Abstract
Objective:
We are studying a new method for estimating blood volume flow that uses 3D ultrasound to measure the total integrated flux through an ultrasound-generated Gaussian surface that intersects the umbilical cord. This method makes none of the assumptions typically required with standard 1D spectral Doppler volume flow estimates. We compared the variations in volume flow estimates between techniques in the umbilical vein.
Methods:
The study was IRB approved and all 12 subjects gave informed consent. Because we had no reference standard for the true umbilical vein volume flow, we compared the variations of the measurements for the two flow measuring techniques. At least 3 separate spectral Doppler and 3 separate Gaussian surface measurements were made along the umbilical vein. Means, standard deviations, and coefficients of variation (standard deviation/mean) for the flow estimation techniques were calculated for each subject. P < 0.05 was considered significant.
Results:
The range of the mean volume flow estimates was 174 – 577 mL/min using the spectral Doppler method and 100 – 341 mL/min using the Gaussian surface integration (GSI) method. The mean standard deviations were 161 ± 95 mL/min and 45 ± 48 mL/min for the spectral Doppler and GSI methods respectively (p < 0.003). The mean coefficients of variation were 0.46 ± 0.17 and 0.18 ± 0.14 for the spectral Doppler and GSI methods respectively (p< 0.002).
Conclusion:
A new volume flow estimation method using 3D ultrasound appears to have significantly less variation in estimates than the standard 1D spectral Doppler method.
Keywords: Doppler, umbilical cord blood flow, umbilical vein volume flow, color Doppler, power Doppler
Introduction:
Umbilical cord blood flow has been considered the physiological analog in fetuses to cardiac output in adults, and studies have shown the potential of true umbilical cord blood flow in the early diagnosis of fetal conditions such as intrauterine growth restriction and pre-eclampsia [1–11]. Unfortunately, umbilical cord blood flow measurements are rarely employed in clinical practice. This is because they are difficult and tedious to perform and require multiple unjustified assumptions to make the flow estimate [12–15]. Since standard blood flow estimates are based on both measurements of vessel diameter from 2D B-mode ultrasound images to calculate cross-sectional area and 1D spectral Doppler for making mean velocity estimates, these flow measurements are angle dependent, flow geometry dependent, and vessel cross-section shape dependent. Accumulation of errors in these measurements lead to large errors in blood flow estimates [13].
We have been developing a method for estimating blood volume flow that uses a process that has none of the limitations described above[11, 16–18]. The method is angle independent, flow profile independent, and vessel geometry independent. It uses a technique developed by the mathematician Gauss which defines blood flow as the integral of the total flux across a vessel. The method requires a three-dimensional (3D) ultrasound acquisition in order to define a C-surface across the ultrasound field that intersects the vessel of interest. The method, originally defined in 1979, has been used to determine cardiac output, flows through transjugular intrahepatic portosystemic shunts (TIPS), and umbilical vein blood flow [18–25]. The C-surface is acquired such that all of the ultrasound Doppler velocity components from the transducer are perpendicular to the C-surface[16].
Given the many sources of error inherent in the spectral Doppler volume flow technique, we wanted to determine if the variations among estimates of umbilical vein volume flow would be different between the two flow measurement techniques. We, therefore, designed a study to test this.
Methods:
This was a University of Michigan IRBMED (HUM00075665) approved prospective study in which all subjects gave written informed consent. All examinations were performed at the University of Michigan Von Voigtlander Women’s Hospital. The study was limited to women who had high risk gestations and were hospitalized during pregnancy. Since all of these patients were hospitalized under observation, they were not pressed for time and were very willing to participate in our study. Twelve women between the gestational ages of 24 and 35 5/7 weeks were included in the study. Each patient had a singleton gestation. The demographics of the included patients are shown in Table 1.
Table 1:
(Subject descriptions):
Composite table showing the clinical conditions, method of delivery, gestational age at delivery, birth weight, and sex of the fetuses included in this study. g = grams, M = male, F = female
| Patient # | Reason(s) for Hospitalization | Method of Delivery | Gestational Age at Delivery (weeks, days) | Birth Weight (g) | Sex |
|---|---|---|---|---|---|
| 1 | autoimmune neutropenia, hx of pre-eclampsia, hx of cervical inompetence, prior C-section | C-Section | 39w2d | 3795 | M |
| 2 | severe pre-eclampsia | C-Section | 34w0d | 2130 | M |
| 3 | placenta accreta, bleeding, hysterectomy | C-Section | 37w0d | 2845 | F |
| 4 | elevated blood pressure | C-Section | 31w1d | 975 | M |
| 5 | Gestational diabetes, at risk for pre-eclampsia, beta thalasemia | C-Section | 39w1d | 2935 | F |
| 6 | severe IUGR, pre-eclampsia | C-Section | 32w3d | 1505 | F |
| 7 | severe IUGR, pre-eclampsia | C-Section | 28w5d | 670 | F |
| 8 | severe preeclampsia, multiple congenital anomalies | C-Section | 33w6d | 1760 | M |
| 9 | systemic lupus | Vaginal | 37w1d | 2730 | M |
| 10 | severe pre-eclampsia | Vaginal | 36w4d | 2075 | M |
| 11 | chronic hypertension | C-Section | 36w6d | 3335 | M |
| 12 | chronic hypertension with pre-eclampsia | C-Section | 36w6d | 2177 | F |
Scans were performed with a Philips EPIQ 7 ultrasound scanner using a 2D array transducer, either an X6-1 or XL14-3. Choice of transducer depended on scanning related issues, such as body habitus and depth to the sampling site along the umbilical vein, and/or the availability of a specific transducer. Across all subjects’ volume flow measurements, there were 25 spectral Doppler estimates made with the XL14-3, 18 spectral Doppler estimates made with the X6-1, 22 Gaussian surface integration (GSI) estimates made with the XL14-3, and 18 GSI estimates made with the X6-1. In one subject measurements were made only with the X6-1, six had only XL14-3 measurements, and five had both X6-1 and XL14-3 measurements. These are shown in Table 2. One spectral Doppler measurement with the X6-1 was excluded due to lack of angle correction and diameter measurement.
Table 2:
(Volume flow data and analysis):
Measured volume flow data from 12 subjects using the Gaussian surface method and the spectral Doppler method. SD = standard deviation, CV = coefficient of variation, Trans = tranducer(s). Each subject had at least 3 Gaussian surface measurements and at least 3 spectral Doppler measurements of the umbilical vein volume flow in their umbilical cord. Four subjects had 4 Gaussian surface measurements and 6 subjects had 4 spectral Doppler measurements. When only 3 measurements were made in a particular category, an “x” is inserted into the empty slot.
| Subject | Trans | Gaussian Surface | Mean | SD | CV | Spectral Doppler | Mean | SD | CV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mL/min | mL/min | mL/min | mL/min | mL/min | mL/min | ||||||||||
| 1 | XL14-3 | 230 | 246 | 229 | x | 235 | 9.5 | 0.041 | 562 | 959 | 209 | x | 577 | 375.2 | 0.651 |
| 2 | X6-1 | 290 | 220 | 240 | x | 250 | 36.1 | 0.144 | 107 | 374 | 417 | x | 299 | 167.9 | 0.561 |
| 3 | XL14-3 | 544 | 209 | 246 | x | 333 | 183.7 | 0.552 | 603 | 285 | 537 | x | 475 | 167.8 | 0.353 |
| 4 | XL14-3 | 186 | 203 | 187 | x | 192 | 9.5 | 0.050 | 94 | 243 | 234 | x | 190 | 83.5 | 0.439 |
| 5 | XL14-3 | 289 | 318 | 417 | x | 341 | 67.1 | 0.197 | 328 | 472 | 370 | 274 | 361 | 83.8 | 0.232 |
| 6 | XL14-3 | 266 | 217 | 331 | x | 271 | 57.2 | 0.211 | 368 | 111 | 396 | x | 292 | 157.1 | 0.539 |
| 7 | XL14-3 | 122 | 110 | 110 | x | 114 | 6.9 | 0.061 | 166 | 235 | 180 | 187 | 192 | 30.0 | 0.156 |
| 8 | X6-1/XL14-3 | 90 | 83 | 123 | 104 | 100 | 17.6 | 0.176 | 207 | 113 | 202 | x | 174 | 52.9 | 0.304 |
| 9 | X6-1/XL14-3 | 120 | 209 | 138 | 166 | 158 | 38.8 | 0.245 | 114 | 137 | 191 | 478 | 230 | 168.5 | 0.732 |
| 10 | X6-1/XL14-3 | 218 | 222 | 263 | x | 234 | 24.9 | 0.106 | 313 | 434 | 156 | 585 | 372 | 182.0 | 0.489 |
| 11 | X6-1/XL14-3 | 233 | 335 | 284 | 323 | 294 | 46.0 | 0.157 | 220 | 856 | 443 | 418 | 484 | 267.2 | 0.552 |
| 12 | X6-1/XL14-3 | 261 | 230 | 218 | 164 | 218 | 40.5 | 0.185 | 591 | 184 | 572 | 354 | 425 | 193.5 | 0.455 |
At least six separate volume flow measurements were made along the umbilical vein in each case. One measurement was made using the standard spectral Doppler technique in which a straight segment of umbilical vein was identified. A Doppler sample volume was placed in the vein with the range-gate extended across the vein’s lumen, an angle-corrected Doppler spectrum was obtained and the mean velocity through the range gate measured over time. The umbilical vein diameter was measured across the vessel perpendicular to the angle-correction marker. When necessary, color Doppler was used to define the margins of the vessel when the vessel was in an orientation not perpendicular to the sound field. Spectral Doppler volume flow was calculated as:
where Q is volume flow, d is the diameter of the umbilical vein as demonstrated along the segment of vein being analyzed, and <v> is the mean velocity of the blood at the site of measurement. This calculation was performed on the ultrasound scanner itself. Each Doppler measurement including vessel diameter, angle correction, and site of measurement was assessed by two observers (JMR and SZP), and both observers had to agree on the measurement before it was recorded. For each of the spectral Doppler volume flow estimates made on the ultrasound machine, the two observers could see what measurement was recorded on screen.
We attempted as best as possible to pair spectral Doppler measurements with GSI measurements at similar sites along the umbilical cord. However, because of differences in the acquisition methods, identical sites for each method could not be used. Fortunately, volume flow should be the same at all locations along the cord such that the variation on measures should be indicative of the associated errors and not the absolute position along the cord.
Spectral Doppler measurements are made in a longitudinal orientation with the direction of the cord positioned parallel to the scan head face (Fig 1). For the GSI method, the cord is more or less directed toward the scan head so the beam could be swept across the cord (Fig 1). The orientation is not absolutely critical, since the method is angle independent as long as a Doppler shift can be obtained across the flow. Changes in fetal position also made it impossible to absolutely scan at the same location for both the spectral Doppler and GSI methods. Ultimately, as mentioned, at least 6 separate measurements of volume flow were made in each case. Three subjects had an additional spectral Doppler measurement not paired with a GSI measurement and one subject had an additional GSI measurement not paired with a spectral Doppler measurement (Table 2).
Fig. 1.
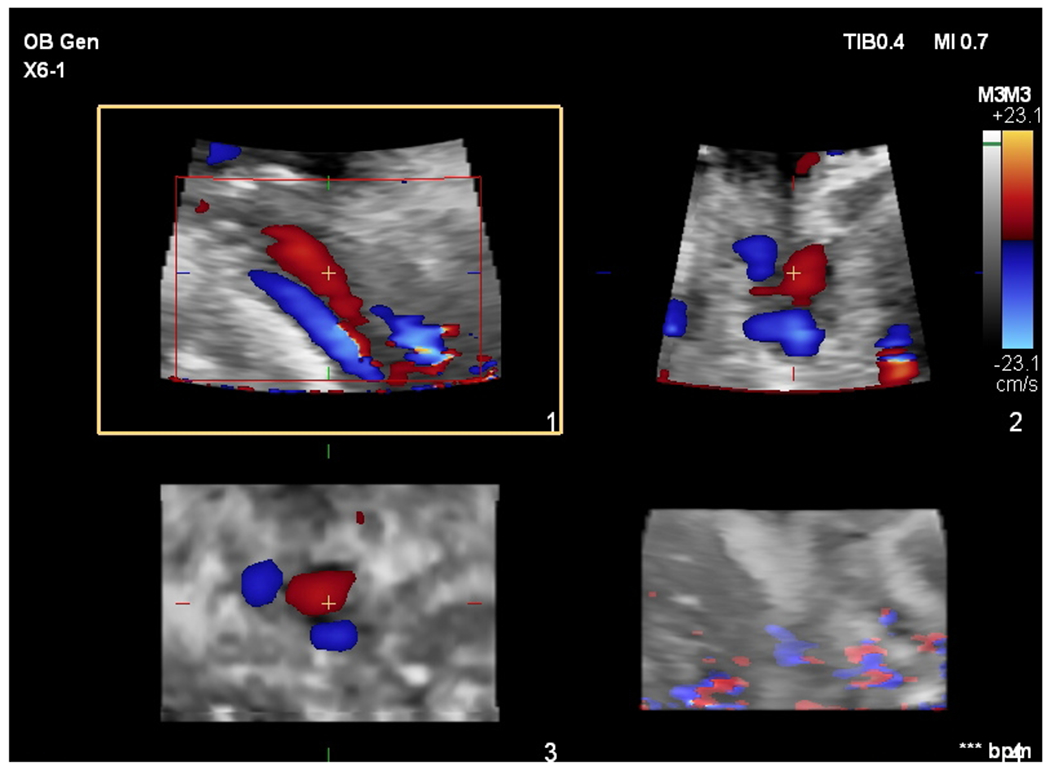
a: Color flow images of the vessels in the umbilical cord in one of the sampling positions for subject 12 in this study. The umbilical arteries are blue and the umbilical vein is red. A “+” is positioned in the umbilical vein identifying a 3D point that coincides in the 3 acquired views. The upper left (axial-lateral) image with a square around it and labeled 1 at the lower right edge is an image along the length of the vein and one of the arteries. The upper right (axial-elevational) image, image 2, is perpendicular to image 1. It would correspond to a transverse image if image 1 is a longitudinal image of the umbilical vein and umbilical arteries. The lower left (elevational-lateral) image, image 3, is the C-surface or Gaussian surface image from which volume flow is calculated. Summing the local flux measurements across the vein (red) in this image produces a volume flow estimate. The lower right image corresponds to a 3D rendering in which the vessels are poorly visualized – this view is not used when positioning the cord in the C-surface, nor for volume flow measurement. Color bar indicates velocity in centimeters per second.
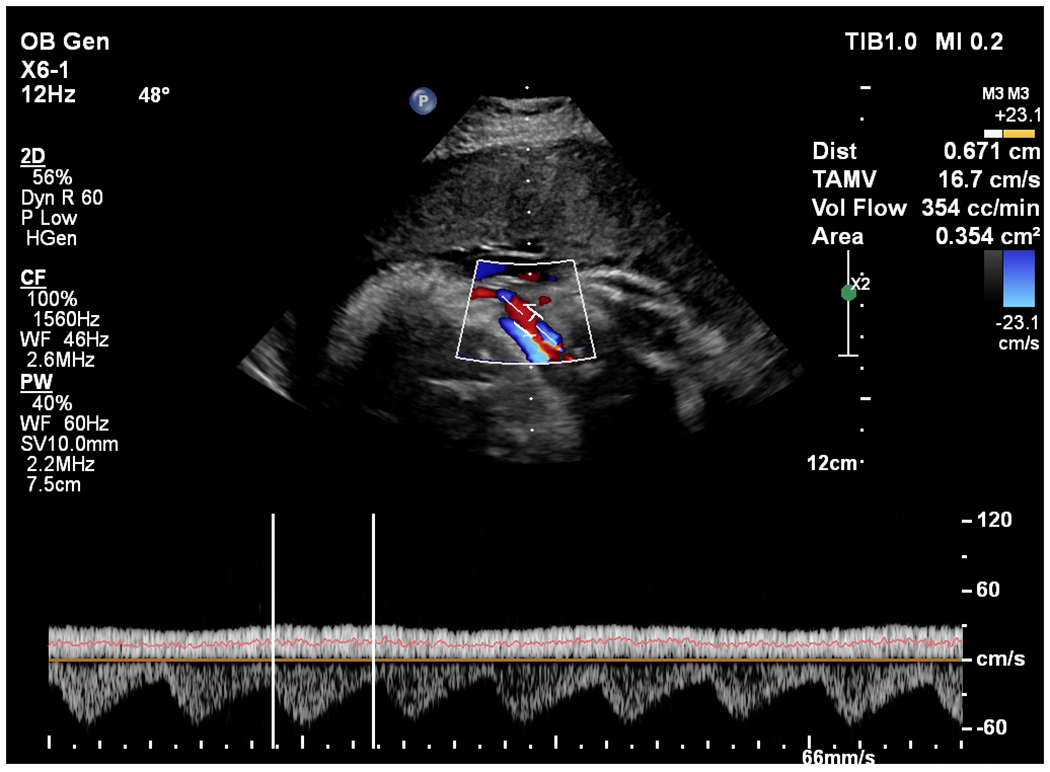
b: Color flow image and angle-corrected spectral Doppler estimate for volume flow in subject 12. The angle correction (48°) and vessel diameter (0.671 cm) estimates are shown. The venous spectral trace with the mean estimate represented by the orange line through the venous trace is shown at the bottom of the image. The two white vertical bars on the trace indicate the time interval used for averaging. The volume flow estimate is 354 cc/min and is computed using the average flow velocity (TAMV = 16.7 cm/s) and the area estimate based on the diameter measurement (0.354 cm2). Color bar indicates velocity in centimeters per second.
The GSI volume flow method itself has been described previously [16]. However briefly, a segment of umbilical cord is identified such that a C-surface can be defined across the ultrasound beam that intersects the umbilical vein, and the surface is defined as being equidistant along all the ultrasound beams from the scan head surface. This particular Gaussian surface is not unique and is defined so that all of the Doppler vectors are perpendicular to the surface. This is perfect for calculating flow using Gauss’s theorem (Eq. 1). In order to do this, a 2D ultrasound array sweeps the beam across the blood vessel making a Doppler estimate for each beam as it intersects the vessel cross-section. The area of each beam’s cross-section where it intersects the umbilical vein multiplied by the mean Doppler shift at that position represents the local flux. The sum of all these local fluxes across the vein is equal to volume flow. This is represented by the following equation and is known as Gauss’s Theorem:
| (Eq. 1) |
where Q is volume flow and v is the velocity of blood passing through a small area component, dA. In this case, dA corresponds to the beam cross-section. “•” is the dot product which ensures that the velocity component being measured is perpendicular to the small area component, and the dot product v • dA is the local flux. For ultrasound, the velocity component in the dot product is along the ultrasound beam which removes the need to angle correct the measurement [16].
The only remaining issue is partial volume correction, which is required since some of the area elements are partly in flowing blood and partly outside of the lumen. To fully count these areas would cause an overestimate of the measurement. Partial volume correction is accomplished by using power Doppler where the power in each area element is normalized by the power in area elements from the center of the vein that are fully in blood. The fraction of flowing blood in the area element is applied as a weighting factor to the flux in order to compensate for partial volume in the area[16, 26, 27]. The distribution of power values that correspond to 100% blood are assigned fractional pixel weighting (w) w=1, partial volume pixel values are assigned fractional pixel weights of 0 < w < 1, and background pixels are assigned w = 0. These weightings are obtained from a histogram composed of power Doppler values produced from several C-surface slices above, below, and including the surface of interest [28]. The partial volume weights (w)i are generated from this histogram.
At least 20 samples, i.e., 20 3D volumes, of umbilical vein flow were acquired at each position; the mean flow calculated from these samples was used as the flow measurement at each position. Using a 2D array ultrasound transducer, it generally took on the order of 5-10 seconds to acquire a multivolume data set at each position.
All of the GSI volume flow estimates were calculated off-line using an algorithm developed by Philips ultrasound (Bothell, WA). The two operators, JMR and SZP, were totally blinded to these results. The spectral Doppler estimates were processed using volume flow software on the EPIQ 7, and the operators knew the results at the time of measurement.
A mean umbilical vein volume flow estimate using the spectral Doppler method and the GSI method was made for each sampling position. Since the mean blood flow in the umbilical cord has to be the same at all positions, we averaged the estimates of each method to get the overall mean estimate for each subject. We then calculated the standard deviation of the overall mean estimate, and finally we calculated a coefficient of variation (standard deviation/overall mean) for each subject.
Comparisons of the mean standard deviations and mean coefficients of variation for the two volume flow determination methods were made using paired t-tests. P values < 0.05 were considered significant.
Results:
Twelve subjects were scanned in this study (Table 2). The range of the mean umbilical vein volume flow estimates in these 12 subjects was 174 – 577 mL/min using the spectral Doppler method and 100 – 341 mL/min using the GSI method. However, since we do not know the true umbilical vein flows in any of these cases, we instead compared the variations in the flow estimates. The mean standard deviation for the spectral Doppler method was 161 ± 95 mL/min, while the mean standard deviation for the GSI method was 45 ± 48 mL/min. This difference was highly significant (p < 0.003). The standard deviation magnitude could vary depending on how large the estimated mean value is, and as stated above, we do not know the true mean flow values. To account for this, we also compared the coefficients of variation of each of the estimates. The mean coefficient of variation for the spectral Doppler method was 0.46 ± 0.17, while the mean coefficient of variation for the GSI method was 0.18 ± 0.14. This difference was again highly significant (p< 0.002).
Discussion:
Umbilical cord volume flow estimates have been referred to as “…a dream comes true…” for fetal assessment [29]. Yet, given the stated significance of the measurement, volume flow measurements are rarely performed during fetal surveys. A continuation of the above quote by Ferrazzi: “…but now for some standardization”[29] and a quote from Parra-Saavedra et al. sum up the problem: “Through the years the repeated attempts to make umbilical flow a relevant clinical parameter have failed, probably due to large measurement variation (particularly in diameter assessment) and the time-consuming technique.”[12] Given that, in order for umbilical cord volume flow estimates to become a standard part of the OB armamentarium, a much more reliable and efficient method needs to be implemented.
We have been working on a method that overcomes many of the problems associated with the standard spectral Doppler estimate of volume flow [16]. The method is angle independent, flow profile independent, and vessel geometry independent. The method also does not require a caliper measurement of the umbilical vein’s diameter. The technique requires a 3D ultrasound acquisition in order to define a 2D Gaussian surface that intersects the umbilical vein. Modern 3D color Doppler (velocity) ultrasound with simultaneous power Doppler have made such measurements possible, and using a 2D ultrasound array transducer, such flow measurements could be performed in near real-time once implemented on a clinical scanner. At a sampling rate of about four volumes per second, a mean volume flow measurement based on 20 flow estimates can be made in five seconds.
Yet, given concerns similar to Parra-Saavedra et al. [12], if the repeatability of the spectral Doppler method is a major issue and if the GSI method could not improve upon spectral Doppler’s poor repeatability, then enthusiasm for the new method would be limited. Based on that, we performed our small study, which definitely suggests that the GSI volume flow quantification method is more reproducible than the spectral Doppler method. In fact, the GSI method had a coefficient of variation that was less than half that of the spectral Doppler method. This is not a surprise, since multiple sources of error that corrupt the spectral Doppler method do not affect the GSI method. In addition, acquisitions are straightforward since the umbilical cord can be intersected in almost any arbitrary orientation and at any location along the cord as long as Doppler shifts are detectable. The potential rapidity of the acquisitions would make annoying problems such as fetal movement during scanning much less of an issue.
One of the advantages of volume flow measurement is that the average volume flow does not vary along the umbilical cord. This has to be the case since there are no feeding or draining vessels entering or leaving the umbilical arteries or vein along the cord[30]. Therefore, any blood that enters and leaves the cord comes in at one end and leaves at the other. There are no branch vessels to divert the flow. There can be variations in instantaneous flow such as pulsations in the arteries, but the average must be the same. Thus, variations in mean volume flow estimates must be due to the measurement technique itself; such as incorrect assumptions, measurement inaccuracies, technical difficulties such as bad Doppler angles, etc. This also holds true on a physiological basis, so it does not matter if the flow is normal or not. Either way, the flow has to be the same along the cord. That is why we felt in this study we could study umbilical vein flows in women with high risk pregnancies.
There are some limitations to this study. First, the number of subjects is relatively small. However, the difference in the mean coefficients of variation between the two methods is large, Cohen’s effect size = 1.81, so we are sufficiently powered even with the twelve subjects studied. The post-hoc power for this study for p < 0.05, our significance threshold, is 0.80. Yet given the small size of this study and the unusual population of high risk patients, the findings herein should be validated in larger studies. Another potential issue is that all of the subjects in this study were inpatients and had complications of pregnancy. This would definitely be an issue if we were investigating and comparing normal umbilical cord flow values between the spectral Doppler method and the GSI method. However, we were only interested in the precision of the flow measurements made using the two techniques, so the absolute flow rates were not an issue. Next, since the spectral Doppler volume flow estimates were calculated on the ultrasound machine, the two operators were not blinded. However, all of the GSI calculations were performed off-line, and both observers were blinded to those. Since the focus of the study was on precision, not accuracy (the correct answer was not known), the observers could not know which measurement set was the more precise, i.e., had the least variation, until after off-line calculation of the GSI estimates. We, therefore, believe that the comparison of the precision of the two techniques is valid. Follow-up studies to confirm this finding might still be in order, however.
Finally, we have no truth data for the flow in the umbilical vein, and frankly multiple studies demonstrating umbilical venous flow by ultrasound did not have truth data in humans either [1–6, 8, 9, 11]. It would be unethical to place a flow cuff around the umbilical cord in humans. Therefore, normal values are typically based on ranges defined by clinical experiences. That is not to say that the GSI flow method is not accurate. Multiple evaluations of the GSI method in phantoms and animals have shown excellent accuracies even in circumstances where the standard Doppler method would likely fail due to flow situations that do not adhere to the strict assumptions made with that technique [17, 31].
In conclusion, this study suggests that the GSI 3D approach to flow quantification is much more precise than the current spectral Doppler method. Further, it is not hard to believe that flow measurements using this method will be easier to perform than those with the spectral method, particularly since the requirements of angle correction and vessel diameter measurement are no longer necessary. Given the improved ease of use and better precision of the GSI measurement, normal and abnormal umbilical vein volume flow ranges will need to be clinically defined just as they have been defined for blood flow parameters such as resistive indices, pulsatility indices, and systolic/diastolic (S/D) ratios [32]. There should be definite interest in defining these ranges, since multiple early clinical studies have shown the ability of volume flow measurements to make accurate diagnostic predictions regarding such conditions as intrauterine growth restriction and pre-eclampsia [1–11]. Hopefully, these incentives will lead to umbilical cord volume flow measurements becoming a part of standard fetal surveys.
Acknowledgments:
This work was partially supported by NIH: R21HD095501-01A1, 5R01HD097756-01. This research was performed in conjunction with and was partially supported by Philips Ultrasound, Bothell, WA, USA.
Contributor Information
Jonathan M. Rubin, University of Michigan Department of Radiology, 3208C Medical Sciences Building 1, 1301 Catherine Street, Ann Arbor, MI, USA 48109
Sibo Li, Philips Research North America
J. Brian Fowlkes, University of Michigan Department of Radiology
Shriram Sethuraman, Philips Research North America
Oliver D. Kripfgans, University of Michigan Department of Radiology
William Shi, Philips Research North America
Marjorie C. Treadwell, University of Michigan Department of Obstetrics and Gynecology
James R. Jago, Philips Corporation
Ronald D. Leichner, Philips Corporation
Stephen Z. Pinter, University of Michigan Department of Radiology
References
- [1].Tchirikov M, Rybadowski C, Huneke B, Schoder V, Schroder HJ. Umbilical vein blood volume flow rate and umbilical artery pulsatility as ‘venous-arterial index’ in the prediction of neonatal compromise. Ultrasound Obstet Gynecol. 2002;20:580–5. [DOI] [PubMed] [Google Scholar]
- [2].Ferrazzi E, Rigano S, Bozzo M, Giovannini N, Galan H, Battaglia FC. Umbilical vein blood flow in growth-restricted fetuses. Ultrasound Obstet Gynecol. 2000;16:432–8. [DOI] [PubMed] [Google Scholar]
- [3].Bellotti M, Pennati G, De Gasperi C, Bozzo M, Battaglia FC, Ferrazzi E. Simultaneous measurements of umbilical venous, fetal hepatic, and ductus venosus blood flow in growth-restricted human fetuses. Am J Obstet Gynecol. 2004;190:1347–58. [DOI] [PubMed] [Google Scholar]
- [4].Lees C, Albaiges G, Deane C, Parra M, Nicolaides KH. Assessment of umbilical arterial and venous flow using color Doppler. Ultrasound Obstet Gynecol. 1999;14:250–5. [DOI] [PubMed] [Google Scholar]
- [5].Rigano S, Bozzo M, Ferrazzi E, Bellotti M, Battaglia FC, Galan HL. Early and persistent reduction in umbilical vein blood flow in the growth-restricted fetus: a longitudinal study. Am J Obstet Gynecol. 2001;185:834–8. [DOI] [PubMed] [Google Scholar]
- [6].Najafzadeh A, Dickinson JE. Umbilical venous blood flow and its measurement in the human fetus. J Clin Ultrasound. 2012;40:502–11. [DOI] [PubMed] [Google Scholar]
- [7].Acharya G, Wilsgaard T, Bernsten GKR, Maltau JM, Kiserud T. Doppler-derived umbilical artery absolute velocities and their relationship to fetoplacental volume blood flow: a longitudinal study. Ultrasound Obstet Gynecol. 2005;25:444–53. [DOI] [PubMed] [Google Scholar]
- [8].Boito SM, Ursem NT, Struijk PC, Stijnen T, Wladimiroff JW. Umbilical venous volume flow and fetal behavioral states in the normally developing fetus. Ultrasound Obstet Gynecol. 2004;23:138–42. [DOI] [PubMed] [Google Scholar]
- [9].Boito S, Struijk PC, Ursem NT, Stijnen T, Wladimiroff JW. Umbilical venous volume flow in the normally developing and growth-restricted fetus. Ultrasound Obstet Gynecol. 2002;19:344–9. [DOI] [PubMed] [Google Scholar]
- [10].Ferrazzi E, Bellotti M, Galan H, Pennati G, Bozzo M, Rigano S, et al. Doppler investigation in intrauterine growth restriction--from qualitative indices to flow measurements: a review of the experience of a collaborative group. Annals of the New York Academy of Sciences. 2001;943:316–25. [DOI] [PubMed] [Google Scholar]
- [11].Pinter SZ, Kripfgans OD, Treadwell MC, Kneitel AW, Fowlkes JB, Rubin JM. Evaluation of umbilical vein blood volume flow in preeclampsia by angle-independent 3D sonography. J Ultrasound Med. 2018;37:1633–40. [DOI] [PubMed] [Google Scholar]
- [12].Parra-Saavedra M, Crovetto F, Triunfo S, Savchev S, Parra G, Sanz M, et al. Added value of umbilical vein flow as a predictor of perinatal outcome in term small-for-gestational-age fetuses. Ultrasound Obstet Gynecol. 2013;42:189–95. [DOI] [PubMed] [Google Scholar]
- [13].Gill R Measurement of blood flow by ultrasound: accuracy and sources of error. Ultrasound in Medicine and Biology. 1985;11:625–41. [DOI] [PubMed] [Google Scholar]
- [14].Eik-Nes SH, Marsal K, Kristoffersen K. Methodology and basic problems related to blood flow studies in the human fetus. Ultrasound Med Biol. 1984;10:329–37. [DOI] [PubMed] [Google Scholar]
- [15].Burns PN. Measuring volume flow with Doppler ultrasound-an old nut. Ultrasound Obstet Gynecol. 1992;2:238–41. [DOI] [PubMed] [Google Scholar]
- [16].Kripfgans OD, Rubin JM, Hall AL, Gordon MB, Fowlkes JB. Measurement of volumetric flow. J Ultrasound Med. 2006;25:1305–11. [DOI] [PubMed] [Google Scholar]
- [17].Richards MS, Kripfgans OD, Rubin JM, Hall AL, Fowlkes JB. Mean volume flow estimation in pulsatile flow conditions. Ultrasound Med Biol. 2009;35:1880–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pinter SZ, Rubin JM, Kripfgans OD, Treadwell MC, Romero VC, Richards MS, et al. Three-dimensional sonographic measurement of blood volume flow in the umbilical cord. J Ultrasound Med. 2012;31:1927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hottinger CF, Meindl JD. Blood flow measurement using the attenuation-compensated volume flowmeter. Ultrasonic Imaging. 1979;1:1–15. [DOI] [PubMed] [Google Scholar]
- [20].Sun Y, Ask P, Janerot-Sjoberg B, Eidenvall L, Loyd D, Wranne B. Estimation of volume flow rate by surface integration of velocity vectors from color Doppler images. J Am Soc Echocardiogr. 1995;8:904–14. [DOI] [PubMed] [Google Scholar]
- [21].Pemberton J, Li X, Karamlou T, Sandquist CA, Thiele K, Shen I, et al. The use of live three-dimensional Doppler echocardiography in the measurement of cardiac output: an in vivo animal study. J Am Coll Cardiol. 2005;45:433–8. [DOI] [PubMed] [Google Scholar]
- [22].Pinter SZ, Rubin JM, Kripfgans OD, Novelli PM, Vargas-Vila M, Hall AL, et al. Volumetric blood flow in transjugular intrahepatic portosystemic shunt revision using 3-dimensional Doppler sonography. J Ultrasound Med. 2015;34:257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moser U, Vieli A, Schumacher P, Pinter P, Basler S, Anliker M. [A Doppler ultrasound device for determining blood volume flow]. Ultraschall Med. 1992;13:77–9. [DOI] [PubMed] [Google Scholar]
- [24].Kim WY, Poulsen JK, Terp K, Staalsen NH. A new Doppler method for quantification of volumetric flow: in vivo validation using color Doppler. J Am Coll Cardiol. 1996;27:182–92. [DOI] [PubMed] [Google Scholar]
- [25].Poulsen JK, Kim WY. Measurement of volumetric flow with no angle correction using multiplanar pulsed Doppler ultrasound. IEEE Trans Biomed Eng. 1996;43:589–99. [DOI] [PubMed] [Google Scholar]
- [26].Rubin JM, Bude RO, Fowlkes JB, Spratt RS, Carson PL, Adler RS. Normalizing fractional moving blood volume estimates with power Doppler US: Defining a stable intravascular point with the cumulative power distribution function. Radiology. 1997;205:757–65. [DOI] [PubMed] [Google Scholar]
- [27].Rubin JM, Adler RS, Fowlkes JB, Spratt S, Pallister JE, Chen J-F, et al. Fractional moving blood volume: estimation with power Doppler US. Radiology. 1995;197:183–90. [DOI] [PubMed] [Google Scholar]
- [28].Kripfgans OD, Rubin JM, Pinter SZ, Jago J, Leichner R, Brian Fowlkes J. Partial volume effect and correction for 3-D color flow acquisition of volumetric blood flow. IEEE Trans Ultrason Ferroelectr Freq Control. 2019;66:1749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ferrazzi E Measurement of venous blood flow in the human fetus: a dream comes true, but now for some standardization. Ultrasound Obstet Gynecol. 2001;18:1–4. [DOI] [PubMed] [Google Scholar]
- [30].Davies JE, Walker JT, Keating A. Concise Review: Wharton’s Jelly: The rich, but enigmatic, source of mesenchymal stromal cells. Stem Cells Transl Med. 2017;6:1620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kripfgans OD, Pinter SZ, Baiu C, Bruce MF, Carson PL, Chen S, Erpelding TN, Gao J, Lockhart ME, Milkowski A, Obuchowski N, Robbin ML, Rubin JM, Zagzebski JA, Fowlkes JB. Three-dimensional volume blood flow – multi-site multi-system results from within the quantitative imaging biomarker alliance (QIBA). Radiology 2020;296:662–670. 10.1148/radiol.2020191332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Acharya G, Wilsgaard T, Berntsen GK, Maltau JM, Kiserud T. Reference ranges for serial measurements of umbilical artery Doppler indices in the second half of pregnancy. Am J Obstet Gynecol. 2005;192:937–44. [DOI] [PubMed] [Google Scholar]


