Abstract
Not 1 year has passed since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19). Since its emergence, great uncertainty has surrounded the potential for COVID-19 to establish as a seasonally recurrent disease. Many infectious diseases, including endemic human coronaviruses, vary across the year. They show a wide range of seasonal waveforms, timing (phase), and amplitudes, which differ depending on the geographical region. Drivers of such patterns are predominantly studied from an epidemiological perspective with a focus on weather and behavior, but complementary insights emerge from physiological studies of seasonality in animals, including humans. Thus, we take a multidisciplinary approach to integrate knowledge from usually distinct fields. First, we review epidemiological evidence of environmental and behavioral drivers of infectious disease seasonality. Subsequently, we take a chronobiological perspective and discuss within-host changes that may affect susceptibility, morbidity, and mortality from infectious diseases. Based on photoperiodic, circannual, and comparative human data, we not only identify promising future avenues but also highlight the need for further studies in animal models. Our preliminary assessment is that host immune seasonality warrants evaluation alongside weather and human behavior as factors that may contribute to COVID-19 seasonality, and that the relative importance of these drivers requires further investigation. A major challenge to predicting seasonality of infectious diseases are rapid, human-induced changes in the hitherto predictable seasonality of our planet, whose influence we review in a final outlook section. We conclude that a proactive multidisciplinary approach is warranted to predict, mitigate, and prevent seasonal infectious diseases in our complex, changing human-earth system.
Keywords: seasonality, infectious diseases, global change, circannual, photoperiod, Anthropocene
COVID-19
This review is written during the coronavirus disease 2019 (COVID-19) pandemic. Not a year (1 seasonal cycle) has passed since its emergence in Asia in late 2019, and great uncertainty still surrounds the potential for COVID-19 to establish seasonality. At the time of writing, the steep autumnal increase in the number of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in Europe and North America may indeed point to seasonality.
Seasonality, characterized by systematic within-year changes that repeat across years, is a feature of many infectious diseases, including respiratory infections such as endemic human coronaviruses (CoV) (Nickbakhsh et al., 2020). The particular shape, timing (phase), and degree of seasonality differ between diseases, climatic regions, and geographic locations (Altizer et al., 2006; Fisman, 2007; Lal et al., 2012; Martinez, 2018) for reasons that are not fully understood. If SARS-CoV-2 (the virus that causes COVID-19) establishes seasonal outbreak patterns in the long term (Cohen, 2020), it will have important implications for public health planning and forecasting. At present, the potential impact of COVID-19 seasonality is being discussed widely within academia, medical organizations, and in politics. These discussions highlight the importance of predicting the cyclical pattern of emerging infectious diseases that are of major societal, public health, and economic concern.
Human civilizations have paid close attention to seasons regarding health, not least in terms of infectious disease. For example, the four humors (i.e., fractions of clotted blood) well characterized by ancient Greek medicine (Hart, 2001), and thought to be responsible for health, growth, and metabolism, were described as seasonal (Jouanna, 2012). Until recently, most humans lived in a highly seasonal environment. Modern westernized lifestyles, including use of artificial light to extend day length and climate control, resulted in eternal summer conditions (Wehr, 2001). For these and further changes in seasonality of humans, such as antibiosis, pathogen control, and a nonseasonal diet (Anderson and Nieman, 2016), the direct and indirect health consequences remain to be fully determined (Stevenson et al., 2015).
Disease-specific seasonal patterns can arise from both ultimate (evolutionary adaptations to the seasonal environment of host and pathogen, for example, internal clocks, photoperiodism, and climate tolerance) and proximate causes (e.g., direct influence of environmental conditions or human behavior). Understanding the drivers of disease seasonality is crucial for the fundamental understanding and control of diseases (Grassly and Fraser, 2006; Fisman, 2007, 2012). If drivers influencing the timing and magnitude of outbreaks are identified, existing disease surveillance methods can be tailored to these seasonal processes to generate appropriate prevention and treatment strategies, develop validated prediction models, and enhance cross-border cooperation. Furthermore, mechanistic understanding of seasonal drivers for specific diseases can help identify medical targets, such as putative molecular pathways that underlie infection, to enable potential future modulation of host susceptibility and resistance.
In this article, we first review the putative environmental drivers of infectious disease seasonality. We then discuss seasonal within-host changes that may affect infectious disease susceptibility, morbidity, and mortality. Such underlying mechanisms may drive the timing of rises in infections at the population level. Based on this, we discuss the potential of COVID-19 to develop seasonality and highlight 2 priority areas to understand and predict such patterns. One area is the emergence of a promising model for drug development in seasonal hamsters. The second is the rapid, human-induced changes in the hitherto predictable seasonality of our planet, which is expected to affect infectious diseases in the future.
Seasonality
The Earth rotates around its axis with a 23.5° tilt relative to the plane of its annual orbit around the sun. This tilt generates predictable seasonal changes in environmental conditions, including day length, UV radiation, and ambient temperatures (Foster and Kreitzman, 2009). These changes are more pronounced at higher latitudes, whereas seasonality in tropical regions is usually more nuanced (e.g., local patterns of dry and rainy seasons). Most living organisms (including humans) have developed adaptations to cope with seasonal changes (Foster and Kreitzman, 2009). Such adaptations enable hosts and pathogens (and vectors and reservoirs) to function, survive, reproduce, or transmit within the seasonal environment (including the seasonal internal environment that occurs within hosts due to seasonal physiology). The seasonal environment may thus directly affect organisms, for example, through annual changes in UV radiation, humidity, and ambient temperature, or do so indirectly through interactions with corresponding internal physiological regulatory mechanisms. The fact that the amplitude and phase of disease seasonality often change with latitude suggests an important role of clines in the environment, particularly changes in day length (photoperiodism). Over evolutionary time, photoperiod has been a noise-free environmental condition with high predictive value to signal future environmental conditions and associated challenges and opportunities. For example, in temperate regions, autumn photoperiods signaled the approach of winter and challenging energetic conditions; similarly, the increasing photoperiod after winter solstice signaled the approach of spring and potentially favorable environmental conditions. Due to this predictive value of photoperiod, organisms have evolved mechanisms to time biological processes either by directly responding to photoperiod or by synchronizing a circannual internal biological clock using photoperiod (Gwinner, 1986; Bradshaw and Holzapfel, 2007; Lincoln, 2019).
Possible Drivers of Infectious Disease Seasonality
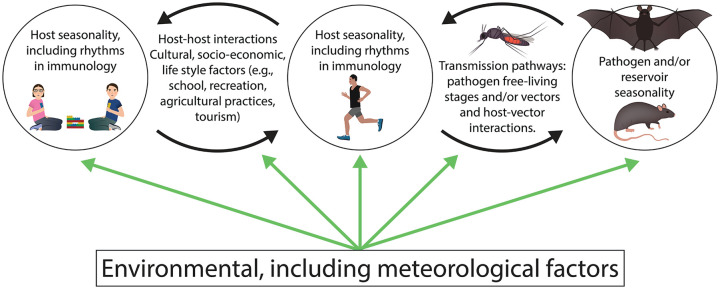
Many factors likely contribute to seasonal patterns of infection, including pathogen survival in the environment and transmissibility, changes over time in pathogen reservoirs (human and non-human) and vectors, frequency of pathogen-host interactions (cultural, socioeconomic, linked to life style), and a relatively understudied factor: host susceptibility to infection (rhythms in immunology) (Lal et al., 2012) (Figure 1).
Figure 1.
Candidate drivers of disease seasonality. Circles show organisms implicated in disease transmission, black arrows indicate their interactions, and green arrows indicate the influence of environmental factors. Color version is available online.
In the context of human respiratory infections, and particularly COVID-19, it is noteworthy that endemic human CoV typically exert the greatest health care burden during winter months in temperate regions (Figure 2), reflecting high community incidence in these periods (Li et al., 2020b; Monto et al., 2020; Nickbakhsh et al., 2020). Meteorological factors are the most commonly studied drivers of such seasonality in host-host transmission of respiratory viruses (Price et al., 2019; Moriyama et al., 2020). Animal experiments have demonstrated, for example, that influenza transmits more readily in conditions of cold temperatures and low relative humidity, possibly as a result of reduced mucociliary clearance or viral stability in the upper respiratory tract (Lowen et al., 2007; Lowen and Steel, 2014). These experimental findings corroborate epidemiological modeling studies which suggest the effect of weather on influenza transmission is sufficient to explain the typical winter timing of outbreaks in temperate regions (Shaman and Kohn, 2009). A common hypothesis is that cold weather leads to crowding indoors and therefore enhanced person-to-person contact, although the role of indoor heating systems that generate conditions of low relative humidity also merits consideration as a mechanism of enhancing transmission (Moriyama et al., 2020).
Figure 2.
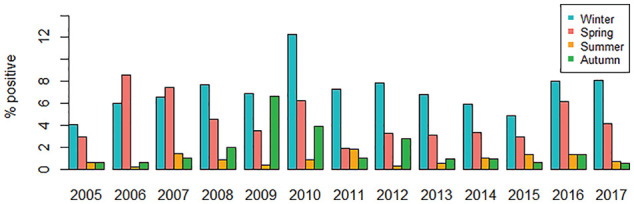
Seasonality of endemic human coronaviruses (CoV-OC43, CoV-NL63, and CoV-229E species) detected in a large urban patient population. The percentage (%) of human coronaviruses detected during routine real-time multiplex RT-PCR diagnostic testing of 84,957 episodes of respiratory illness in NHSGGC (primary and secondary care services), Scotland, UK, by calendar month categories from 2005 to 2017. Winter = December-February; Spring = March-May; Summer = June-August; Autumn = September-November. Note: Years 2009 and 2010 must be viewed with caution as only influenza-negative patients at risk of severe illness were tested for human coronaviruses in NHSGGC during the three waves of the influenza A/H1N1 pandemic in the United Kingdom; see also Nickbakhsh et al. (2020). Abbreviations: CoV = coronaviruses; NHSGGC = NHS Greater Glasgow and Clyde; RT-PCR = reverse transcription polymerase chain reaction.
Additional hypotheses regarding the autumn/winter occurrence of respiratory infections include the magnitude of temperature fluctuations impacting transmission. In particular, the correlation between indoor and outdoor temperatures is strong during warmer months, yet substantially weakens when the weather gets cooler (Nguyen et al., 2014). This suggests that people may experience greater temperature swings during a given day in the winter, compared to in the summer, especially in cold/temperate areas. Temperature impinges upon metabolism across taxa, and while temperature swings in theory could weaken or strengthen the immune system (Shampo and Kyle, 1987), several negative consequences are apparent. Temperature drops of 5 °C in the nose can weaken antiviral defenses and have been linked to a higher likelihood of catching a cold virus (Foxman et al., 2015). Temperature swings also tend to be associated with spending more time indoors, which can facilitate viral transmission at a higher rate than during time spent outdoors (Jayaweera et al., 2020), especially if using public transport or during social events.
Other aspects of weather and the environment may also affect behavior and immune responses. For instance, vitamin D levels are influenced by exposure to sunlight. They tend to be higher during the summer (Klingberg et al., 2015) and could bolster the immune system when fighting viral infections. The impact of weather on infection is understudied in tropical and subtropical regions compared to temperate regions. Some locations experience regular outbreaks coinciding with rainy seasons, often with multiple peaks (Bloom-Feshbach et al., 2013), while other locations under similar climatic conditions experience low year-round variability in disease incidence (Nguyen et al., 2009).
In addition to environmental conditions, host behavioral changes can create seasonality in the frequency of host-pathogen interactions (Martinez-Bakker and Helm, 2015). For example, the mixing patterns of school-aged children are recognized as important for the transmission dynamics of several infectious diseases, including measles, varicella, influenza, and other common respiratory infections. However, the role of this school-term forcing of disease transmission in explaining the timing of outbreaks of seasonal infectious disease is not fully established (Cauchemez et al., 2008; Eames et al., 2011; Luca et al., 2018). Similarly, seasonal changes in outdoor activities including tourism, recreation, and management of crops and livestock may drive seasonal exposure to vectors and/or environmental reservoirs of pathogens, resulting in disease seasonality (Altizer et al., 2006).
Another, albeit largely understudied, factor likely contributing to infection seasonality is host seasonality, including seasonality of the immune system (discussed at length below), and other biological functions, such as birth seasonality. Birth seasonality affects disease incidence and timing by replenishing the pool of susceptible individuals. For wildlife hosts, birth seasonality also affects population density and hence disease transmission (He and Earn, 2007; Begon et al., 2009; Duke-Sylvester et al., 2011; Dorélien et al., 2013). Large amplitudes of seasonal birth pulses can induce corresponding increases in childhood disease, while changes in seasonal phase can influence the timing of the outbreaks. In both cases, the effect is larger at higher birth rates (He and Earn, 2007; Dorélien et al., 2013).
A well-described driver of infectious disease seasonality is vector biology. For example, dengue incidence in Bali is highly seasonal, with most cases reported during the rainy season between December and April. Evidently, the many water bodies and occurrence of lowland flooding provide excellent conditions for the vector, Aedes mosquitoes, to reproduce and undergo their larval aquatic life stage (Dhewantara et al., 2019). Similarly, Anopheles mosquitos are required for the malaria parasite to conclude its life cycle prior to human transmission, so seasonality of the disease in humans is primarily caused by seasonality in climatic conditions required for the vector to breed (Herekar et al., 2020). Malaria has been shown to exert significant selective pressure on the human genome, thereby potentially implicating seasonal mechanisms (Kwiatkowski, 2005).
It is important to note that the drivers of disease seasonality are not mutually exclusive, and that the proximate mechanisms driving seasonality for a specific pathogen may involve several organisms and be affected by the ecological context. For example, disease seasonality could result from epidemiological interactions with other pathogens sensitive to environmental conditions, as studied for influenza and non-influenza respiratory viruses (Nickbakhsh et al., 2019). Moreover, broad-acting host immune responses (e.g., shifting between Th1 and Th2 immune response) caused by one seasonal pathogen may change the host susceptibility to other pathogens and could lead to seasonality in risk, duration, or severity of infections and co-infections (Cizauskas et al., 2014). Thus, diseases resulting from non-seasonal pathogens may exhibit seasonality as a result of immunomodulation by co-circulating seasonal pathogens. Seasonal immunomodulation and other within-host factors, such as circannual rhythms, are not well-integrated topics within epidemiological research but may hold important clues as to disease seasonality, as discussed below.
Seasonal Within-Host Changes: Photoperiodism And Circannual Rhythms
Seasonal changes in the vertebrate immune system have long been widely documented (Nelson et al., 2002). Recently, such data are also becoming available for humans with unprecedented data depth and sample sizes (Sailani et al., 2020; Wyse et al., 2020). Whereas we will review some emerging patterns in this rapidly growing research field in a subsequent section, here we focus on underlying mechanisms that generate seasonal within-host changes. Principally, changes in the immune system can arise as direct responses to seasonal immune challenges and from seasonally changing modulating factors, for example, nutrition. Alternatively, such changes can arise from biological time-keeping programs that are triggered by changes in photoperiod or oscillate endogenously as circannual rhythms (Gwinner, 1986; Nelson et al., 2002). Distinguishing direct responses to the seasonal environment from photoperiodism and circannual cycles requires experimental approaches whereby animals are tested under controlled laboratory conditions. Under these conditions, pervasive changes in the immune system persist in many species. Strikingly, different immune parameters vary independently across the seasons (Nelson et al., 2002) so that, in effect, the immune system is reconfigured, rather than simply upregulated or downregulated. The discovery of programmed changes in the immune system, on annual as well as on diel time-scales (Borrmann et al., 2021), is of fundamental importance for understanding the etiologies of diseases. Once rhythmicity is established, its features can be adjusted through various modifications, for example, population-level changes in photo-responsiveness within a murine species (Heideman and Pittman, 2009) or switches between short-day (SD) and long-day (LD) breeding within closely related mammalian or avian taxa (e.g., Helm, 2009). Such modifications can adjust the timing (phase), waveform, amplitude, and robustness of particular aspects or of the entire annual cycle, as well as the degree of photoperiodism.
Direct effects of photoperiod on the immune system were demonstrated by evidence that acclimation to SD or LD induces enhancement and suppression of several components within the immune system in vertebrates (Nelson et al., 1995; Baillie and Prendergast, 2008; Stevenson and Prendergast, 2015; Weil et al., 2015; Onishi et al., 2020). Circannual rhythms, on the contrary, oscillate endogenously under constant photoperiodic, thermal, and dietary conditions, with period lengths that may differ slightly from 1 year (from Latin: circa—about, annus—year). Strong evidence for circannual rhythms requires observing animals for two or more annual cycles, but even cyclic changes over 1 year indicate a high level of endogenous control (Gwinner, 1986). Circannual rhythms are evident for many processes, including hibernation, reproduction, metabolism, molt, and migration (Gwinner, 1986; Visser et al., 2010; Stevenson et al., 2015). In most species, these rhythms entrain readily to photoperiod, but the extent of their persistence in the absence of photoperiodic change differs between species and even within taxonomic groups such as ungulates and rodents (Lincoln, 2019).
Circannual studies of the immune system have been scarce but have confirmed that changes in the host can be hard-wired, independent of photoperiodic change. Major circannual changes in the immune system are reported for hibernators, where lymphoid tissue can entirely regress during hibernation, but recrudesce spontaneously in anticipation of arousal (Shivatcheva and Hadjioloff, 1987). Similar, but weaker cycles have been reported for nonhibernating rodents. Even in laboratory mice, which in some strains have retained some seasonality despite rigid breeding against it, immune cycles have been repeatedly reported. For example, cultured spleen lymphocytes harvested at different times of year from BALB/c mice showed marked annual cycles in proliferation response to several mitogens, across sex and age groups (Planelles et al., 1994). Similarly, the blastogenic response in C57BL/6 mice, in different age groups, also showed circannual cycles under strictly controlled conditions, which the authors found relevant for mortality patterns and seasonal virus infections (Brock, 1983, 1987). An alternative interpretation of such immune cycles, as arising from cyclic pathogen exposure, is unlikely because circannual period lengths deviate from 1 year (Brock, 1983, 1987) and because animals with different genetic backgrounds may show different immune cycles under identical conditions (Versteegh et al., 2014).
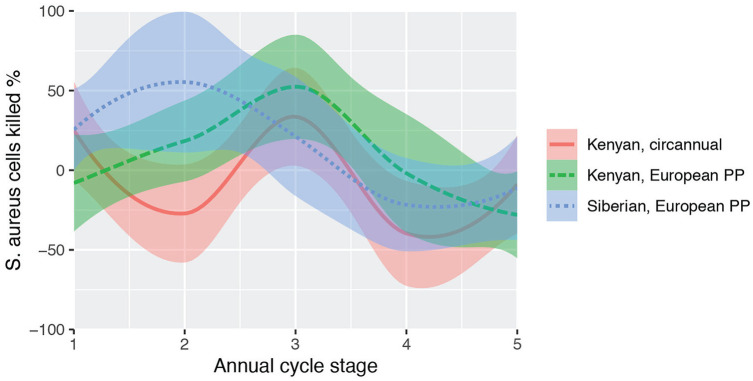
An example of population-specific immune cycles is shown in Figure 3. Several populations of a songbird taxon, stonechats (genus Saxicola), were kept under identical conditions in mixed groups. At defined phases of the annual cycle, such as migration, molt, or reproductive activation, several measures were taken to assess immunity (Versteegh et al., 2014). The study focused on constitutive immunity as a general, first line of defense, including against viral and bacterial pathogens (Paludan et al., 2020). Most measures differed across the birds’ annual cycle, as well as between populations. Figure 3 shows annual patterns of bacteria-killing ability of whole blood, an activity that combines various mechanisms of the innate immune system, for example, phagocytic activities of leukocytes and microbicidal activities of humoral proteins (Millet et al., 2007; Versteegh et al., 2014). In addition to published data from birds kept under simulated European photoperiod, we include unpublished data of a circannual control group that was kept under constant photoperiod (LD 12.25:11.75 h). These birds showed highly similar cycles in bacterial killing, although individual differences between free-running individuals slightly damped the amplitude.
Figure 3.
Annual and circannual cycle in an immune parameter. The capacity of whole blood to kill cultures of Staphylococcus aureus is shown for 3 experimental groups of songbirds, stonechats (genus Saxicola). Siberian (blue) and Kenyan (green) stonechats were kept in a common garden setup of annually changing European photoperiod (PP) over 1 year, where they showed distinct, population-specific annual cycles (Versteegh et al., 2014). Groups were measured per life cycle stage because the populations differed in duration of phases such as migration or molt (annual cycle stages 1-5: spring migration; breeding season; molt; autumn migration; winter). An additional Kenyan (red) group (Versteegh, Tieleman & Helm, unpubl.) that was kept under constant photoperiod showed similar, circannual cycles; curves indicate loess smoothing. Color version is available online.
Pervasive evidence of host immune cycles brings up the question why immune defense is not simply consistently upregulated. A possible answer may lie in trade-offs between different seasonal functions, including reproduction, migration, hibernation and molt, with different immune parameters (Nelson et al., 2002). Changes in vertebrate immunity often, but not necessarily, associate with such major physiological changes. For example, some arms of the immune system are depressed specifically during times of reproduction (Nelson et al., 1995; Weil et al., 2015). Lymphoid tissues in mammals express androgen and estrogen receptors, and may regress during photoperiodic reproductive activation, resulting in T-lymphocyte reduction (Nelson et al., 2002). However, castrated individuals also show some photoperiodic change, indicating steroid-independent components of immune cycles (Prendergast et al., 2008).
Within-Host Changes That May Affect Susceptibility, Morbidity, and Mortality
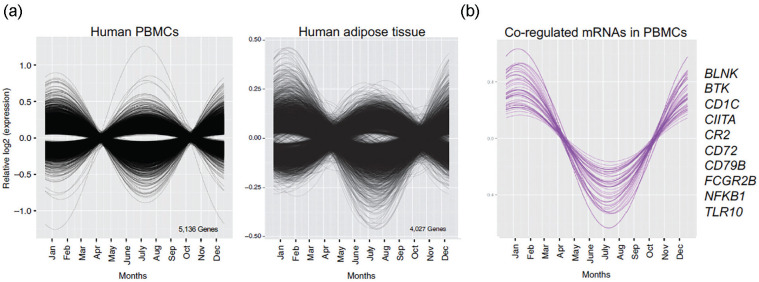
In the human immune system, seasonal molecular immunological phenotypes have been widely described (Dopico et al., 2015; Aguirre-Gamboa et al., 2016; Lockett et al., 2016; Ter Horst et al., 2016; Thysen et al., 2016; Ucar et al., 2017; Calov et al., 2020; Sailani et al., 2020; Wyse et al., 2020). Several of these studies highlight seasonal effects on the transcriptome, cytokine signaling, and cell numbers and ratios (Figure 4; Dopico et al., 2015). The results show that despite the widespread modification of putative seasonal cues in modern environments, seasonal genetic networks continue to impinge upon immune function. Different vaccine responses, for example, have been found to show seasonal variation in humans (World Health Organization, 1995; Deming et al., 1997; Moore et al., 2006; Lalor et al., 2009).
Figure 4.
Seasonal gene expression changes in humans. (a) Peripheral white blood cells (PBMCs, from children) and subcutaneous adipose tissue (adults). (b) A group of 68 coregulated messenger RNAs (mRNAs) with winter tropism in the human immune system, enriched for genes associated with B-lymphocyte activation (KEGG). A cosinor model was used to analyze seasonality in mRNA expression (Dopico et al., 2015). Abbreviation: PBMC = peripheral blood mononuclear cell.
An understanding of seasonally responsive genetic systems could broadly inform clinical medicine, as many common diseases of modernity are typified by both immunological and metabolic dysregulation (Herder et al., 2007; Pedersen, 2007; Insull, 2009; Mathis, 2013; Bauer and Teixeira, 2019). This is especially important as an increasing number of health conditions are known to have a seasonal component to diagnosis and disease activity, including different cancers (Moan et al., 2010), cardiovascular diseases (Nguyen et al., 2016), multiple sclerosis (Harding et al., 2017), type 1 diabetes (Moltchanova et al., 2009), and psychological disorders (Quera Salva et al., 2011).
Applied to an emerging disease pandemic, seasonal changes in immune function can be important, for example, by altering the permeability of within-host barriers, contributing to zoonotic spillover (Plowright et al., 2017). Both SARS-CoV and SARS-CoV-2 recently emerged during winter (February 2003 and December 2019), when human CoV exhibit increased incidence and health care burden in temperate regions (Plowright et al., 2017; Nickbakhsh et al., 2020). Notably, interleukin 6 (IL-6) signaling is increased in humans during winter and is associated with mortality in COVID-19 (Grifoni et al., 2020). Coronaviruses are not alone in their predilection for winter, whereas some other pathogens display preferences for other seasons and environmental conditions, putatively exploiting differences in host biology.
Due to ethical, logistical, and technical difficulties of performing appropriately powered studies in humans, quantification of seasonal phenotypes in controlled experiments is lacking. Systems analyses of the interactions between diet (including timing of meal intake), photoperiod, ambient temperature, and immune challenge in animal models will provide greater understanding of the evolutionary mechanisms at work. In mice, for example, herpes and influenza viruses replicate more efficiently in the absence of the key circadian protein, ARNTL (BMAL1) (Bunger et al., 2000; Nguyen et al., 2013; Edgar et al., 2016), whose expression is reduced in the human immune system during winter (Dopico et al., 2015), perhaps contributing to increased virus dissemination at this time.
Furthermore, recent developments highlight a central role for molecular metabolism in immune function. Elegant studies have demonstrated how various small molecules (such as itaconate) and different metabolic pathways are critical to separate anti-pathogen responses (Buck et al., 2015; West et al., 2015; Mills et al., 2018; Peruzzotti-Jametti et al., 2018; Weisel et al., 2020). In humans, seasonal metabolic phenotypes that could impinge upon the immune system, or vice versa, include adiposity (Bartness et al., 2002), osmoregulation (Yoshimura, 1958; Dopico et al., 2015), body mass index (Visscher and Seidell, 2004), cognition (Meyer et al., 2016; Lim et al., 2017), hair growth rate (Randall and Ebling, 1991), and vitamin D metabolism (Norman, 1998). Amazingly, UV skin exposure cues skin nitric oxide synthase (NOS)–independent nitrate metabolism, reducing blood pressure (Liu et al., 2014), which is itself associated with inflammation (Chae et al., 2001). How the mammalian immune system aligns itself with whole-organism metabolic needs and processes, and at different times, is largely unknown at the molecular level.
As demonstrated by investigations of humans and seasonal animal species, dietary metabolism is critical for immune competence (Luca et al., 2010; Singh et al., 2013; Carrillo et al., 2016; Singh et al., 2017; Zandkarimi et al., 2018). Worryingly, humans globally suffer from an increasing and considerable metabolic disease burden, where chronic inflammation is a major morbidity factor and immune competence is impaired (Brouwer et al., 2015). This includes various cancers, cardiovascular diseases, and diabetes and is largely attributed to obesity, diet-associated metabolic impairment, circadian disruption (Zimmet et al., 2019), and a lack of physical exercise. Preliminary findings suggest that comorbidities such as obesity are associated with an 86%-142% higher risk of developing severe pneumonia, another major risk factor for COVID-19-associated mortality (Stefan et al., 2020). Therefore, further research into the interactions between human immune and metabolic networks, biological rhythms, and numerous environmental factors that impinge upon them will contribute to a more complete understanding of human molecular physiology (Renz et al., 2017; West and Wood, 2018), with implications for improved societal health and well-being (Naumova, 2006).
Potential Effects of Host Immune Seasonality on Sars-Cov-2 Infection
It is possible that changes in the immune system are directly relevant for susceptibility, morbidity, replication, and transmission dynamics of SARS-CoV-2. For example, early infection steps involving the spike glycoprotein (S) may be affected by host seasonality. In vitro analyses have identified that SARS-CoV-2 infects cells by the coordinated action of 2 domains of its surface spike glycoprotein: S1 and S2. Enzymes such as transmembrane protease serine 2 (TMPRSS2) and Furin cause conformational changes separating the S1 and S2 domains to allow cell entry. The catalyzed separation facilitates S1 binding to angiotensin-converting enzyme 2 (ACE2) and S2 to the cell membrane, leading to endocytosis into the cytoplasm (Tay et al., 2020).
Recent genome-wide transcriptomics of human leukocytes identified that Furin transcripts exhibit seasonal rhythmicity in children, with high expression in summer and low levels in winter (Dopico et al., 2015). These findings raise an exciting hypothesis that there may be endogenous seasonal variation in immune defense against SARS-CoV-2. Here, the availability of leukocyte Furin expression may underlie increased endocytosis of viral mRNA in a seasonal-dependent manner. As SARS-CoV-2 RNA is detectable in the blood of patients (Young et al., 2020), longitudinal analyses of samples could be a significant avenue to understand seasonal disease dynamics. A second lead to possible effects of seasonal within-host changes comes from genome-wide analyses of UK Biobank patients who developed severe COVID-19. Through analyses of multi-SNP (single nucleotide polymorphism) genotype signatures compared to controls, these patients were found to possess 68 risk-associated protein-coding genes (Taylor et al., 2020). Of these, 9 were linked to host responses to viral infections including SARS-CoV-2. One potentially exciting gene of these 9 robust markers was Anthrax toxin receptor 1 (ANTXR1). In hamsters, Antxr1 shows robust photoperiodic regulation with high leukocyte expression in long summer-like days (Figure 4). Other transcriptome analyses of leukocytes in humans have also identified ANTXR2 expression as seasonally dependent (Dopico et al., 2015).
Anthrax receptors are potentially important for seasonal disease dynamics as Furin has the capacity to regulate viral propagation (e.g., SARS-CoV-2) and bacterial toxin (e.g., Anthrax) activation. The site of action for TMPRSS2- and Furin-mediated SARS-CoV-2 endocytosis is homologous to the processing site of anthrax toxin PA protein (Barile et al., 2020). One conjecture is that seasonal variation in immune responses to SARS-CoV-2 may entail the inadvertent activation of other pathogenic pathways (e.g., anthrax receptor) and increase the incidence of severe cases. If confirmed, these findings would indicate that at least one component of the molecular driver of seasonal cycles in disease includes the co-activation of multiple antigen pathways and not necessarily a “singular” immune response pathway.
Is covid-19 expected to be seasonal and why?
The knowledge on drivers of seasonal respiratory viral infections, summarized above, can inform cautious considerations of the potential future seasonality of COVID-19. For SARS-CoV-2, studies have highlighted moderate temperature and dry environmental conditions under which the virus appears to thrive most optimally (Brassey et al., 2020), with studies describing the influence of every 1 °C increase in temperature and 1% increase in relative humidity as lowering the effective R0 by 0.0383 and 0.0224, respectively (where the base reproduction number R0 of SARS-CoV-2 is estimated to fall between 1.5 and 3.5 (Brassey et al., 2020; Li et al., 2020a)). A recent cohort study of 50 cities identified a corridor roughly between 30 °N to 50 °N latitude and with consistent mean temperatures of 5-11 °C, combined with low humidity, as the most conducive to large COVID-19 community outbreaks, further implicating seasonality of the virus (Sajadi et al., 2020). A systematic review of global surveillance data found seasonal patterns of endemic human CoV in many temperate regions, as anticipated (Kissler et al., 2020; Li et al., 2020b). Indeed, the incidence of SARS-CoV-2 infections has risen in autumn 2020 (European Centre for Disease Prevention and Control [ECDC], European Union [EU]) as predicted for temperate regions (Li et al., 2020b; Scafetta, 2020).
The preference for cool and dry conditions (e.g., typical air-conditioned environments) of SARS viruses has previously been described during the 2002-2003 SARS-CoV outbreak (Chan et al., 2011). However, other factors that correlate with such environmental conditions have been largely neglected thus far, including behavioral changes described above. Moderately cool temperatures below 10 °C or 11 °C are conducive to persons spending time indoors, and differences between indoor and outdoor temperatures can impact transmission through physiological and behavioral factors. Thus, for COVID-19, which is transmitted via droplets with a lingering debate regarding its potential for aerosol-based transmission (Klompas et al., 2020), spending time indoors and closer to each other has likely contributed to a second wave of the COVID-19 pandemic in the fall in the absence of stringent social distancing and lockdown interventions. Current evidence for SARS-CoV-2 does not provide support for low vitamin D—beyond its known impact on human immune response (Bordon, 2017)—as a causal factor for heightened receptivity of the infection (Lanham-New et al., 2020), but rather as a potential surrogate marker surrounding the optimal conditions to leverage spread and impact of the virus (i.e., cooler temperatures, more time spent indoors, comorbidities, older age).
Overall, there is good reason to believe SARS-CoV-2 may display seasonality in the long run, although the relative contribution of the weather, behavior, and seasonal immunity to SARS-CoV-2 replication and transmission warrants further investigation.
Outlook: Priority Areas for Understanding the Seasonality of Infectious Diseases
Our assessment of the current knowledge base for predicting seasonality of COVID-19 indicates promising avenues but also major deficiencies. Below, we highlight 2 areas that we consider particularly important for understanding and mitigating seasonal infectious diseases more broadly. First, from a physiological perspective, we endorse the need for animal models that can inform human disease seasonality. Second, from an environmental perspective, we emphasize the importance of understanding the causes of disease spillovers and outbreaks to be able to predict, prepare for, and even prevent new emerging pandemics. Importantly, we note that predicting the net impact of climate change on global infectious disease burden, whether in the form of increased or decreased infection risks, is particularly challenging when considering likely interactions with other geographically varying anthropogenic factors.
Animal Models and the Potential Mechanisms of Seasonal COVID-19
A major challenge limiting our knowledge of SARS-CoV-2 infection and pathology is developing a broad range of suitable animal models. Small animal models are vital to identify potential mechanisms of transmission and immune responses. Due to the ability of respiratory transmission and similar SARS-CoV-2 infection, animals such as hamsters, ferrets, and cats have emerged as valuable investigative models (Imai et al., 2020; Richard et al., 2020; Shi et al., 2020; Sia et al., 2020). Other domesticated animals such as dogs, pigs, chickens, and ducks show low or absent susceptibility to infection (Shi et al., 2020). Common biomedical models (e.g., rats and mice), while being well positioned to address some aspects of nonrespiratory-based SARS-CoV-2, typically exhibit low seasonal immune dynamics and critically lack the ACE2 homology required for SARS-CoV-2 infection (Lutz et al., 2020). One advantage of mouse models is the ability to create transgenic animals to explore SARS-CoV-2 viral replication and pathology. Transgenic mice that produce human ACE2 are susceptible to SARS-CoV-2 infection and show some viral-induced pathologies observed in humans (Lutz et al., 2020). Unfortunately, the low homology in human-mouse respiratory inflammatory responses (Seok et al., 2013) limits our ability to develop from these models solid interpretations for seasonal immune dynamics associated with COVID-19.
The rodent subfamily of hamsters (Cricetinae) contains 19 species, of which the Syrian hamster (Mesocricetus auratus) and Siberian hamster (Phodopus sungorus) are 2 models that exhibit seasonality, respond to changes in photoperiod, and express circannual rhythms, including robust seasonal variation in immunity. These animal models provide a rewarding approach to examine the potential seasonal basis of SARS-CoV-2 due to reliable immune responses to changes in day lengths (Stevenson and Prendergast, 2015). Recently, Syrian hamsters were identified as an excellent model due to the capability of SARS-CoV-2 to infect respiratory epithelium and macrophages in a manner similar to human pathologies (Imai et al., 2020; Sia et al., 2020). Moreover, there is a high level of transmission between hamsters providing a valuable opportunity to examine between-subject transmission (Imai et al., 2020; Sia et al., 2020). A recent study reported high vaccination success even against severe clinical forms of the disease in this species (Tostanoski et al., 2020). These findings indicate that hamster models provide a powerful approach to examine seasonal variation in SARS-CoV-2 transmission, mechanisms of disease progression, and potentially vaccination success (Tostanoski et al., 2020).
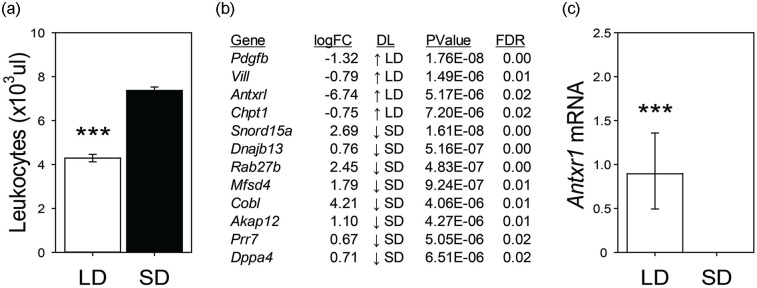
For both Syrian and Siberian hamsters, a simple switch in laboratory day length (e.g., 1600:0800 h light:dark schedule to 0800:1600 h light:dark schedule) induces a wide range of changes in innate and adaptive immunity. Exposure to winter-like short days (SD) increases spleen mass and numbers of macrophages and lymphocytes in Syrian (Brainard et al., 1987) and Siberian hamsters (Bilbo et al., 2002) (Figure 5a). These physiological changes in immune markers are thought to enhance fitness, as SD hamsters show enhanced innate (Stevenson et al., 2014) and adaptive (Bilbo et al., 2002) immune responses when challenged. The Siberian hamster is a key model species due to the broad range of documented photoperiodic changes in immune function (reviewed in Stevenson and Prendergast, 2015). In contrast, Syrian hamsters do not exhibit photoperiodic differences in some adaptive immune responses including delayed-type hypersensitivity reactions or antibody production, which limits the viability of this species for some immune measures (Zhou et al., 2002). To date, there is no evidence that either TMPRSS2 or ACE2, key molecules involved in SARS-CoV-2-induced immune reactions, changes across seasonal phenotypes in any mammal studied. However, in hamsters housed in SD conditions, the leukocyte expression of anthrax receptor 1 is significantly downregulated (unpublished data, Figure 5b and 5c), in a manner potentially consistent with findings of human leukocyte ANTXR2 (Dopico et al., 2015). These data indicate that seasonal rhythms in immune responses might be associated with lower anthrax receptor signaling and subsequent reduction in infection incidence.
Figure 5.
Photoperiodic regulation of Siberian hamster blood leukocytes. Siberian hamsters housed in summer-like long-day (LD) photoperiods have lower levels of circulating leukocytes compared to winter-like short-day (SD) housed animals. (a) Unpublished RNA sequencing of blood leukocytes revealed several transcripts that are differentially expressed between LD and SD conditions. (b) Adult male hamsters were kept in LD (15L:9D) or SD (9D:15L) for 12 weeks. At the termination of the study, leukocytes were obtained from a retroorbital sample and cells were separated as described previously (Stevenson et al., 2014). Illumina sequencing and statistical analyses were conducted using the same procedures described in Bao et al. (2019). In LD, hamster leukocytes express the anthrax receptor 1 transcript, whereas there is a complete absence of its expression in SD. (c) Asterisks denote p < 0.001. Abbreviations: Pdgfb = Platelet-Derived Growth Factor Subunit B; Vill = Villin-like; Antxr1 = Anthrax receptor 1; Chpt1 = Choline Phosphotransferase 1; Snord15a = Small Nucleolar RNA, C/D Box 15A; Hsp40 = DnaJ Heat Shock Protein Family; Dnajb13 = Member B13; Rab27b = RAB27B Member RAS Oncogene Family; Mfsd4 = Major facilitator superfamily domain–containing protein 4; Cobl = Cordon-Bleu WH2 Repeat Protein; Akap12 = A-Kinase Anchoring Protein 12; Prr7 = Proline Rich 7 Synaptic; Dppa4 = Developmental Pluripotency Associated 4; DL = daylength; FDR = false discovery rate. ***p < 0.005.
Changing Seasonality in the Anthropocene
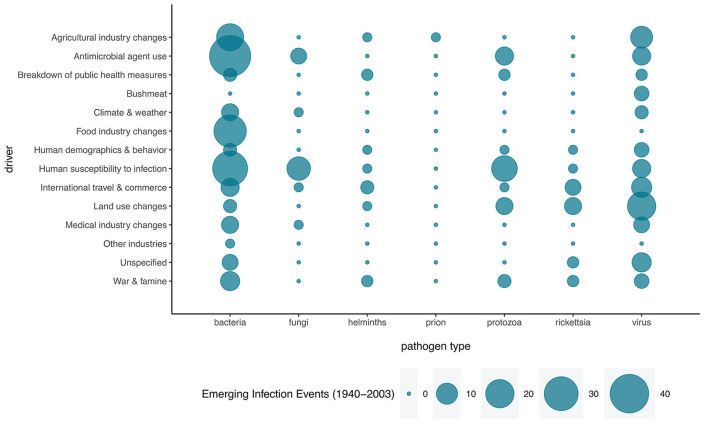
Infectious diseases have plagued humanity since the earliest days, and human activities are accelerating their emergence. Hence, in this section we will broadly explore potential drivers of such changes, widening our perspective to include a wide range of pathogens. The majority of recently emerging human infectious diseases are zoonotic, and most of these originate in wildlife (Johnson et al., 2015), indicating that as wildlife populations are increasingly affected by anthropogenic impacts, the incidence of emerging diseases is expected to rise (Figure 6; for example, Jones et al., 2008; Guo et al., 2019; Rizzoli et al., 2019). These rapidly increasing threats develop against the backdrop of equally rapid change in the formerly highly predictable seasonality of our planet, under human-induced drivers of global change. Changes in patterns of Earth’s seasonality may thereby play a significant role in changing emerging disease burden, through increases or decreases in risk of emergence and spread, depending on geographical location.
Figure 6.
Drivers of emerging infectious disease events during 1940-2003. Data from Jones et al. (2008).
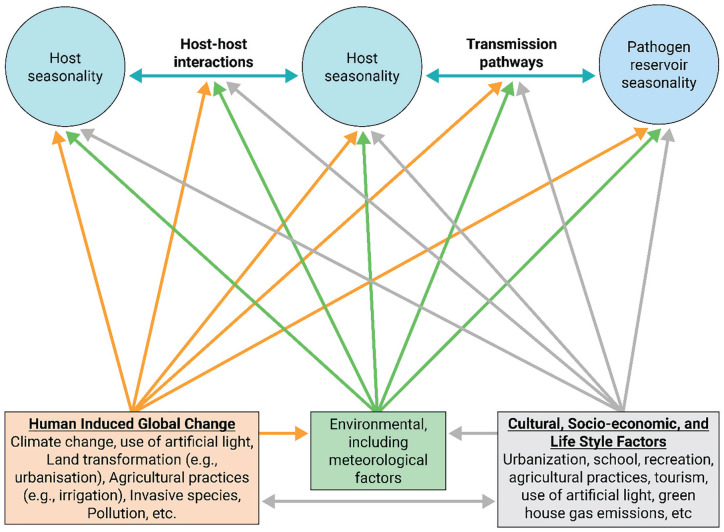
Anthropogenic impacts on seasonality are particularly evident for climate change, land-use change, and exposure of wild organisms to artificial light at night (ALAN). These factors may render the cues for diel and seasonal rhythms unintelligible or misleading (e.g., ALAN interfering with photoperiodism). Through their impact, the biotic and abiotic environment may differ substantially from that expected at a given time under the prevalent cues. Climate change may thus impact the seasonality of certain infectious diseases, making it more difficult to predict when surges of cases may appear (Figure 7; for example, Smith, 2019). The above factors may act in concert with changes in species distributions, including the global increase in invasive species, which are often disease vectors (Figure 7; for example, Stuart et al., 2020). The results of anthropogenic changes are therefore expected to be complex, interdependent, and differ between species. Thereby, they may, for example, result in mismatches between an individual’s physiology and the environment, between individuals within a species, and between species (Visser and Gienapp, 2019; Sanders et al., 2020).
Figure 7.
Effects of anthropogenic global changes on drivers of infectious disease seasonality. Global change of the environment (orange box and arrows), and cultural and socioeconomic changes (gray), can affect the seasonality of infectious diseases directly, but also indirectly through their effects on environmental conditions (green). Color version is available online.
Combinations of the main parameters that characterize environmental rhythmicity (i.e., level, variation, amplitude, phase, and waveform) have all been reported to change. The global level of ambient temperature is steadily increasing, affecting infectious disease (e.g., Marcogliese, 2008; Altizer et al., 2013), for example, through changes in species distributions, where range shifts and dispersal of disease vectors are well documented (e.g., Iwamura et al., 2020). In addition, increases in variability occur depending on time of year (Dillon and Woods, 2016), and episodic climate events such as heat waves, common under climate change, may also have a complex impact on disease dynamics (Claar and Wood, 2020). The phenology of many biological processes advances with climate change (Thackeray et al., 2010), affecting given species and those that ecologically interact with them, including the phenology of parasites (Rizzoli et al., 2019). Altered timing of vector emergence can increase the impact of viruses on hosts, for example, if infections coincide with sensitive developmental periods, as shown for Zika (Martinez, 2016). Moreover, through the changes in level, temperature-sensitive phases of phenology, such as the growing season, are extended in many global habitats (McCabe et al., 2015) but shortened in others, for example, depending on aridity (Sarr, 2012), with consequences for activity phases of vectors, synchrony of birth pulses, and many other aspects of seasonality. These changes are not limited to ambient temperature: seasonal rhythms of precipitation are also changing (Dunning et al., 2018). Such changes may impact pest populations and disease vectors but possibly also fomite transmission pathways. A recent study of respiratory syncytial virus in current and future climates found consistent patterns of climate drivers at a continental scale explaining latitudinal differences in the dynamics and seasonality of local epidemics, suggesting that temperature-driven increases to humidity may lead to a northward shift in the dynamic patterns observed (Baker et al., 2019).
In addition to effects of climate change, environmental cycles are also modified by changes in land use, including freshwater use, agricultural practice, and human settlement, which can affect the likelihood of transmission (e.g., Johnson et al., 2020; White and Razgour, 2020). For example, irrigation and other provision of year-round access to open water (Mackenzie et al., 2004; Govoetchan et al., 2014) may affect distributions, abundances, and phenology of species, including those that are disease vectors (Wasserberg et al., 2003a, 2003b). In areas where irrigation gives year-round access to open water, Japanese encephalitis is markedly increased (Mackenzie et al., 2004), and water containers have been shown to serve as dry-season refugia for Dengue vectors (Govoetchan et al., 2014). On the contrary, more than 50% of global wetlands have been lost in the past century (e.g., Davidson, 2014), groundwater overexploitation causes the disappearance of freshwater springs (e.g., Rödiger et al., 2020), and dams cause river fragmentation and dewatering (e.g., Farah-Pérez et al., 2020); these, in turn, may affect species patterns of distribution, abundance, and seasonality.
Another important form of land-use change that raises disease risk is urbanization, where multiple aspects of seasonality are buffered, for example, by year-around availability of open water and food (Govoetchan et al., 2014; Becker et al., 2015). Also given high human population density and disproportionately high zoonotic capacity of species that tolerate human land use (Gibb et al., 2020), it is little wonder that cities are hot spots for emerging infectious diseases (Santiago-Alarcon and MacGregor-Fors, 2020).
Disconcertingly, even the putatively most stable and reliable environmental rhythm, the alternation between light and darkness, is changing through increasing ALAN (Kyba et al., 2017). ALAN disrupts circadian biology in humans and many other organisms, with cascading effects on seasonal processes (Knop et al., 2017). For humans, additional to circadian disruption, indoor ALAN exposure masks the annual change in photoperiod through continuously available LD. Outdoors, wild species may show altered seasonality when exposed to ALAN, which physiologically elicits LD responses (Robert et al., 2015). Consequences of ALAN for immunity have been well established in laboratory species, notably in Siberian hamsters (Bedrosian et al., 2011), and are now suggested to also increase risk from viral diseases. Recent research on West Nile virus, a disease that can be transmitted from birds to humans, has shown that ALAN increased exposure risk and vector competence of avian hosts (Kernbach et al., 2019, 2020a, 2020b, 2020c). Furthermore, ALAN also increased avian host morbidity, indicating that risks of rhythm disruption are not limited to effects on vectors. Bats have been described as natural host reservoirs for several recently emerged viruses (Marburg, Ebola, SARS (Moratelli and Calisher, 2015; Olival et al., 2017)) and they, too, are adversely impacted by artificial light on multiple scales, potentially contributing to enhanced viral spread (Stone et al., 2015).
The effects of global change in ambient temperature, rainfall, daylight hours, diet, social activity, and land use on respiratory virus infection risks and spread, including SARS-CoV-2, and their manifestation as increases or decreases in disease burden, are likely to vary geographically and require further research attention. For instance, climate change is thought to lead to both warmer and dryer conditions in some tropical regions, with expected opposing effects on SARS-CoV-2 transmissibility. Thus, rather than postulating net effects, our intention has been to provide the base material for researchers to use when addressing these important problems in particular context.
Conclusions
We have presented a brief overview of environmental and physiological seasonality, which may contribute to potential future seasonality of COVID-19, as well as of other infectious diseases. On the side of host physiology, we encourage further studies on seasonal restructuring of the immune system in relevant animal models and through the generation of seasonal omics data in humans. We propose that such research should complement current efforts to understand climatic and behavioral drivers of infectious disease seasonality. Even in the absence of host adaptations, seasonal predictability of diseases has major advantages for medical applications (Kissler et al., 2020), as evidenced by the success of seasonal influenza vaccination campaigns (Chung et al., 2020). To the extent that host physiology, too, is seasonal, such consistent patterns can be further exploited, analogous to the growing importance of circadian research for medical intervention. Based on the broad impact of Earth’s seasonality, it is reasonable to assume that global change can affect host immunity and susceptibility; enhance or reduce pathogen survival and proliferation depending on region; modify pathogen load in animal reservoirs; and alter transmission season and human-pathogen interactions. A proactive, multidisciplinary approach to disease control and prevention aiming to better understand these complex interactions, with a greater emphasis on all aspects of health—human, environmental, animal—as exemplified by the notion of One Health, could mitigate some of the expected impacts of global change.
Acknowledgments
This review was written as a continuation of a CAREebrs workshop on Chronobiology of COVID-19 (https://careconferences.org/our-concept/7-blog/82-care-chrono-covid19.html) organized by the European Biological Rhythms Society. We particularly thank the driving force behind it, Martha Merrow. We thank Rory Gunson (West of Scotland Specialist Virology Centre, NHS Greater Glasgow and Clyde) for providing the data underlying Figure 2, Maaike Versteegh and Irene Tieleman for sharing data underlying Figure 3, and Brian Prendergast for sharing data underlying Figure 5. We also thank Fernando Cazarez-Marquez and two anonymous reviewers for helpful comments.
Footnotes
Conflict of Interest Statement: The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: N. Kronfeld-Schor  https://orcid.org/0000-0002-5224-3341
https://orcid.org/0000-0002-5224-3341
References
- Aguirre-Gamboa R, Joosten I, Urbano PC, van der Molen RG, van Rijssen E, van Cranenbroek B, Oosting M, Smeekens S, Jaeger M, Zorro M. (2016) Differential effects of environmental and genetic factors on T and B cell immune traits. Cell Rep 17:2474-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. (2006) Seasonality and the dynamics of infectious diseases. Ecol Lett 9:467-484. [DOI] [PubMed] [Google Scholar]
- Altizer S, Ostfeld RS, Johnson PT, Kutz S, Harvell CD. (2013) Climate change and infectious diseases: from evidence to a predictive framework. Science 341:514-519. [DOI] [PubMed] [Google Scholar]
- Anderson JJ, Nieman DC. (2016) Diet quality—the Greeks had it right! Nutrients 8:636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie SR, Prendergast BJ. (2008) Photoperiodic regulation of behavioral responses to bacterial and viral mimetics: a test of the winter immunoenhancement hypothesis. J Biol Rhythms 23:81-90. [DOI] [PubMed] [Google Scholar]
- Baker RE, Mahmud AS, Wagner CE, Yang W, Pitzer VE, Viboud C, Vecchi GA, Metcalf CJE, Grenfell BT. (2019) Epidemic dynamics of respiratory syncytial virus in current and future climates. Nat Commun 10:5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao R, Onishi KG, Tolla E, Ebling FJ, Lewis JE, Anderson RL, Barrett P, Prendergast BJ, Stevenson TJ. (2019) Genome sequencing and transcriptome analyses of the Siberian hamster hypothalamus identify mechanisms for seasonal energy balance. Proc Natl Acad Sci U S A 116:13116-13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile E, Baggio C, Gambini L, Shiryaev SA, Pellecchia AYS. (2020) Potential therapeutic targeting of coronavirus spike glycoprotein priming. Molecules 25:2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Demas GE, Song CK. (2002) Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp Biol Med 227:363-376. [DOI] [PubMed] [Google Scholar]
- Bauer ME, Teixeira AL. (2019) Inflammation in psychiatric disorders: what comes first? Ann N Y Acad Sci 1437:57-67. [DOI] [PubMed] [Google Scholar]
- Becker DJ, Streicker DG, Altizer S. (2015) Linking anthropogenic resources to wildlife-pathogen dynamics: a review and meta-analysis. Ecol Lett 18:483-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian TA, Fonken LK, Walton JC, Nelson RJ. (2011) Chronic exposure to dim light at night suppresses immune responses in Siberian hamsters. Biol Lett 7:468-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begon M, Telfer S, Smith MJ, Burthe S, Paterson S, Lambin X. (2009) Seasonal host dynamics drive the timing of recurrent epidemics in a wildlife population. Proc Biol Sci 276:1603-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Dhabhar FS, Viswanathan K, Saul A, Yellon SM, Nelson RJ. (2002) Short day lengths augment stress-induced leukocyte trafficking and stress-induced enhancement of skin immune function. Proc Natl Acad Sci U S A 99:4067-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom-Feshbach K, Alonso WJ, Charu V, Tamerius J, Simonsen L, Miller MA, Viboud C. (2013) Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PLoS ONE 8:e54445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordon Y. (2017) Vitamin D primes neonatal immune system. Nat Rev Immunol 17:467-467. [DOI] [PubMed] [Google Scholar]
- Borrmann H, McKeating JA, Zhuang X. (2021) The circadian clock and viral infections. J Biol Rhythms 36:9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. (2007) Evolution of animal photoperiodism. Annu Rev Ecol Evol Syst 38:1-25. [Google Scholar]
- Brainard GC, Knobler RL, Podolin PL, Lavasa M, Lublin FD. (1987) Neuroimmunology: modulation of the hamster immune system by photoperiod. Life Sci 40:1319-1326. [DOI] [PubMed] [Google Scholar]
- Brassey J, Heneghan C, Mahtani K, Aronson J. (2020) Do weather conditions influence the transmission of the coronavirus (SARS-CoV-2). Oxford (UK): The Centre for Evidence-Based Medicine. [Google Scholar]
- Brock MA. (1983) Seasonal rhythmicity in lymphocyte blastogenic responses of mice persists in a constant environment. J Immunology 130:2586-2588. [PubMed] [Google Scholar]
- Brock MA. (1987) Age-related changes in circannual rhythms of lymphocyte blastogenic responses in mice. Am J Physiol 252:R299-R305. [DOI] [PubMed] [Google Scholar]
- Brouwer A, van Raalte DH, Diamant M, Rutters F, van Someren EJW, Snoek FJ, Beekman ATF, Bremmer MA. (2015) Light therapy for better mood and insulin sensitivity in patients with major depression and type 2 diabetes: a randomised, double-blind, parallel-arm trial. BMC Psychiatry 15:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck MD, O’Sullivan D, Pearce EL. (2015) T cell metabolism drives immunity. J Exp Med 212:1345-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. (2000) Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103:1009-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calov M, Alinaghi F, Hamann CR, Silverberg J, Egeberg A, Thyssen JP. (2020) The Association between season of birth and atopic dermatitis in the Northern Hemisphere: a systematic review and meta-analysis. J Allergy Clin Immunol Pract 8:674-680. e675. [DOI] [PubMed] [Google Scholar]
- Carrillo JA, He Y, Li Y, Liu J, Erdman RA, Sonstegard TS, Song J. (2016) Integrated metabolomic and transcriptome analyses reveal finishing forage affects metabolic pathways related to beef quality and animal welfare. Sci Rep 6:25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauchemez S, Valleron A-J, Boëlle P-Y, Flahault A, Ferguson NM. (2008) Estimating the impact of school closure on influenza transmission from Sentinel data. Nature 452:750-754. [DOI] [PubMed] [Google Scholar]
- Chae CU, Lee RT, Rifai N, Ridker PM. (2001) Blood pressure and inflammation in apparently healthy men. Hypertension 38:399-403. [DOI] [PubMed] [Google Scholar]
- Chan KH, Peiris JSM, Lam SY, Poon LLM, Yuen KY, Seto WH. (2011) The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol 2011:734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JR, Flannery B, Gaglani M, Smith ME, Reis EC, Hickey RW, Jackson ML, Jackson LA, Belongia EA, McLean HQ, et al. (2020) Patterns of influenza vaccination and vaccine effectiveness among young US children who receive outpatient care for acute respiratory tract illness. JAMA Pediatr 174:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizauskas CA, Turner WC, Wagner B, Küstersrs M, Vance RE, Getz WM. (2014) Gastrointestinal helminths may affect host susceptibility to anthrax through seasonal immune trade-offs. BMC Ecol 14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claar DC, Wood CL. (2020) Pulse heat stress and parasitism in a warming world. Trends Ecol Evol 35:704-715. [DOI] [PubMed] [Google Scholar]
- Cohen J. (2020) Sick time. Science 367:1294-1297. [DOI] [PubMed] [Google Scholar]
- Davidson NC. (2014) How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar Freshw Res 65:934-941. [Google Scholar]
- Deming M, Linkins R, Jaiteh K, Hull H. (1997) The clinical efficacy of trivalent oral polio vaccine in The Gambia by season of vaccine administration. J Infect Dis 175:S254-S257. [DOI] [PubMed] [Google Scholar]
- Dhewantara PW, Marina R, Puspita T, Ariati Y, Purwanto E, Hananto M, Hu W, Magalhaes RJS. (2019) Spatial and temporal variation of dengue incidence in the island of Bali, Indonesia: an ecological study. Travel Med Infect Dis; 32:101437. [DOI] [PubMed] [Google Scholar]
- Dillon ME, Woods HA. (2016) Introduction to the symposium: beyond the mean: biological impacts of changing patterns of temperature variation. Integr Comp Biol 56:11-13. [DOI] [PubMed] [Google Scholar]
- Dopico XC, Evangelou M, Ferreira RC, Guo H, Pekalski ML, Smyth DJ, Cooper N, Burren OS, Fulford AJ, Hennig BJ. (2015) Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat Commun 6:7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorélien AM, Ballesteros S, Grenfell BT. (2013) Impact of birth seasonality on dynamics of acute immunizing infections in Sub-Saharan Africa. PLoS ONE 8:e75806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke-Sylvester SM, Bolzoni L, Real LA. (2011) Strong seasonality produces spatial asynchrony in the outbreak of infectious diseases. J R Soc Interface 8:817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning CM, Black E, Allan RP. (2018) Later wet seasons with more intense rainfall over Africa under future climate change. J Clim 31:9719-9738. [Google Scholar]
- Eames KT, Tilston NL, Edmunds WJ. (2011) The impact of school holidays on the social mixing patterns of school children. Epidemics 3:103-108. [DOI] [PubMed] [Google Scholar]
- Edgar RS, Stangherlin A, Nagy AD, Nicoll MP, Efstathiou S, O’Neill JS, Reddy AB. (2016) Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proc Natl Acad Sci U S A 113:10085-10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah-Pérez A, Umaña-Villalobos G, Picado-Barboza J, Anderson EP. (2020) An analysis of river fragmentation by dams and river dewatering in Costa Rica. River Res Appl 36:1442-1448. [Google Scholar]
- Fisman DN. (2007) Seasonality of infectious diseases. Ann Rev Pub Health 28:127-143. [DOI] [PubMed] [Google Scholar]
- Fisman DN. (2012) Seasonality of viral infections: mechanisms and unknowns. Clin Microbiol Infect 18:946-954. [DOI] [PubMed] [Google Scholar]
- Foster RG, Kreitzman L. (2009) Seasons of life. New Haven (CT): Yale University Press. [Google Scholar]
- Foxman EF, Storer JA, Fitzgerald ME, Wasik BR, Hou L, Zhao H, Turner PE, Pyle AM, Iwasaki A. (2015) Temperature-dependent innate defense against the common cold virus limits viral replication at warm temperature in mouse airway cells. Proc Natl Acad Sci U S A 112:827-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb R, Redding DW, Chin KQ, Donnelly CA, Blackburn TM, Newbold T, Jones KE. (2020) Zoonotic host diversity increases in human-dominated ecosystems. Nature 584:398-402. [DOI] [PubMed] [Google Scholar]
- Govoetchan R, Gnanguenon V, Ogouwalé E, Oké-Agbo F, Azondekon R, Sovi A, Attolou R, Badirou K, Youssouf R, Osse R, et al. (2014) Dry season refugia for anopheline larvae and mapping of the seasonal distribution in mosquito larval habitats in Kandi, northeastern Benin. Parasi Vectors 7:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassly NC, Fraser C. (2006) Seasonal infectious disease epidemiology. Proc Biol Sci 273:2541-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni E, Valoriani A, Cei F, Lamanna R, Gelli AMG, Ciambotti B, Vannucchi V, Moroni F, Pelagatti L, Tarquini R. (2020) Interleukin-6 as prognosticator in patients with COVID-19: IL-6 and COVID-19. J Infect 81:452-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Bonebrake TC, Gibson L. (2019) Land-use change alters host and vector communities and may elevate disease risk. Ecohealth 16:647-658. [DOI] [PubMed] [Google Scholar]
- Gwinner E. (1986) Circannual rhythms. Heidelberg (Berlin): Springer, p. 154. [Google Scholar]
- Harding K, Tilling K, MacIver C, Willis M, Joseph F, Ingram G, Hirst C, Wardle M, Pickersgill T, Ben-Shlomo Y. (2017) Seasonal variation in multiple sclerosis relapse. J Neurol 264:1059-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart GD. (2001) Descriptions of blood and blood disorders before the advent of laboratory studies. Br J Haematol 115:719-728. [DOI] [PubMed] [Google Scholar]
- He D, Earn DJ. (2007) Epidemiological effects of seasonal oscillations in birth rates. Theor Popul Biol 72:274-291. [DOI] [PubMed] [Google Scholar]
- Heideman PD, Pittman JT. (2009) Microevolution of neuroendocrine mechanisms regulating reproductive timing in Peromyscus leucopus. Integr Comp Biol 49:550-562. [DOI] [PubMed] [Google Scholar]
- Helm B. (2009) Geographically distinct reproductive schedules in a changing world: costly implications in captive stonechats. Integr Comp Biol 49:563-579. [DOI] [PubMed] [Google Scholar]
- Herder C, Schneitler S, Rathmann W, Haastert B, Schneitler H, Winkler H, Bredahl R, Hahnloser E, Martin S. (2007) Low-grade inflammation, obesity, and insulin resistance in adolescents. J Clin Endocrinol Metab 92:4569-4574. [DOI] [PubMed] [Google Scholar]
- Herekar F, Iftikhar S, Nazish A, Rehman S. (2020) Malaria and the climate in Karachi: an eight year review. Pak J Med Sci 36:S33-S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M, Iwatsuki-Horimoto K, Hatta M, Loeber S, Halfmann PJ, Nakajima N, Watanabe T, Ujie M, Takahashi K, Ito M. (2020) Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci U S A 117:16587-16595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insull W., Jr (2009) The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med 122:S3-S14. [DOI] [PubMed] [Google Scholar]
- Iwamura T, Guzman-Holst A, Murray KA. (2020) Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat Commun 11:2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaweera M, Perera H, Gunawardana B, Manatunge J. (2020) Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res 188:109819-109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CK, Hitchens PL, Pandit PS, Rushmore J, Evans TS, Young CC, Doyle MM. (2020) Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proc Biol Sci 287:20192736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PT, De Roode JC, Fenton A. (2015) Why infectious disease research needs community ecology. Science 349:1259504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. (2008) Global trends in emerging infectious diseases. Nature 451:990-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanna J. (2012) The legacy of the Hippocratic treatise the nature of man: the theory of the four humours. In: Scarborough J, van der Eijk PJ, Hanson AE, Ziegler J, editors. Greek medicine from Hippocrates to Galen. Leiden (the Netherlands): Brill, p. 335-359. [Google Scholar]
- Kernbach ME, Cassone VM, Unnasch TR, Martin LB. (2020. a) Broad-spectrum light pollution suppresses melatonin and increases West Nile virus-induced mortality in House Sparrows (Passer domesticus). Condor 122:1-13. [Google Scholar]
- Kernbach ME, Martin LB, Unnasch TR, Hall RJ, Jiang RH, Francis CD. (2020. b) Light pollution affects West Nile virus exposure risk across Florida. bioRxiv. doi: 10.1101/2020.05.08.082974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernbach ME, Newhouse DJ, Miller JM, Hall RJ, Gibbons J, Oberstaller J, Selechnik D, Jiang RH, Unnasch TR, Balakrishnan CN. (2019) Light pollution increases West Nile virus competence of a ubiquitous passerine reservoir species. Proc Biol Sci 286:20191051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernbach ME, Unnasch TR, Martin LB. (2020. c) Differential effects of spectral composition of nighttime lighting on west nile virus resistance and mortality in house sparrows. Integr Comp Biol 60:E122-E122. [Google Scholar]
- Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. (2020) Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 368:860-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg E, Olerod G, Konar J, Petzold M, Hammarsten O. (2015) Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine 49:800-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompas M, Baker MA, Rhee C. (2020) Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA 324:441-442. [DOI] [PubMed] [Google Scholar]
- Knop E, Zoller L, Ryser R, Gerpe C, Hörler M, Fontaine C. (2017) Artificial light at night as a new threat to pollination. Nature 548:206-209. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DP. (2005) How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet 77:171-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyba CCM, Kuester T, Sánchez de Miguel A, Baugh K, Jechow A, Hölker F, Bennie J, Elvidge CD, Gaston KJ, Guanter L. (2017) Artificially lit surface of Earth at night increasing in radiance and extent. Sci Adv 3:e1701528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A, Hales S, French N, Baker MG. (2012) Seasonality in human zoonotic enteric diseases: a systematic review. PLoS ONE 7:e31883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalor MK, Ben-Smith A, Gorak-Stolinska P, Weir RE, Floyd S, Blitz R, Mvula H, Newport MJ, Branson K, McGrath N. (2009) Population differences in immune responses to Bacille Calmette-Guerin vaccination in infancy. J Infect Dis 199:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanham-New SA, Webb AR, Cashman KD, Buttriss JL, Fallowfield JL, Masud T, Hewison M, Mathers JC, Kiely M, Welch AA, et al. (2020) Vitamin D and SARS-CoV-2 virus/COVID-19 disease. BMJ Nutr Prev Health 3:106-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KS, Lau EH, Wong JY. (2020. a) Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382:1199-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang X, Nair H. (2020. b) Global seasonality of human seasonal coronaviruses: a clue for postpandemic circulating season of severe acute respiratory syndrome coronavirus 2? J Infect Dis 222:1090-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AS, Klein H-U, Yu L, Chibnik LB, Ali S, Xu J, Bennett DA, De Jager PL. (2017) Diurnal and seasonal molecular rhythms in human neocortex and their relation to Alzheimer’s disease. Nat Commun 8:14931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln G. (2019) A brief history of circannual time. J Neuroendocrinol 31:e12694. [DOI] [PubMed] [Google Scholar]
- Liu D, Fernandez BO, Hamilton A, Lang NN, Gallagher JM, Newby DE, Feelisch M, Weller RB. (2014) UVA irradiation of human skin vasodilates arterial vasculature and lowers blood pressure independently of nitric oxide synthase. J Invest Dermatol 134:1839-1846. [DOI] [PubMed] [Google Scholar]
- Lockett GA, Soto-Ramírez N, Ray MA, Everson TM, Xu CJ, Patil VK, Terry W, Kaushal A, Rezwan FI, Ewart SL. (2016) Association of season of birth with DNA methylation and allergic disease. Allergy 71:1314-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen AC, Steel J. (2014) Roles of humidity and temperature in shaping influenza seasonality. J Virol 88:7692-7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen AC, Mubareka S, Steel J, Palese P. (2007) Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog 3:1470-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca F, Perry GH, Di Rienzo A. (2010) Evolutionary adaptations to dietary changes. Annu Rev Nutr 30:291-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca GD, Kerckhove KV, Coletti P, Poletto C, Bossuyt N, Hens N, Colizza V. (2018) The impact of regular school closure on seasonal influenza epidemics: a data-driven spatial transmission model for Belgium. BMC Infect Dis 18:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C, Maher L, Lee C, Kang W. (2020) COVID-19 preclinical models: human angiotensin-converting enzyme 2 transgenic mice. Hum Genomics 14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J, Gubler D, Petersen LR. (2004) Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med 10:98-109. [DOI] [PubMed] [Google Scholar]
- Marcogliese D. (2008) The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev Sci Tech 27:467-484. [PubMed] [Google Scholar]
- Martinez ME. (2016) Preventing Zika virus infection during pregnancy using a seasonal window of opportunity for conception. PLoS Biol 14:e1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez ME. (2018) The calendar of epidemics: seasonal cycles of infectious diseases. PLoS Pathog 14:e1007327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Bakker M, Helm B. (2015) The influence of biological rhythms on host-parasite interactions. Trends Ecol Evol 30:314-326. [DOI] [PubMed] [Google Scholar]
- Mathis D. (2013) Immunological goings-on in visceral adipose tissue. Cell Metab 17:851-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe GJ, Betancourt JL, Feng S. (2015) Variability in the start, end, and length of frost-free periods across the conterminous United States during the past century. Int J Climatol 35:4673-4680. [Google Scholar]
- Meyer C, Muto V, Jaspar M, Kussé C, Lambot E, Chellappa SL, Degueldre C, Balteau E, Luxen A, Middleton B. (2016) Seasonality in human cognitive brain responses. Proc Natl Acad Sci U S A 113:3066-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet S, Bennett J, Lee KA, Hau M, Klasing KC. (2007) Quantifying and comparing constitutive immunity across avian species. Dev Comp Immunol 31:188-201. [DOI] [PubMed] [Google Scholar]
- Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, Jedrychowski MP, Costa AS, Higgins M, Hams E. (2018) Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556:113-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moan JE, Lagunova Z, Bruland Ø, Juzeniene A. (2010) Seasonal variations of cancer incidence and prognosis. Dermatoendocrinol 2:55-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moltchanova E, Schreier N, Lammi N, Karvonen M. (2009) Seasonal variation of diagnosis of Type 1 diabetes mellitus in children worldwide. Diabet Med 26:673-678. [DOI] [PubMed] [Google Scholar]
- Monto AS, DeJonge P, Callear AP, Bazzi LA, Capriola S, Malosh RE, Martin ET, Petrie JG. (2020) Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis 222:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SE, Collinson AC, Fulford AJ, Jalil F, Siegrist CA, Goldblatt D, Hanson LÅ, Prentice AM. (2006) Effect of month of vaccine administration on antibody responses in The Gambia and Pakistan. Trop Med Int Health 11:1529-1541. [DOI] [PubMed] [Google Scholar]
- Moratelli R, Calisher CH. (2015) Bats and zoonotic viruses: can we confidently link bats with emerging deadly viruses? Mem Inst Oswaldo Cruz 110:1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M, Hugentobler WJ, Iwasaki A. (2020) Seasonality of respiratory viral infections. Annu Rev Virol 7:83-101. [DOI] [PubMed] [Google Scholar]
- Naumova EN. (2006) Mystery of seasonality: getting the rhythm of nature. J Pub Health Policy 27:2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE, Klein SL, Kriegsfeld LJ. (1995) Minireview the influence of season, photoperiod, and pineal melatonin on immune function. J Pineal Res 19:149-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE, Klien SL, Kriegsfeld LJ. (2002) Seasonal patterns of stress, immune function and disease. Cambridge (UK): Cambridge University Press. [Google Scholar]
- Nguyen HT, Dharan NJ, Le MTQ, Nguyen NB, Nguyen CT, Hoang DV, Tran HN, Bui CT, Dang DT, Pham DN, et al. (2009) National influenza surveillance in Vietnam, 2006-2007. Vaccine 28:398-402. [DOI] [PubMed] [Google Scholar]
- Nguyen JL, Schwartz J, Dockery DW. (2014) The relationship between indoor and outdoor temperature, apparent temperature, relative humidity, and absolute humidity. Indoor Air 24:103-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JL, Yang W, Ito K, Matte TD, Shaman J, Kinney PL. (2016) Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol 1:274-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. (2013) Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 341:1483-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickbakhsh S, Ho A, Marques DF, McMenamin J, Gunson RN, Murcia PR. (2020) Epidemiology of seasonal coronaviruses: establishing the context for the emergence of coronavirus disease 2019. J Infect Dis 222:17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickbakhsh S, Mair C, Matthews L, Reeve R, Johnson PCD, Thorburn F, von Wissmann B, Reynolds A, McMenamin J, Gunson RN, et al. (2019) Virus-virus interactions impact the population dynamics of influenza and the common cold. Proc Natl Acad Sci U S A 116:27142-27150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AW. (1998) Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system. Oxford (UK): Oxford University Press. [DOI] [PubMed] [Google Scholar]
- Olival KJ, Hosseini PR, Zambrana-Torrelio C, Ross N, Bogich TL, Daszak P. (2017) Host and viral traits predict zoonotic spillover from mammals. Nature 546:646-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi KG, Maneval AC, Cable EC, Tuohy MC, Scasny AJ, Sterina E, Love JA, Riggle JP, Malamut LK, Mukerji A, et al. (2020) Circadian and circannual timescales interact to generate seasonal changes in immune function. Brain Behav Immun 83:33-43. [DOI] [PubMed] [Google Scholar]
- Paludan SR, Pradeu T, Masters SL, Mogensen TH. (2020) Constitutive immune mechanisms: mediators of host defence and immune regulation. Nat Rev Immunol. Epub ahead of print August. doi: 10.1038/s41577-020-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen B. (2007) IL-6 signalling in exercise and disease. London (UK): Portland Press. [DOI] [PubMed] [Google Scholar]
- Peruzzotti-Jametti L, Bernstock JD, Vicario N, Costa AS, Kwok CK, Leonardi T, Booty LM, Bicci I, Balzarotti B, Volpe G. (2018) Macrophage-derived extracellular succinate licenses neural stem cells to suppress chronic neuroinflammation. Cell Stem Cell 22:355-368. e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planelles D, Hernández-Godoy J, Montoro A, Montoro J, González-Molina A. (1994) Seasonal variation in proliferative response and subpopulations of lymphocytes from mice housed in a constant environment. Cell Prolif 27:333-341. [DOI] [PubMed] [Google Scholar]
- Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, Lloyd-Smith JO. (2017) Pathways to zoonotic spillover. Nature Rev Microbiol 15:502-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Baillie SR, Dhabhar FS. (2008) Gonadal hormone-dependent and -independent regulation of immune function by photoperiod in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 294:R384-R392. [DOI] [PubMed] [Google Scholar]
- Price RHM, Graham C, Ramalingam S. (2019) Association between viral seasonality and meteorological factors. Sci Rep 9:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quera Salva MA, Hartley S, Barbot FC, Alvarez J, Lofaso F, Guilleminault C. (2011) Circadian rhythms, melatonin and depression. Curr Pharm Des 17:1459-1470. [DOI] [PubMed] [Google Scholar]
- Randall VA, Ebling F. (1991) Seasonal changes in human hair growth. Br J Dermatol 124:146-151. [DOI] [PubMed] [Google Scholar]
- Renz H, Holt PG, Inouye M, Logan AC, Prescott SL, Sly PD. (2017) An exposome perspective: early-life events and immune development in a changing world. J Allergy Clin Immunol 140:24-40. [DOI] [PubMed] [Google Scholar]
- Richard M, Kok A, de Meulder D, Bestebroer TM, Lamers MM, Okba NM, van Vlissingen MF, Rockx B, Haagmans BL, Koopmans MP. (2020) SARS-CoV-2 is transmitted via contact and via the air between ferrets. bioRxiv. https://www.biorxiv.org/content/10.1101/2020.04.16.044503v1. [DOI] [PMC free article] [PubMed]
- Rizzoli A, Tagliapietra V, Cagnacci F, Marini G, Arnoldi D, Rosso F, Rosà R. (2019) Parasites and wildlife in a changing world: the vector-host-pathogen interaction as a learning case. Int J Parasitol Parasites Wildl 9:394-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert KA, Lesku JA, Partecke J, Chambers B. (2015) Artificial light at night desynchronizes strictly seasonal reproduction in a wild mammal. Proc Biol Sci 282:20151745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rödiger T, Magri F, Geyer S, Mallast U, Odeh T, Siebert C. (2020) Calculating man-made depletion of a stressed multiple aquifer resource on a national scale. Sci Total Environ 725:138478. [DOI] [PubMed] [Google Scholar]
- Sailani MR, Metwally AA, Zhou W, Rose SMS-F, Ahadi S, Contrepois K, Mishra T, Zhang MJ, Kidziński Ł, Chu TJ. (2020) Deep longitudinal multiomics profiling reveals two biological seasonal patterns in California. Nat Commun 11:4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadi MM, Habibzadeh P, Vintzileos A, Shokouhi S, Miralles-Wilhelm F, Amoroso A. (2020) Temperature, humidity, and latitude analysis to estimate potential spread and seasonality of coronavirus disease 2019 (COVID-19). JAMA Netw Open 3:e2011834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Frago E, Kehoe R, Patterson C, Gaston KJ. (2020) A meta-analysis of biological impacts of artificial light at night. Nat Ecol Evol 5:74-81. [DOI] [PubMed] [Google Scholar]
- Santiago-Alarcon D, MacGregor-Fors I. (2020) Cities and pandemics: urban areas are ground zero for the transmission of emerging human infectious diseases. J Urban Ecol 6:juaa012. [Google Scholar]
- Sarr B. (2012) Present and future climate change in the semi-arid region of West Africa: a crucial input for practical adaptation in agriculture. Atmosph Sci Lett 13:108-112. [Google Scholar]
- Scafetta N. (2020) Distribution of the SARS-CoV-2 pandemic and its monthly forecast based on seasonal climate patterns. Int J Environ Res Public Health 17:3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L. (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 110:3507-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J, Kohn M. (2009) Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci U S A 106:3243-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shampo MA, Kyle RA. (1987) Hydrotherapy (Kneippism). Mayo Clin Proc 62:929. [DOI] [PubMed] [Google Scholar]
- Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, Liu R, He X, Shuai L, Sun Z. (2020) Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science 368:1016-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivatcheva TM, Hadjioloff AI. (1987) Seasonal involution of gut-associated lymphoid tissue of the European ground squirrel. Dev Comp Immunol 11:791-799. [DOI] [PubMed] [Google Scholar]
- Sia SF, Yan L-M, Chin AW, Fung K, Choy K-T, Wong AY, Kaewpreedee P, Perera RA, Poon LL, Nicholls JM. (2020) Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature 583:834-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu TH. (2017) Influence of diet on the gut microbiome and implications for human health. J Transl Med 15:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Pattanaik AK, Goswami T, Sharma K. (2013) Effect of varying the energy density of protein-adequate diets on nutrient metabolism, clinical chemistry, immune response and growth of Muzaffarnagari lambs. Asian-Australas J Anim Sci 26:1089-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. (2019) The effect of potential climate change on infectious disease presentation. J Nurse Pract 15:405-409. [Google Scholar]
- Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. (2020) Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol 16:341-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, Prendergast BJ. (2015) Photoperiodic time measurement and seasonal immunological plasticity. Front Neuroendocrinol 37:76-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, Onishi KG, Bradley SP, Prendergast BJ. (2014) Cell-autonomous iodothyronine deiodinase expression mediates seasonal plasticity in immune function. Brain Behav Immun 36:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, Visser ME, Arnold W, Barrett P, Biello S, Dawson A, Denlinger DL, Dominoni D, Ebling FJ, Elton S, et al. (2015) Disrupted seasonal biology impacts health, food security and ecosystems. Proc Biol Sci 282:20151453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EL, Harris S, Jones G. (2015) Impacts of artificial lighting on bats: a review of challenges and solutions. Mamm Biol 80:213-219. [Google Scholar]
- Stuart P, Paredis L, Henttonen H, Lawton C, Torres CAO, Holland CV. (2020) The hidden faces of a biological invasion: parasite dynamics of invaders and natives. Int J Parasitol Parasites Wildl 50:111-123. [DOI] [PubMed] [Google Scholar]
- Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. (2020) The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20:363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K, Das S, Pearson M, Kozubek J, Pawlowski M, Jensen CE, Skowron Z, Møller GL, Strivens M, Gardner S. (2020) Analysis of genetic host response risk factors in severe COVID-19 patients. medRxiv. https://www.medrxiv.org/content/10.1101/2020.06.17.20134015v2.
- Ter Horst R, Jaeger M, Smeekens SP, Oosting M, Swertz MA, Li Y, Kumar V, Diavatopoulos DA, Jansen AF, Lemmers H. (2016) Host and environmental factors influencing individual human cytokine responses. Cell 167:1111-1124. e1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackeray SJ, Sparks TH, Frederiksen M, Burthe S, Bacon PJ, Bell JR, Botham MS, Brereton TM, Bright PW, Carvalho L. (2010) Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob Change Biol 16:3304-3313. [Google Scholar]
- Thysen AH, Rasmussen MA, Kreiner-Møller E, Larsen JM, Følsgaard NV, Bønnelykke K, Stokholm J, Bisgaard H, Brix S. (2016) Season of birth shapes neonatal immune function. J Allergy Clin Immunol 137:1238-1246. e1213. [DOI] [PubMed] [Google Scholar]
- Tostanoski LH, Wegmann F, Martinot AJ, Loos C, McMahan K, Mercado NB, Yu J, Chan CN, Bondoc S, Starke CE. (2020) Ad26 vaccine protects against SARS-CoV-2 severe clinical disease in hamsters. Nat Med 26:1694-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucar D, Márquez EJ, Chung C-H, Marches R, Rossi RJ, Uyar A, Wu T-C, George J, Stitzel ML, Palucka AK. (2017) The chromatin accessibility signature of human immune aging stems from CD8+ T cells. J Exp Med 214:3123-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteegh MA, Helm B, Kleynhans EJ, Gwinner E, Tieleman BI. (2014) Genetic and phenotypically flexible components of seasonal variation in immune function. J Exp Biol 217:1510-1518. [DOI] [PubMed] [Google Scholar]
- Visscher T, Seidell J. (2004) Time trends (1993-1997) and seasonal variation in body mass index and waist circumference in the Netherlands. Int J Obes 28:1309-1316. [DOI] [PubMed] [Google Scholar]
- Visser ME, Gienapp P. (2019) Evolutionary and demographic consequences of phenological mismatches. Nat Ecol Evol 3:879-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser ME, Caro SP, van Oers K, Schaper SV, Helm B. (2010) Phenology, seasonal timing and circannual rhythms: towards a unified framework. Philos Trans R Soc Lond B Biol Sci 365:3113-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserberg G, Abramsky Z, Kotler B, Ostfeld R, Yarom I, Warburg A. (2003. a) Anthropogenic disturbances enhance occurrence of cutaneous Leishmaniasis in Israel deserts: patterns and mechanisms. Ecol Appl 13:868-881. [Google Scholar]
- Wasserberg G, Yarom I, Warburg A. (2003. b) Seasonal abundance patterns of the sandfly Phlebotomus papatasi in climatically distinct foci of cutaneous leishmaniasis in Israeli deserts. Med Vet Entomol 17:452-456. [DOI] [PubMed] [Google Scholar]
- Wehr TA. (2001) Photoperiodism in humans and other primates: evidence and implications. J Biol Rhythms 16:348-364. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Borniger JC, Cisse YM, Salloum BAA, Nelson RJ. (2015) Neuroendocrine control of photoperiodic changes in immune function. Front Neuroendocrinol 37:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel FJ, Mullett SJ, Elsner RA, Menk AV, Trivedi N, Luo W, Wikenheiser D, Hawse WF, Chikina M, Smita S. (2020) Germinal center B cells selectively oxidize fatty acids for energy while conducting minimal glycolysis. Nature Immunol 21:331-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AC, Wood SH. (2018) Seasonal physiology: making the future a thing of the past. Curr Opin Physiol 5:1-8. [Google Scholar]
- West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA. (2015) Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520:553-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RJ, Razgour O. (2020) Emerging zoonotic diseases originating in mammals: a systematic review of effects of anthropogenic land-use change. Mam Rev 50:336-352. 10.1111/mam.12201 [DOI] [PMC free article] [PubMed]
- World Health Organization (1995) Factors affecting the immunogenicity of oral poliovirus vaccine: a prospective evaluation in Brazil and The Gambia. J Infect Dis 171:1097-1106. [DOI] [PubMed] [Google Scholar]
- Wyse C, O’Malley G, Coogan A, Smith D. (2020) Seasonal and daytime variation in multiple immune parameters in humans: evidence from 329,261 participants of the UK Biobank cohort. medRxiv. https://www.medrxiv.org/content/10.1101/2020.10.23.20218305v1.full [DOI] [PMC free article] [PubMed]
- Yoshimura h. (1958) Seasonal changes in human body fluids. Jpn J Physiol 8:165-179. [DOI] [PubMed] [Google Scholar]
- Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng O-T, Marimuthu K, Ang LW, Mak TM. (2020) Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 323:1488-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandkarimi F, Vanegas J, Fern X, Maier C, Bobe G. (2018) Metabotypes with elevated protein and lipid catabolism and inflammation precede clinical mastitis in prepartal transition dairy cows. J Dairy Sci 101:5531-5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Cagampang FR, Stirland JA, Loudon AS, Hopkins SJ. (2002) Different photoperiods affect proliferation of lymphocytes but not expression of cellular, humoral, or innate immunity in hamsters. J Biol Rhythms 17:392-405. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Alberti K, Stern N, Bilu C, El-Osta A, Einat H, Kronfeld-Schor N. (2019) The circadian syndrome: is the metabolic syndrome and much more! J Intern Med 286:181-191. [DOI] [PMC free article] [PubMed] [Google Scholar]