In this systematic review and meta-analysis, we synthesize the effects of umbilical cord management strategies (timing of clamping, cord milking) in preterm infants <34 weeks’ gestational age.
Abstract
Video Abstract
CONTEXT:
The International Liaison Committee on Resuscitation prioritized scientific review of umbilical cord management strategies at preterm birth.
OBJECTIVE:
To determine the effects of umbilical cord management strategies (including timing of cord clamping and cord milking) in preterm infants <34 weeks’ gestation.
DATA SOURCES:
Cochrane Central Register of Controlled Trials, Medline, PubMed, Embase, CINAHL, and trial registries were searched through July 2019 for randomized controlled trials assessing timing of cord clamping and/or cord milking.
STUDY SELECTION:
Two authors independently assessed trial eligibility, extracted data, appraised risk of bias, and assessed evidence certainty (GRADE).
DATA EXTRACTION:
We identified 42 randomized controlled trials (including 5772 infants) investigating 4 different comparisons of cord management interventions.
RESULTS:
Compared to early cord clamping, delayed cord clamping (DCC) and intact-cord milking (ICM) may slightly improve survival; however, both are compatible with no effect (DCC: risk ratio: 1.02, 95% confidence interval: 1.00 to 1.04, n = 2988 infants, moderate certainty evidence; ICM: risk ratio: 1.02, 95% confidence interval: 0.98 to 1.06, n = 945 infants, moderate certainty evidence). DCC and ICM both probably improve hematologic measures but may not affect major neonatal morbidities.
LIMITATIONS:
For many of the included comparisons and outcomes, certainty of evidence was low. Our subgroup analyses were limited by few researchers reporting subgroup data.
CONCLUSIONS:
DCC appears to be associated with some benefit for infants born <34 weeks. Cord milking needs further evidence to determine potential benefits or harms. The ideal cord management strategy for preterm infants is still unknown, but early clamping may be harmful.
Immaturity of multiple organ systems puts preterm infants born at <34 weeks’ gestation at high risk for mortality and morbidities, such as intraventricular hemorrhage (IVH), and they are more likely to need resuscitation and stabilization at birth compared with those born late preterm or at term.1 They therefore require different policies and management than infants born late preterm or term.
Umbilical cord management affects every one of the 15 million infants born preterm annually.2,3 There is growing evidence that umbilical cord management at birth may influence survival, and major neonatal morbidities associated with preterm birth.4–8 Currently, there are several alternative cord management strategies, including deferring clamping on the basis of timing or consideration of the infants’ respiratory status (from here on referred to as delayed cord clamping [DCC]) or milking the intact or cut cord.9
Several mechanisms are proposed to explain how cord management might influence infant mortality and morbidity. At the time of birth ∼30% of the fetal-placental circulation is outside the fetus.10 If the cord is not clamped immediately at birth, blood flow between the placenta and the infant may continue, which may increase placental transfusion, the net transfer of blood from the placenta to the infant. Cord management at birth impacts not only the volume of placental transfusion to the infant but also the cardiovascular transition around the onset of breathing and/or ventilation.11–13 Early cord clamping (ECC) before establishment of respiration may be associated with major hemodynamic consequences especially in extremely preterm and nonvigorous infants who are at high risk of brain injuries.12,14–16
In a statement in 2015, the International Liaison Committee on Resuscitation (ILCOR) gave a weak recommendation for delayed umbilical cord clamping for preterm infants not requiring immediate resuscitation after birth.17 In the statement, they identified many knowledge gaps regarding cord management for both infant and maternal outcomes. To derive stronger recommendations, more evidence is required on existing strategies (such as DCC and milking of the intact or cut cord) and innovative techniques (such as resuscitation with intact cord) in a variety of neonatal populations. There have been many randomized controlled trials (RCTs) published since the latest ILCOR recommendations in 2015, including the largest to date addressing DCC at preterm birth.18
This systematic review and meta-analysis includes this latest evidence. Simultaneously, the ILCOR Consensus on Science with Treatment Recommendations was completed in collaboration with the Cochrane Neonatal group. This will be published separately.
Objective
To determine the effects of different umbilical cord management strategies (including timing of clamping and cord milking) at preterm birth <34 weeks’ gestational age.
Methods
This review was conducted by following the methodology outlined in the Cochrane Handbook for Systematic Reviews of Interventions and adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines.19,20 The protocol was registered prospectively with the International Prospective Register of Systematic Reviews (PROSPERO, CRD42019155475). Full methods are detailed in Appendix 1 in Supplemental Information.
Eligibility Criteria
We considered all RCTs and cluster RCTs in which researchers compared alternative umbilical cord management strategies at preterm birth <34 weeks’ gestational age or with low birth weight <2500 g. Studies were included if the authors reported a mean gestational age of <34 weeks or if >80% of the births were <34 weeks’ gestation.
Studies in which researchers compare the following umbilical cord management interventions were included in this review:
ECC, defined as application of a clamp to the cord <30 seconds after birth, without cord milking;
DCC, defined as application of a clamp to the cord ≥30 seconds after birth or based on physiologic parameters (such as when cord pulsation has ceased or breathing has been initiated), without cord milking;
intact-cord milking (ICM) (also referred to as “stripping”), defined as repeated compression of the cord from the placental side toward the infant with the connection to the placenta intact at any time point within the first few minutes after birth; and
cut-cord milking (CCM) (also referred to as “stripping”), defined as drainage of the cord by compression from the cut end toward the infant after clamping and cutting a long segment.
Outcomes
Review outcomes were selected in consultation with representatives from the World Health Organization and ILCOR. They comprised infant and maternal outcomes that were seen as clinically relevant and therefore likely to change clinical practice.21 All outcomes and their definitions have been summarized in Table 1. Prespecified subgroup analyses, search strategy, study selection, data extraction, risk of bias evaluation, certainty of evidence assessment, and data synthesis are detailed in Appendix 1 in Supplemental Information.
TABLE 1.
Outcome Measures Included in the Systematic Review
| Outcome Measures | |
|---|---|
| Primary outcomes | |
| Neonatal | Survival to discharge from hospital; survival without moderate to severe neurodevelopmental impairment in early childhood (see definitions below); severe IVH: ultrasound diagnosis grades III and IV (Papile et al45) |
| Maternal | PPH: clinically estimated blood loss of at least 500 mL, or as defined by the trial authors |
| Key secondary outcomes | |
| Neonatal | Chronic lung disease (supplemental oxygen at 36 wk’ postmenstrual age)46; NEC (Bell ≥stage II)47; hyperbilirubinemia requiring phototherapy; peak hematocrit or hemoglobin concentrations at 24 h after birth; peak hematocrit or hemoglobin concentrations at 7 d after birth |
| Infant and early childhood | Moderate to severe neurodevelopmental impairment in early childhood; components of moderate to severe neurodevelopmental impairment in early childhood including: (1) cerebral palsy, (2) significant mental developmental delay (Bayley Scales of Infant Development Mental Developmental Index <70; Bayley48), (3) legal blindness (<20/200 visual acuity), and (4) hearing deficit (aided or <60 dB on audiometric testing) |
| Maternal | Severe PPH: clinically estimated blood loss of at least 1000 mL; maternal death or severe morbidity composite (eg, organ failure, ICU admission, or as defined by trial authors); use of therapeutic uterotonic agent/s; blood transfusion; manual removal of the placenta; additional treatment of PPH (uterine tamponade, embolization); postpartum infection |
| Other secondary outcomes | |
| Neonatal | Condition at birth: Apgar score at 5 min of age; resuscitation (need for positive pressure ventilation, intubation, chest compressions); temperature <36° within 1 h of birth |
| Respiratory: respiratory distress syndrome; respiratory support (use of mechanical ventilation or CPAP); duration of respiratory support (days of mechanical ventilation or CPAP); surfactant treatment; home oxygen | |
| Cardiovascular: treatment of patent ductus arteriosus (medical and/or surgical); inotropic support for hypotension during the first 24 h of life; lowest mean arterial blood pressure in the first 12 h of life | |
| Central nervous system: any IVH (grade 1 or greater) on cranial ultrasound, as per Papile classification45; periventricular leukomalacia (any grade [grade ≥1], on basis of ultrasound or MRI49) | |
| Gastrointestinal: NEC requiring surgery | |
| Hematologic: Blood transfusion (any); total No. blood transfusions | |
| Other: late sepsis (positive blood or fungal culture after 3 d of life); retinopathy of prematurity in infants examined (all stages [stage ≥1] and severe [defined as stage ≥3])50; treatment of retinopathy of prematurity; length of infant stay in NICU (d); fully breastfed or mixed feeding at infant discharge; resource use | |
| Maternal | Maternal death; individual components of severe morbidity (as listed above or as defined by the trial authors); prolonged third stage (>30 min); length of third stage of labor; postnatal anemia (defined by trial authors, absolute or relative drop in hemoglobin); maternal length of hospital stay after birth; mother’s or partner’s views regarding the intervention and control |
CPAP, continuous positive airway pressure; PPH, postpartum hemorrhage.
Results
Literature Search and Study Selection
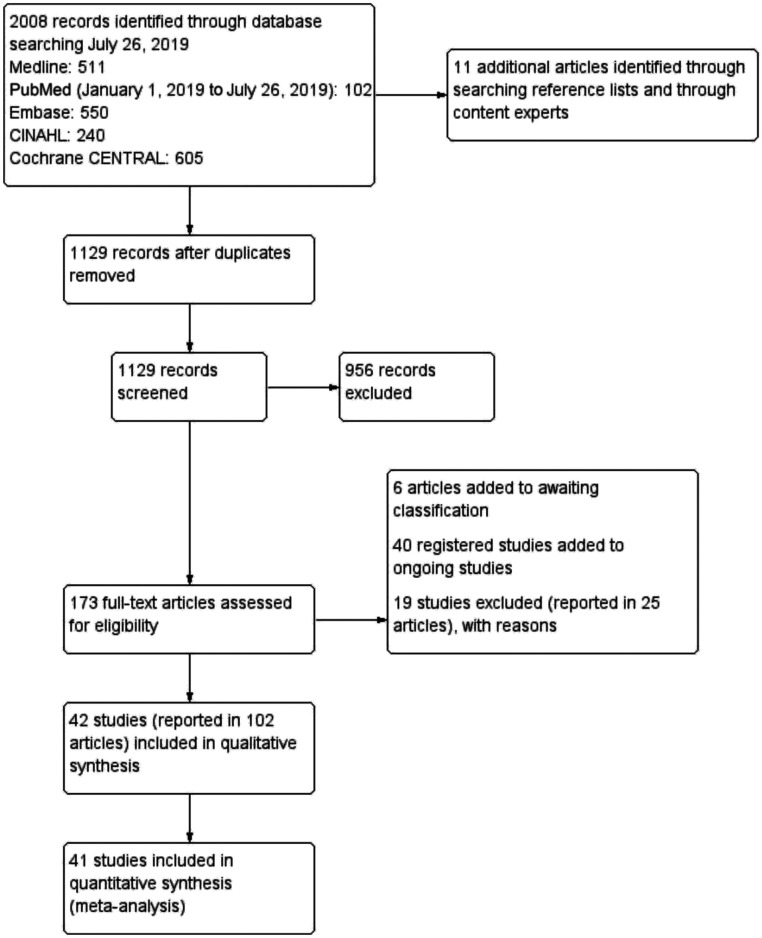
Forty-two studies (reported in 102 articles) including 5772 infants met the inclusion criteria for the review, of which 41 studies (including 5676 infants) had data that could be included in the meta-analysis (Fig 1, Appendix 3 in Supplemental Information: full list of included studies per comparison).
FIGURE 1.
PRISMA study flow diagram. CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Cumulative Index to Nursing and Allied Health Literature.
Study and Participant Characteristics of Included Studies
Study characteristics and participant characteristics for the included studies are outlined for each comparison in Tables 1a–1d in the Supplemental Information and Tables 2–5, respectively.
TABLE 2.
ILCOR Preterm Cord Management Comparison 1: DCC Versus ECC Participant Characteristics
| Study | Intervention (DCC) and Control (ECC) | No. Infants | Gestational Age (Mean ± SD), wk | Birth Weight (Mean ± SD), g | Antenatal Steroid Administration, % | Cesarean Delivery, % |
|---|---|---|---|---|---|---|
| Aladangady et al51 2006 | DCC ≥30–90 s | 23 | NR | NR | NR | 48 |
| ECC (immediate) | 23 | NR | NR | NR | 39 | |
| Armanian et al52 2017 | DCC 30–45 s | 32 | 31.9 ± 1.58 | 1597 ± 282 | 33 | 83 |
| ECC 5–10 s | 31 | 31.0 ± 2.09 | 1518 ± 327 | 47 | 67 | |
| Backes et al532016 | DCC 30–45 s | 18 | 24.4 ± 1.2 | 645 ± 193 | 100 | NR |
| ECC 5–10 s | 22 | 24.6 ± 1.1 | 634 ± 160 | 100 | NR | |
| Baenziger et al54 2007 | DCC 60–90 s | 15 | 30 3/7 ± 2.3 | 1115 ± 344 | NR | 73.3 |
| ECC <20 s | 24 | 29 5/7 ± 2.4 | 1330 ± 484 | NR | 66.7 | |
| Das et al55 2018 | DCC 60 s | 233 | 31.9 ± 1.1 | 1540 ± 374 | 89 | 42 |
| ECC <10 s | 228 | 31.8 ± 1.1 | 1550 ± 336 | 86 | 38 | |
| Dipak et al56 2017 | DCC 1>60 s | 26 | 30.1 ± 1.2 | 1316 ± 163 | NR | 15.9 |
| DCC 2>60 s + ergometrine | 25 | 30.2 ± 1.2 | 1298 ± 178 | NR | 16 | |
| ECC <10 s | 27 | 29.9 ± 1.4 | 1284 ± 176 | NR | 14.8 | |
| Dong et al57 2016 | DCC 45 s | 46 | NR | NR | NR | NR |
| ECC <10 s | 44 | NR | NR | NR | NR | |
| Duley et al58 2018 | DCC >120 s | 137 | 28.9a | 1108 (880–1360)a | 87 | 61 |
| ECC <20 s | 139 | 29.2a | 1180 (900–1418)a | 94 | 67 | |
| Finn et al25 2019 | DCC >60 s (with respiratory support if needed) | 14 | 28.0 (26.4–29.6)a | 925 (630–1490)a | NR | NR |
| ECC <20 s | 12 | 28.5 (25.7–30.5)a | 1080 (755–1613)a | NR | NR | |
| Gokmen et al59 2011 | DCC 30–45 s | 21 | 29.3 ± 1.2 | 1360 ± 413 | 95 | NR |
| ECC 5–10 s | 21 | 29.4 ± 1.5 | 1323 ± 358 | 86 | NR | |
| Hofmeyr et al60 1988 | DCC >60 s ± ergometrine | 24 | NR | NR | NR | NR |
| ECC (immediate) | 14 | NR | NR | NR | NR | |
| Hofmeyr et al61 1993 | DCC 60–120 s | 40 | 31.9 ± 0.33 (SE) | 1761 ± 65 (SE) | NR | 18 |
| ECC (immediate) | 46 | 32.1 ± 0.36 (SE) | 1734 ± 75 (SE) | NR | 26 | |
| Kazemi et al27 2017 | DCC 30–45 s | 35 | 30.1 ± 1.7 | 1261 ± 213 | NR | 100 |
| ECC <10 s | 35 | 29.8 ± 1.8 | 1241 ± 234 | NR | 100 | |
| Kinmond et al62 1993 | DCC 30 s | 17 | 30 (27–32)b | 1500 (1010–2330)b | NR | 0 |
| ECC (at attendant discretion) | 19 | 30 (27–32)b | 1600 (1070–2410)b | NR | 0 | |
| Kugelman et al63 2007 | DCC 30–45 s | 30 | 32.0 ± 2.5 | 1616 ± 497 | 53 | 67 |
| ECC 5–10 s | 35 | 31.9 ± 2.5 | 1676 ± 475 | 62 | 66 | |
| McDonnell et al64 1997 | DCC 30 s | Total enrolled | 30 (28–33)b | 1350 (755–2290)b | NR | NR |
| ECC (immediate) | 46 | 30 (26–33)b | 1505 (865–2110)b | NR | NR | |
| Mercer et al65 2003 | DCC 30–45 s | 16 | 28.0 ± 2.0 | 1064 ± 290 | 94 | 56 |
| ECC 5–10 s | 16 | 27.0 ± 2.2 | 1005 ± 260 | 94 | 37.5 | |
| Mercer et al66 2006 | DCC 30–45 s | 36 | 28.3 ± 2.1 | 1175 ± 346 | 42 | 43 |
| ECC 5–10 s | 36 | 28.2 ± 2.4 | 1151 ± 379 | 47 | 39 | |
| Oh et al67 2011 | DCC 30–45 s | 16 | 26.0 ± 1.4 | 854 ± 222 | NR | NR |
| ECC <10 s | 17 | 26.0 ± 1.1 | 767 ± 243 | NR | NR | |
| Rabe et al68 2000 | DCC 45 s | 20 | 30.01 ± 1.57 | 1185 ± 394 | NR | 78.9 |
| ECC 20 s | 20 | 29.48 ± 1.96 | 1080 ± 340 | NR | 95 | |
| Rana et al69 2018 | DCC 120 s | 50 | 32.3 ± 1.1 | 1818 ± 282 | NR | 16 |
| ECC <30 s | 50 | 32.4 ± 1.0 | 1679 ± 373 | NR | 18 | |
| Ruangkit et al31 2018 | DCC 30–60 s | 51 | 33.6 ± 2.2 | 1895 ± 431 | NR | 100 |
| ECC 3–5 s | 50 | 33.4 ± 2.0 | 1916 ± 402 | NR | 100 | |
| Tarnow-Mordi et al18 2017 | DCC ≥60 s | 818 | 28 ± 2 | 1018 ± 281 | NR | 66.3 |
| ECC ≤10 s | 816 | 28 ± 2 | 1000 ± 269 | NR | 65.1 |
NR, not reported.
Median (interquartile range).
Median (range).
TABLE 5.
ILCOR Preterm Cord Management Comparison 4: DCC Versus ICM, Participant Characteristics
| Study | Intervention | No. Infants | Gestational Age (Mean ± SD) | Birth Weight (Mean ± SD) | Antenatal Steroid Administration, % | Cesarean Delivery, % |
|---|---|---|---|---|---|---|
| Finn et al25 2019 | DCC >60 s (with respiratory support if needed) | 14 | 28 (26.4–29.6)a | 925 (630–1490)a | NR | NR |
| ICM | 19 | 28.4 (25.7–29.6)a | 930 (700–1545)a | NR | NR | |
| Katheria et al79 2015 | DCC 45–60 s | 99 | 28 ± 2 | 1132 ± 392 | 75 | 100 |
| ICM ×4 | 98 | 28 ± 2 | 1255 ± 413 | 69 | 100 | |
| Katheria et al26 2019 | DCC >60 s | 238 | 28.4 ± 2.5 | NR | 88 | 67 |
| ICM ×4 | 236 | 28.4 ± 2.4 | NR | 89 | 76 | |
| Krueger et al80 2015 | DCC 30 s | 32 | 28.3 ± 2.3 | 1087 ± 406 | NR | NR |
| ICM ×4 | 35 | 28.5 ± 2.4 | 1111 ± 363 | NR | NR | |
| Pratesi et al30 2018 | DCC 180 s | 20 | 27.1 ± 1.3 | 955 ± 211 | 92.8 | 42.8 |
| ICM ×4 | 20 | 26.7 ± 1.7 | 960 ± 305 | 91.6 | 54.1 | |
| Rabe et al11 2011 | DCC 30 s | 31 | 29.2 ± 2.3 | 1263 ± 428 | 77 | 58 |
| ICM ×4 | 27 | 29.5 ± 2.7 | 1235 ± 468 | 52 | 78 | |
| Shirk et al32 2019 | DCC 60 s | 104 | 32.0 (29.2–34.0)a | 1579 ± 576 | NR | 49 |
| ICM ×4 | 100 | 32.1 (29.5–34.0)a | 1620 ± 587 | NR | 54 |
NR, not reported.
Median and interquartile range.
TABLE 4.
ILCOR Preterm Cord Management Comparison 3: CCM Versus ECC, Participant Characteristics
| Study | Intervention (CCM) and Control (ECC) | No. Infants | Gestational Age (Mean ± SD) | Birth Weight (Mean ± SD) | Antenatal Steroid Administration, % | Cesarean Delivery, % |
|---|---|---|---|---|---|---|
| Ram Mohan et al78 2018 | CCM ×3 | 30 | 33 (27–36)a | 1400 (945–3750)a | 53 | NR |
| ECC | 30 | 33 (29–36)a | 1516 (760–2370)a | 50 | NR |
NR, not reported.
Median and interquartile range.
All of the included studies were individual RCTs (unit of randomization was either the mother or the infant). Studies were undertaken in a range of countries (although most were high income by World Bank country classifications22). Most studies excluded infants with complications such as major malformations or congenital anomalies.
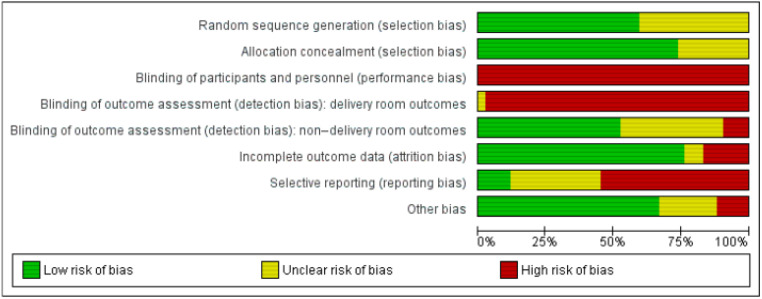
Risk of Bias
Risk of bias is summarized in Fig 2. The majority of studies were at low risk of selection bias (62% low for random sequence generation, 71% low for allocation concealment). All included studies were at high risk of performance bias, because it is difficult, if not impossible, to blind the clinicians managing the infant’s care. Blinding of outcome assessment was rated separately for delivery room outcomes and outcomes assessed at a later stage. Although risk of bias was high across all studies for delivery room outcomes (because of the nature of the intervention), it was low for most studies (55%) for other outcomes. Most studies were at low risk of attrition bias. There were some concerns regarding selective outcome reporting bias. Evidence profile tables were collated for primary and key secondary outcomes applying the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework. These include details on risk of bias (Tables 2–5 in Supplemental Information).
FIGURE 2.
Risk of bias summary. Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
Synthesis of Results
Comparison 1: DCC Compared to ECC
We identified 23 studies including 3514 infants comparing DCC to ECC. Studies were undertaken in a range of countries, mostly high-income. Most studies included births before 32 to 34 weeks’ gestation and were conducted at a single center (78%), but the largest RCTs were multicenter (22%). The studies covered a variety of timings of cord clamping and positioning of the infant. Timing of DCC ranged between 30 and ≥120 seconds, with half the studies (52%) delayed by 30 to 45 seconds. Timing of early or immediate cord clamping ranged from within 5 seconds to within 30 seconds across studies; in most studies (69%), clamping was within 10 seconds.
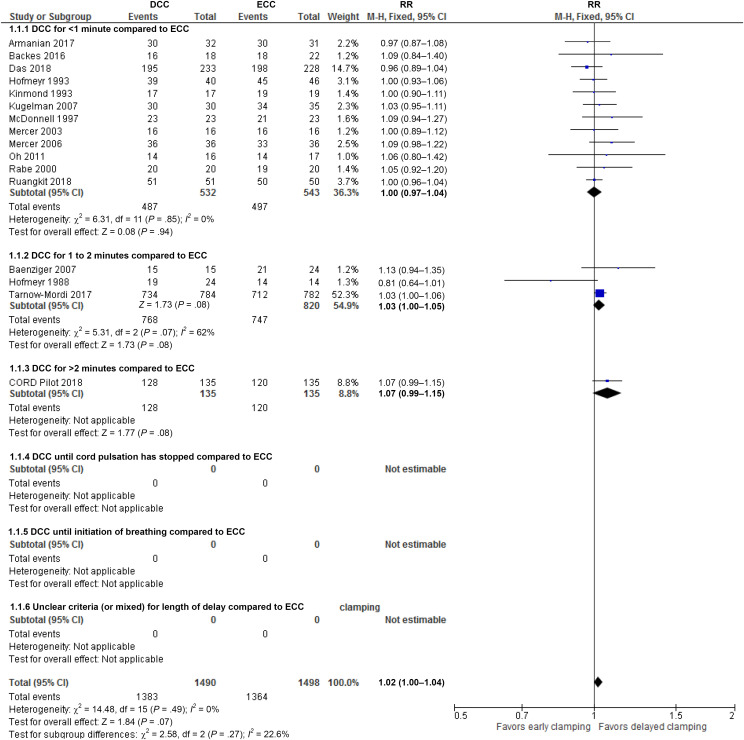
Results for all primary and key outcomes are summarized in Table 6. Compared to ECC, DCC may improve neonatal survival (or reduce neonatal mortality) or may make no difference (survival: risk ratio [RR]: 1.02, 95% confidence interval [CI]: 1.00 to 1.04 (Fig 3); Number needed to benefit: 50, 95% CI: 25 to no benefit; 16 studies, 2988 infants; I2 = 0%, certainty of evidence moderate). This translates into an RR of 0.80 (95% CI: 0.63 to 1.02) for the inverse outcome of mortality (post hoc analysis, Table 6 in Supplemental Information).
TABLE 6.
Key Outcomes for Comparison 1: DCC Versus ECC
| Outcomes | No. Participants (Studies) Follow-up | Certainty of the Evidence | RR (95% CI) | Absolute Risk Difference/MD (95% CI) | I2, % |
|---|---|---|---|---|---|
| Neonatal outcomes | |||||
| Survival to discharge from hospital | 2988 (16 RCTs) | ⊕⊕⊕⊝ Moderatea,b | RR: 1.02 (0.993 to 1.04) | RD: 0.02 (−0.00 to 0.04) | 0 |
| Severe IVH: ultrasound diagnosis grades III, IV | 2972 (14 RCTs) | ⊕⊕⊝⊝ Lowc,d | RR: 0.98 (0.67 to 1.42) | RD: −0.00 (−0.01 to 0.01) | 0 |
| Chronic lung disease: oxygen at 36 wk PMA | 2427 (10 RCTs) | ⊕⊕⊕⊕ Higha | RR: 1.03 (0.94 to 1.13) | RD: 0.01 (−0.02 to 0.04) | 0 |
| NEC (Bell’s stage ≥II or any grade47) | 2745 (14 RCTs) | ⊕⊕⊕⊝ Moderatea,e | RR: 0.83 (0.61 to 1.13) | RD: −0.01 (−0.03 to 0.01) | 0 |
| Peak Hb concentrations within the first 24 h after birth | 196 (4 RCTs) | ⊕⊕⊕⊝ Moderatea,f | Continuous outcome | MD: 1.24 (0.01 to 2.47) | 79 |
| Peak Hct within the first 24 h after birth | 1100 (14 RCTs) | ⊕⊕⊕⊕ Higha | Continuous outcome | MD: 2.63 (1.85 to 3.42) | 5 |
| Peak Hb concentrations within 7 d after birth | 100 (1 RCT) | ⊕⊕⊕⊝ Moderatea,g | Continuous outcome | MD: 9.50 (8.27 to 10.28) | Not estimable |
| Peak Hct within 7 d after birth | 1550 (1 RCT) | ⊕⊕⊕⊕ Higha,h | Continuous outcome | MD: 2.70 (1.88 to 3.52) | Not estimable |
| Hyperbilirubinemia (treated by phototherapy) | 908 (6 RCTs) | ⊕⊕⊕⊕ Higha | RR: 0.99 (0.95 to 1.03) | RD: −0.01 (−0.04 to 0.03) | 0 |
| Infant outcomes | |||||
| Moderate to severe neurodevelopmental impairment in early childhood | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Cerebral palsy in early childhood | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Significant mental developmental delay in early childhood | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Legal blindness in early childhood (<20/200 visual acuity) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Hearing deficit in early childhood (aided or <60 dB on audiometric testing) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Maternal outcomes | |||||
| PPH (clinically estimated blood loss of ≥500 mL) | 1477 (3 RCTs) | ⊕⊝⊝⊝ Very lowd,i,j | RR: 0.93 (0.54 to 1.62) | RD: 0.02 (−0.08 to 0.12) | 52 |
| Maternal death or severe morbidity | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Severe PPH (blood loss ≥1000 mL) | 254 (1 RCT) | ⊕⊝⊝⊝ Very lowi,k,l | RR: 0.81 (0.38 to 1.73) | −0.02 (−0.09 to 0.05) | Not estimable |
| Use of therapeutic uterotonic agents | 1566 (1 RCT) | ⊕⊕⊕⊕ Highk | RR: 1.00 (0.97 to 1.04) | 0.00 (−0.02 to 0.03) | Not estimable |
| Blood transfusion (maternal) | 715 (2 RCTs) | ⊕⊕⊝⊝ Lowl,m | RR: 1.82 (0.78 to 4.23) | 0.02 (−0.01 to 0.04) | 0 |
| Manual removal of the placenta | 105 (1 RCT) | ⊕⊕⊝⊝ Lowk,l,m | RR: 0.99 (0.32 to 3.04) | −0.00 (−0.12 to 0.12) | Not estimable |
| Additional treatment of PPH (uterine tamponade, embolization) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Postpartum infection | 254 (1 RCT) | ⊕⊕⊝⊝ Lowk,m,n | RR: 1.12 (0.73 to 1.72) | 0.03 (−0.08 to 0.13) | Not estimable |
Hb, hemoglobin; Hct, hematocrit; NA, not applicable; PMA, postmenstrual age; PPH, postpartum hemorrhage; RD, risk difference. —, not applicable; ⊕, positive; ⊝, negative.
Some concerns from lack of participant and personnel blinding in most studies. No downgrade for risk of bias because outcome unlikely to be influenced by this. This is a borderline decision.
CI includes null effect, or clinically important outcome of 36 more survivals per 1000. Downgrade by 1 for imprecision. This is a borderline decision.
Largest study (>50% wt) unblinded for outcome assessment. Severe IVH assessment can be subjective. Downgrade by 1 for risk of bias.
CI includes clinically important increase and clinically important decrease. Downgrade by 1 for imprecision.
CI includes clinically important decrease and no effect. Downgrade by 1 for imprecision.
Substantial heterogeneity. Direction of effect the same across all studies. Downgrade by 1 for inconsistency.
Only one 100-ppt single-center study impairs generalizability. Downgrade by 1 for indirectness.
Unable to assess inconsistency (only 1 study). No downgrade.
All studies unblinded for intervention and outcome assessment. Subjective outcome; may have been influenced by lack of blinding. Downgrade by 1 for risk of bias.
Moderate heterogeneity. Downgrade by 1 for inconsistency.
Unable to assess inconsistency (only 1 study). No downgrade.
Very large CI and low event rates. Downgrade by 2 for imprecision.
Some concerns due to lack of participant and personnel blinding. No downgrade for risk of bias because outcome unlikely to be influenced by this. This is a borderline decision.
Only 1 study, large CI, low event rates. Downgrade by 2 for imprecision. (Borderline decision whether to downgrade by 1 or 2).
FIGURE 3.
Forest plot: comparison 1. DCC versus ECC (based on timing of delaying clamping); outcome: survival to discharge from hospital. df, degrees of freedom; M-H, Mantel-Haenszel.
There was no clear difference in the number of infants with severe IVH (RR: 0.98, 95% CI: 0.67 to 1.42) and necrotizing enterocolitis (NEC) (RR: 0.83, 95% CI: 0.61 to 1.13). There was little to no difference for chronic lung disease (RR: 1.03, 95% CI: 0.94 to 1.13) and hyperbilirubinemia treated by phototherapy (RR: 0.99, 95% CI: 0.95 to 1.03).
DCC probably improves hematologic measures. Peak hemoglobin and hematocrit (%) were probably higher for DCC compared to ECC within 24 hours after birth (peak hemoglobin: mean difference [MD]: 1.24 g/dL, 95% CI: 0.01 to 2.47; peak hematocrit: MD: 2.63%, 95% CI: 1.85 to 3.42), and peak hematocrit was higher within 7 days after birth (MD: 2.70%, 95% CI: 1.88 to 3.52).
The evidence was unclear for survival without moderate or severe neurodevelopmental impairment in early childhood (RR: 0.96, 95% CI: 0.78 to 1.17). None of the included studies assessed other early childhood outcomes. Compared to ECC, DCC may make little or no difference to maternal complications, including any postpartum hemorrhage ≥500 mL (RR: 0.93, 95% CI: 0.54 to 1.62), severe postpartum hemorrhage ≥1000 mL, use of therapeutic uterotonic agents, blood transfusion, manual removal of the placenta, or postpartum infection (Table 6). No researchers reported on maternal deaths, severe morbidity, or additional treatment of postpartum hemorrhage. Authors of 1 study reported on mothers’ views and experiences.23,24
Other outcomes are detailed in Table 7a in Supplemental Information. Few differences were found except for hematologic outcomes. Compared with infants in the ECC group, infants in the DCC group had less inotropic support for hypotension during the first 24 hours of life (RR: 0.36, 95% CI: 0.17 to 0.75), a higher measurement of lowest mean arterial blood pressure in the first 12 hours of life (MD: 1.79 mm Hg, 95% CI: 0.53 to 3.05), lower incidence of any blood transfusion (RR: 0.83, 95% CI: 0.77 to 0.90), and a lower total number of blood transfusions per infant (MD: −0.63, 95% CI: −1.08 to −0.17) during hospital course.
Comparison 2: ICM Compared to ECC
We identified 13 studies comparing ICM to ECC (Table 3). Studies in comparison 2 included 1170 infants, and all were single center. Two studies (18%) included only preterm births <30 weeks. Timing of ECC ranged between clamping immediately and within 20 seconds of birth, and in most studies (69%), clamping was immediately. For ICM, the cord was milked between 2 and 4 times, with most studies (54%) reporting milking 3 times.
TABLE 3.
ILCOR Preterm Cord Management Comparison 2: ICM Versus ECC, Participant Characteristics
| Study | Intervention (ICM) and Control (ECC) | No. Infants | Gestational Age (Mean ± SD) | Birth Weight (Mean ± SD) | Antenatal Steroid Administration, % | Cesarean Delivery, % |
|---|---|---|---|---|---|---|
| Alan et al70 2014 | ICM ×3 | 24 | 28.4 ± 1.8 | 1103 ± 236 | 68.2 | 86.4 |
| ECC <10 s | 24 | 28.0 ± 1.9 | 1101 ± 262 | 63.6 | 81.8 | |
| Elimian et al71 2014 | ICM 30 s ×3 | 99 | 30.8 ± 3.1 | 1661 ± 598 | 93.9 | NR |
| ECC <5 s | 101 | 30.7 ± 2.8 | 1542 ± 555 | 97 | NR | |
| El-Naggar et al72 2016 | ICM ×3 | 37 | 27.6 ± 1.8 | 1061 ± 383 | 100 | 56.8 |
| ECC <10 s | 36 | 27.2 ± 2.0 | 1019 ± 282 | 100 | 66.7 | |
| Finn et al25 2010 | ICM ×3 | 19 | 28.4 (25.7–29.6)a | 930 (700–1545) | NR | NR |
| ECC <20 s | 12 | 28.5 (25.7–30)a | 1080 (755–1613) | NR | NR | |
| Hosono et al73 2008 | ICM ×2–3 | 20 | 27.0 ± 1.5 | 836 ± 223 | 35 | 70 |
| ECC (immediate) | 20 | 26.6 ± 1.2 | 846 ± 171 | 35 | 70 | |
| Katheria et al74,75 | ICM ×3 | 30 | 28 ± 2 | 1170 ± 356 | 100 | 60 |
| ECC (immediate) | 30 | 28 ± 3 | 1131 ± 396 | 100 | 44 | |
| Kilicdag et al76 2016 | ICM ×4 | 29 | 30.2 ± 1.9 | 1495 ± 409 | 82.8 | NR |
| ECC (immediate) | 25 | 31.0 ± 1.4 | 1661 ± 351 | 84 | NR | |
| Leal et al28 2018 | ICM ×4 | 69 | NR | 1817 ± 637 | NR | NR |
| ECC <20 s | 69 | NR | 2043 ± 637 | NR | NR | |
| Li et al29 2018 | ICM ×4 | 48 | 33 (28.5–36.4)b | 1940 ± 478 | 85.4 | NR |
| ECC (immediate) | 54 | 33.9 (29.3–36.2)b | 1893 ± 511 | 92.6 | NR | |
| March et al77 2013 | ICM ×3 | 36 | 27.0 (25.5–28.1)a | 755 (688–980)a | NR | 55.6 |
| ECC (immediate) | 39 | 26.3 (25.1–27.1)a | 770 (650–940)a | NR | 66.7 | |
| Mercer et al7 2016 | ICM ×1+ DCC (30–45 s) or ICM ×2–3 | 103 | 28.3 ± 2 | 1203 ± 352 | NR | NR |
| ECC (immediate) | 105 | 28.4 ± 2 | 1136 ± 350 | NR | NR | |
| Silahli et al33 2018 | ICM ×3 | 38 | NR | 1885 (620–2990)b | 51.9 | 56.1 |
| ECC (immediate) | 37 | NR | 1860 (820–2640)b | 48.1 | 43.9 | |
| Song et al34 2012 | ICM ×4 | 34 | 30.1 ± 2.5 | 1256 ± 271 | 70.6 | 70.6 |
| ECC (immediate) | 32 | 29.0 ± 2.6 | 1256 ± 288 | 59.4 | 78.1 |
NR, not reported.
Median and interquartile range.
Median and range.
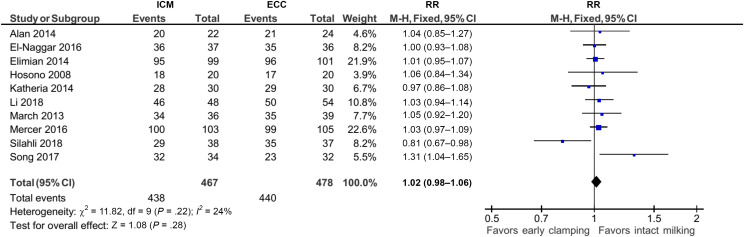
Compared to ECC, ICM may make no difference, slightly decrease, or slightly improve survival to discharge (RR: 1.02, 95% CI: 0.98 to 1.06; I2 = 24%, 10 studies, 945 infants; certainty of evidence moderate) (Fig 4). This translates into an RR of 0.77 (95% CI: 0.49 to 1.23) for the inverse outcome of mortality (post hoc analysis, Table 6 in Supplemental Information).
FIGURE 4.
Forest plot: comparison 2. ICM versus ECC (based on timing of delaying clamping); outcome: survival to discharge from hospital. df, degrees of freedom; M-H, Mantel-Haenszel.
We found no clear difference for severe IVH (RR: 0.72, 95% CI: 0.44 to 1.19), chronic lung disease (RR: 1.02, 95% CI: 0.63 to 1.65), and NEC (RR: 0.80, 95% CI: 0.55 to 1.18), and there was little or no difference for hyperbilirubinemia treated by phototherapy (RR: 1.04, 95% CI: 0.94 to 1.16).
ICM probably improves hematologic measures within 24 hours after birth. Peak hemoglobin and hematocrit (%) were higher for ICM compared to ECC within 24 hours after birth (peak hemoglobin: MD: 1.18 g/dL, 95% CI: 0.65 to 1.71; peak hematocrit: MD: 3.04%, 95% CI: 1.28 to 4.80). Evidence was uncertain for peak hematocrit and hemoglobin within 7 days after birth.
Limited data are available regarding outcomes in later infancy. Certainty of evidence was very low for moderate to severe neurodevelopmental impairment in early childhood (RR: 0.75, 95% CI: 0.21 to 2.71) and cerebral palsy in early childhood (RR: 2.65, 95% CI: 0.88 to 7.97). There were no researchers assessing sensory outcomes in later infancy.
The evidence is uncertain about maternal complications, including severe postpartum hemorrhage ≥1000 mL or blood transfusion, and there were no researchers assessing other maternal complications such as postpartum hemorrhage ≥500 mL (Table 7).
TABLE 7.
Key Outcomes for Comparison 2: ICM Versus ECC
| Outcomes | No. Participants (Studies) Follow-up | Certainty of the Evidence | Relative Effect (95% CI) | RD/MD (95% CI) | I2, % |
|---|---|---|---|---|---|
| Neonatal outcomes | |||||
| Survival to discharge from hospital | 945 (10 RCTs) | ⊕⊕⊕⊝ Moderatea,b,c | RR: 1.02 (0.98 to 1.06) | RD: 0.02 (−0.01 to 0.05) | 24 |
| Severe IVH: ultrasound diagnosis grades III, IV | 889 (10 RCTs) | ⊕⊕⊝⊝ Lowd,e | RR: 0.72 (0.44 to 1.19) | RD: −0.02 (−0.05 to 0.01) | 0 |
| CLD: oxygen at 36 wk PMA | 685 (7 RCTs) | ⊕⊕⊝⊝ Lowf,g | RR: 1.02 (0.63 to 1.65) | RD: −0.02 (−0.12 to 0.08) | 60 |
| NEC (Bell’s stage ≥II or any grade47; requiring surgery) | 843 (9 RCTs) | ⊕⊕⊕⊝ Moderatea,h | RR: 0.80 (0.55 to 1.18) | RD: (−0.06 to 0.02) | 0 |
| Peak Hb concentrations within the first 24 h after birth | 914 (10 RCTs) | ⊕⊕⊕⊝ Moderatei,j | Continuous outcome | MD: 1.18 (0.65 to 1.71) | 71 |
| Peak Hct within the first 24 h after birth | 774 (7 RCTs) | ⊕⊕⊕⊝ Moderatei,j | Continuous outcome | MD: 3.04 (1.28 to 4.80) | 69 |
| Peak Hb concentrations within 7 d after birth | 54 (1 RCT) | ⊕⊕⊝⊝ Lowa,k,l,m | Continuous outcome | MD: 0.60 (−0.57 to 1.77) | Not estimable |
| Peak Hct within 7 d after birth | 54 (1 RCT) | ⊕⊕⊝⊝ Lowa,k,l,m | Continuous outcome | MD: 1.00 (−2.32 to 4.32) | Not estimable |
| Hyperbilirubinemia (treated by phototherapy) | 480 (5 RCTs) | ⊕⊕⊕⊕ Higha | RR: 1.04 (0.94 to 1.16) | RD: 0.03 (−0.04 to 0.10) | 10 |
| Infant outcomes | |||||
| Moderate to severe neurodevelopmental impairment in early childhood | 26 (1 RCT) | ⊕⊝⊝⊝ Very lown,o,p,q | RR: 0.75 (0.21 to 2.71) | RD: −0.08 (−0.42 to 0.26) | Not estimable |
| Cerebral palsy in early childhood | 161 (1 RCT) | ⊕⊝⊝⊝Very lown,o,p,q | RR: 2.65 (0.88 to 7.97) | RD: 0.08 (−0.00 to 0.17) | Not estimable |
| Significant mental developmental delay in early childhood | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Legal blindness in early childhood (<20/200 visual acuity) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Hearing deficit in early childhood (aided or <60 dB on audiometric testing) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Maternal outcomes | |||||
| PPH (clinically estimated blood loss of ≥ 500 mL) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Maternal death or severe morbidity | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Severe PPH (blood loss ≥1000 mL) | 266 (2 RCTs) | ⊕⊝⊝⊝ Very lowr,s | RR: 2.83 (0.12 to 67.01) | RD: 0.01 (−0.02 to 0.03) | Not estimable |
| Use of therapeutic uterotonic agents | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Blood transfusion (maternal) | 66 (1 RCT) | ⊕⊝⊝⊝ Very lowr,t,u | RR: 2.83 (0.12 to 67.01) | RD: 0.03 (−0.05 to 0.11) | Not estimable |
| Manual removal of the placenta | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Additional treatment of PPH (uterine tamponade, embolization) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Postpartum infection | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
CLD, chronic lung disease; Hb, hemoglobin; Hct, hematocrit; PMA, postmenstrual age; PPH, postpartum hemorrhage; RD, risk difference; —, not applicable; ⊕, positive; ⊝, negative.
No downgrade despite concerns due to blinding of intervention and selective outcome reporting bias. Blinding less likely to affect this outcome. Selective outcome reporting bias less likely to affect this estimate because this is a null result. This is a borderline decision.
Some inconsistency, but not sufficient to downgrade. I2 = 24%.
Effect ranges from clinically important reduction to clinically important increase of survival. Downgrade by 1 for imprecision.
Effect ranges from clinically important reduction to clinically important increase. Low events and low participants. Downgrade by 2 for imprecision.
No downgrade despite concerns because of blinding of intervention and selective outcome reporting bias. Blinding less likely to affect this outcome. Biggest and majority of studies were blinded for outcome assessment. Selective outcome reporting bias less likely to affect this estimate because this is a null result. This is a borderline decision.
Effect ranges from clinically important reduction to clinically important increase. Downgrade by 1 for imprecision.
Moderate heterogeneity downgrade by 1 for inconsistency.
Wide CI and relatively low event rates. Downgrade by 1 for imprecision.
No downgrade despite concerns due to blinding of intervention and selective outcome reporting bias. Blinding less likely to affect this outcome. This is a borderline decision.
Substantial heterogeneity, all but one effect estimates point in the same direction. Downgrade by 1 for inconsistency.
Unable to assess inconsistency (only 1 study). No downgrade.
Only 1 small study, wide CI. Downgrade by 1 for imprecision.
Only 1 single-center study impairs generalizability. Downgrade by 1 for indirectness.
No downgrade despite concerns due to blinding of intervention and selective outcome reporting bias. Blinding less likely to affect this outcome. Selective outcome reporting bias less likely to affect this estimate because this is a null result. This is a borderline decision.
Unable to assess inconsistency (only 1 study). No downgrade.
Only 1 small study, low event numbers, very wide CI. Downgrade by 2 for imprecision.
Only 1 single-center study impairs generalizability. Downgrade by 1 for indirectness.
Very wide CI, only 1 event. Downgrade by 2 for imprecision.
All studies unblinded for intervention and outcome assessment. Subjective outcome, may have been influenced by lack of blinding. Downgrade by 1 for risk of bias.
No downgrade despite concerns due to blinding of intervention and selective outcome reporting bias. Blinding less likely to affect this outcome. Selective outcome reporting bias less likely to affect this estimate because this is a null result. This is a borderline decision.
Only 1 single-center study, this impairs generalizability. Downgrade by 1 for indirectness.
Other outcomes are detailed in Table 7b in Supplemental Information. In infants, few differences were found, with the exception of less inotropic support for hypotension during the first 24 hours of life (RR: 0.61, 0.44 to 0.84) and fewer infants receiving ≥1 blood transfusion (RR: 0.73, 95% CI: 0.56 to 0.94) in the ICM group.
Comparison 3: CCM Compared to ECC
We identified 1 single-center study of 60 infants evaluating CCM compared to ECC. The evidence was uncertain for the incidence of survival or its inverse mortality to hospital discharge, with no deaths in either group (Table 8). Evidence was also uncertain for severe IVH (RR: 0.33, 95% CI: 0.01 to 7.87), chronic lung disease (RR: 1.00, 95% CI: 0.07 to 15.26), and NEC (RR: 0.50, 95% CI: 0.05 to 5.22). CCM may increase peak hematocrit concentrations within 24 hours after birth (MD: 3.34%, 95% CI: 0.60 to 6.08). The authors of the study did not report other hematologic measures and did not assess any of the included early childhood or maternal outcomes. Other outcomes are detailed in Table 7c in Supplemental Information.
TABLE 8.
Key Outcomes for Comparison 3: CCM Versus ECC
| Outcomes | No. Participants (Studies) Follow-up | Certainty of the Evidence | Relative Effect (95% CI) | RD/MD (95% CI) | I2 |
|---|---|---|---|---|---|
| Neonatal outcomes | |||||
| Survival to discharge from hospital | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c,d | RR: 1.00 (0.94 to 1.07) | RD: 0.00 (−0.06 to 0.06) | Not estimable |
| Severe IVH: ultrasound diagnosis grades III, IV | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c,d,e | RR: 0.33 (0.01 to 7.87) | RD: −0.03 (−0.12 to 0.05) | Not estimable |
| CLD: oxygen at 36 wk PMA | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowf | RR: 1.00 (0.07 to 15.26) | RD: 0.00 (−0.09 to 0.09) | Not estimable |
| NEC (Bell’s stage ≥II or any grade47; requiring surgery) | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c,d,f | RR: 0.50 (0.05 to 5.22) | RD: −0.03 (−0.14 to 0.08) | Not estimable |
| Peak Hb concentrations within the first 24 h after birth | 0 (0 studies) | — | — | Not estimable | Not estimable |
| Peak Hct within the first 24 h after birth | 60 (1 RCT) | ⊕⊕⊝⊝ Lowa,c,d,g | Continuous outcome | MD: 3.34 (0.60 to 6.08) | Not estimable |
| Peak Hb concentrations within 7 d after birth | 0 (0 studies) | — | — | Not estimable | Not estimable |
| Peak Hct within 7 d after birth | 0 (0 studies) | — | — | Not estimable | Not estimable |
| Hyperbilirubinemia (treated by phototherapy) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Infant outcome | |||||
| Moderate to severe neurodevelopmental impairment in early childhood | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Cerebral palsy in early childhood | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Significant mental developmental delay in early childhood | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Legal blindness in early childhood (<20/200 visual acuity) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Hearing deficit in early childhood (aided or <60 dB on audiometric testing) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Maternal outcomes | |||||
| PPH (clinically estimated blood loss of ≥500 mL) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Maternal death or severe morbidity | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Severe PPH (blood loss ≥1000 mL) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Use of therapeutic uterotonic agents | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Blood transfusion (maternal) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Manual removal of the placenta | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Additional treatment of PPH (uterine tamponade, embolization) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Postpartum infection | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
CLD, chronic lung disease; Hb, hemoglobin; Hct, hematocrit; PMA, postmenstrual age; PPH, postpartum hemorrhage; —, not applicable; ⊕, positive; ⊝, negative.
Some concerns because of lack of blinding. No downgrade, because this outcome unlikely to be influenced by lack of blinding. This is a borderline decision.
No death in either of the intervention groups. Effect could range from clinically meaningful reduction to clinically meaningful increase in survival. Downgrade by 2 for imprecision.
Only 1 study, so not possible to assess inconsistency. No downgrade.
Only 1 single-center study with 60 participants. Impairs generalizability. Downgrade by 1 for indirectness.
Effect could range from very large reduction to very large increase in outcome. Only 1 event. Downgrade by 2 for imprecision.
Effect could range from very large reduction to very large increase in outcome. Low event number. Downgrade by 2 for imprecision.
Low event number, wide CI. Downgrade by 1 for imprecision.
Comparison 4: DCC Compared to ICM
We identified 7 studies including 1073 infants comparing DCC to ICM. The studies were published between 2011 and 2019, and most were single center (71%). Timing of DCC ranged between 30 and 180 seconds, and most studies (71%) reported delay of 30 to 60 seconds. For ICM, the cord was milked between 3 and 4 times, with most studies (71%) reporting milking 4 times.
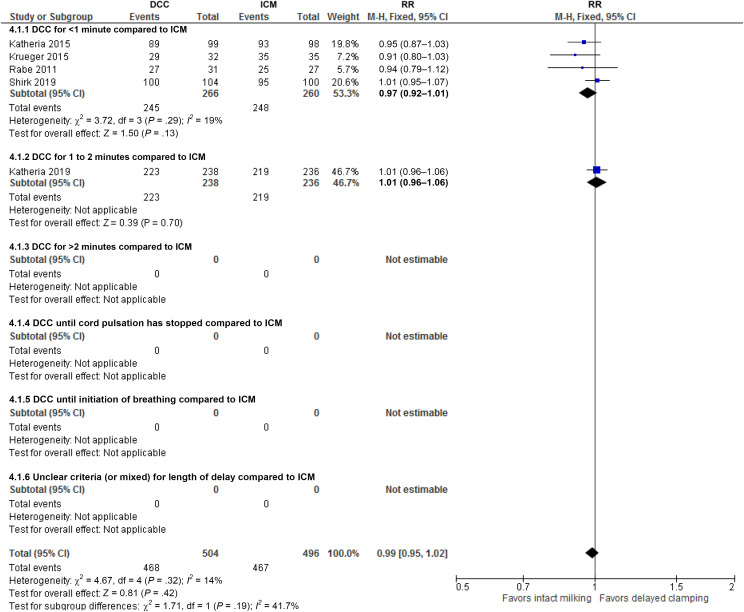
Compared to ICM, DCC may make no difference, slightly decrease, or slightly improve survival to discharge (RR: 0.99, 95% CI: 0.95 to 1.02; I2 = 14%; 5 studies, 1000 infants, certainty of evidence moderate) (Fig 5, Table 9). This translates into an RR of 1.21 (95% CI: 0.76 to 1.94) for the inverse outcome of mortality (post hoc analysis, Table 6 in Supplemental Information).
FIGURE 5.
Forest plot: comparison 4. DCC compared to ICM (based on timing of delaying clamping); outcome: survival to discharge from hospital. df, degrees of freedom; M-H, Mantel-Haenszel.
TABLE 9.
Key Outcomes for Comparison 4: DCC Versus ICM
| Outcomes | No. Participants (Studies) | Certainty of the Evidence (GRADE) | Relative Effect (95% CI) | Risk Difference/MD (95% CI) | I2, % |
|---|---|---|---|---|---|
| Neonatal outcomes | |||||
| Survival to discharge from hospital | 1000 (5 RCTs) | ⊕⊕⊕⊝ Moderatea,b | RR: 0.99 (0.95 to 1.02) | −0.01 (−0.04 to 0.02) | 14 |
| Severe IVH: ultrasound diagnosis grades III, IV | 761 (4 RCTs) | ⊕⊕⊕⊝ Moderatec,d | RR: 0.60 (0.32 to 1.12) | −0.03 (−0.06 to 0.00) | 23 |
| CLD: oxygen at 36 wk PMA | 734 (4 RCTs) | ⊕⊕⊕⊝ Moderatea,d | RR: 0.91 (0.67 to 1.25) | −0.02 (−0.07 to 0.04) | 0 |
| NEC (Bell’s stage ≥II or any grade47; requiring surgery) | 922 (5 RCTs) | ⊕⊕⊕⊝ Moderatea,d | RR: 1.57 (0.83 to 2.97) | 0.02 (−0.01 to 0.04) | 0 |
| Peak Hb concentrations within the first 24 h after birth | 941 (6 RCTs) | ⊕⊕⊕⊝ Moderatea,e | Continuous outcome | MD: −0.02 (−0.56 to 0.53) | 52 |
| Peak Hct within the first 24 h after birth | 841 (5 RCTs) | ⊕⊕⊕⊝ Moderatea,f | Continuous outcome | MD: −0.18 (−1.90 to 1.54) | 51 |
| Peak Hb concentrations within 7 d after birth | 0 (0 studies) | — | — | Not estimable | Not estimable |
| Peak Hct within 7 d after birth | 0 (0 studies) | — | — | Not estimable | Not estimable |
| Hyperbilirubinemia (treated by phototherapy) | 236 (2 RCTs) | ⊕⊕⊕⊝ Moderatea,g | RR: 1.02 (0.92 to 1.13) | 0.06 (−0.10 to 0.22) | 43 |
| Infant outcomes | |||||
| Moderate to severe neurodevelopmental impairment in early childhood | 135 (1 RCT) | ⊕⊕⊝⊝ Lowh,i,j | RR: 0.22 (0.01 to 4.40) | −0.03 (−0.08 to 0.02) | Not estimable |
| Cerebral palsy in early childhood | 193 (2 RCTs) | ⊕⊕⊝⊝ Lowh,k | RR: 0.36 (0.01 to 8.65) | −0.01 (−0.04 to 0.02) | Not estimable |
| Significant mental developmental delay in early childhood | 39 (1 RCT) | ⊕⊝⊝⊝ Very lowh,l,m | RR: 14.06 (0.83 to 237.84) | 0.29 (0.07 to 0.52) | Not estimable |
| Legal blindness in early childhood (<20/200 visual acuity) | 58 (1 study) | — | Continuous outcome | 0.00 (−0.07 to 0.07) | Not estimable |
| Hearing deficit in early childhood (aided or <60 dB on audiometric testing) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Maternal outcomes | |||||
| PPH (clinically estimated blood loss of ≥500 mL) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Maternal death or severe morbidity | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Severe PPH (blood loss ≥1000 mL) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Use of therapeutic uterotonic agents | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Blood transfusion (maternal) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Manual removal of the placenta | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Additional treatment of PPH (uterine tamponade, embolization) | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
| Postpartum infection | 0 (0 studies) | — | Not estimable | Not estimable | Not estimable |
CLD, chronic lung disease; Hb, hemoglobin; Hct, hematocrit; PMA,postmenstrual age; PPH, postpartum hemorrhage; —, not applicable; ⊕, positive; ⊝, negative.
Risk of bias no downgrade, although there were some concerns due to lack of blinding in most studies, because this outcome unlikely to be influenced by lack of blinding. This is a borderline decision.
Effect ranges from clinically important increase to clinically important decrease. Downgrade by 1 for imprecision.
Risk of bias no downgrade, although there were some concerns because of lack of intervention delivery blinding in most studies, because this outcome unlikely to be influenced by lack of blinding of intervention delivery. Outcome assessment blinded in all but 1 small study. This is a borderline decision.
Wide CI, relatively low event rate. Downgrade by 1 for imprecision.
Moderate heterogeneity (I2 = 52%). Downgrade by 1 for inconsistency.
Moderate heterogeneity (I2 = 51%). Downgrade by 1 for inconsistency.
Moderate heterogeneity (I2 = 43%). Downgrade by 1 for inconsistency.
Risk of bias no downgrade, although there were some concerns due to lack of blinding in most studies, because this outcome is unlikely to be influenced by lack of blinding. This is a borderline decision.
Unable to assess inconsistency (only 1 study). No downgrade.
Very wide CI, only 2 events. Downgrade by 2 for inconsistency.
Very wide CI, only 1 event. Downgrade by 2 for inconsistency.
Only 1 single-center study, this impairs generalizability. Downgrade by 1 for indirectness.
Very wide CI, very low event rate. Downgrade by 2 for imprecision.
There were no clear differences for key neonatal morbidities of severe IVH (RR: 0.60, 95% CI: 0.32 to 1.12), chronic lung disease (RR: 0.91, 95% CI: 0.67 to 1.25), NEC (RR: 1.57, 95% CI: 0.83 to 2.97), and hyperbilirubinemia treated phototherapy (RR: 1.05, 95% CI: 0.90 to 1.24).
There were also no clear differences between DCC and ICM for hematologic measures within 24 hours (peak hemoglobin concentrations [g/dL]: MD: −0.02, 95% CI: −0.56 to 0.53, peak hematocrit concentrations [%] MD: −0.18, 95% CI: −1.90 to 1.54). No study authors reported data on peak hemoglobin or peak hematocrit concentration within 7 days after birth.
Limited data were available regarding outcomes in later infancy. Certainty of evidence was low for moderate to severe neurodevelopmental impairment (RR: 0.22, 95% CI: 0.01 to 4.40), cerebral palsy in early childhood (RR: 0.36, 95% CI: 0.01 to 8.65), and significant developmental delay in early childhood (RR: 14.06, 95% CI: 0.83 to 237.84). Researchers of 1 study assessed legal blindness and reported no events, and no researchers assessed hearing deficits.
No researchers reported the included maternal outcomes. Other outcomes are detailed in Table 7d in Supplemental Information. Few differences were found between ICM and DCC.
Comparisons 5 to 8
No studies were identified for any of these comparisons (DCC versus CCM, ICM versus CCM, DCC <60 seconds versus DCC ≥60 seconds, time-based DCC versus physiologic DCC).
Subgroup Analyses
No patterns were identified in the subgroup analyses (Table 8 in Supplemental Information). The number of prespecified subgroup analyses was large, and P values were not adjusted for multiple comparisons. Researchers of only 2 studies reported data by subgroup, limiting the ability to perform subgroup analyses.
Discussion
Summary of Main Findings
In this systematic review and meta-analysis, we identified 42 eligible studies with 5722 infants comparing cord management interventions. Compared to early clamping, delayed clamping may slightly improve infant survival but may make no difference (moderate quality evidence). We found moderate- to high-quality evidence that delayed clamping does not reduce or increase major neonatal morbidities, but it probably improves hematologic measures and may reduce the use of inotropes and blood transfusions in infants.
Compared to early clamping, intact milking may result in increased survival, slightly reduced survival, or make no difference. We found low to moderate quality evidence indicating no clear difference in major neonatal morbidities such as chronic lung disease, IVH, and NEC. Intact milking probably improves hematologic measures.
For the 1 study in which researchers compared ECC to CCM, the evidence was uncertain for infant survival and major morbidities. CCM may increase peak hematocrit within 24 hours after birth.
Compared to ICM, delayed clamping probably results in little to no difference in survival, major neonatal morbidities, and hematologic measures.
Across all comparisons, many of the infants could not be classified into the correct subgroup categories, and thus, meaningful subgroup differences are not possible to detect with the current data.
Agreement and Disagreement With Previous Research
The latest comprehensive review in this area was a Cochrane review with searches conducted in November 2017.8 Authors of that review found a reduction in infant death for delayed compared to early clamping, a slight reduction in any IVH, but no reduction in severe IVH. There was insufficient evidence to derive conclusions for cord milking. With our review, we add new information, because we identified and included 11 additional recently published trials.25–35
Although previous reviews included preterm infants born at less than 37 weeks’ gestational age,4,8 our review is limited to infants born at less than 34 weeks’. Although late preterm infants have increased risk for admission to neonatal intensive care and poor developmental outcome compared with term infants, they do not have the same serious morbidities experienced by less mature preterm infants.36 Therefore, 18 studies included in the Cochrane review were excluded from the current review, leading to a slightly smaller total number of infants (188 less), despite the 11 additional trials.
Previous reviews included infant mortality as a primary outcome, whereas in this review, we assess the inverse of mortality, survival, because this is the standard ILCOR approach. This changes the relative effect measures, as shown in our post hoc sensitivity analysis comparing RRs for survival and mortality using the same data (Table 6 in Supplemental Information). The reason for this is that relative risk depends on the incidence of an event, which is higher for survival than mortality. Thus, the same absolute number of deaths can translate into different relative risk estimates for survival or mortality. For instance, in comparison 1, in the delayed clamping group, 1383 (93%) infants survived and 107 (7%) died. In the early clamping group, 1364 (91%) infants survived and 134 (9%) died. This equals a 2% absolute difference for both survival (93% to 91% = 2%) and mortality (9% to 7% = 2%). However, because survival was more common than mortality, the relative risk indicates a small 2% increase in survival (RR: 0.93/0.91 = 1.02) but a much larger 20% relative risk reduction for mortality (RR: 0.07/0.09 = 0.80).
For comparison 1 (early versus delayed clamping), the relative risk for mortality (indicating a 20% reduction) is similar to that reported in previous reviews (eg, 27% relative risk reduction in the Cochrane review).8 Although for previous reviews, this finding was statistically significant, in the current review, the CI touches the line of no effect. This may be due to different eligibility criteria for gestational age (as outlined above) or to the more-recent studies included in the current review. We did not find a difference in survival between ICM and delayed clamping (comparison 4). Point estimates for survival with intact milking compared to early clamping (comparison 2) are similar to point estimates for delayed compared to early clamping (comparison 1), but CIs are wider in comparison 2 because of fewer included studies. This suggests that intact milking may be comparable to delayed clamping for the outcome of survival, but more evidence is needed to confirm this.
In this review, we find improved hematologic measures and reduced use of inotropes for delayed clamping, and intact and cut milking compared to early clamping, in accordance with previous reviews.4,8,13 This supports the proposed mechanism of placental transfusion (ie, increased net transfer of blood from the placenta to the infant) through delayed clamping or milking.10,37 Our findings did not suggest a difference between delayed clamping and milking with respect to hematologic measures.
Although authors of previous reviews report differences in IVH rates for different cord management strategies,8 we did not find evidence for this in the current review. Animal models have been used to demonstrate that during umbilical cord milking, there was an increase in carotid blood flow and pressure.26 In addition, a recent trial comparing delayed clamping to milking was stopped early in the subgroup of very preterm infants (<28 weeks’ gestation), because of a higher incidence of severe IVH in the milking group.26 Thus, there may be different IVH risks related to cord management strategies depending on gestational age. Further evidence is required to resolve this question. In addition, not all studies in the current review were blinded for assessment of IVH, which is problematic because ultrasound diagnosis of IVH can be rater-dependent.38 Consequently, we downgraded certainty of evidence for this outcome.
Few researchers reported developmental outcomes in early childhood, and the evidence was uncertain for all comparisons. One study published outcomes in early childhood for early clamping compared to delayed clamping (comparison 1) shortly after our search date and was therefore not included in the analysis.39 Authors of this study found that delayed clamping may reduce the risk of death or adverse neurodevelopmental outcome at 2 years of age for children born <32 weeks, but confirmation in larger studies is needed.
Implications for Practice and Research
Cord management at preterm birth is an active research field, evidenced by the number of additional studies included in this review compared to previous reviews. The searches for the latest Cochrane update were conducted in November 2017.8 In <2 years (search to July 2019), we identified 11 new studies. Still, more evidence is being generated; a search in February 2019 identified an additional 62 ongoing trials evaluating cord management strategies in preterm infants.40
Ultimately, we want to answer the question: “which cord management strategy is the best and for whom?” With the current study, we take a step toward answering this question by looking at different comparisons analyzed in pairwise meta-analyses. Yet, there is insufficient evidence, when using aggregate data, to derive a definite answer, particularly when assessing differences for key infant subgroups. Once ongoing trials are completed, a network meta-analysis will be possible, which allows comparing and ranking of multiple interventions simultaneously.41 For assessing differential treatment effects across subgroups, the use of individual participant data can increase statistical power and reduce the risk of ecological bias.42 The individual participant data on Cord Management at Preterm Birth (iCOMP) Collaboration is collating individual participant data from ongoing and completed trials to perform network meta-analysis and subgroup analyses to resolve remaining questions.40 Investigators planning future trials in this area should follow a prospective meta-analysis framework in collaboration with the iCOMP Collaboration to target evidence gaps and avoid research waste.43
Strengths and Limitations
Strengths of this review include its rigorous methods, including a prospectively registered protocol, a comprehensive search strategy, two reviewers independently completing each step of the review process, and the use of GRADE to determine certainty of evidence.44 The author team constitutes a collaboration of world experts in systematic reviews, neonatology, and obstetrics, including the ILCOR taskforce, the Cochrane Neonatal and Pregnancy and Childbirth groups, and independent experts in cord management.
Yet, there are several limitations. For many reported comparisons and outcomes, certainty of evidence was low or very low, or no studies were available. This was mainly due to imprecision and, in some cases, due to inconsistency and risk of bias. For four of the prespecified comparisons, no studies were identified. In this review, only pairwise comparisons are presented; we did not conduct analyses comparing all available comparisons simultaneously (network meta-analysis). Our subgroup analyses were limited by authors of most studies not reporting outcomes separately by subgroup, highlighting the need for individual participant data to resolve these questions. Definitions for early and delayed clamping and milking varied across studies. Delayed clamping ranged from 30 seconds to >2 minutes, and early clamping ranged from within 5 seconds to within 30 seconds. Thus, in some instances, early and delayed clamping groups may have received similar interventions.
Conclusions
DCC at preterm birth may be beneficial compared to early clamping, and these benefits appear to be hemodynamic, but additional evidence is required to confirm this. There is some evidence that ICM may be similarly beneficial, but this needs further study. Additional evidence from ongoing trials and individual participant data network meta-analysis is required to determine which cord management strategies are the most advantageous and for whom.
Acknowledgments
We thank Carol Friesen (Cochrane Neonatal) for developing and conducting literature searches. We thank the following members of the ILCOR Scientific Advisory Committee and Neonatal Life Support Task Force for their input and assistance in developing the protocol and offering feedback on the review: Myra H. Wyckoff, MD, chair; Jonathan Wyllie MBChB, BSC, vice chair; Maria Fernanda de Almeida, MD; Jorge W. Fabres, MD; Joe Fawke, MD; Ruth Guinsburg, MD, PhD; Shigeharu Hosono, MD, PhD; Tetsuya Isayama, MD, MSc, PhD; Vishal S. Kapadia, MD, MSCS; Han-Suk Kim, MD, PhD; Helen G. Liley, MBChB; Chris JD McKinlay, MBChB, PhD; Lindsay Mildenhall, MBChB; Jeffrey M. Perlman, MBChB; Yacov Rabi, MD; Charles C. Roehr, MD, PhD; Edgardo Szyld, MD, MSc; Daniele Trevisanuto, MD; Sithembiso Velaphi, MBChB, FC Ped, PhD; and Gary Weiner, MD. We also thank Slavica Berber, PhD, Sol Libesman, BSc, Kylie Hunter, MSc, Angie Barba, MSc, Mason Aberoumand, MSc, and Hannah Ahern, MSc (National Health and Medical Research Council Clinical Trials Centre, University of Sydney) for assistance in the review process.
Glossary
- CCM
cut-cord milking
- CI
confidence interval
- DCC
delayed cord clamping
- ECC
early cord clamping
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- ICM
intact-cord milking
- ILCOR
International Liaison Committee on Resuscitation
- IVH
intraventricular hemorrhage
- MD
mean difference
- NEC
necrotizing enterocolitis
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
randomized controlled trial
- RR
risk ratio
Footnotes
Ms Seidler conceptualized the protocol, designed the data collection forms, selected studies for inclusion, extracted data, assessed risk of bias and certainty of evidence, conducted the analyses, drafted the initial manuscript, and reviewed and revised the manuscript; Ms Gyte conceptualized the protocol, designed the data collection forms, selected studies for inclusion, extracted data, assessed risk of bias and certainty of evidence, conducted the analyses and reviewed the manuscript; Ms Ovelman assisted with protocol development and study selection, checked data extractions, risk of bias assessments, conducted subgroup analyses and reviewed and prepared the manuscript; Dr Soll conceptualized the protocol, supervised study selection, data extraction, risk of bias and certainty of evidence assessments, and data analyses, and drafted, reviewed and revised the manuscript; Drs Rabe, Díaz-Rossello, Duley, Aziz, Testoni Costa-Nobre, Davis, Schmölzer, and Askie conceptualized the protocol and critically reviewed the manuscript for important intellectual content; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This protocol has been registered with the International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/prospero/) (identifier CRD42019155475).
FINANCIAL DISCLOSURE: The following authors received payment from the American Heart Association, on behalf of the International Liaison Committee on Resuscitation to complete this systematic review: Ms Gyte, Prof Rabe, and Drs Díaz-Rossello and Duley received honorariums as expert systematic reviewers for the Knowledge Synthesis Unit; Ms Seidler received payment as research associate with the Knowledge Synthesis Unit; Ms Ovelman and Dr Soll are employees of the Vermont Oxford Network; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the American Heart Association, on behalf of the International Liaison Committee on Resuscitation for article submission to the editor. This review has also been supported in part by the Vermont Oxford Network. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Ms Seidler is the chair of the individual participant data on Cord Management at Preterm Birth (iCOMP) Collaboration, a meta-analysis on cord clamping management using individual participant data. Dr Duley was chief investigator for the Cord Pilot Trial, collaborator for Australian Placental Transfusion Study, and a member of the secretariat for individual participant data on Cord Management at Preterm Birth. She was awarded a National Institute for Health Research grant for applied research for a program of work on care at very preterm birth, which included the Cord Pilot Trial. Ms Gyte was an investigator for the Cord Pilot Trial. Prof Rabe is the main author for 2 included studies in this review. In the event that an author of this review was also an author on an included study, that author did not assess eligibility, extract data, or assess risk of bias for the study on which he or she was an author. Dr Soll and Ms Ovelman work in the editorial office for Cochrane Neonatal, which received a contract from the American Heart Association as a Knowledge Synthesis Unit to undertake this systematic review for International Liaison Committee on Resuscitation. Dr Soll was a collaborator for the Australian Placental Transfusion Study; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Weiner GM, Zaichkin J; American Academy of Pediatrics; American Heart Association . Textbook of Neonatal Resuscitation (NRP), 7th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2016 [Google Scholar]
- 2.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization; March of Dimes; The Partnership for Maternal, Newborn & Child Health; Save the Children . Born Too Soon: The Global Action Report on Preterm Birth. Geneva, Switzerland: World Health Organization; 2012 [Google Scholar]
- 4.Fogarty M, Osborn DA, Askie L, et al. Delayed vs early umbilical cord clamping for preterm infants: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(1):1–18 [DOI] [PubMed] [Google Scholar]
- 5.Rabe H, Sawyer A, Amess P, Ayers S; Brighton Perinatal Study Group . Neurodevelopmental outcomes at 2 and 3.5 years for very preterm babies enrolled in a randomized trial of milking the umbilical cord versus delayed cord clamping. Neonatology. 2016;109(2):113–119 [DOI] [PubMed] [Google Scholar]
- 6.Al-Wassia H, Shah PS. Efficacy and safety of umbilical cord milking at birth: a systematic review and meta-analysis. JAMA Pediatr. 2015;169(1):18–25 [DOI] [PubMed] [Google Scholar]
- 7.Mercer JS, Erickson-Owens DA, Vohr BR, et al. Effects of placental transfusion on neonatal and 18 month outcomes in preterm infants: a randomized controlled trial. J Pediatr. 2016;168:50–55.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabe H, Gyte GML, Díaz‐Rossello JL, Duley L. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2019;(9):CD003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katheria A, Hosono S, El-Naggar W. A new wrinkle: umbilical cord management (how, when, who). Semin Fetal Neonatal Med. 2018;23(5):321–326 [DOI] [PubMed] [Google Scholar]
- 10.Farrar D, Airey R, Law GR, Tuffnell D, Cattle B, Duley L. Measuring placental transfusion for term births: weighing babies with cord intact. BJOG. 2011;118(1):70–75 [DOI] [PubMed] [Google Scholar]
- 11.Rabe H, Jewison A, Alvarez RF, et al.; Brighton Perinatal Study Group . Milking compared with delayed cord clamping to increase placental transfusion in preterm neonates: a randomized controlled trial. Obstet Gynecol. 2011;117(2 pt 1):205–211 [DOI] [PubMed] [Google Scholar]
- 12.Bhatt S, Alison BJ, Wallace EM, et al. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J Physiol. 2013;591(8):2113–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao AC, Moinian M, Lind J. Distribution of blood between infant and placenta after birth. Lancet. 1969;2(7626):871–873 [DOI] [PubMed] [Google Scholar]
- 14.Hooper SB, Te Pas AB, Lang J, et al. Cardiovascular transition at birth: a physiological sequence. Pediatr Res. 2015;77(5):608–614 [DOI] [PubMed] [Google Scholar]
- 15.Kluckow M, Hooper SB. Using physiology to guide time to cord clamping. Semin Fetal Neonatal Med. 2015;20(4):225–231 [DOI] [PubMed] [Google Scholar]
- 16.Niermeyer S, Velaphi S. Promoting physiologic transition at birth: re-examining resuscitation and the timing of cord clamping. Semin Fetal Neonatal Med. 2013;18(6):385–392 [DOI] [PubMed] [Google Scholar]
- 17.Perlman JM, Wyllie J, Kattwinkel J, et al.; Neonatal Resuscitation Chapter Collaborators . Part 7: neonatal resuscitation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2015;132(16 suppl 1):S204–S241 [DOI] [PubMed] [Google Scholar]
- 18.Tarnow-Mordi W, Morris J, Kirby A, et al.; Australian Placental Transfusion Study Collaborative Group . Delayed versus immediate cord clamping in preterm infants. N Engl J Med. 2017;377(25):2445–2455 [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Altman DG, Sterne JAC; on Behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group . Assessing Risk of Bias in Included Studies. In: Higgins JPT, Churchill R, Chandler J, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0. 2017 [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012 [DOI] [PubMed] [Google Scholar]
- 21.Strand ML, Simon WM, Wyllie J, Wyckoff MH, Weiner G. Consensus outcome rating for international neonatal resuscitation guidelines. Arch Dis Child Fetal Neonatal Ed. 2020;105(3):328–330 [DOI] [PubMed] [Google Scholar]
- 22.The World Bank . World Bank country and lending groups. 2019. Available at: http://data.worldbank.org/about/country-classifications/countryand-lending-groups. Accessed January 12, 2020
- 23.Bradshaw L, Sawyer A, Armstrong-Buisseret L, Mitchell E, Ayers S, Duley L. Cord pilot trial, comparing alternative policies for timing of cord clamping before 32 weeks gestation: follow-up for women up to one year. BMC Pregnancy Childbirth. 2019;19(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradshaw L, Sawyer A, Mitchell E, Armstrong-Buisseret L, Ayers S, Duley L. Women’s experiences of participating in a randomised trial comparing alternative policies for timing of cord clamping at very preterm birth: a questionnaire study. Trials. 2019;20(1):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn D, Ryan DH, Pavel A, et al. Clamping the Umbilical Cord in Premature Deliveries (CUPiD): neuromonitoring in the immediate newborn period in a randomized, controlled trial of preterm infants born at <32 weeks of gestation. J Pediatr. 2019;208:121–126.e2 [DOI] [PubMed] [Google Scholar]
- 26.Katheria A, Reister F, Essers J, et al. Association of umbilical cord milking vs delayed umbilical cord clamping with death or severe intraventricular hemorrhage among preterm infants. JAMA. 2019;322(19):1877–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazemi MV, Akbarianrad Z, Zahedpasha Y, Haghshenas Mojaveri M, Mehraein R. Effects of delayed cord clamping on intraventricular hemorrhage in preterm infants. Iran J Pediatr. 2017;27(5):e6570 [Google Scholar]
- 28.Leal VL, Bueno LP, Vilaplana LC, et al. Effect of milking maneuver in preterm infants: a randomized controlled trial. Fetal Diagn Ther. 2019;45(1):57–61 [DOI] [PubMed] [Google Scholar]
- 29.Li J, Yu B, Wang W, Luo D, Dai QL, Gan XQ. Does intact umbilical cord milking increase infection rates in preterm infants with premature prolonged rupture of membranes? J Matern Fetal Neonatal Med. 2020;33(2):184–190 [DOI] [PubMed] [Google Scholar]
- 30.Pratesi S, Montano S, Ghirardello S, et al. Placental circulation intact trial (PCI-T)-Resuscitation with the placental circulation intact vs. cord milking for very preterm infants: a feasibility study. Front Pediatr. 2018;6:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruangkit C, Bumrungphuet S, Panburana P, Khositseth A, Nuntnarumit P. A randomized controlled trial of immediate versus delayed umbilical cord clamping in multiple-birth infants born preterm. Neonatology. 2019;115(2):156–163 [DOI] [PubMed] [Google Scholar]
- 32.Shirk SK, Manolis SA, Lambers DS, Smith KL. Delayed clamping vs milking of umbilical cord in preterm infants: a randomized controlled trial. Am J Obstet Gynecol. 2019;220(5):482.e1-482.e8 [DOI] [PubMed] [Google Scholar]
- 33.Silahli M, Duman E, Gokmen Z, Toprak E, Gokdemir M, Ecevit A. The relationship between placental transfusion, and thymic size and neonatal morbidities in premature infants - a randomized control trial. J Pak Med Assoc. 2018;68(11):1560–1565 [PubMed] [Google Scholar]
- 34.Song SY, Kim Y, Kang BH, Yoo HJ, Lee M. Safety of umbilical cord milking in very preterm neonates: a randomized controlled study. Obstet Gynecol Sci. 2017;60(6):527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engle WA, Tomashek KM, Wallman C; Committee on Fetus and Newborn, American Academy of Pediatrics . “Late-preterm” infants: a population at risk. Pediatrics. 2007;120(6):1390–1401 [DOI] [PubMed] [Google Scholar]
- 36.Boere I, Roest AA, Wallace E, et al. Umbilical blood flow patterns directly after birth before delayed cord clamping. Arch Dis Child Fetal Neonatal Ed. 2015;100(2):F121–F125 [DOI] [PubMed] [Google Scholar]
- 37.Blank DA, Polglase GR, Kluckow M, et al. Haemodynamic effects of umbilical cord milking in premature sheep during the neonatal transition. Arch Dis Child Fetal Neonatal Ed. 2018;103(6):F539–F546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hintz SR, Slovis T, Bulas D, et al. Interobserver reliability and accuracy of cranial ultrasound scanning interpretation in premature infants. J Pediatr. 2007;150(6):592–596.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armstrong-Buisseret L, Powers K, Dorling J, et al. Randomised trial of cord clamping at very preterm birth: outcomes at 2 years. Arch Dis Child Fetal Neonatal Ed. 2020;105(3):292–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seidler AL, Duley L, Katheria A, et al. Systematic review and network meta-analysis with individual participant data on Cord Management at Preterm Birth (iCOMP): study protocol. BMJ Open. 2020;10(3):e034595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutton A, Ades AE, Cooper N, Abrams K. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics. 2008;26(9):753–767 [DOI] [PubMed] [Google Scholar]
- 42.Stewart L, Tierney J, Clarke M. Reviews of Individual Patient Data. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. London, United Kingdom: The Cochrane Collection; 2011 [Google Scholar]
- 43.Seidler AL, Hunter KE, Cheyne S, Ghersi D, Berlin JA, Askie L. A guide to prospective meta-analysis. BMJ. 2019;367:l5342. [DOI] [PubMed] [Google Scholar]
- 44.Schünemann HBJ, Brożek J, Guyatt G, Oxman A, eds.. GRADE Handbook. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. The GRADE Working Group; 2013. Available at: https://gdt.gradepro.org/app/handbook/handbook.html#h.svwngs6pm0f2 [Google Scholar]
- 45.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights <1500 gm. J Pediatr. 1978;92(4):529–534 [DOI] [PubMed] [Google Scholar]
- 46.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729 [DOI] [PubMed] [Google Scholar]
- 47.Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bayley N. Bayley Scales of Infant Development (BSID-II). San Antonio, TX: Psychological Corporation; 1993 [Google Scholar]
- 49.de Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49(1):1–6 [DOI] [PubMed] [Google Scholar]
- 50.International Committee for the Classification of Retinopathy of Prematurity . The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123(7):991–999 [DOI] [PubMed] [Google Scholar]
- 51.Aladangady N, McHugh S, Aitchison TC, Wardrop CAJ, Holland BM. Infant’s blood vol in a controlled trial of placental transfusion at preterm delivery. Pediatrics. 2006;117:93–98 [DOI] [PubMed] [Google Scholar]
- 52.Armanian AM, Ghasemi Tehrani H, Ansari M, Ghasemi S. Is “delayed umbilical cord clamping” beneficial for premature newborns? Int J Pediatr. 2017;5:4909–4918 [Google Scholar]
- 53.Backes CH, Huang H, Iams JD, Bauer JA, Giannone PJ. Timing of umbilical cord clamping among infants born at 22 through 27 weeks’ gestation. J Perinatol. 2016;36(1):35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baenziger O, Stolkin F. , Keel, M, et al. The influence of the timing of cord clamping on postnatal cerebral oxygenation in preterm neonates: a randomized, controlled trial. Pediatrics 2007;119:455–459 [DOI] [PubMed] [Google Scholar]
- 55.Das B, Sundaram V, Kumar P, Mordi WT, Dhaliwal LK, Das R. Effect of placental transfusion on iron stores in moderately preterm neonates of 30–33 weeks gestation. Indian J Pediatr. 2018;85(3):172–178 [DOI] [PubMed] [Google Scholar]
- 56.Dipak KN, Nanavati RN, Kabra NK, Srinivasan A, Ananthan A. Effect of delayed cord clamping on hematocrit, and thermal and hemodynamic stability in preterm neonates: A randomized controlled trial. Indian P. 2017;54(2):112–115 [DOI] [PubMed] [Google Scholar]
- 57.Dong XY, Sun XF, Li MM, Yu ZB, Han SP. Influence of delayed cord clamping on preterm infants with a gestational age of <32 weeks [in Chinese]. Zhongguo Dang Dai Er Ke Za Zhi. 2016;18(7):635–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duley L, Dorling J, Pushpa-Rajah A, et al.; Cord Pilot Trial Collaborative Group . Randomised trial of cord clamping and initial stabilisation at very preterm birth. Arch Dis Child Fetal Neonatal Ed. 2018;103(1):F6–F14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gokmen Z, Ozkiraz S, Tarcan A, Kozanoglu I, Ozcimen EE, Ozbek N. Effects of delayed umbilical cord clamping on peripheral blood hematopoietic stem cells in premature neonates. J Perinat Med. 2011;39:323–329 [DOI] [PubMed] [Google Scholar]
- 60.Hofmeyr GJ, Bolton KD, Bowen DC, Govan JJ. Periventricular/intraventricular haemorrhage and umbilical cord clamping. S Afr Med. 1988;73:104–106 [PubMed] [Google Scholar]
- 61.Hofmeyr GJ, Gobetz L, Bex PJM, et al. Periventricular/intraventricular hemorrhage following early and delayed umbilical cord clamping: a randomized trial. Online J Curr Clin Trials. 1993;Doc No 110 [PubMed] [Google Scholar]
- 62.Kinmond S, Aitchison TC, Holland BM, Jones JG, Turner TL, Wardrop CA. Umbilical cord clamping and preterm infants: a randomised trial. BMJ. 1993;306:172–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kugelman A, Borenstein-Levin L, Riskin A, et al. Immediate versus delayed umbilical cord clamping in premature neonates born <35 weeks: a prospective, randomized, controlled study. Am J Perinatol. 2007;24:307–315 [DOI] [PubMed] [Google Scholar]
- 64.McDonnell M, Henderson Smart DJ. Delayed umbilical cord clamping in preterm infants: a feasibility study. J Paediatr Child Health. 1997;33(4):308–310 [DOI] [PubMed] [Google Scholar]
- 65.Mercer JS, McGrath MM, Hensman A, Silver H, Oh W. Immediate and delayed cord clamping in infants born between 24 and 32 weeks: a pilot randomized controlled trial. J Perinatol. 2003;23:466–472 [DOI] [PubMed] [Google Scholar]
- 66.Mercer JS, Vohr BR, Erickson-Owens DA, Padbury JF, Oh W. Seven-month developmental outcomes of very low birth weight infants enrolled in a randomized controlled trial of delayed versus immediate cord clamping. J Perinatol. 2010;30(1):11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oh W, Fanaroff AA, Carlo WA, et al. Effects of delayed cord clamping in very-low-birth weight infants. J Perinatol. 2011;31:S68–S71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rabe H, Wacker A, Hulskamp G, et al. A randomised controlled trial of delayed cord clamping in very low birth weight preterm infants. Eur J Pediatr. 2000;159(10):775–777 [DOI] [PubMed] [Google Scholar]
- 69.Rana A, Agarwal K, Ramji S, Gandhi G, Sahu L. Safety of delayed umbilical cord clamping in preterm neonates of less than 34 weeks of gestation: a randomized controlled trial. Obstet Gynecol Sci. 2018;61(6):655–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alan S, Arsan S, Okulu E, et al. Effects of umbilical cord milking on the need for packed red blood cell transfusions and early neonatal hemodynamic adaptation in preterm infants born ≤1500 g: a prospective, randomized, controlled trial. J Pediatr Hematol Oncol. 2014;36(8):e493–e498 [DOI] [PubMed] [Google Scholar]
- 71.Elimian A, Goodman J, Escobedo M, Nightingale L, Knudtson E, Williams M. Immediate compared with delayed cord clamping in the preterm neonate. Obstet Gynecol. 2014;124(6):1075–1079 [DOI] [PubMed] [Google Scholar]
- 72.El-Naggar W, Simpson D, Hussain A, et al. The effect of umbilical cord milking on hemodynamic status of preterm infants: a randomized controlled trial. J Paediatr Child Health. 2016;21:e88 [Google Scholar]
- 73.Hosono S, Mugishima H, Fujita H, et al. Umbilical cord milking reduces the need for red cell transfusions and improves neonatal adaptation in infants born less than 29 weeks’ gestation: a randomized controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93:F14–F19 [DOI] [PubMed] [Google Scholar]
- 74.Katheria A, Blank D, Rich W, Finer N. Umbilical cord milking improves transition in premature infants at birth. Plos One. 2014;9(4):e94085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katheria AC, Leone TA, Woelkers D, Garey DM, Rich W, Finer NN. The effects of umbilical cord milking on hemodynamics and neonatal outcomes in premature neonates. J Pediatr. 2014;164(5):1045–1050 [DOI] [PubMed] [Google Scholar]
- 76.Kilicdag H, Gulcan H, Hanta D, et al. Is umbilical cord milking always an advantage? J Matern Fetal Neonatal Med. 2016;29(4):615–618 [DOI] [PubMed] [Google Scholar]
- 77.March MI, Hacker MR, Parson AW, Modest AM, De M. The effects of umbilical cord milking in extremely preterm infants: a randomized controlled trial. J Perinatol. 2013;33(10):763–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ram Mohan G, Shashidhar A, Chandrakala BS, Nesargi S, Suman Rao PN. Umbilical cord milking in preterm neonates requiring resuscitation: a randomized controlled trial. Resuscitation. 2018;130:88–91 [DOI] [PubMed] [Google Scholar]
- 79.Katheria AC, Truong G, Cousins L, Oshiro B, Finer NN. Umbilical cord milking versus delayed cord clamping in preterm infants. Pediatrics. 2015;136(1):61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krueger MS, Eyal FG, Peevy KJ, Hamm CR, Whitehurst RM, Lewis DF. Delayed cord clamping with and without cord stripping: a prospective randomized trial of preterm neonates. Am J Obstetr Gynecol. 2015;212(3):394.e1. [DOI] [PubMed] [Google Scholar]