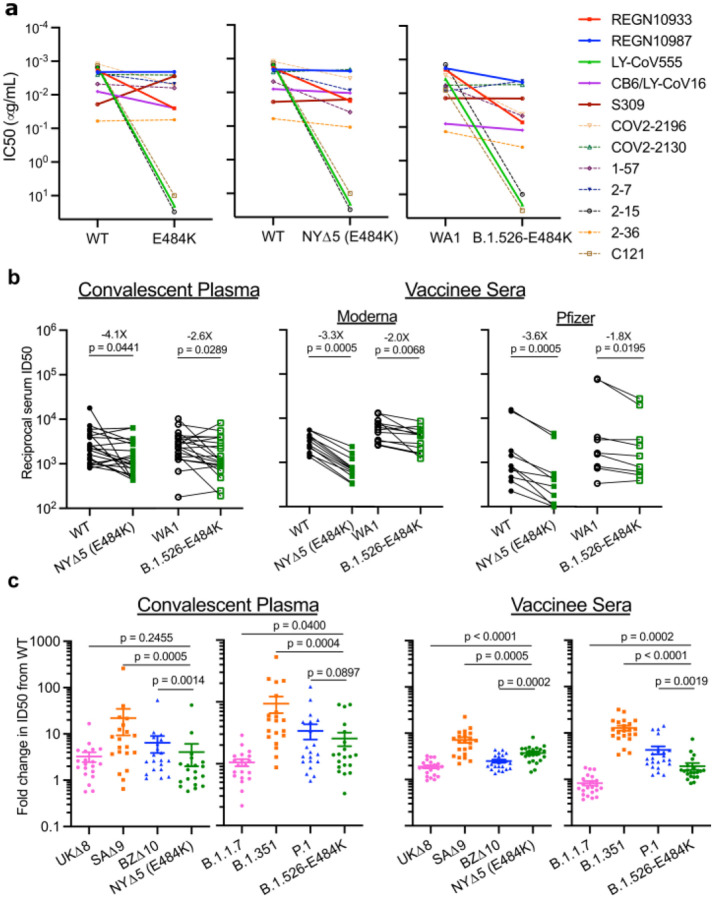
Figure 3. Neutralization studies of B.1.526-E484K and comparative analyses.
(a) Neutralizing activities of 12 monoclonal antibodies against pseudoviruses containing E484K alone or all five signature B.1.526 mutations (L5F, T95I, D253G, A701V, and E484K), termed NYΔ5(E484K) as well as against the authentic B.1.526-E484K. Antibodies with emergency use authorization are shown in bold solid lines. Data are represented as mean ± SEM of technical triplicates and represent one of two independent experiments. (b) Neutralizing activities of convalescent plasma (n=20) and vaccinee sera (n=22) against the NYΔ5(E484K) pseudovirus compared to wildtype pseudovirus as well as against authentic B.1.526-E484K and wildtype virus (WA1). (c) Fold change in convalescent plasma and vaccinee sera neutralization ID50 of different variant pseudoviruses and live viruses compared to wildtype counterparts. The data on B.1.1.7, B.1.351 and P.1 were derived from our prior publications4,18. Data from 20 convalescent patients or 22 vaccinated individuals were averaged and are represented as arithmetic mean ± SEM (individual data points also shown). Statistical comparisons were made using the Wilcoxon matched-pairs signed rank test; two-tailed p-values are reported.