 300°/s (kJ) | 855.39 ± 413.86 | 755.93 ± 521.66* | 0.004 | 102.94 ± 308.95 |
| Peak torque of elbow flexion 60°/s (N m) | 34.71 ± 13.93 | 32.57 ± 14.70 | 0.098 | 1.64 ± 8.79 |
| Total work of elbow flexion 300°/s (kJ) | 735.35 ± 274.89 | 724.10 ± 388.98 | 0.338 | −74.00 ± 686.19 |
| Isometric peak torque of knee extension (N m) | 159.12 ± 59.94 | 164.30 ± 60.63 | 0.109 | −4.27 ± 38.51 |
| Isometric peak torque of elbow flexion (N m) | 41.60 ± 18.11 | 41.67 ± 26.43 | 0.281 | −5.92 ± 48.77 |
Data are reported as means ± SD or frequency (%).
p < 0.05 versus baseline.
The cross-sectional correlations between HGS and isokinetic muscle function indexes were moderate-to-strong, with a range r = 0.41 to r = 0.76 (p < 0.01). However, the prospective associations between HGS and 1-year absolute change in isokinetic muscle function of the knee were weak-to-moderate (r = 0.26 to r = 0.34), whereas the correlations with muscle function indexes of elbow were non-significant (Table 3).
Table 3.
Bivariate correlations between handgrip strength and isokinetic muscle function indexes (n = 780).
| Total sample (n = 780) |
1-year absolute change (n = 242) |
|
|---|---|---|
| r | r | |
| Peak torque of knee extension 60°/s (N m) | 0.71* | 0.34* |
| Peak torque of knee flexion 60°/s (N m) | 0.69* | 0.29* |
| Total work of knee extension 300°/s (kJ) | 0.68* | 0.33* |
| Total work of knee flexion 300°/s (kJ) | 0.64* | 0.31* |
| Isometric peak torque of knee extension (N m) | 0.70* | 0.09 |
| Peak torque of elbow extension 60°/s (N m) | 0.71* | 0.19 |
| Peak torque of elbow flexion 60°/s (N m) | 0.76* | 0.19 |
| Total work of elbow extension 300°/s (kJ) | 0.45* | 0.18 |
| Total work of elbow flexion 300°/s (kJ) | 0.41* | 0.26* |
| Isometric peak torque of elbow flexion (N m) | 0.41* | 0.06 |
p < 0.01.
In the cross-sectional multivariate models, the HGS was selected as a strong predictor for isokinetic muscle function, explaining alone 17.1% to 58.3% of the total variability of isokinetic muscle function. Therefore, the HGS was the main independent predictor of the isokinetic muscle function for both elbow and knee joints. Regarding the isokinetic muscle function of the knee, the anthropometric variables were included and, at least, one comorbidity and one variable representative of physical activity and fitness (Table 4).
Table 4.
Results of multiple linear regressions with handgrip strength and its 1-year absolute change as the main predictor and isokinetic muscle function variables as outcomes.
| Outcomes | Cross-sectional |
|||
|---|---|---|---|---|
| B (SE) | R 2 | ∆R2 | Other significant predictors | |
| Cross-sectional | ||||
| Peak torque of knee extension 60°/s (N m) | 1.223 (0.341) | 0.650 | 0.504 | Age, height, lean body mass and sex |
| Peak torque of knee flexion 60°/s (N m) | 0.740 (0.188) | 0.617 | 0.484 | Age, sex, height, moderate-to-vigorous physical activity |
| Total work knee extension 300°/s (kJ) | 16.530 (3.963) | 0.629 | 0.463 | Age, sex, weight and dyslipidemia |
| Total work of knee flexion 300°/s (kJ) | 17.710 (3.287) | 0.549 | 0.416 | Age, sex and moderate-to-vigorous physical activity |
| Isometric peak torque of knee extension (N m) | 1.291 (0.4) | 0.621 | 0.065 | Age, lean body mass and sex |
| Peak torque of elbow extension 60°/s (N m)a | 0.687 (0.120) | 0.563 | 0.511 | Sex, age and lean body mass |
| Peak torque of elbow flexion 60°/s (N m)a | 0.545 (0.082) | 0.668 | 0.583 | Sex, age, dyslipidemia and weight |
| Total work of elbow extension 300°/s (kJ)a | 20.175 (5.040) | 0.220 | 0.207 | Sex |
| Total work of elbow flexion 300°/s (kJ)a | 17.978 (2.922) | 0.187 | 0.171 | Weight |
| Isometric peak torque of elbow flexion (N m)a | 1.35 (0.19) | 0.174 | 0.174 | |
| 1-year follow-up | ||||
| Peak torque of knee extension 60°/s (N m) | 1.97 (0.377) | 0.134 | 0.117 | Arterial hypertension |
| Peak torque of knee flexion 60°/s (N m) | 1.026 (0.202) | 0.113 | 0.113 | – |
| Total work knee extension 300°/s (kJ) | 19.734 (4.670) | 0.108 | 0.088 | Age |
| Total work of knee flexion 300°/s (kJ) | 17.308 (3.642) | 0.121 | 0.099 | Arterial hypertension |
| Isometric peak torque of knee extension (N m) | 1.895 (0.498) | 0.07 | 0.07 | – |
| Peak torque of elbow extension 60°/s (N m)a | – | – | – | Weight |
| Peak torque of elbow flexion 60°/s (N m)a | – | – | – | – |
| Total work of elbow extension 300°/s (kJ)a | – | – | – | Weight |
| Total work of elbow flexion 300°/s (kJ)a | – | – | – | – |
| Isometric peak torque of elbow flexion (N m)a | – | – | – | – |
The absolute change in HGS was not selected as a significant predictor of the absolute change of the predictive models of isokinetic muscle function of the elbow. The models were adjusted by hand grip strength, height, age, sex, body mass, lean mass, lean body mass, moderate-to-vigorous physical activity, diabetes, dyslipidemia, obesity and hypertension.
Regarding the prospective multivariate models, we found that the HGS was selected as the main predictor only for the isokinetic muscle function of the knee, but not of the elbow. However, HGS could not explain more than 13.4% of the variability of the isokinetic muscle function of the knee (Table 4).
Discussion
In this study, we investigated the cross-sectional and prospective associations between HGS and isokinetic muscle function of elbow and knee joints. Despite the moderate-to-strong cross-sectional correlations found, we observed that these correlations became weaker for the knee muscle function and lost the statistical significance for the elbow after 1-year follow-up. Although it was selected as one of the main predictors in the multivariate models developed, the HGS did not explain the variability of the isokinetic muscle function of the knee, especially in prospective analysis. When considering the elbow muscle function, the HGS was not even included in the multivariate models.
Our sample was composed mainly of middle-aged and overweight women in agreement with the Brazilian population (Table 1). Also, we found higher prevalence of obesity and diabetes in our sample, but lesser arterial hypertension when compared to Brazilian population.20 When compared to women, men are still in an unfavorable health situation.21 This fact may be related to the lower demand for health services by this population, leading to the identification of diseases in later stages. Mortality indicators show that men die by preventable deaths more than women. Other studies with populations with chronic diseases show that women have more self-care when compared to men.22,23 These facts can contribute to explain our predominantly female sample.
The relative decrease in muscle strength was greater on elbow function in contrast to the increase in HGS (Table 2), which is consistent with previously described. Previously, Vidt et al.24 observed muscle mass decreased in abductors of the shoulder and flexors and extensors of the elbow. In addition, instead of the decreased muscle mass in the proximal muscles, the authors found that wrist extensors presented muscle mass increased. It is important to consider the significant differences between the peak isometric torque measurements of the shoulder in comparison with the elbow muscle function and, especially, when compared to the wrist. This fact suggests that the shoulder muscles are relatively weaker than the muscles from proximal to distal segments of the upper limb in older adults.24 Thus, these findings complies with our results, suggesting that the decline of the muscle strength and muscle mass is greater in the proximal compared to distal segments and, hence, contributing to explain the absence of HGS in the predictive models of elbow muscle function prospectively.
Regarding 1-year absolute change in isokinetic muscle function, we found significant decrease just for PT of knee flexion while HGS increased (Table 2), which agrees with previous literature.25,26 We attributed these differences to our brief follow-up and our sample characteristics. Despite this, we associated this result to the loss of muscle mass26 and also the impact that sedentary behavior secondary to aging may present on lower limbs muscle function, while the muscle strength and muscle mass of the upper limbs remain preserved because of its functional demand in daily living activities.27 According to Xue et al.,7 this may occur in an attempt to compensate for the decrease in functionality resulting from the loss of muscle strength through the modification of lifestyle, suppressing the physical activity to maintain the baseline performance and, hence, slowing up the subsequent decline. With a similar sample to ours, Ferreira et al.28 showed that the activity level for the upper limbs grows, while the activity of lower limbs decreases, when comparing older adults with young subjects.28 Also, the reduction of fine motor function of the hand precedes the decline of the muscle strength, leading to the older adults to raise the force control to compensate for the precision loss and, thereby, keep their functionality stable until they reach the age of 65 years.29,30 These previous findings in addition to our results imply not only the need to evaluate HGS but also the importance to use specific and accurate measurements to predict and monitor upper and lower limbs muscle function decline over time.
As expected, the cross-sectional correlations between HGS and the isokinetic muscle function were moderate-to-strong2,3,25 (Table 3). The previous literature2,3 reported moderate-to-strong correlations (r = 0.51–0.80) in elderly, similarly to our findings. Nevertheless, these correlations showed moderate-to-weak coefficients when submitted to the prospective analysis, reinforcing the results of previous studies5–7,31 regarding the lower limb strength. In addition, Xue et al.7 obtained even weaker correlations in a prospective analysis (r = 0.10). Thus, our results are in agreement with previous literature.
Also, we were able to observe that the HGS was selected as the main predictor for the isokinetic muscle function, notably for the knee and in the cross-sectional models (Table 4). In contrast, the best model developed was not able to explain the major variability of our outcomes. The ISCOPE study, with follow-up similar to ours, reported, based on two linear regression models (with and without adjustment for sex and age), a coefficient of determination closed to those obtained in this study (R2 = 0.16 and R2 = 0.17, respectively).31 Despite the cross-sectional design, Akbari and Mousavikhatir8 observed that, in asymptomatic women aged 20–80 years, stratified into six age groups, the muscle strength of hip extensors and dorsiflexors suffer reduction from the fourth decade, while hip abductors and knee extensors start to present significant lower values from the fifth decade. Although there was a different progression of the decline of muscle strength between muscle groups, Xue et al.7 observed in a 10-year follow-up study that there is a plateau on such decline, especially for HGS. It has been suggested that HGS declines faster up to 75 years when a plateau can be observed. Therefore, we attributed our prospective results to these previous studies, which may explain the loss of significance of the association between HGS and isokinetic muscle function and the absence in the predictive models, especially prospective and for the prediction of elbow muscle function.
In addition, we found that sociodemographic and anthropometric variables were the main predictor of isokinetic muscle function in cross-sectional and prospective analysis.18,32 In cross-sectional models, age, sex, height and weight were selected as significant predictor of elbow and/or knee muscle function in accordance with previous literature. MVPA was only included in the models for cross-sectional prediction of knee muscle function, which may be attributed to physical activity level related to age span of our sample as previously discussed.6,33,34 Regarding isometric PT of knee extension and PT of elbow extension, the LBM was also selected for inclusion in cross-sectional models. Previous studies35 shows that measurements of lean mass and skeletal muscle quality can assist in better predicting skeletal muscle strength, which could explain this finding. Among the risk factors for cardiovascular disease, only dyslipidemia was included (TW of knee extension and PT of elbow flexion) in the cross-sectional models and only hypertension was included (PT of knee extension) in the 1-year follow-up models. This can be credited to a decrease in strength in hypertensive individuals, as previously discussed in the literature.36 As widely addressed, obesity can affect muscle function due to bearing additional weight during daily activities, but, as described by Tallis et al.,37 the obesity effect may be attenuated after adjustment to weight. These findings contributed to explain the inclusion of weight, but not obesity, in our multivariate models, especially in the prospective analysis.
This study has some limitations that should be considered. The convenience sample composed mainly of middle-aged subjects and the short period follow-up are the main limitations, as well as the great loss of follow-up. Our sample characteristics, especially the mean age, largely explain our main results since the muscle strength decline for some muscle groups starts from this age span for instance. But even in a short period of follow-up, we are able to observe that the change in muscle function of upper and lower limbs cannot be predicted through HGS. Therefore, our study also has strengths. To our knowledge, the literature about this topic is still scarce, which makes our study even more relevant. In addition, the larger proportion of women, as well as the prevalence of comorbidities, is due to the convenience sample, but it has similarities to our population. The evaluation protocol also is an important strength of our study, highlighting the use of isokinetic dynamometer to obtain precise measurements of muscle function, triaxial accelerometer to quantify physical activity level and bioelectrical impedance to provide body composition. Also, our general health screening must be considered, which allowed us to adjust our main analysis to the risk factors for cardiovascular disease. Finally, although we had a great loss of follow-up, our sample size was enough to perform the statistical analysis. Thus, we are confident about the generalizability of the results presented here.
It is evident the relevance of HGS as indicative of global muscle strength, proving useful in initial clinical evaluations and health screening. However, HGS cannot be used as a parameter of force variation in other body segments to avoid misinterpretation, such as the muscle function of the knee and elbow. Therefore, HGS does not replace isokinetic dynamometry to obtain more precise measures of muscle function and its monitoring over time. In addition, their values should be associated with considerations about the level of physical activity of the subjects and the presence of risk factors for cardiovascular disease.
Conclusion
We can conclude that, despite these moderate-to-strong cross-sectional correlations between the HGS and the isokinetic muscle function of upper and lower limbs, the absolute change of HGS over time seems not to be able to predict the absolute change of the isokinetic muscle function of the elbow and the knee expressively, even in a short period follow-up. Therefore, in addition to HGS, specific strength measures are required for assessing muscle function in epidemiological studies, as well as to ensure an adequate and more complete clinical assessment routine and health screening.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_1049732320931430 for Association between the handgrip strength and the isokinetic muscle function of the elbow and the knee in asymptomatic adults by Thatiane Lopes Valentim Di Paschoale Ostolin, Bárbara de Barros Gonze, Wesley de Oliveira Vieira, André Luiz Silva de Oliveira, Matheus Bibian Nascimento, Rodolfo Leite Arantes, Marcello Romiti, Evandro Fornias Sperandio and Victor Zuniga Dourado in SAGE Open Medicine
Supplemental material, sj-docx-2-smo-10.1177_1049732320931430 for Association between the handgrip strength and the isokinetic muscle function of the elbow and the knee in asymptomatic adults by Thatiane Lopes Valentim Di Paschoale Ostolin, Bárbara de Barros Gonze, Wesley de Oliveira Vieira, André Luiz Silva de Oliveira, Matheus Bibian Nascimento, Rodolfo Leite Arantes, Marcello Romiti, Evandro Fornias Sperandio and Victor Zuniga Dourado in SAGE Open Medicine
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Research Ethics Committee of human beings of Federal University of São Paulo (CEP: 186.796).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received financial support in the form of a research grant from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation; Grant No. 2011/07282-6).
Informed consent: All participants included in the study provided written informed consent.
ORCID iD: Thatiane Lopes Valentim Di Paschoale Ostolin  https://orcid.org/0000-0002-8492-2840
https://orcid.org/0000-0002-8492-2840
Supplemental material: Supplemental material for this article is available online.
References
- 1. Bohannon RW. Muscle strength: clinical and prognostic value of hand-grip dynamometry. Curr Opin Clin Nutr Metab Care 2015; 18(5): 465–470. [DOI] [PubMed] [Google Scholar]
- 2. Bohannon RW, Magasi SR, Bubela DJ, et al. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve 2012; 46: 555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc 2016; 64: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Porto JM, Nakaishi APM, Cangussu-Oliveira LM, et al. Relationship between grip strength and global muscle strength in community-dwelling older people. Arch Gerontol Geriatr 2019; 82: 273–278. [DOI] [PubMed] [Google Scholar]
- 5. Felicio DC, Pereira DS, Assumpcao AM, et al. Poor correlation between handgrip strength and isokinetic performance of knee flexor and extensor muscles in community-dwelling elderly women. Geriatr Gerontol Int 2014; 14(1): 185–189. [DOI] [PubMed] [Google Scholar]
- 6. Jenkins NDM, Buckner SL, Bergstrom HC, et al. Reliability and relationships among handgrip strength, leg extensor strength and power, and balance in older men. Exp Gerontol 2014; 58: 47–50. [DOI] [PubMed] [Google Scholar]
- 7. Xue QL, Beamer BA, Chaves PHM, et al. Heterogeneity in rate of decline in grip, hip, and knee strength and the risk of all-cause mortality: the women’s health and aging study II. J Am Geriatr Soc 2010; 58(11): 2076–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akbari M, Mousavikhatir R. Changes in the muscle strength and functional performance of healthy women with aging. Med J Islam Repub Iran 2012; 26(3): 125–131. [PMC free article] [PubMed] [Google Scholar]
- 9. Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q). Can J Sport Sci 1992; 17(4): 338–345. [PubMed] [Google Scholar]
- 10. de Oliveira Luz L, Neto GAM, Farinatti PTV. Validity of the physical activity readiness questionnaire (Par-q) in elder subjects. Rev Bras Cineantropom Desempenho Hum 2007; 9(4): 366–371. [Google Scholar]
- 11. Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008; 40(1): 181–188. [DOI] [PubMed] [Google Scholar]
- 12. Brooks AG, Gunn SM, Withers RT, et al. Predicting walking METs and energy expenditure from speed or accelerometry. Med Sci Sports Exerc 2005; 37(7): 1216–1223. [DOI] [PubMed] [Google Scholar]
- 13. ACSM’s Guidelines for exercise testing prescription 9th ed. 2014. J Can Chiropr Assoc 2014; 58: 328. [Google Scholar]
- 14. Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr 2004; 23: 1226–1243. [DOI] [PubMed] [Google Scholar]
- 15. Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis—part II: utilization in clinical practice. Clin Nutr 2004; 23: 1430–1453. [DOI] [PubMed] [Google Scholar]
- 16. Kyle UG, Genton L, Karsegard L, et al. Single prediction equation for bioelectrical impedance analysis in adults aged 20-94 years. Nutrition 2001; 17: 248–253. [DOI] [PubMed] [Google Scholar]
- 17. Rondelli RR, Dal Corso S, Simões A, et al. Métodos de avaliação da fadigabilidade muscular periférica e seus determinantes energético-metabólicos na DPOC. J Bras Pneumol 2009; 35: 1125–1135. [DOI] [PubMed] [Google Scholar]
- 18. Neder JA, Nery LE, Shinzato GT, et al. Reference values for concentric knee isokinetic strength and power in nonathletic men and women from 20 to 80 years old. J Orthop Sports Phys Ther 1999; 29: 116–126. [DOI] [PubMed] [Google Scholar]
- 19. Mathiowetz V, Kashman N, Volland G, et al. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil 1985; 66: 69–74. [PubMed] [Google Scholar]
- 20. Ministério da Saúde. Vigitel Brasil, 2019, http://bvsms.saude.gov.br/
- 21. Ministério da Saúde. Sistema De Informações Sobre Mortalidade (SIM). Datasus, 2017, http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sim/cnv/obt10uf.def
- 22. Rossaneis MA, Haddad M, do CFL, et al. Differences in foot self-care and lifestyle between men and women with diabetes mellitus. Rev Lat Am Enfermagem 2016; 24: e2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heo S, Moser DK, Lennie TA, et al. Gender differences in and factors related to self-care behaviors: a cross-sectional, correlational study of patients with heart failure. Int J Nurs Stud 2008; 45: 1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vidt ME, Daly M, Miller ME, et al. Characterizing upper limb muscle volume and strength in older adults: a comparison with young adults. J Biomech 2012; 45: 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Samuel D, Wilson K, Martin HJ, et al. Age-associated changes in hand grip and quadriceps muscle strength ratios in healthy adults. Aging Clin Exp Res 2012. [DOI] [PubMed] [Google Scholar]
- 26. Hughes VA, Frontera WR, Wood M, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 2001; 56: B209–B217. [DOI] [PubMed] [Google Scholar]
- 27. Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull 2010; 95: 139–159. [DOI] [PubMed] [Google Scholar]
- 28. Ferrreira L, Gobbi S, Gobbi LTB. An explanatory mechanism for the different decline in limb strength in older women. Arch Gerontol Geriatr 2009; 49: 373–377. [DOI] [PubMed] [Google Scholar]
- 29. Carmeli E, Patish H, Coleman R. The aging hand. J Gerontol A Biol Sci Med Sci 2003; 58: 146–152. [DOI] [PubMed] [Google Scholar]
- 30. Scherder E, Dekker W, Eggermont L. Higher-level hand motor function in aging and (preclinical) dementia: its relationship with (instrumental) activities of daily life—a mini-review. Gerontology 2008; 54: 333–341. [DOI] [PubMed] [Google Scholar]
- 31. Chan OYA, van Houwelingen AH, Gussekloo J, et al. Comparison of quadriceps strength and handgrip strength in their association with health outcomes in older adults in primary care. Age 2014; 36: 9714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pereira JC, Neri SGR, Vainshelboim B, et al. A reference equation for normal standards for knee extensor isokinetic strength in Brazilian older women. Aging Clin Exp Res 2019; 31: 1531–1537. [DOI] [PubMed] [Google Scholar]
- 33. Reed RL, Den Hartog R, Yochum K, et al. A comparison of hand-held isometric strength measurement with isokinetic muscle strength measurement in the elderly. J Am Geriatr Soc 1993; 41: 53–56. [DOI] [PubMed] [Google Scholar]
- 34. Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the health, aging and body composition study. J Am Geriatr Soc 2003; 51: 323–330. [DOI] [PubMed] [Google Scholar]
- 35. Bourgeois B, Fan B, Johannsen N, et al. Improved strength prediction combining clinically available measures of skeletal muscle mass and quality. J Cachexia Sarcopenia Muscle 2019; 10: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mainous AG, 3rd, Tanner RJ, Anton SD, et al. Grip strength as a marker of hypertension and diabetes in healthy weight adults. Am J Prev Med 2015; 49: 850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tallis J, James RS, Seebacher F. The effects of obesity on skeletal muscle contractile function. J Exp Biol 2018; 221: jeb163840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_1049732320931430 for Association between the handgrip strength and the isokinetic muscle function of the elbow and the knee in asymptomatic adults by Thatiane Lopes Valentim Di Paschoale Ostolin, Bárbara de Barros Gonze, Wesley de Oliveira Vieira, André Luiz Silva de Oliveira, Matheus Bibian Nascimento, Rodolfo Leite Arantes, Marcello Romiti, Evandro Fornias Sperandio and Victor Zuniga Dourado in SAGE Open Medicine
Supplemental material, sj-docx-2-smo-10.1177_1049732320931430 for Association between the handgrip strength and the isokinetic muscle function of the elbow and the knee in asymptomatic adults by Thatiane Lopes Valentim Di Paschoale Ostolin, Bárbara de Barros Gonze, Wesley de Oliveira Vieira, André Luiz Silva de Oliveira, Matheus Bibian Nascimento, Rodolfo Leite Arantes, Marcello Romiti, Evandro Fornias Sperandio and Victor Zuniga Dourado in SAGE Open Medicine