Abstract
Zika virus (ZIKV) causes Congenital Zika Syndrome (CZS) in individuals exposed prenatally. Here, we investigated polymorphisms in VEGFA, PTGS2, NOS3, TNF, and NOS2 genes as risk factors to CZS. Forty children with CZS and forty-eight children who were in utero exposed to ZIKV infection, but born without congenital anomalies, were evaluated. Children with CZS were predominantly infected by ZIKV in the first trimester (p < 0.001) and had mothers with lower educational level (p < 0.001) and family income (p < 0.001). We found higher risk of CZS due the allele rs2297518[A] of NOS2 (OR = 2.28, CI 95% 1.17–4.50, p = 0.015). T allele and TT/CT genotypes of the TNF rs1799724 and haplotypes associated with higher expression of TNF were more prevalent in children with CZS and severe microcephaly (p = 0.029, p = 0.041 and p = 0.030, respectively). Our findings showed higher risk of CZS due ZIKV infection in the first trimester and suggested that polymorphisms in NOS2 and TNF genes affect the risk of CZS and severe microcephaly.
Keywords: maternal exposure, Zika virus, Zika virus infection, teratogens, congenital abnormalities, genetic variation, genetic polymorphism, disease susceptibility, inflammation
1. Introduction
Zika virus (ZIKV) is a human teratogen capable of causing neurological and ocular malformations in fetuses with in utero exposure to ZIKV infection [1,2]. Congenital Zika Syndrome (CZS) does not occur in all embryos or fetuses exposed, but in less than 42% [3,4]. The congenital anomalies present in children with CZS include brain calcifications, microcephaly, joint abnormalities, and ocular abnormalities, among others [4].
In order to understand the mechanisms associated with the development of congenital anomalies due to ZIKV infection, environmental and molecular variables have been investigated in humans, in vivo and in vitro; however, risk and protection factors still need to be better elucidated. The timing of ZIKV infection during pregnancy, for example, is a variable that has been discussed in studies as relevant to the occurrence and severity of congenital anomalies [5,6]. The investigation of molecular changes caused by ZIKV during infection, such as in the gene expression, is one approach used to understand its molecular mechanisms and factors associated with its teratogenesis. Studies in this context have shown an exacerbated activity of genes/proteins involved in the immune and inflammatory response during ZIKV infection [7,8,9,10].
It has been reported that some genes, such as TNF, NOS2, PTGS2, and VEGFA, as well as their proteins, are overexpressed during ZIKV infection, acting on the inflammatory response mechanism [11,12,13,14]. This neuroinflammatory profile in the central nervous system has been suggested as impairing the cell differentiation and proliferation—especially of neuroprogenitor cells—inducing the CZS [10,11,12]. The inefficient immune response as well as a highly inflammatory environment are, therefore, harmful scenarios for the developing brain, probably related to the increased cell death associated with the CZS development. Based on these data, the investigation of genes that act in this pathway and in developmental process, such as VEGFA, PTGS2, NOS3, TNF, and NOS2, as well as functional polymorphisms that affect the expression of these genes and their proteins activity may lead to the discovery of genetic factors of susceptibility to teratogenesis of ZIKV.
Hence, the aim of this study was to assess environmental variables, such as sociodemographic and clinical characteristics, as well as genetic variants in genes involved in the inflammatory process of response to ZIKV as risk or protective factors for CZS. Therefore, we investigated a sample of children who were in utero exposed to ZIKV infection and later developed CZS or, some of them, were born without congenital anomalies.
2. Materials and Methods
2.1. Ethical Issues
This study was carried out following the rules of the Declaration of Helsinki and approved by the Ethics and Research Committee of the Hospital de Clínicas de Porto Alegre, the institution responsible for this study (nº 170619–CAAE 78735817910015327), and by all participating institutions. All legal guardians of individuals recruited for this study gave their informed consent for inclusion before they participated in the study.
2.2. Sample
In this case-control study, we included 40 children diagnosed with CZS whose mothers had evidence of ZIKV infection during pregnancy (case group) and 48 children without congenital anomalies whose mothers also had evidence of ZIKV infection (control group). Evidence of ZIKV exposure was defined as positive RT-PCR or specific symptoms of infection ZIKV during a ZIKV outbreak in the region during the pregnancy (e.g., rash, fever and/or joint pain). Case children were recruited between June 2018 to November 2019 from reports of microcephaly in five Brazilian research and/or assistance centers: North region (Fundação Hospital do Acre, n = 4), Northeast region (Fundação Universidade Estadual do Ceará, n = 21), Midwest region (Universidade do Estado de Mato Grosso, n = 2 and Universitário Júlio Muller, n = 12), and South region (Hospital de Clínicas de Porto Alegre, n = 1). Control children were recruited in the same research and/or assistance centers from the North region (n = 1), Midwest (n = 46, from a cohort of women that gave birth in 2016, in the city of Tangará da Serra), and South region (n = 1).
Children with CZS included in this study were, in the first year of life, attended by a team of doctors, including geneticists, neuropediatricians, neurologists, ophthalmologists, physiotherapists, and (or) dentists. Subsequently, according to the needs related to the neurodevelopment of each child, they continued to be accompanied by some professionals, including pediatricians, physiotherapists, ophthalmologists, dentists, and (or) neurologists, among others, who are part of this study. Therefore, the clinical characteristics of these individuals, as well as ophthalmological and neuroimaging tests were obtained, when available, through the medical records of these consultations or through questionnaires applied to the mothers. Likewise, sociodemographic data were obtained through questionnaires applied during the medical consultation.
2.3. Genetic Analysis
A blood or saliva sample was collected from individuals and DNA extraction was performed by the FlexiGene DNA Kit (Qiagen, Hilden, Germany) or Oragene Kit (DNA Genotek, Ottawa, Ontario, Canada). Eleven polymorphisms in VEGFA, PTGS2, NOS3, TNF, and NOS2 genes were selected to be evaluated in this study. Information about the polymorphisms evaluated and the TaqMan assays used are found in Appendix A. The criteria for genetic variants selection were based on: (1) Minor Allele Frequency (MAF) > 1% (based on gnomAD database information for non-Finnish European and/or AbraOM database for Brazilian), and (2) functional description as modifiers of their gene’s expression or protein function. The genotyping was performed through the TaqMan Genotyping Assay method in Step One PlusTM Real-Time PCR Systems (Thermo Fisher Scientific, Waltham, Massachusetts, EUA).
2.4. Statistical Analyses
A descriptive analysis of the congenital anomalies of individuals with CZS was performed. Quantitative variables were tested through the Shapiro–Wilk test to verify their normality and according to the distribution found, Student’s t test or Mann–Whitney U test were applied. The Hardy–Weinberg equilibrium was tested for all polymorphisms. Categorical variables were compared between the groups by Chi-square test or Fisher’s Exact Test. Through a univariate logistic regression analysis, we looked for associations between individual variables and the occurrence of CZS. p-values lower than 0.05 were considered to be significant. The SPSS v.18 software was used to perform the statistical analyses (IBM, Armonk, New York, NY, USA).
Linkage disequilibrium (LD) analysis was inferred with Haploview v.4.2. software (Broad Institute, Cambridge, MA, USA). The haplotypes were obtained with the Bayesian algorithm in Phase 2.1.1 software.
3. Results
3.1. Sociodemographic and Clinical Profile of Children Who Were In Utero Exposed to ZIKV Infection
Eighty-eight children who were in utero exposed to ZIKV infection were recruited for this study. Forty children developed Congenital Zika Syndrome (CZS) (case group) and forty-eight were born without alterations (control group). The sociodemographic characteristics of the children are presented in Table 1. There was a higher prevalence of black individuals in both case (77%) and control (65%) groups and, therefore, this variable did not present a statistically significant difference between the groups. As expected, weight, height, and the cephalic perimeter were higher in the control group (p < 0.001). Most mothers of children with CZS were exposed to ZIKV infection in the first trimester (80%) while most mothers of children without CZS were exposed to ZIKV infection the third trimester (44%) (p < 0.001). Cesarean section had a significantly higher prevalence in the control group (90%) (p < 0.001). Mothers of children with CZS had a lower educational (p < 0.001) and lower monthly family income (p = 0.008).
Table 1.
Evaluation of Clinical, Gestational, and Sociodemographic Characteristics in the Case and Control Groups.
| Variables | Case † (n = 40) | Control (n = 48) | p-Value ‡ |
|---|---|---|---|
| Sex (n, %) | |||
| Male | 23 (57%) | 26 (54%) | 0.754 |
| Female | 17 (43%) | 22 (46%) | |
| Ethnicity (n, %) | |||
| Black | 31 (77%) | 31 (65%) | 0.242 |
| White | 9 (23%) | 17 (35%) | |
| Weight (kg) | 2.5 (2.2–2.9) | 3.2 (2.8–3.5) | <0.001 * |
| Height (cm) | 45.0 (44.0–48.0) | 49.0 (47.0–50.0) | 0.001 * |
| Cephalic perimeter (cm) | 29.0 (27.3–31.0) | 35.0 (34.0–36.0) | <0.001 * |
| Gestational age at birth (weeks) | 38.0 (37.0–39.0) | 38.0 (37.0–38.7) | 0.522 |
| Types of delivery (n, %) | |||
| Vaginal delivery | 16/30 (53%) | 5 (10%) | <0.001 * |
| Cesarean section | 14/30 (47%) | 43 (90%) | |
| Mother’s age (years) | 28.0 (22.5–35.5) | 29.5 (22.0–33.0) | 0.824 |
| Father’s age (years) | 28.0 (24.0–37.8) | 31.0 (26.0–35.0) | 0.693 |
| Trimester of ZIKV infection (n, %) | |||
| 1st | 24/30 (80%) | 13 (27%) | <0.001 * |
| 2nd | 4/30 (13%) | 14 (29%) | |
| 3rd | 2/30 (7%) | 21 (44%) | |
| Exposure during pregnancy (n, %) | |||
| Alcohol | 3/35 (9%) | 14 (29%) | 0.028 * |
| Smoke | 0/35 | 1 (2%) | 1.000 |
| Drugs | 0/35 | 1 (2%) | 1.000 |
| Maternal yellow fever vaccine (n, %) | 18/24 (75%) | 35 (73%) | 1.000 |
| Maternal educational level (n, %) | |||
| Elementary school | 13/38 (34%) | 0 | <0.001 * |
| High school | 13/38 (34%) | 2 (4%) | |
| Incomplete or complete higher education | 12/38 (32%) | 46 (96%) | |
| Monthly family income (n, %) | |||
| Less than 3 minimum wages | 23/27 (85%) | 12/25 (46%) | 0.008 * |
| Between 3 and 9 minimum wages | 4/27 (15%) | 12/25 (46%) | |
| More than 9 minimum wages | 0 | 2/25 (8%) |
† In the case group, some information was not available or was not answered for all mothers. ‡ Quantitative variables were compared between the groups through the Student’s t test or Mann–Whitney U test and categorical variables through the Chi-squared test or Fisher’s exact test; Quantitative variables are presented as median and quartiles; * Statistically significant.
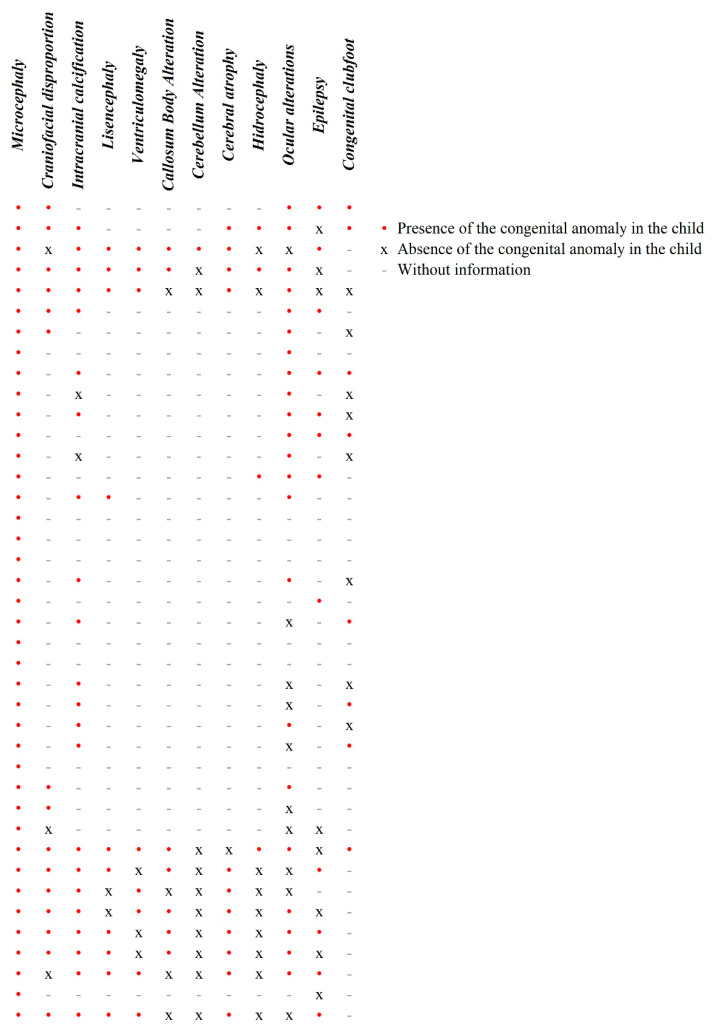
Congenital anomalies of the children with CZS are presented in Figure 1. Such anomalies were identified during the children’s first year of life by a team of doctors and through dysmorphological, neuroimaging, and ophthalmic examinations that are available in some of the research and assistance centers in which the children have been treated.
Figure 1.
Descriptive analysis of the clinical findings of each individual of the case group.
For those whom the exams were performed and the information was available, the most prevalent clinical findings were microcephaly (n = 40–100%), calcifications (n = 22/24–92%), cerebral atrophy (n = 11/12–92%), lissencephaly (n = 10/12–83%), craniofacial disproportion (n = 15/18–83%), ventriculomegaly (n = 8/11–73%), and ocular alterations (n = 22/32–69%).
Given the neurological impairment of most of these children, they continued to be accompanied by specific doctors, such as pediatricians and physiotherapists (more regularly), or ophthalmologists, dentists, and neurologists (more sporadically). Such monitoring varied again depending on the research and assistance center in which the children have been treated.
3.2. Genetic Susceptibility to CZS and Severe Microcephaly
The allelic and genotypic frequencies of polymorphisms in the VEGFA, PTGS2, NOS3, TNF, and NOS2 genes in both groups are presented in Table 2. The genotypic frequencies of the rs1799983 (NOS3), rs1799724 (TNF), rs2779249, and rs2297518 (NOS2) in the control group were not consistent with the Hardy–Weinberg equilibrium as well as the genotypic frequencies of the rs2297518 (NOS2) in the case group.
Table 2.
Allelic and genotypic frequencies of polymorphisms in VEGFA, PTGS2, NOS3, TNF, and NOS2 genes in the case and control groups.
| Gene | Polymorphism | Allele/Genotype | Case (n = 40) | Control (n = 48) | p-Value † |
|---|---|---|---|---|---|
| VEGFA (n, %) | rs1570360 | G | 70 (87%) | 72 (75%) | 0.054 |
| A | 10 (13%) | 24 (25%) | |||
| GG | 30 (75%) | 28 (58%) | 0.109 | ||
| GA | 10 (25%) | 16 (33%) | |||
| AA | 0 | 4 (9%) | |||
| rs2010963 | G | 39 (49%) | 47 (49%) | 1.000 | |
| C | 41 (51%) | 49 (51%) | |||
| GG | 11 (27%) | 11 (23%) | 0.821 | ||
| GC | 18 (46%) | 25 (52%) | |||
| CC | 11 (27%) | 12 (25%) | |||
| rs3025039 | C | 61 (76%) | 84 (87%) | 0.073 | |
| T | 19 (24%) | 12 (13%) | |||
| CC | 25 (63%) | 36 (75%) | 0.084 | ||
| CT | 11 (27%) | 12 (25%) | |||
| TT | 4 (10%) | 0 | |||
| PTGS2 (n, %) | rs689465 | T | 68 (85%) | 77 (80%) | 0.434 |
| C | 12 (15%) | 19 (20%) | |||
| TT | 29 (73%) | 30 (63%) | 0.746 | ||
| TC | 10 (25%) | 17 (35%) | |||
| CC | 1 (2%) | 1 (2%) | |||
| NOS3 (n, %) | rs2070744 | T | 57 (71%) | 63 (66%) | 0.516 |
| C | 23 (29%) | 33 (34%) | |||
| TT | 20 (50%) | 21 (44%) | 0.679 | ||
| TC | 17 (42%) | 21 (44%) | |||
| CC | 3 (8%) | 6 (12%) | |||
| rs1799983 | G | 65 (81%) | 73 (76%) | 0.464 | |
| T | 15 (19%) | 23 (24%) | |||
| GG | 27 (67%) | 25 (52%) | 0.045 * | ||
| TG | 11 (28%) | 23 (48%) | |||
| TT | 2 (5%) | 0 | |||
| TNF (n, %) | rs1799724 | C | 70 (87%) | 86 (90%) | 0.812 |
| T | 10 (13%) | 10 (10%) | |||
| CC | 31 (78%) | 40 (83%) | 0.606 | ||
| CT | 8 (20%) | 6 (13%) | |||
| TT | 1 (2%) | 2 (4%) | |||
| rs361525 | G | 76 (95%) | 93 (97%) | 0.703 | |
| A | 4 (5%) | 3 (3%) | |||
| GG | 36 (90%) | 45 (94%) | 0.694 | ||
| GA | 4 (10%) | 3 (6%) | |||
| rs1799964 | T | 63 (79%) | 75 (80%) | 1.000 | |
| C | 17 (21%) | 19 (20%) | |||
| TT | 25 (62%) | 31 (66%) | 0.939 | ||
| TC | 13 (33%) | 13 (28%) | |||
| CC | 2 (5%) | 3 (6%) | |||
| NOS2 (n, %) | rs2779249 | C | 28 (35%) | 40 (42%) | 0.437 |
| A | 52 (65%) | 56 (58%) | |||
| CC | 6 (15%) | 12 (25%) | 0.521 | ||
| CA | 16 (40%) | 16 (33%) | |||
| AA | 18 (45%) | 20 (42%) | |||
| rs2297518 | G | 50 (62%) | 76 (79%) | 0.019 * | |
| A | 30 (38%) | 20 (21%) | |||
| GG | 20 (50%) | 33 (69%) | 0.144 | ||
| GA | 10 (25%) | 10 (21%) | |||
| AA | 10 (25%) | 5 (10%) |
† Chi-squared test or Fisher’s exact test; * Statistically significant.
The genotypic frequencies of the rs1799983 (NOS3) were different between the groups (p = 0.045). Moreover, there was a higher frequency of the A allele of the rs2297518 (NOS2) in the case group (p = 0.019). The allelic and genotypic frequencies of the other polymorphisms showed no statistically significant difference between the groups.
The frequencies of haplotypes generated by combining alleles of variants in the same gene were also compared between groups (Table 3). The frequencies of the VEGFA haplotypes were statistically different between cases and controls (p = 0.002). Although, NOS2 haplotypes also presented a differential frequency between the groups, these were not statistically significant (p = 0.054). The haplotypic frequencies of the other genes showed no statistically significant difference between the groups. In the TNF gene, the TCG haplotype (rs1799964, rs1799724, and rs361525, respectively) contained risk alleles associated with low TNF expression [15,16,17,18]. The frequency of this haplotype was compared between cases and controls, but there was no statistically significant difference.
Table 3.
Frequencies of the haplotypes generated by the combination of alleles of the polymorphisms in VEGFA, NOS3, TNF, and NOS2 genes in case and control groups.
| Gene | Haplotypes † | Cases (n = 40) | Controls (n = 48) | p-Value § |
|---|---|---|---|---|
| VEGFA (n, %) | GGC | 30 (38%) | 23 (24%) | 0.002 * |
| GCC | 25 (31%) | 40 (42%) | ||
| GCT | 14 (17%) | 9 (9%) | ||
| AGC | 4 (5%) | 21 (22%) | ||
| AGT | 4 (5%) | 3 (3%) | ||
| ACC | 2 (3%) | 0 | ||
| GGT | 1 (1%) | 0 | ||
| NOS3 (n, %) | TG | 55 (69%) | 55 (57%) | 0.156 |
| CT | 14 (17%) | 15 (16%) | ||
| CG | 9 (11%) | 18 (19%) | ||
| TT | 2 (3%) | 8 (8%) | ||
| TNF (n, %) | TCG | 54 (66%) | 68 (71%) | 0.831 |
| CCG | 14 (16%) | 16 (17%) | ||
| TTG | 10 (13%) | 9 (9%) | ||
| CCA | 4 (5%) | 3 (3%) | ||
| TCG ‡ | 53 (70%) | 68 (73%) | 0.732 | |
| others ‡ | 23 (30%) | 25 (27%) | ||
| NOS2 (n, %) | GA | 37 (46%) | 51 (53%) | 0.054 |
| AA | 15 (19%) | 7 (7%) | ||
| AC | 15 (19%) | 13 (14%) | ||
| GC | 13 (16%) | 25 (26%) |
† VEGFA haplotypes: variants rs1570360, rs2010963, and rs3025039, respectively; NOS3: rs2070744 and rs1799983, respectively; TNF: rs1799964, rs1799724, and rs361525, respectively; NOS2: rs2297518, and rs2779249, respectively; ‡ Comparison of the frequencies of a haplotype containing all alleles associated with low expression of the TNF against the other haplotypes; § Chi-squared test or Fisher’s exact test; * Statistically significant.
Table 4 presents the results of a univariate logistic regression analysis of significant risk variables for the CZS. The first trimester of exposure to the ZIKV infection was related to a higher risk to CZS development (OR: 19.38, CI 95% 4.70–133.78, p < 0.001). Furthermore, the presence of the A allele of the rs2297518 (NOS2) was related to a higher risk to the CZS (OR: 2.28, CI 95% 1.17–4.50, p = 0.015).
Table 4.
Univariate logistic regression showing risk variables for the Congenital Zika Syndrome.
| Risk Variables | Odds Ratio (95% IC) | p-Value |
|---|---|---|
| 1st trimester of ZIKV infection | 19.38 (95% IC 4.70–133.78) | <0.001 * |
| NOS2 rs2297518[A] | 2.28 (95% IC 1.17–4.50) | 0.015 * |
[A]: allele A of the NOS2 rs2297518 genetic variant; * Statistically significant.
A specific phenotype of the CZS, the severity of the microcephaly, was evaluated in the case group. Mild or moderate microcephaly was defined if the head circumference was between 2 and 3 standard deviation (SD) below the mean for sex and age, and severe if it was 3 or more SD below the mean [19]. Children with 2SD of microcephaly were compared to children with 3SD of microcephaly for the frequency of two variables already reported in the literature as related to these phenotypes—trimester of ZIKV infection and TNF gene [20,21]. The results of such analysis are presented in Table 5. The frequency of ZIKV infection in the first trimester was higher in children with severe microcephaly (100%), compared with children with moderate microcephaly (33%) (p = 0.019). Children with severe microcephaly presented higher frequency of the T allele and genotypes TT or CT of the TNF rs1799724, compared with children with moderate microcephaly (p = 0.029 and p = 0.041, respectively). Moreover, the two groups presented a differential frequency of TNF haplotypes (p = 0.030), with an especially higher frequency of the TCG haplotype, which contains all the alleles related to the lower expression of TNF [15,16,17,18]. This haplotype is found in 88% of the children with moderate microcephaly (<2SD) against 58% in children with severe microcephaly (<3SD) (p = 0.079).
Table 5.
Association between trimester of Zika virus (ZIKV) infection during pregnancy or TNF polymorphisms and the severity of microcephaly in cases with CZS.
| Variables | Allele/ Genotype/ Haplotype |
Severity of Microcephaly | ||
|---|---|---|---|---|
| Mild (n = 8) | Severe (n = 12) | p-Value | ||
| Trimester of ZIKV infection † (n, %) | ||||
| 1st | 2 (33%) | 7 (100%) | 0.019 | |
| 2nd | 3 (50%) | 0 | ||
| 3rd | 1 (17%) | 0 | ||
| TNF (n, %) | ||||
| rs1799724 | T | 0 | 7 (29%) | 0.029 * |
| C | 16 (100%) | 17 (71%) | ||
| CC | 8 (100%) | 6 (50%) | 0.041 * | |
| CT | 0 | 5 (42%) | ||
| TT | 0 | 1 (8%) | ||
| rs361525 | A | 1 (6%) | 0 | 0.400 |
| G | 15 (94%) | 24 (100%) | ||
| GG | 7 (87%) | 12 (100%) | 0.400 | |
| AG | 1 (13%) | 0 | ||
| rs1799964 | C | 2 (13%) | 3 (13%) | 1.000 |
| T | 14 (87%) | 21 (87%) | ||
| TT | 6 (75%) | 9 (75%) | 1.000 | |
| CT | 2 (25%) | 3 (25%) | ||
| Haplotypes ‡ | CCG | 1 (6%) | 3 (13%) | 0.030 * |
| CCA | 1 (6%) | 0 | ||
| TCG | 14 (88%) | 14 (58%) | ||
| TTG | 0 | 7 (29%) | ||
| TCG § | 14 | 14 | 0.079 | |
| others § | 2 | 10 | ||
† The information about the trimester of exposure to ZIKV infection during pregnancy was not available for all children with data of the severity of the microcephaly; ‡ TNF haplotypes: variants rs1799964, rs1799724, and rs361525, respectively; § Comparison of the frequencies of a haplotype containing all the alleles associated with low expression of the TNF against the other haplotypes; Chi-squared test or Fisher’s exact test; * Statistically significant.
4. Discussion
Zika virus teratogenic potential has been discovered in the recent years; thus, little is known about the susceptibility factors and mechanisms related to the adverse effects caused by its exposure in embryos and fetuses in development so far. It is known that around 1 to 42% of embryos or fetuses with in utero exposure to ZIKV infection developed the CZS [4]. Discordant twins for CZS have been shown to be not uncommon [9]. Based on this, it is important to take into account the environmental and genetic factors that may confer susceptibility to ZIKV teratogenesis. In this work, we evaluated some sociodemographic, clinical, and genetic variants as possible risk factors to CZS in a sample of children who were in utero exposed to ZIKV infection, comparing children who were born with and without CZS.
Regarding some gestational risk factor to CZS, the exposure to ZIKV in the first trimester of pregnancy has been reported as a risk factor to CZS, presenting more severe congenital anomalies than later exposures [2,6]. Our results corroborate these findings, since we found a higher prevalence of children with CZS who were exposed in utero to ZIKV in the first trimester of pregnancy while children born without congenital anomalies were predominantly exposed in second and third trimester of gestation. Moreover, we found a higher frequency of the first trimester exposure in children with severe microcephaly.
Still, in this context of environmental variables possibly involved with the teratogenic effects of ZIKV, the socioeconomic level has been discussed as a possible environmental factor associated with this asymmetric distribution of CZS in Brazil [22]. That discussion is important, since this variable was also associated with the availability and quality of mothers’ diets during pregnancy, a condition recently also associated with the development of CZS [23]. In the present sample, we observed that families of children with CZS had a lower socioeconomic level compared to families of children without CZS. Mothers in the case group had a lower educational level and reported lower family income. This finding this is not new in the literature, since low socioeconomic level has been described as a risk factor for congenital anomalies. Therefore, it is probably that this population presents a higher risk for negative outcomes after ZIKV infection during pregnancy [24,25].
In relation to genetic risk factors, through the analyses of functional genetic variants in VEGFA, PTGS2, NOS3, TNF, and NOS2, genes related to immune and inflammatory response, we found different allelic, genotypic, and haplotypic frequencies between children with and without CZS. Regarding the NOS2 gene, we found a higher prevalence of the rs2297518 allele A in children with CZS, being that this allele is associated with an increased risk to CZS (OR: 2.28 (95% IC 1.17–4.50). NOS2 rs2297518 is a functional polymorphism that affects the iNOS protein activity, with the A allele related to increased protein activity and higher nitric oxide (NO) production [26,27]. NO has been reported as playing major roles in neurogenesis and neurodevelopment, and its dosage is extremely important [28,29]. The dysregulation of NO has been involved in the progression of many neurodevelopmental, neurobehavioral, and neurodegenerative disorders [29]. In this sense, the dysregulation of NO during ZIKV infection, caused both by the host response to the virus and by the individual’s genotype, could affect the development process causing a congenital anomaly.
Similarly, the haplotypic frequencies of two genes, VEGFA and NOS2, were different between children with and without CZS. The comparison of haplotype frequencies between cases and controls has been suggested as helping to identify overlapping haplotypes among affected individuals, representing a shared region that contains a genetic risk factor [30]. We highlight that the NOS2 gene haplotypic frequency was different between the two groups of children who were in utero exposed to ZIKV infection; however, it has not reached statistical significance, probably due to the sample size of the groups. On the other hand, VEGFA presented a statistically significant difference of its haplotype frequencies between the two groups. VEGFA is an important gene during neurodevelopment, acting on the neurogenesis, neuronal differentiation, and angiogenesis processes [31,32]. Genetic variants and haplotypes that affect the expression of this gene have the potential to impact and impair these developmental processes [31]. In the context of ZIKV infection during the developing brain, where the expression of this gene seems to be already affected, the presence of genetic variants and haplotypes that decreased its expression could modulate the impact of ZIKV for a worse scenario, further damaging neurogenesis and processing angiogenesis.
Focusing on a specific phenotype of the CZS, the severity of the microcephaly, we found an association of it and the TNF gene. The frequency of the rs1799724[T] was higher in children with a severe microcephaly. This allele has been related to the higher expression of the TNF gene [15,16]. The haplotypes of TNF also showed a different frequency between children with CZS and moderate or severe microcephaly, but in this scenario, a higher frequency of the TCG haplotype was found in children with a moderate microcephaly. This haplotype is composed by the three alleles of the variants rs1799964[T], rs1799724[C], and rs361525[G] of TNF related to the low expression of the gene [15,16,17,18]. TNF is a cytokine that, among many functions, has pro-inflammatory effects during a viral infection [33]. The immunological and inflammatory effects caused by TNF act in the eradication of infectious agents, but they can cause tissue damage, cell death, and systemic effects [33]. In this sense, the higher frequency of the rs1799724[T] in children with severe microcephaly, leading to a greater expression of TNF, combined with the natural increase of TNF expression to combat the ZIKV infection could be associated to this most severe phenotype in these individuals due to an exacerbated immunological and inflammatory response in the neural cells. On the other hand, the higher frequency of the TCG haplotype of TNF in individuals with moderate microcephaly, which is associated with the lower expression of TNF, could also explain this less severe phenotype in these individuals.
It is important to highlight that the polymorphisms evaluated in this study affect the expression of their genes or function of the proteins, which act on the fetus’ brain, both in the context of development and inflammatory response [34,35,36,37,38,39]. Since the aim of this study was evaluating the role that these polymorphisms could have, in the context of the ZIKV infection, on the fetal brain development, we considered that it would be interesting to evaluate their frequency only in individuals with CZS and individuals who have been exposed in utero to the ZIKV infection but were born normal and, in this sense, the parents were not evaluated, since this analysis would not add information to answer to the aim of the study.
In addition, although these genes and polymorphisms have an important role in neurogenesis or neuroinflammation, they have not been described as capable of causing the phenotypes seen in CZS, such as microcephaly, among others outside the context of the ZIKV infection [34,35,36,37,38,39,40,41]. This could mean that ZIKV infection potentiates the effect of these polymorphisms and, for this reason, they act on individuals’ susceptibility to CZS in the context of ZIKV infection.
Despite the interesting results of the present study, some limitations of should be considered for the results to be interpreted in a clear and unbiased manner. Firstly, the sample size of our sample is a limiting factor in the study, as it restricts our power to make strong associations and prevents us from performing more robust statistical analyses. Moreover, it is important to highlight that the number of children with CZS coming from a given region was not matched with the number of children without CZS coming from the same region. These two aspects of the sample may have been responsible for the fact that some genotypic frequencies found in this work were not consistent with the Hardy–Weinberg equilibrium. In this sense, the associations observed in the present study need to be confirmed by other studies in order better understand their real impact on CZS. Although the sample was obtained in partnership with several Brazilian institutions, the recruitment of new and well documented cases is difficult, taking into account that the ZIKV outbreak in Brazil has decreased considerably since 2017. In addition, there are a great number of cases where the confirmation of infection has been lost and, therefore, they were not included in this study. Regarding the clinical description of the individuals in the case group, the data were not available to all individuals due to the resources of the institution where they were attended and their possibility of carrying out exams.
The investigation for risk factors for the ZIKV teratogenesis and for the understanding of its molecular mechanisms still has much to advance, since little is known about this. Teratogenesis is a complex event and, therefore, there is not only one factor that can explain the susceptibility to damage caused by a teratogen exposure. In this study, we reported an interesting association of alleles and haplotypes of NOS2, VEGFA, and TNF genes with the CZS development and severity of the microcephaly in the CZS. Moreover, our results corroborate that the exposure to ZIKV in the first trimester of pregnancy is associated with the CZS as well as with the severity of the microcephaly in the affected individuals. Similarly, we found the socioeconomic level as a possible environmental risk factor to CZS. Future studies must explore such variables in larger samples as well as explore other possible risk factors to the ZIKV teratogenesis.
Acknowledgments
We appreciate all parents and children for their participation in this study.
Appendix A
Table A1.
General information of the polymorphisms evaluated.
| Gene | Polymorphism | Commercial Assay Codes † | Allelic Frequency ‡ | Allelic Frequency § | Gene Location | Impact on the Gene or Protein |
|---|---|---|---|---|---|---|
| VEGFA | rs1570360 | C_1647379_10 | A = 34% | A = 24% | promoter | VEGFA gene expression |
| rs2010963 | C_8311614_10 | C = 29% | C = 36% | 5′UTR | VEGFA gene expression | |
| rs3025039 | C_16198794_10 | T = 15% | T = 13% | 3′UTR | VEGFA mRNA concentration | |
| PTGS2 | rs689465 | C_2517146_10 | C = 12% | - | promoter | In combination with other genetic variants in haplotypes, it affects PTGS2 expression |
| NOS3 | rs2070744 | C_15903863_10 | C = 37% | - | intron | NOS3 gene expression |
| rs1799983 | C_3219460_20 | T = 32% | T = 29% | missense | eNOS enzyme activity | |
| TNF | rs1799724 | C_11918223_10 | T = 9% | - | promoter | TNF gene expression |
| rs361525 | C_2215707_10 | A = 4% | A = 5% | promoter | TNF gene expression | |
| rs1799964 | C_7514871_10 | C = 20% | C = 24% | promoter | TNF gene expression | |
| NOS2 | rs2779249 | C_2593689_10 | A = 29% | - | intron | iNOS protein activity |
| rs2297518 | C_11889257_10 | A = 19% | A = 17% | missense | iNOS protein activity |
† TaqMan assays used to genotype the polymorsphisms in this study; ‡ Data from gnomAD database (European non-Finnish population); § Data from AbraOM database (Brazilian population).
Author Contributions
Conceptualization of the study, J.A.G., L.S.-F. and F.S.L.V.; acquisition, analysis, and interpretation of the data, J.A.G., J.A.B., E.S., A.C.P.T.-T., J.H.d.S., B.F.R.R., M.F.G., T.M.d.O., M.D.F.C.d.A. and I.F.C.; writing—original draft preparation, J.A.G.; writing—review and editing, J.A.G., J.A.B., F.S.L.V.; supervision, L.S.-F. and F.S.L.V.; project administration, J.A.G. and F.S.L.V.; funding acquisition J.A.G., L.S.-F. and F.S.L.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Instituto Nacional de Genética Médica Populacional (INAGEMP), grant numbers CNPq 573993/2008-4 and FAPERGS 17/2551.0000521-0; Hospital de Clínicas de Porto Alegre (HCPA)-Fundo de Incentivo a Pesquisa e Eventos (FIPE), grant numbers 2017-0619 and 2019-0295; and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grant number Chamada MCTIC/FNDCT-CNPq/MEC-CAPES/MS-Decit/Nº 14/2016–Prevenção e Combate ao vírus Zika. The APC was funded by Hospital de Clínicas de Porto Alegre (HCPA)-Fundo de Incentivo a Pesquisa e Eventos (FIPE), grant numbers 2017-0619, 2019-0295. FSLV is recipient of a CNPq scholarship grant [grant number CNPq 312993/2017-0].
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Hospital de Clínicas de Porto Alegre (protocol code 170619—CAAE 78735817910015327, approved in 22 October 2017).
Informed Consent Statement
Informed consent was obtained from all legal guardians of the subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schuler-Faccini L., Sanseverino M.T.V., Vianna F.S.L., Da Silva A.A., Larrandaburu M., Marcolongo-Pereira C., Abeche A.M. Zika virus: A new human teratogen? Implications for women of reproductive age. Clin. Pharmacol. Ther. 2016;100:28–30. doi: 10.1002/cpt.386. [DOI] [PubMed] [Google Scholar]
- 2.França G.V.A., Schuler-Faccini L., Oliveira W.K., Henriques C.M.P., Carmo E.H., Pedi V.D., Nunes M.L., Castro M.C., Serruya S., Silveira M.F., et al. Congenital Zika virus syndrome in Brazil: A case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388:891–897. doi: 10.1016/S0140-6736(16)30902-3. [DOI] [PubMed] [Google Scholar]
- 3.Jaenisch T., Rosenberger K.D., Brito C., Brady O., Brasil P., Marques E.T.A. Risk of microcephaly after Zika virus infection in Brazil, 2015 to 2016. Bull. World Health Organ. 2017;95:191–198. doi: 10.2471/BLT.16.178608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nithiyanantham S.F., Badawi A. Maternal infection with Zika virus and prevalence of congenital disorders in infants: Systematic review and meta-analysis. Can. J. Public Health. 2019;110:638–648. doi: 10.17269/s41997-019-00215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werner H., Fazecas T., Guedes B., Lopes Dos Santos J., Daltro P., Tonni G., Campbell S., Araujo Júnior E. Intrauterine Zika virus infection and microcephaly: Correlation of perinatal imaging and three-dimensional virtual physical models. Ultrasound Obstet. Gynecol. 2016;47:657–660. doi: 10.1002/uog.15901. [DOI] [PubMed] [Google Scholar]
- 6.Werner H., Sodré D., Hygino C., Guedes B., Fazecas T., Nogueira R., Daltro P., Tonni G., Lopes J., Araujo Júnior E. First-trimester intrauterine Zika virus infection and brain pathology: Prenatal and postnatal neuroimaging findings. Prenat. Diagn. 2016;36:785–789. doi: 10.1002/pd.4860. [DOI] [PubMed] [Google Scholar]
- 7.Zhang F., Hammack C., Ogden S.C., Cheng Y., Lee E.M., Wen Z., Qian X., Nguyen H.N., Li Y., Yao B., et al. Molecular signatures associated with ZIKV exposure in human cortical neural progenitors. Nucleic Acids Res. 2016;44:8610–8620. doi: 10.1093/nar/gkw765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcez P.P., Nascimento J.M., De Vasconcelos J.M., Madeiro Da Costa R., Delvecchio R., Trindade P., Loiola E.C., Higa L.M., Cassoli J.S., Vitória G., et al. Zika virus disrupts molecular fingerprinting of human neurospheres. Sci. Rep. 2017;7 doi: 10.1038/srep40780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caires-Júnior L.C., Goulart E., Melo U.S., Araujo B.S.H., Alvizi L., Soares-Schanoski A., De Oliveira D.F., Kobayashi G.S., Griesi-Oliveira K., Musso C.M., et al. Discordant congenital Zika syndrome twins show differential in vitro viral susceptibility of neural progenitor cells. Nat. Commun. 2018;9 doi: 10.1038/s41467-017-02790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lima M.C., de Mendonça L.R., Rezende A.M., Carrera R.M., Aníbal-Silva C.E., Demers M., D’Aiuto L., Wood J., Chowdari K.V., Griffiths M., et al. The transcriptional and protein profile from human infected neuroprogenitor cells is strongly correlated to zika virus microcephaly cytokines phenotype evidencing a persistent inflammation in the CNS. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayless N.L., Greenberg R.S., Swigut T., Wysocka J., Blish C.A. Zika Virus Infection Induces Cranial Neural Crest Cells to Produce Cytokines at Levels Detrimental for Neurogenesis. Cell Host Microbe. 2016;20:423–428. doi: 10.1016/j.chom.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Sousa J.R., Azevedo R. do S. da S.; Martins Filho, A.J.; de Araujo, M.T.F.; Cruz, E. do R.M.; Vasconcelos, B.C.B.; Cruz, A.C.R.; de Oliveira, C.S.; Martins, L.C.; Vasconcelos, B.H.B.; et al. In situ inflammasome activation results in severe damage to the central nervous system in fatal Zika virus microcephaly cases. Cytokine. 2018;111:255–264. doi: 10.1016/j.cyto.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Diop F., Vial T., Ferraris P., Wichit S., Bengue M., Hamel R., Talignani L., Liegeois F., Pompon J., Yssel H., et al. Zika virus infection modulates the metabolomic profile of microglial cells. PLoS ONE. 2018;13:e0206093. doi: 10.1371/journal.pone.0206093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiwari S.K., Dang J., Qin Y., Lichinchi G., Bansal V., Rana T.M. Zika virus infection reprograms global transcription of host cells to allow sustained infection. Emerg. Microbes Infect. 2017;6 doi: 10.1038/emi.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi K.Q., Cai X.H., Xiao D.D., Wu S.J., Peng M.M., Lin X.F., Liu W.Y., Fan Y.C., Chen Y.P., Zheng M.H. Tumour necrosis factor-α-857T allele reduces the risk of hepatitis B virus infection in an Asian population. J. Viral Hepat. 2012;19 doi: 10.1111/j.1365-2893.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang P., Wang J., Yu M., Li Z. Tumor necrosis Factor-α T-857C (rs1799724) polymorphism and risk of cancers: A meta-analysis. Dis. Markers. 2016;2016:4580323. doi: 10.1155/2016/4580323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nourian M., Chaleshi V., Pishkar L., Azimzadeh P., Baradaran Ghavami S., Balaii H., Alinaghi S., Shahrokh S., Asadzadeh Aghdaei H., Zali M.R. Evaluation of tumor necrosis factor (TNF)-α mRNA expression level and the rs1799964 polymorphism of the TNF-α gene in peripheral mononuclear cells of patients with inflammatory bowel diseases. Biomed. Rep. 2017;6:698–702. doi: 10.3892/br.2017.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu T., Kong Z., Zhao H. Relationship between tumor necrosis factor-α rs361525 polymorphism and gastric cancer risk: A meta-analysis. Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carvalho F.H.C., Cordeiro K.M., Peixoto A.B., Tonni G., Moron A.F., Feitosa F.E.L., Feitosa H.N., Araujo Júnior E. Associated ultrasonographic findings in fetuses with microcephaly because of suspected Zika virus (ZIKV) infection during pregnancy. Prenat. Diagn. 2016;36:882–887. doi: 10.1002/pd.4882. [DOI] [PubMed] [Google Scholar]
- 20.Santos C.N.O., Ribeiro D.R., Cardoso Alves J., Cazzaniga R.A., Magalhães L.S., De Souza M.S.F., Fonseca A.B.L., Bispo A.J.B., Porto R.L.S., Dos Santos C.A., et al. Association between Zika Virus Microcephaly in Newborns with the rs3775291 Variant in Toll-Like Receptor 3 and rs1799964 Variant at Tumor Necrosis Factor-α Gene. J. Infect. Dis. 2019;220:1797–1801. doi: 10.1093/infdis/jiz392. [DOI] [PubMed] [Google Scholar]
- 21.Antoniou E., Orovou E., Sarella A., Iliadou M., Rigas N., Palaska E., Iatrakis G., Dagla M. Zika virus and the risk of developing microcephaly in infants: A systematic review. Int. J. Environ. Res. Public Health. 2020;17:3806. doi: 10.3390/ijerph17113806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbeito-Andrés J., Schuler-Faccini L., Garcez P.P. Why is congenital Zika syndrome asymmetrically distributed among human populations? PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.2006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbeito-Andrés J., Pezzuto P., Higa L.M., Dias A.A., Vasconcelos J.M., Santos T.M.P., Ferreira J.C.C.G., Ferreira R.O., Dutra F.F., Rossi A.D., et al. Congenital Zika syndrome is associated with maternal protein malnutrition. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aaw6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J., Carmichael S.L., Canfield M., Song J., Shaw G.M. Socioeconomic status in relation to selected birth defects in a large multicentered US case-control study. Am. J. Epidemiol. 2008;167:145–154. doi: 10.1093/aje/kwm283. [DOI] [PubMed] [Google Scholar]
- 25.Carmichael S.L., Nelson V., Shaw G.M., Wasserman C.R., Croen L.A. Socio-economic status and risk of conotruncal heart defects and orofacial clefts. Paediatr. Perinat. Epidemiol. 2003;17:264–271. doi: 10.1046/j.1365-3016.2003.00498.x. [DOI] [PubMed] [Google Scholar]
- 26.Dhillon S.S., Mastropaolo L.A., Murchie R., Griffiths C., Thöni C., Elkadri A., Xu W., Mack A., Walters T., Guo C., et al. Higher activity of the inducible nitric oxide synthase contributes to very early onset inflammatory bowel disease. Clin. Transl. Gastroenterol. 2014;5 doi: 10.1038/ctg.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikkari S.T., Määttä K.M., Kunnas T.A. Functional inducible nitric oxide synthase gene variants associate with hypertension a case-control study in a finnish population-the TAMRISK study. Medicine (United States) 2015;94:e1958. doi: 10.1097/MD.0000000000001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garry P.S., Ezra M., Rowland M.J., Westbrook J., Pattinson K.T.S. The role of the nitric oxide pathway in brain injury and its treatment—From bench to bedside. Exp. Neurol. 2015;263:235–243. doi: 10.1016/j.expneurol.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Tripathi M.K., Kartawy M., Amal H. The role of nitric oxide in brain disorders: Autism spectrum disorder and other psychiatric, neurological, and neurodegenerative disorders. Redox Biol. 2020;34:101567. doi: 10.1016/j.redox.2020.101567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman E.K. Haplotype thinking in lung disease. Proc. Am. Thorac. Soc. 2007;4:4–8. doi: 10.1513/pats.200607-145JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raab S., Beck H., Gaumann A., Yüce A., Gerber H.P., Plate K., Hammes H.P., Ferrara N., Breier G. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb. Haemost. 2004;91:595–605. doi: 10.1160/TH03-09-0582. [DOI] [PubMed] [Google Scholar]
- 32.Rosenstein J.M., Krum J.M., Ruhrberg C. VEGF in the nervous system. Organogenesis. 2010;6:107–114. doi: 10.4161/org.6.2.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waters J.P., Pober J.S., Bradley J.R. Tumour necrosis factor in infectious disease. J. Pathol. 2013;230:132–147. doi: 10.1002/path.4187. [DOI] [PubMed] [Google Scholar]
- 34.Jin K., Zhu Y., Sun Y., Mao X.O., Xie L., Greenberg D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: An update. Trends Immunol. 2015;36:161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Ricciotti E., Fitzgerald G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chavez G.G., Taylor G., Garaliene J., Richardson G.P., Korneev S.A. The temporal expression profile of a Nos3-related natural antisense RNA in the brain suggests a possible role in neurogenesis. Nitric Oxide Biol. Chem. 2017;71:27–31. doi: 10.1016/j.niox.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muhammad M. Cytokines. IntechOpen; London, UK: 2020. Tumor Necrosis Factor Alpha: A Major Cytokine of Brain Neuroinflammation. [DOI] [Google Scholar]
- 39.Olivera G.C., Ren X., Vodnala S.K., Lu J., Coppo L., Leepiyasakulchai C., Holmgren A., Kristensson K., Rottenberg M.E. Nitric Oxide Protects against Infection-Induced Neuroinflammation by Preserving the Stability of the Blood-Brain Barrier. PLOS Pathog. 2016;12:e1005442. doi: 10.1371/journal.ppat.1005442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J., Liu J., Zhou R., Ding X., Zhang Q., Zhang C., Li L. Zika virus infected primary microglia impairs NPCs proliferation and differentiation. Biochem. Biophys. Res. Commun. 2018;497:619–625. doi: 10.1016/j.bbrc.2018.02.118. [DOI] [PubMed] [Google Scholar]
- 41.Ensembl Genome Browser 102. [(accessed on 4 February 2021)]; Available online: https://www.ensembl.org/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.