Abstract
Background
Aloperine can regulate miR-296-5p/Signal Transducer and Activator of Transcription 3 (STAT3) pathway to inhibit the malignant development of colorectal cancer (CRC), but the regulatory mechanism is unclear. This study explored the upstream mechanism of Aloperine in reducing CRC damage from the perspective of the circRNA-miRNA-mRNA regulatory network.
Methods
After treatment with gradient concentrations of Aloperine (0.1 mmol/L, 0.2 mmol/L, 0.4 mmol/L, 0.8 mmol/L and 1 mmol/L) for 24 hours, changes in CRC cell proliferation and apoptosis were detected by functional experiments. Data of the differential expression of miR-296-5p in CRC patients and healthy people were obtained from Starbase. The effects of Aloperine on 12 differentially expressed circRNAs were detected. The binding of miR-296-5p with NOP2/Sun RNA methyltransferase 2 (circNSUN2) and STAT3 was predicted by TargetScan and confirmed through dual-luciferase experiments. The expressions of circNSUN2, miR-296-5p and STAT3 as well as apoptosis-related genes in CRC cells were detected by qRT-PCR and Western blot as needed. Rescue experiments were conducted to test the regulatory effects of circNSUN2, miR-296-5p and STAT3 on CRC cells.
Results
Aloperine at a concentration gradient inhibited proliferation and promoted apoptosis in CRC cells. The abnormally low expression of miR-296-5p in CRC could be upregulated by Aloperine. Among the differentially expressed circRNAs in CRC, only circNSUN2 not only targets miR-296-5p, but also can be regulated by Aloperine. The up-regulation of circNSUN2 offset the inhibitory effect of Aloperine on cancer cells. The rescue experiments finally confirmed the regulation of circNSUN2/miR-296-5p/STAT3 axis in CRC cells.
Conclusion
By regulating the circNSUN2/miR-296-5p/STAT3 pathway, Aloperine prevents the malignant development of CRC cells.
Keywords: colorectal cancer, aloperine, NOP2/Sun RNA methyltransferase 2, miR-296-5p, signal transducer and activator of transcription 3
Introduction
Cancer has become the first killer in the world and the biggest obstacle for extending human life expectancy. It is estimated that there were 18.1 million new cancer cases and 9.6 million cancer deaths in 2018.1 Colorectal cancer (CRC) is one of the most common gastrointestinal tumors.2 According to the data from the International Agency for Research on Cancer (IARC),1 the number of new CRC cases worldwide in 2018 was approximately 1.09 million, making CRC the fourth most prevalent malignant tumor after lung, breast and prostate cancers; meantime, CRC had a high mortality rate of about 9.2%, ranking only after lung cancer. Epidemiological studies have shown that the incidence of CRC varies significantly in different countries, and is higher in developed areas.3 More importantly, as the incidence of CRC rises rapidly in people under 50, the age of onset of the disease is getting lower.4,5 Therefore, it is urgent to improve the diagnosis rate and treatment effect of CRC.
Currently, the main clinical treatment of CRC is surgery combined with adjuvant radiotherapy, chemotherapy or molecular targeted therapy. Surgery is generally considered as the first choice for comprehensive treatment of CRC. This method is suitable for patients whose tumors are confined to the intestinal wall while penetrating the intestinal wall and invading the serous or extraserous membrane without lymph node metastasis.6,7 However, the availability of radical resection is limited as most patients with CRC have adenocarcinoma, which generally develops from polyps and is metastatic by nature.8 As a result, CRC patients tend to undergo simple resection, which leads to a high recurrence rate. Chemotherapy, therefore, is still necessary for postoperative and late-stage CRC patients.9,10 At present, the commonly used chemotherapeutic drugs in clinic mainly include 5-fluorouracil (5-Fu), oxaliplatin and its derivatives.9 Chemotherapy is highly risky because it indiscriminately kills tumor cells and immune cells and thereby reduces the anti-tumor immune effect.11 Likewise, radiotherapy greatly weakens the body’s immunity.12 Hence, researchers have proposed to find effective treatments or drugs that cause less damage to patients.
With advances in the research and development of traditional Chinese medicine, more attention has been paid to the roles of traditional Chinese medicine and its active ingredients in disease prevention and treatment. At the same time, new targets for molecular targeted therapy of diseases have been discovered in the exploration of the molecular mechanisms of traditional Chinese medicine and its monomers. Aloperine (ALO) has been confirmed to have a significant anti-cancer activity. ALO is a component of the traditional Chinese medicine Sophora alopecuroides L.13 It is also one of the main alkaloids separated and extracted in the lab, with a molecular formula of C15H24N2.14 Recent studies have found that ALO has the effects of anti-inflammation, immunosuppression, redox suppression, cardiovascular protection and anti-cancer, among which its tumor-suppressing activity has been extensively reported. For example, Yu et al revealed that ALO inhibited the carcinogenesis process by regulating excessive autophagy in thyroid cancer cells;15 Liu et al demonstrated that ALO inhibited the PI3K/Akt pathway, increased apoptosis and caused cell cycle block in liver cancer cells;16 besides, ALO was also found to exert an anti-cancer effect in prostate cancer and breast cancer.17,18 In the previous study, our research group found that ALO up-regulated miR-296-5p and inhibited the activity of its target gene STAT3, thereby inhibiting the proliferation and inducing the apoptosis of CRC cells. However, the activation pathway of miR-296-5p is unclear. In order to clarify the upstream activation pathway of ALO-upregulated miR-296-5p, this study will explore the mechanism of ALO in inhibiting CRC from the perspective of the circRNA-miRNA-mRNA regulatory network.
Method
Cell Purchase and ALO Processing
CCD-18Co and CRC cell lines SW480 (CCL-228™) and HT29 (HTB-38™) used in this research were provided by the American Type Culture Collection (ATCC). According to the culture requirements, CCD-18Co, SW480 and HT29 cells were separately inoculated in DMEM medium (30–2007, ATCC) containing 10% fetal bovine serum and incubated in a D180-P cell incubator (RWD, China) containing 5% CO2 at 37 °C.
ALO (DK0052, CAS NO: 56293-29-9, HPLC≥98%), the research object of this study, was provided by Chengdu DESITE Biological Company, China. ALO was dissolved in fresh DMEM to prepare 0.1 mmol/L, 0.2 mmol/L, 0.4 mmol/L, 0.8 mmol/L and 1 mmol/L ALO solutions, which were separately used to treat the CCD-18Co, SW480 and HT29 cells for 24 hours (h).
MTT Assay
One hundred µL of SW480 or HT29 cell suspension (1×104 cells/mL) was transferred to each well of a 96-well plate. Of note, three holes were added to the same treatment group. After treatment with the ALO solutions for 24 h, SW480 or HT29 cells were added with MTT reagent (10 µL/well, PB180519, Procell, USA), which could react with mitochondria in living cells. After 4 h of binding, a HBS-1096A enzyme label analyzer (DeTie, China) was used to detect the absorbance of SW480 or HT29 cells in the mixed solutions at 490 nm, and then cell viability was calculated based on the absorbance.
EdU (5-Ethynyl-2ʹ-Deoxyuridine) Cell Proliferation Experiment
SW480 or HT29 cells in logarithmic growth phase were seeded in 96-well plates at 1×104 cells/well. EdU reagent (ST067) purchased from Beyotime was diluted to 50 μmol/L with fresh DMEM medium. The EdU dilution was then used to incubate the cells at 100 μL/well for 2 h. After removing the culture medium, the SW480 or HT29 cells were fixed with methanol at room temperature for 15 minutes (min). Positive fluorescence expression (red) in the collected SW480 or HT29 cells were observed, photographed and recorded under a Leica DMi8 inverted fluorescence microscope (magnification 100 ×). Then DAPI reagent was used to stain the nuclei, and the stains were observed under a microscope.
Colony Formation Assay
Two hundred SW480 or HT29 cells were transferred to petri dishes containing complete medium (10 mL). In order to distribute the cells evenly, the petri dishes were rotated slowly for 1 min after cell inoculation. Next, the culture dishes containing cells were placed in an incubator for routine culture. During the culture, the medium was changed every 2 days (d). After 14 d, the SW480 or HT29 cells were soaked with Giemsa dye for 20 min and then the colony formation of the cells was observed under a microscope.
Apoptosis Experiment
An Annexin V-FITC Apoptosis Detection Kit (CA1020, Solarbio, China) and a flow cytometer (CytoFLEX, BECKMAN COULTER, USA) were used to detect changes in the apoptosis of SW480 or HT29 cells. In brief, SW480 or HT29 cells were prepared into 1×106 cells/mL cell suspension with 1 mL 1× Binding Buffer. One hundred μL of SW480 or HT29 cells and 5 μL of Annexin V-FITC reagent were added to a centrifuge tube and mixed for 10 min in the dark at room temperature. Next, 5 μL of PI reagent was added to the centrifuge tube and incubated in the dark at room temperature for 5 min. Afterwards, PBS was added to adjust the mixture to a final volume of 500 μL. After half an hour, the cells in the centrifuge tube were transferred to the detection instrument to calculate the number of apoptotic cells.
Bioinformatics Analysis and Target Gene Binding Verification
Starbase (http://starbase.sysu.edu.cn/index.php) was used to retrieve and analyze information of differential expression of miR-296-5p in Colon adenocarcinoma (COAD) patients and healthy volunteers. TargetScan (http://www.targetscan.org) was used to predict the circRNA that contained a binding sequence for miR-296-5p and the downstream target genes of miR-296-5p. The binding sequences predicted by TargetScan were designed by COBIOER (China) and used to construct the following reporter plasmids for dual luciferase experiments: NOP2/Sun RNA methyltransferase 2 wild type (circNSUN2-WT), circNSUN2-mutant type (MUT), Signal Transducer and Activator of Transcription 3 (STAT3)-WT and STAT3-MUT. The reporter plasmids were separately co-transfected with miR-296-5p mimic or mimic control into SW480 and HT29 cells using liposome for 48 h. Luciferase activity in the transfected SW480 and HT29 cells was detected using Dual-Luciferase® Reporter Assay System (E1960, Promega, USA) with a GloMax 20/20 luminometer (Promega, USA).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
qRT-PCR is a reliable method for rapid detection of gene mRNA levels. Briefly, total RNA of SW480 and HT29 cells was fully isolated by the conventional RNA extraction reagent TRIzol (15,596,018, Invitrogen, USA), and subsequently reverse transcribed into cDNA using the RNA reverse transcription reagent (RR047A) developed by TaKaRa (Japan). The qRT-PCR reaction system which consisted of cDNA, gene primers (Sangon synthesis), SYBR® Green (S4438-20RXN, Sigma-Aldrich, Germany) and DEPC water was added to a 96-well plate and put into the detection instrument (Bio-Rad thermal cycler T100). The reaction conditions were set as follows: pre-denaturation at 95 °C for 10 min, denaturation at 95 °C for 15 seconds (s), and annealing at 58 °C for 1 min, for a total of 40 cycles. According to Anita Ciesielska’s report,19 gene mRNA levels were quantified by the 2−ΔΔCT method. GAPDH and U6 were internal references in the experiment. The specific sequences of synthetic primers were shown in Table 1.
Table 1.
Primers for qRT-PCR
| Gene | Forward Primer (5ʹ-3ʹ) | Reverse Primer (5ʹ-3ʹ) |
|---|---|---|
| miR-296-5p | CCTGTGTCGTATCCAGTGCAA | GTCGTATCCAGTGCGTGTCG |
| circDDX17 | TGCCAACCACAACATCCTCCA | CGCTCCCCAGGATTACCAAAT |
| circHIPK3 | TATGTTGGTGGATCCTGTTCGGCA | TGGTGGGTAGACCAAGACTTGTGA |
| circZFR | AACCACCACAGATTCACTAT | AACCACCACAGATTCACTAT |
| circRNA_100290 | GTCATTCCCTCTTTAATGGTG | CAGAACTTCCGCTCTAACATAC |
| Has_circ_0026344 | CTCAGCCTCTAGCATAAGCTC | AGGCAAGAGAATGATTTGAAC |
| circVAPA | TGGATTCCAAATTGAGATGCGTATT | CACTTTTCTATCCGATGGATTTCGC |
| Hsa_circ_0009361 | AGAACCAGATTCGAGACGCC | GTGCTCTTCAATGCCACCTTC |
| circNSUN2 | CTTGAGAAAATCGCCACACTT | GTTGAGGAGCAGTGGTGG |
| circACAP2 | TCAGAGCATCTGCCCAAAGTT | AAATGAACCCCAAGGCTCCG |
| circITGA7 | GTGTGCACAGGTCCTTCCAA | TGGAAGTTCTGTGAGGGACG |
| circEIF4G3 | CCTACCCCATCCCCTTATTC | ACCGTGCTGTAGACTGCTGAG |
| STAT3 | CCCCATACCTGAAGACCAAG | GGACTCAAACTGCCCTCCT |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| GAPDH | TGTGGGCATCAATGGATTTGG | ACACCATGTATTCCGGGTCAAT |
Cell Transfection
Exogenous up-regulation or knockdown of test genes is a common method to observe their regulatory effects on cells or tissues. Liposome transfection with Lipofectamine 3000 (L3000008, ThermoFisher Scientific, USA) is the most common and convenient method for exogenous interference. Therefore, we commissioned Guangzhou GENESEED Biological Company to synthesize circNSUN2 overexpressed plasmid, circNSUN2 silenced plasmid (sh-circNSUN2), STAT3 overexpressed plasmid and their respective negative controls. MiR-296-5p mimic (miR10000690-1-5) and mimic control (miR1N0000001-1-5), as well as miR-296-5p inhibitor (miR20000690-1-5) and inhibitor control (miR2N0000001-1-5), were purchased directly from Guangzhou RIOBOBIO Company. The plasmids were transfected into SW480 and HT29 cells according to the grouping using Lipofectamine 3000. After 48 h, the success rate of transfection of the cells was determined by qRT-PCR.
Western Blot
RIPA lysate (tissue/cell, R0010) produced by Solarbio (China) was used to separate protein from SW480 and HT29 cells, and its concentration was determined with BCA protein concentration determination reagent (PC0020, Solarbio, China). Then the protein was subjected to high temperature denaturation to maintain a relatively stable state. After being electrophoresed by SDS-PAGE, the protein was transferred to the membrane carrier (PVDF membrane). The membrane was soaked with the same BSA sealant (SW3015) produced by Solarbio for 2 h at room temperature. Next, the membrane was incubated with Abcam (USA) antibodies (anti-STAT3, ab68153, 88 kDa; Bax, ab32503, 21 kDa; Bcl-2, ab59348, 26 kDa) that could bind to the corresponding antigens in the protein on the membrane overnight (4 °C), with GAPDH (ab8245, 36KD) as an internal reference. To identify the antigen-antibody complex, a secondary antibody labeled with HRP (Goat Anti-Rabbit antibody, 1:10,000, ab6721; Goat Anti-Mouse antibody, 1:10000, ab205719) was used to treat the PVDF membrane for 1.5 h at room temperature. Fluorescent signal of the complex was enhanced using the ECL Western Blotting Substrate (PE0010, Solarbio) and detected using the Gel Doc™ XR+ imaging system (BIO-RAD, USA). The gray value of the bands was calculated by Image Lab software to determine the final gene protein level.
Statistical Analysis
SPSS 20.0 software (IBM, USA) was employed for statistical analysis of the data obtained in the experiments. Differences between two groups were compared by independent sample t-test, and those between multiple groups were compared by one-way ANOVA followed by Tukey’s test. When p<0.05, the difference was considered statistically significant.
Result
ALO at a Concentration Gradient Inhibited Proliferation Yet Promoted Apoptosis in CRC Cells
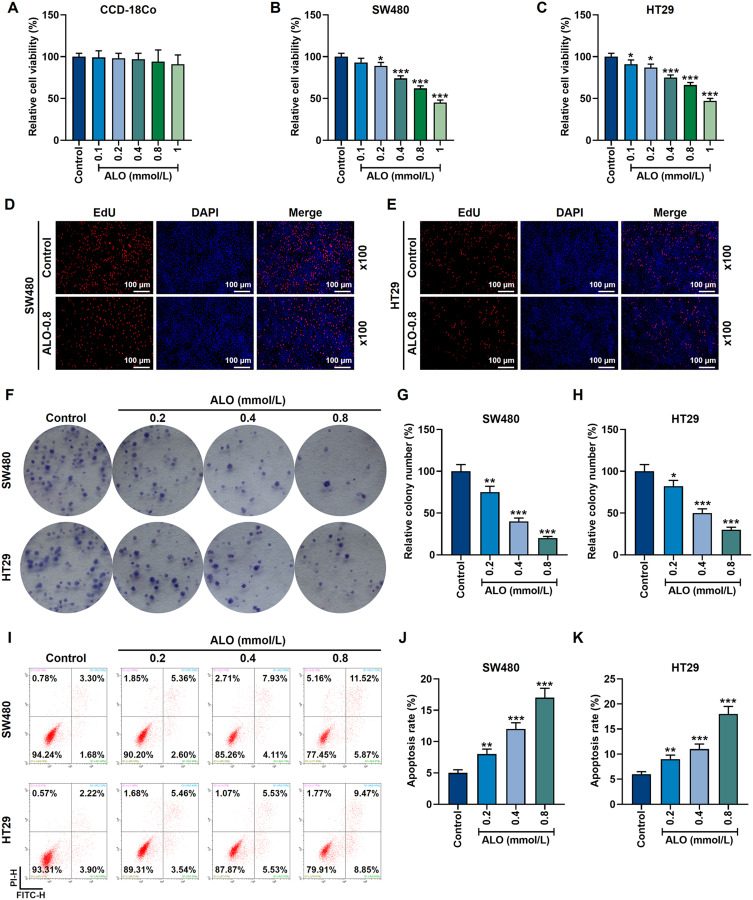
In this study, the toxicity of ALO in normal cells (CCD-18Co) was detected, ALO showed no significant toxicity to CCD-18Co cells (Figure 1A). In order to study the effects of ALO on the growth and basic physiological functions of CRC cells, we used different concentrations of ALO to treat SW480 and HT29 cells. The results showed that 0.2 mmol/L, 0.4 mmol/L, 0.8 mmol/L and 1 mmol/L ALO solutions all inhibited the viability of SW480 and HT29 cells (p<0.05, Figure 1B and C). Moreover, the viability of SW480 and HT29 cells was close to 50% under the treatment of 0.8 mmol/L ALO solution, but was reduced to less than 50% when the ALO concentration was increased to 1 mmol/L (Figure 1B and C). In the following EdU cell proliferation experiment, the red fluorescence expression was reduced in SW480 and HT29 cells under the treatment of 0.8 mmol/L ALO (Figure 1D and E). Consistently, the clone formation experiment also showed that different concentrations of ALO (0.2 mmol/L, 0.4 mmol/L, 0.8 mmol/L) reduced the number of cell clones and inhibited the proliferation of SW480 and HT29 cells (p<0.05, Figure 1F–H). At the same time, the increase in the concentration of the ALO solution accelerated the death of CRC cells. As shown in Figure 1I–K, the number of apoptotic SW480 and HT29 cells increased significantly after treatment with different concentrations of ALO (p<0.01, Figure 1I–K).
Figure 1.
ALO at a concentration gradient inhibited proliferation yet promoted apoptosis in CRC cells. (A) The effect of ALO on the normal colonic tissue cells (CCD-18Co) was detected by MTT experiments. (B and C) MTT experiments showed that ALO at a concentration gradient (0.1 mmol/L, 0.2 mmol/L, 0.4 mmol/L, 0.8 mmol/L and 1 mmol/L) inhibited the activity of SW480 and HT29 cells. (D and E) EdU cell proliferation experiments showed that 0.8 mmol/L ALO inhibited the proliferation of SW480 and HT29 cells. The magnification was 100×. (F–H) The clone formation experiment showed that ALO at a concentration gradient (0.2 mmol/L, 0.4 mmol/L and 0.8 mmol/L) inhibited the proliferation of SW480 and HT29 cells. (I–K) Flow cytometry experiments showed that ALO at a concentration gradient (0.2 mmol/L, 0.4 mmol/L and 0.8 mmol/L) promoted the apoptosis of SW480 and HT29 cells. All experiments were repeated three times to obtain average values. *p<0.05, **p<0.01, ***p<0.001 vs Control.
Abbreviations: ALO, aloperine; CRC, colorectal cancer.
The Abnormally Low Expression of miR-296-5p in Colon Cancer Could Be Up-Regulated by ALO
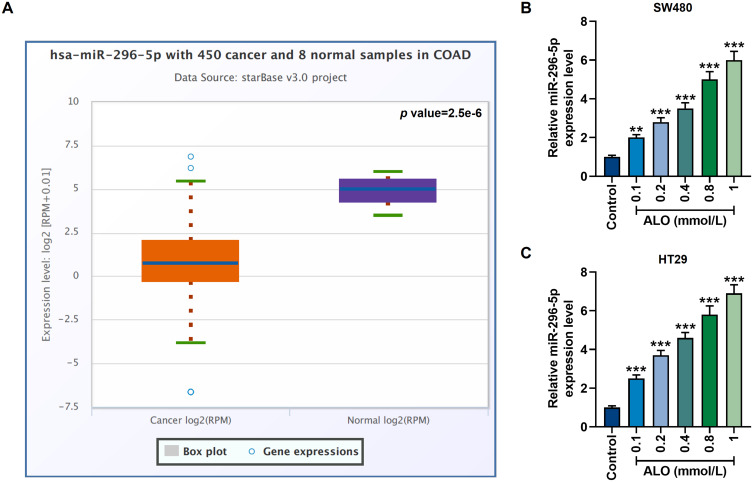
Data from the Starbase database showed that the expression of miR-296-5p in colon cancer patients was significantly lower than that in healthy people (p=2.5e-6, Figure 2A). In this study, however, after treatment with different concentrations of ALO for 24 h, miR-296-5p activity in SW480 and HT29 cells was significantly increased (p<0.01, Figure 2B and C), suggesting that ALO can stimulate the up-regulation of miR-296-5p.
Figure 2.
The abnormally low expression of miR-296-5p in colon cancer could be upregulated by ALO. (A) Starbase (http://starbase.sysu.edu.cn/index.php) was used to retrieve the information of differential expression of miR-296-5p in colon adenocarcinoma (COAD, n=450) patients and healthy volunteers (n=8) in vivo. P=2.5e-6. (B and C) The qRT-PCR experiment showed that ALO at a concentration gradient (0.1 mmol/L, 0.2 mmol/L, 0.4 mmol/L, 0.8 mmol/L and 1 mmol/L) up-regulated miR-296-5p expression in SW480 and HT29 cells. All experiments were repeated three times to obtain average values. **p<0.01, ***p<0.001 vs Control.
Abbreviation: qRT-PCR, quantitative real-time polymerase chain reaction.
Among the Differentially Expressed circRNAs in CRC, Only circNSUN2 Not Only Had a Target Site for miR-296-5p, but Also Could Be Regulated by ALO
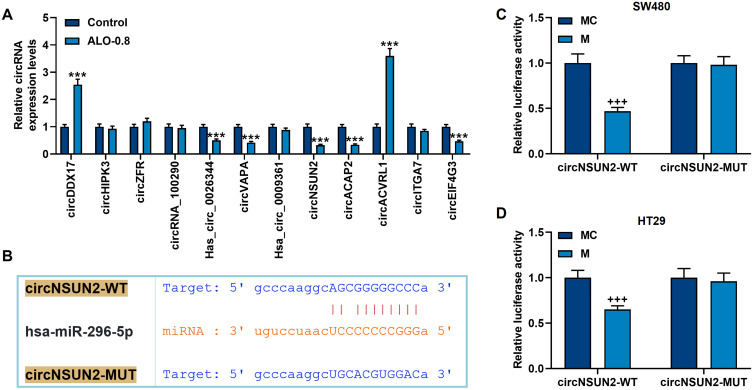
Through literature review, we screened 12 circRNAs that were reported to be associated with CRC: circDDX17, circHIPK3, circZFR, circRNA_100290, Has_circ_0026344, circVAPA, Hsa_circ_0009361, circNSUN2, circACAP2, circACVRL1, circITGA7 and circEIF4G3. We first detected the regulatory effect of ALO on these differentially expressed circRNAs by qRT-PCR to perform preliminary screening (Figure 3A). The results showed that the expressions of circDDX17, Has_circ_0026344, circVAPA, circNSUN2, circACAP2, circACVRL1 and circEIF4G3 were regulated by 0.8 mmol/L ALO (p<0.001, Figure 3A). Through subsequent screening of target genes, we found that only circNSUN2 and miR-296-5p have targeted binding sites (Figure 3B). After further verification, it was confirmed that circNSUN2-WT and miR-296-5p mimic did reduce the luciferase activity in SW480 and HT29 cells (p<0.001, Figure 3C and D). Therefore, we chose circNSUN2 as the next research object.
Figure 3.
Among the differentially expressed circRNAs in CRC, only circNSUN2 not only had a target site for miR-296-5p, but also could be regulated by ALO. (A) qRT-PCR detected the regulation of 0.8 mmol/L ALO on the differentially expressed circRNAs in CRC. GAPDH played the role of internal reference. (B) The TargetScan database (http://www.targetscan.org) was used to predict the target sites of circNSUN2 and miR-296-5p. (C and D) The dual luciferase experiment confirmed that circNSUN2 can bind to miR-296-5p in SW480 and HT29 cells. All experiments were repeated three times to obtain average values. ***p<0.001 vs Control; +++p<0.001 vs MC.
Abbreviation: MC, miR-296-5p mimic control.
Overexpression of circNSUN2 Partially Offset the Suppression of ALO on the Biological Behavior of Cancer Cells
In SW480 and HT29 cells, ALO suppressed the mRNA level of circNSUN2, whereas transfection of circNSUN2 overexpression significantly increased the expression of circNSUN2 (p<0.001, Figure 4A and B). The following basic cell function experiments showed that the upregulation of circNSUN2 partially offset the inhibitory effect of ALO on CRC cell viability, proliferation and apoptosis (p<0.001, Figure 4C–L).
Figure 4.
Overexpression of circNSUN2 partially offset the suppression of ALO on the biological behavior of cancer cells. (A and B) The qRT-PCR experiment showed that ALO (0.8 mmol/L) inhibited the expression of circNSUN2 in SW480 and HT29 cells. GAPDH played the role of internal reference. (C and D) The MTT experiment showed that overexpression of circNSUN2 partially offset the inhibition of ALO on cell viability in SW480 and HT29 cells. (E and F) EdU cell proliferation experiments showed that overexpression of circNSUN2 partially offset the inhibition of ALO on proliferation in SW480 and HT29 cells. The magnification was 100×. (G–I) The clone formation experiment showed that overexpression of circNSUN2 partially offset the inhibition of ALO on proliferation in SW480 and HT29 cells. (J–L) Flow cytometry experiments showed that overexpression of circNSUN2 partially offset the promotion of ALO on apoptosis in SW480 and HT29 cells. All experiments were repeated three times to obtain average values. ***p<0.001 vs Control; ^p<0.05, ^^p<0.01, ^^^p<0.001 vs ALO+NC.
Abbreviations: CircNSUN2, NOP2/Sun RNA methyltransferase 2; NC, negative control.
Sh-circNSUN2 Inhibited the Malignant Development of CRC Cells, Which Was Neutralized by miR-296-5p Inhibitor
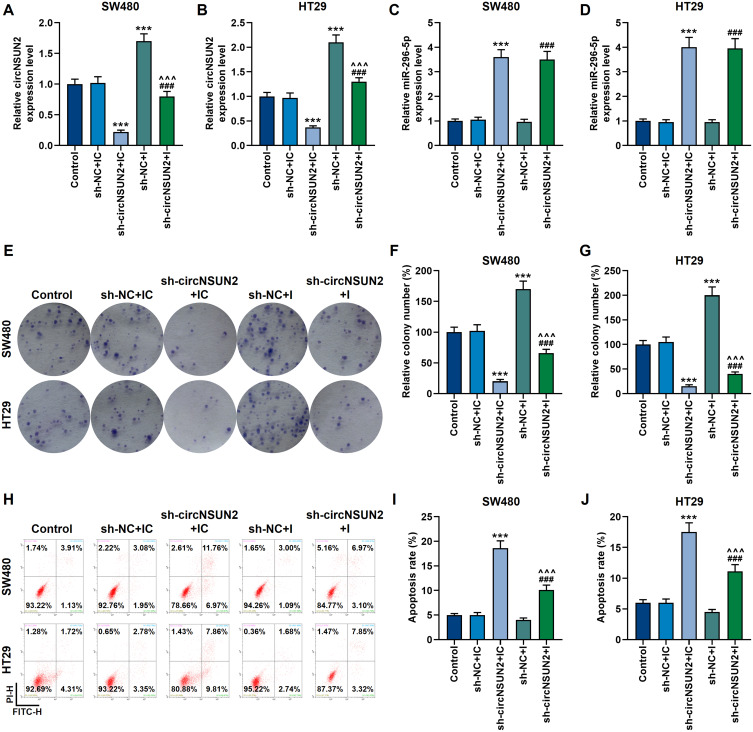
We also tested the mutual regulation of circNSUN2 and miR-296-5p in SW480 and HT29 cells. Sh-circNSUN2 could down-regulate the expression of circNSUN2, and besides, it inhibited the proliferation of SW480 and HT29 cells, while promoting their apoptosis and the expression of miR-296-5p (p<0.001, Figure 5A–J). However, the inhibitory effect of sh-circNSUN2 on the malignant development of the two cancer cells was neutralized by miR-296-5p inhibitor (p<0.001, Figure 5A–J).
Figure 5.
Sh-circNSUN2 inhibited the malignant development of CRC cells, which was neutralized by miR-296-5p inhibitor. (A and B) The qRT-PCR experiment showed that miR-296-5p inhibitor neutralized the inhibitory effect of sh-circNSUN2 on circNSUN2 expression. GAPDH played the role of internal reference. (C and D) The qRT-PCR experiment showed that sh-circNSUN2 up-regulated the mRNA level of miR-296-5p. U6 played the role of internal reference. (E–G) The clone formation experiment showed that the effect of sh-circNSUN2 on the proliferation of SW480 and HT29 cells was neutralized by miR-296-5p inhibitor. (H-J) Flow cytometry experiments showed that the effect of sh-circNSUN2 on promoting the apoptosis of SW480 and HT29 cells was neutralized by miR-296-5p inhibitor. All experiments were repeated three times to obtain average values. ***p<0.001 vs sh-NC+IC; ^^^p<0.001 vs sh-circNSUN2+IC; ###p<0.001 vs sh-NC+I.
Abbreviations: Sh-circNSUN2, silent circNSUN2; sh-NC, silent negative control; IC, miR-296-5p inhibitor control; I, miR-296-5p inhibitor.
MiR-296-5p Bound to STAT3 and Upregulation of Mir-296-5p Inhibited the Expression of STAT3 in CRC Cells
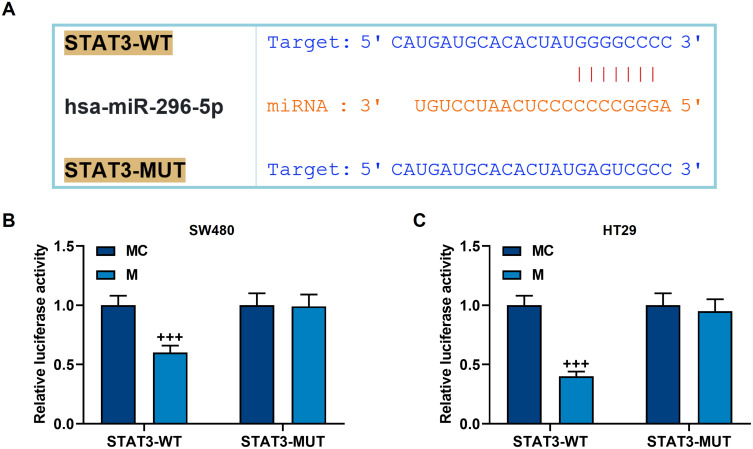
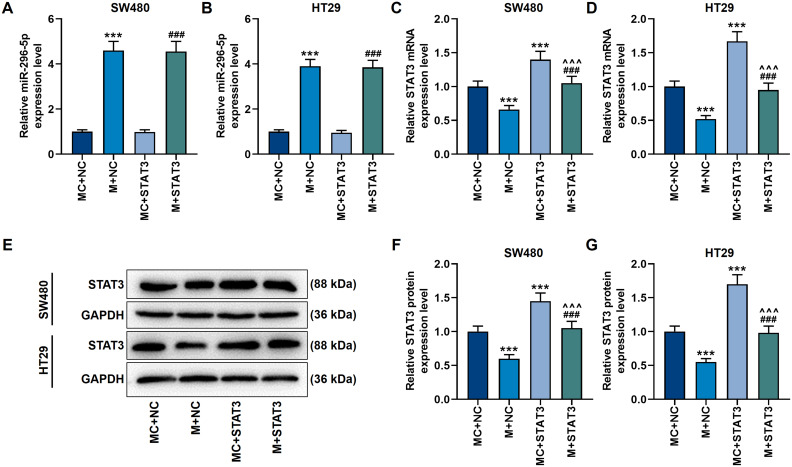
We screened the target genes of miR-296-5p, and found that miR-296-5p and STAT3 have targeted binding sites (Figure 6A), and this binding relation was further confirmed by a dual-luciferase verification test (p<0.001, Figure 6B and C). Next, we used qRT-PCR and Western blot to analyze the mRNA and protein levels of miR-296-5p and STAT. It was found that miR-296-5p mimic up-regulated miR-296-5p expression while preventing the activation of STAT3 (p<0.001, Figure 7A–G). However, the overexpression of STAT3 reversed the regulation of miR-296-5p mimic on the two genes (p<0.001, Figure 7A–G).
Figure 6.
The targeted binding of miR-296-5p to STAT3. (A) TargetScan was used to predict the binding sequence of miR-296-5p and STAT3. (B and C) Dual luciferase experiment confirmed that miR-296-5p can bind to STAT3 in SW480 and HT29 cells. All experiments were repeated three times to obtain average values. +++p<0.001 vs MC.
Figure 7.
Up-regulation of miR-296-5p inhibited the expression of STAT3 in CRC cells. (A and B) The qRT-PCR experiment showed that miR-296-5p mimic up-regulated the mRNA level of miR-296-5p. U6 played the role of internal reference. (C and D) The qRT-PCR experiment showed that miR-296-5p mimic suppressed the mRNA level of STAT3, while overexpression of STAT3 reversed this effect. GAPDH played the role of internal reference. (E–G) The Western blot experiment showed that miR-296-5p mimic inhibited the protein level of STAT3, while over-expression of STAT3 reversed this effect. GAPDH played the role of internal reference. All experiments were repeated three times to obtain average values. ***p<0.001 vs MC+NC; ^^^p<0.001 vs M+NC; ###p<0.001 vs MC+STAT3.
Abbreviation: STAT3, signal transducer and activator of transcription 3.
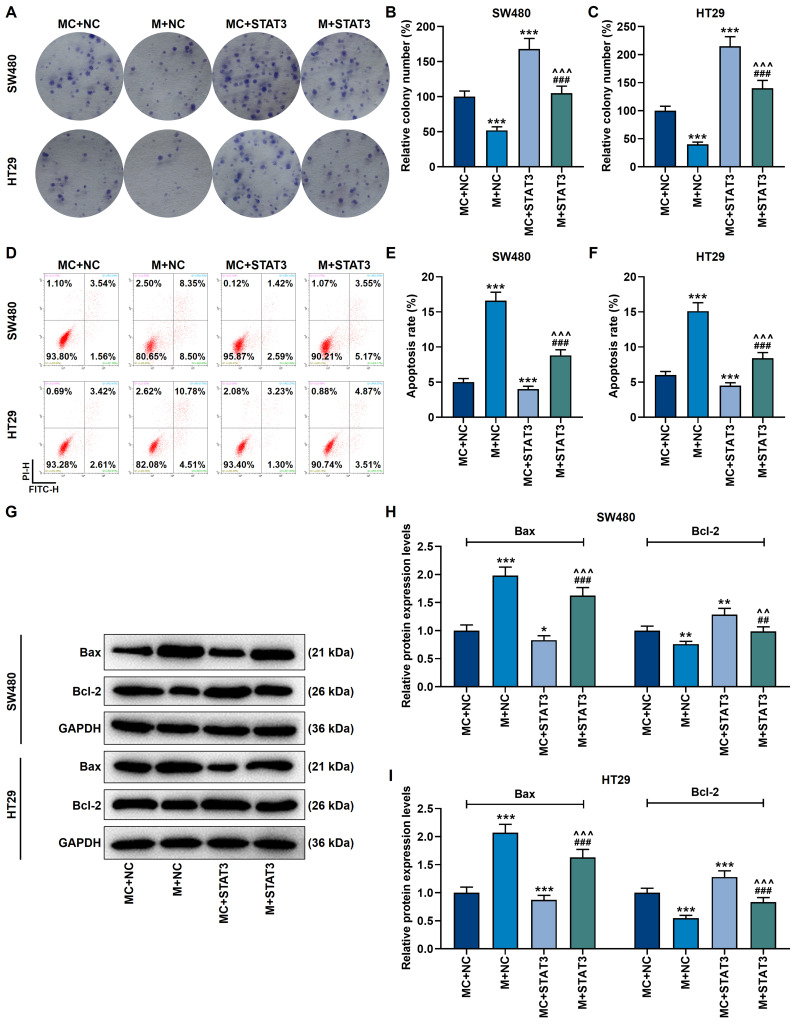
MiR-296-5p Mimic Inhibited Proliferation and Promoted Apoptosis in CRC Cells by Regulating Apoptosis-Related Genes, Which Was Partially Reversed by Overexpressed STAT3
The following cell experiments showed that by activating Bax and blocking the protein activity of Bcl-2, miR-296-5p mimic inhibited proliferation yet accelerated apoptosis in cancer cells (p<0.001, Figure 8A–I). However, up-regulation of STAT3 produced a completely opposite regulatory effect (p<0.001, Figure 8A–I). More importantly, overexpression of STAT3 neutralized the regulation of miR-296-5p mimic on CRC cells (p<0.001, Figure 8A–I).
Figure 8.
MiR-296-5p mimic inhibited proliferation and promoted apoptosis in CRC cells by regulating apoptosis-related genes, which was partially reversed by overexpression of STAT3. (A–C) The clone formation experiment showed that the inhibitory effect of miR-296-5p mimic on the proliferation of SW480 and HT29 cells was neutralized by overexpressed STAT3. (D–F) Flow cytometry experiments showed that miR-296-5p mimic promoted the apoptosis of SW480 and HT29 cells, which was neutralized by overexpressed STAT3. (G–I) Western blot experiments showed that the regulation of apoptosis-related protein expression by miR-296-5p mimic was neutralized by overexpressed STAT3. GAPDH played the role of internal reference. All experiments were repeated three times to obtain average values. *p<0.05, **p<0.01, ***p<0.001 vs MC+NC; ^^p<0.01, ^^^p<0.001 vs M+NC; ##p<0.01, ###p<0.001 vs MC+STAT3.
Discussion
CRC is a malignant lesion of the mucosal epithelium of the colon or rectum under the action of various carcinogenic factors such as environment or heredity.2 Most CRC patients suffer from adenocarcinoma, which usually develops from polyps. Tumors developed from polyps can infiltrate in a circular shape along the horizontal axis of the intestinal tube, develop into the deep layer of the intestinal wall, and finally penetrate the intestinal wall and metastasize to blood vessels or lymphatic vessels.20 This is one of the important reasons for the high recurrence and metastasis rates of CRC. The ultimate goals of CRC research are to improve the current treatment of CRC, save patients’ lives and improve their quality of life. Based on the role of traditional Chinese medicine in disease prevention and treatment, this study explored the effects of ALO, an alkaloid exhibiting strong anticancer activity, on the proliferation and apoptosis of CRC cells. The results showed that after ALO treatment, the viability and proliferation of CRC cells were significantly inhibited; on the contrary, the number of apoptotic cancer cells was significantly increased. This is consistent with the previous experimental results.21 In order to probe deeper into the upstream mechanism of ALO in alleviating CRC carcinogenesis through the miR-296-5p/STAT3 axis, we screened and verified the upstream targeting circRNA of miR-296-5p.
CircRNAs are a type of covalently closed circular non-coding RNA formed by back-splicing of mRNA precursors (pre-mRNA).22 CircRNA was considered to be a useless splicing by-product in early research. With the deepening of research, it is found that circRNA has a wide range of sources and plays a variety of functional roles in the growth and development of organisms, with the characteristics of conservative, stable, and tissue-specific.22,23 The most familiar and extensively studied function of circRNA is its sponging effect on miRNA.24 For example, circRNA ciRS-7 which contains more than 70 miR-7 binding sites can achieve competitive adsorption of miR-7 through the AGO2 protein.25 Besides, increasing reports on cancer indicate that circRNAs, such as circRNA-cTFRC, circPSMC3 and circSETD3, act as sponges of miRNAs and participate in transcriptional regulation.26–28 Based on these literature reports, we screened circRNAs that were reported to be abnormally expressed in CRC, and finally obtained 12 circRNAs. After detecting their expressions in ALO-treated cancer cells and predicting their binding sequence for miR-296-5p, circNSUN2 was finally identified as the research object of this study.
CircNSUN2, which maps to the 5p15 amplicon in CRCs, was confirmed by Chen et al to regulate cytoplasmic output and promote liver metastasis of CRC through N 6-methyladenosine modification.29 Similarly, our experimental results uncovered that knockdown of circNSUN2 inhibited proliferation and accelerated apoptosis in CRC cells. More importantly, we for the first time revealed that ALO can prevent the activation of circNSUN2 and offset the promotion effect of overexpression of circNSUN2 on esophageal cancer progression. Since circNSUN2 and miR-296-5p have targeting sequences, we verified the cellular regulatory effects of circNSUN2 and miR-296-5p/STAT3 through dual luciferase experiments and rescue experiments. The final result confirmed our conjecture that regulating the circNSUN2/miR-296-5p/STAT3 axis can reduce the proliferation rate and increase the apoptosis rate of CRC cells.
At present, blocking proliferation and increasing apoptosis in cancer cells are the intensively discussed mechanisms in CRC study. Tumor cells can proliferate quickly and unrestrictedly, and thus, inhibiting their proliferation can produce an anti-tumor effect.30 Apoptosis, alternatively called programmed cell death, is an autonomous cell death process strictly controlled by multiple genes. Apoptosis is an important part of cell life cycle, and it is also an important link in regulating the development of the body and maintaining the stability of the internal environment in organisms.31,32 Our research shows that ALO inhibits the proliferation and promotes the apoptosis of CRC cells by regulating the circNSUN2/miR-296-5p/STAT3 pathway, and ultimately prevents the tumorigenesis of CRC.
There are still certain limitations in our research. Although we have confirmed the effect of ALO on inducing apoptosis and reducing proliferation in CRC cells and clarified the underlying mechanism, the circNSUN2/miR-296-5p/STAT3 axis and the pathways related to proliferation and apoptosis have not been analyzed yet, which will be further explored in future research. In addition, the anticancer effect of ALO on CRC and its experimental concentration needs to be further tested in animal and clinical experiments. Whether ALO has an impact on drug resistance in CRC is also a direction of future research.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Brody H. Colorectal cancer. Nature. 2015;521(7551):S1. doi: 10.1038/521S1a [DOI] [PubMed] [Google Scholar]
- 3.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394(10207):1467–1480. doi: 10.1016/s0140-6736(19)32319-0 [DOI] [PubMed] [Google Scholar]
- 4.The Lancet O. Colorectal cancer: a disease of the young? Lancet Oncol. 2017;18(4):413. doi: 10.1016/s1470-2045(17)30202-4 [DOI] [PubMed] [Google Scholar]
- 5.Patel SG, Ahnen DJ. Colorectal cancer in the young. Curr Gastroenterol Rep. 2018;20(4):15. doi: 10.1007/s11894-018-0618-9 [DOI] [PubMed] [Google Scholar]
- 6.Costas-Chavarri A, Nandakumar G, Temin S, et al. Treatment of patients with early-stage colorectal cancer: ASCO resource-stratified guideline. J Glob Oncol. 2019;5(5):1–19. doi: 10.1200/jgo.18.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zellweger M, Abdelnour-Berchtold E, Krueger T, Ris HB, Perentes JY, Gonzalez M. Surgical treatment of pulmonary metastasis in colorectal cancer patients: current practice and results. Crit Rev Oncol Hematol. 2018;127:105–116. doi: 10.1016/j.critrevonc.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 8.Chakedis J, Schmidt CR. Surgical treatment of metastatic colorectal cancer. Surg Oncol Clin N Am. 2018;27(2):377–399. doi: 10.1016/j.soc.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 9.Kim JH. Chemotherapy for colorectal cancer in the elderly. World J Gastroenterol. 2015;21(17):5158–5166. doi: 10.3748/wjg.v21.i17.5158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sileri P, D’Ugo S, Benavoli D, et al. Metachronous splenic metastasis from colonic carcinoma five years after surgery: a case report and literature review. South Med J. 2009;102(7):733–735. doi: 10.1097/SMJ.0b013e3181a93c39 [DOI] [PubMed] [Google Scholar]
- 11.Koi M, Carethers JM. The colorectal cancer immune microenvironment and approach to immunotherapies. Future Oncol. 2017;13(18):1633–1647. doi: 10.2217/fon-2017-0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrobel P, Ahmed S. Current status of immunotherapy in metastatic colorectal cancer. Int J Colorectal Dis. 2019;34(1):13–25. doi: 10.1007/s00384-018-3202-8 [DOI] [PubMed] [Google Scholar]
- 13.Fu X, Sun F, Wang F, et al. Aloperine protects mice against DSS-induced colitis by PP2A-mediated PI3K/Akt/mTOR signaling suppression. Mediators Inflamm. 2017;2017:5706152. doi: 10.1155/2017/5706152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolkachev ON, Monakhova TE, Sheichenko VI, Kabanov VS, Fesenko OG, Proskurnina NF. Alkaloids of a new type from Sophora alopecuroides L. Chem Nat Compd. 1975;11(1):29–34. doi: 10.1007/BF00567025 [DOI] [Google Scholar]
- 15.Yu HI, Shen HC, Chen SH, et al. Autophagy modulation in human thyroid cancer cells following aloperine treatment. Int J Mol Sci. 2019;20(21):5315. doi: 10.3390/ijms20215315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu JS, Huo CY, Cao HH, et al. Aloperine induces apoptosis and G2/M cell cycle arrest in hepatocellular carcinoma cells through the PI3K/Akt signaling pathway. Phytomedicine. 2019;61:152843. doi: 10.1016/j.phymed.2019.152843 [DOI] [PubMed] [Google Scholar]
- 17.Ling Z, Guan H, You Z, et al. Aloperine executes antitumor effects through the induction of apoptosis and cell cycle arrest in prostate cancer in vitro and in vivo. Onco Targets Ther. 2018;11:2735–2743. doi: 10.2147/ott.s165262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian D, Li Y, Li X, Tian Z. Aloperine inhibits proliferation, migration and invasion and induces apoptosis by blocking the Ras signaling pathway in human breast cancer cells. Mol Med Rep. 2018;18(4):3699–3710. doi: 10.3892/mmr.2018.9419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciesielska A, Stączek P. Selection and validation of reference genes for qRT-PCR analysis of gene expression in microsporum canis growing under different adhesion-inducing conditions. Sci Rep. 2018;8(1):1197. doi: 10.1038/s41598-018-19680-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller MF, Ibrahim AE, Arends MJ. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016;469(2):125–134. doi: 10.1007/s00428-016-1956-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Zheng Y, Deng H, Liang L, Peng J. Aloperine induces G2/M phase cell cycle arrest and apoptosis in HCT116 human colon cancer cells. Int J Mol Med. 2014;33(6):1613–1620. doi: 10.3892/ijmm.2014.1718 [DOI] [PubMed] [Google Scholar]
- 22.Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12(4):381–388. doi: 10.1080/15476286.2015.1020271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Teng S, Xu J, et al. Microarray is an efficient tool for circRNA profiling. Brief Bioinform. 2019;20(4):1420–1433. doi: 10.1093/bib/bby006 [DOI] [PubMed] [Google Scholar]
- 24.Conn SJ, Pillman KA, Toubia J, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160(6):1125–1134. doi: 10.1016/j.cell.2015.02.014 [DOI] [PubMed] [Google Scholar]
- 25.Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495(7441):384–388. doi: 10.1038/nature11993 [DOI] [PubMed] [Google Scholar]
- 26.Xu L, Feng X, Hao X, et al. CircSETD3 (Hsa_circ_0000567) acts as a sponge for microRNA-421 inhibiting hepatocellular carcinoma growth. J Exp Clin Cancer Res. 2019;38(1):98. doi: 10.1186/s13046-019-1041-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye CY, Chen L, Liu C, Zhu QH, Fan L. Widespread noncoding circular RNAs in plants. New Phytol. 2015;208(1):88–95. doi: 10.1111/nph.13585 [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Yang M, Wei S, Qin F, Zhao H, Suo B. Identification of circular RNAs and their targets in leaves of triticum aestivum l. under dehydration stress. Front Plant Sci. 2016;7:2024. doi: 10.3389/fpls.2016.02024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen RX, Chen X, Xia LP, et al. N(6)-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat Commun. 2019;10(1):4695. doi: 10.1038/s41467-019-12651-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fletcher JS, Springer MG, Choi K, et al. STAT3 inhibition reduces macrophage number and tumor growth in neurofibroma. Oncogene. 2019;38(15):2876–2884. doi: 10.1038/s41388-018-0600-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammad RM, Muqbil I, Lowe L, et al. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol. 2015;35:S78–s103. doi: 10.1016/j.semcancer.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev. 2015;16(6):2129–2144. doi: 10.7314/apjcp.2015.16.6.2129 [DOI] [PubMed] [Google Scholar]