Abstract
Polychlorinated biphenyls (PCBs) are persistent toxic chemicals with both legacy sources (e.g., Aroclors) and new sources (e.g., unintentional contaminants in some pigments and varnishes). PCB sulfates are derived from further metabolism of hydroxylated PCBs (OH-PCBs), which are oxidative metabolites of PCBs. While OH-PCBs and PCB sulfates are implicated in multiple toxicological effects, studies of PCB sulfates in human serum have been limited by available analytical procedures. We have now developed a method for extraction of PCB sulfates from serum followed by differential analysis with, and without, sulfatase-catalyzed hydrolysis to OH-PCBs. A sulfatase from Helix pomatia was purified by affinity chromatography, and it displayed broad specificity for PCB sulfates without contaminant glucuronidase activity. Following sulfatase-catalyzed hydrolysis of the PCB sulfates extracted from serum, the corresponding OH-PCBs were derivatized to methoxy-PCBs and quantitated by GC-MS/MS. In a pooled sample of human serum, we identified 10 PCB sulfates, with three PCB sulfate congeners exhibiting the highest concentrations from 1200–3970 pg/g of serum. In conclusion, we have developed a sensitive and specific method for the determination of PCB sulfates in human serum.
Graphical Abstract

INTRODUCTION
Polychlorinated biphenyls (PCBs) are a class of toxic industrial chemicals comprising 209 congeners that are named according to the different positions of chlorine atoms on the biphenyl ring system.1 PCBs were synthesized in large quantities for use in a wide range of applications that included plasticizers, transformers, caulking materials, and many others.2, 3 The industrial production of PCBs was banned in the 1970s by the United States and most other countries because of their various adverse health effects.4–8 Even though they are no longer intentionally produced, a large amount of traditionally manufactured PCBs remain in the environment worldwide. Moreover, new sources of PCBs (e.g., unintentionally produced contaminants in some pigments and varnishes) are currently adding to human exposures and their resulting health impacts.9–12
Metabolism of PCBs in humans can lead to both stable and unstable metabolites, some of which have biological/toxicological activities. PCBs are often initially metabolized in reactions catalyzed by cytochrome P450s (CYPs) to form hydroxylated PCBs (OH-PCBs) or PCB epoxides.5, 6, 13 PCB epoxides may be further metabolized by conjugation with glutathione (GSH), or they may rearrange to mono-OH-PCBs.6, 13 Further metabolism of OH-PCBs can include conversion to PCB sulfates catalyzed by sulfotransferases6, 14–16 and/or formation of PCB-glucuronides catalyzed by UDP-glucuronosyl transferases.5, 6, 17 Many studies have shown that PCBs and their metabolites elicit toxicities that include neurologic, carcinogenic, endocrine, and other effects.7, 18–22 Among those PCB metabolites with potential toxicological effects, PCB sulfates have been demonstrated to be high affinity ligands for transthyretin, a protein involved in the transport of L-thyroxine.14, 23 This transthyretin-binding of PCB sulfates may potentially have significant toxicological effects through thyroid hormone disruption. In addition, OH-PCBs and some PCB sulfates also inhibit sulfotransferases (SULT1E1 and SULT2A1), and as a result, they may interfere with the sulfation of endogenous steroid hormones as well as xenobiotic molecules.24, 25 Furthermore, PCB sulfates can serve as ligands for albumin, a major protein in human serum that functions as a carrier of many drugs and other molecules.26 This selective and reversible binding to serum albumin might lead to retention and/or transport of PCB sulfates to target sites.
While there have been extensive studies to determine the levels of PCBs and OH-PCBs in human serum samples, little research has been conducted to examine the concentrations of PCB sulfates in human serum4, 6, 8. The first PCB sulfate to be identified and quantitated in human serum was 4-PCB 11 sulfate (3,3′-dichloro-4′-sulfooxy-biphenyl). This PCB sulfate congener was found in 10 out of 46 human serum samples from subjects in Columbus Junction, Iowa, and East Chicago, Indiana.27 The discovery of 4-PCB 11 sulfate in human serum suggested that, if more PCB sulfates were present, the total exposure to PCBs in humans might have been underestimated by only measuring PCBs and OH-PCBs.27 Even though PCB 11 sulfate has been detected in human serum samples using an LC/MS technique, a combination of the lack of a generally applicable method and the relatively limited numbers of experimental standards available for PCB sulfates (e.g., the theoretical number of mono-hydroxylated PCBs forming PCB sulfates might be as high as 837 congeners) has limited our understanding of the prevalence and importance of these metabolites.
We have now successfully developed a method to identify and quantify up to 74 PCB sulfate congeners using a GC-MS/MS technique. The central concept of our method involves using a highly purified sulfatase from Helix pomatia to convert PCB sulfates to their related OH-PCBs, and this is followed by use of a well-documented procedure28–30 for the extraction and quantification of OH-PCBs in human serum samples. Parallel experiments with and without incubation with the sulfatase yield a determination of the identity and amount of PCB sulfates in serum samples. The method was validated by the analysis of a commercial mixture of pooled human serum. In this pooled serum sample, we found ten PCB sulfate congeners above the limit of quantitation with statistical evidence to validate their significance. Thus, the methodology presented will enable a more detailed understanding of the identity and prevalence of individual PCB sulfate congeners in serum.
MATERIALS AND METHODS
Materials
The following were purchased from Sigma-Aldrich (St. Louis, MO): Helix pomatia arylsulfatase (Type H-2, ≥2,000 units/mL), NHS-Activated Sepharose 4 Fast Flow (GE17-096-01), L-tyrosine ethyl ester hydrochloride, sodium metavanadate (anhydrous, 99.9%), Centriprep centrifugal filter units (Millipore, 10K MWCO, cellulose), p-nitrophenyl sulfate, p-nitrophenyl glucuronide, and human serum from male AB clotted whole blood (USA origin, sterile filtered, H6914, batch number SLBW5405). Tris-HCl (Ultrapure, Molecular Biology Grade) and Mops (Molecular Biology Grade) were from RPI (Mt. Prospect, IL). All other reagents for enzyme purification and assay were from commercial sources and were ACS reagent grade or higher. Diazomethane was prepared by the Synthesis Core of the Iowa Superfund Research Program (ISRP).
The PCB sulfates used for determining the specificity of the purified sulfatase (i.e., 2’-PCB 3 sulfate, 4’-PCB 9 sulfate, 4’- PCB 3 sulfate, 4’- PCB 26 sulfate, 4-PCB 52 sulfate, 4’-PCB 8 sulfate, 4’-PCB 12 sulfate, 4-PCB 11 sulfate, 4’-PCB 33 sulfate, and 4’-PCB 25 sulfate) were synthesized and authenticated as described previously14, 26, 31, 32. The 13C-labelled chlorinated biphenylol mixture used as surrogate standard for OH-PCB analysis (3’,4’-dichloro-4-[13C12]biphenylol, 2’,4’,5’-trichloro-4-[13C12]biphenylol, 2’,3’,4’,5’-tetrachloro-4-[13C12]biphenylol, 2’,3,4’,5,5’-pentachloro-4-[13C12]biphenylol, 2’,3,3’,4’,5,5’-hexachloro-4-[13C12]biphenylol, 2,2’,3,3’,4’,5,5’-heptachloro-4-[13C12]biphenylol, and 2,2’,3,4’,5,5’,6-heptachloro-4-[13C12]biphenylol) was purchased from Wellington Laboratories (Guelph, ON, Canada; product code MHPCB-MXA). PCB 204 (2,2′,3,4,4′,5,6,6′-octachlorobiphenyl) was from AccuStandard (New Haven, CT) and deuterated-PCB 30 (2,4,6-trichlorinatedbiphenyl-d5) was from Cambridge Isotope Laboratories (Tewksbury, MA). Methoxy-PCB standards for GC-MS/MS quantitation were purchased from Wellington Laboratories (Guelph, ON), AccuStandard (New Haven, CT), CDN Isotopes (Pointe-Claire, QC) or Cambridge Isotope Laboratories (Tewksbury, MA), as indicated in Table S1, Supporting Information. All solvents for extraction and analysis of OH-PCBs were of pesticide residue analysis grade.
Purification of Helix pomatia Sulfatase
Helix pomatia sulfatase was purified by affinity chromatography using a modification of the procedure developed by Skorey et al.33 The affinity gel column was initially prepared by washing NHS-activated sepharose (25 mL) with 200 mL of ice-cold 1 mM HCl. The sepharose gel was then placed in 100 mL of a solution containing 0.1 M tyrosine ethyl ester (TEE), 0.1 M NaHCO3, and 0.5 M NaCl at pH 8.0 and incubated in a rotary shaker at room temperature for 1h. The TEE solution was removed, and excess reactive groups were blocked by the addition of 80 mL of 0.1 M ice-cold Tris-HCl, pH 8.0, followed by incubation for 1 h at room temperature. The Tris-HCl buffer was removed, and the TEE-Sepharose was washed with 80 mL 0.1 M NaHCO3 followed by a wash with 80 mL 0.05 M Tris-HCl buffer containing 0.5 M NaCl. The TEE-Sepharose was then washed with 80 mL of buffer containing 0.05 M sodium acetate and 0.5 M NaCl, pH 4.0. Finally, the TEE-Sepharose gel was washed with 80 mL of 20 mM Mops buffer containing 0.1 M KCl at pH 7.0 (Buffer A). The amount of TEE bound to the affinity matrix was determined by the absorbance at 280 nm of a suspension of the gel in ethylene glycol, as described previously.33 Using a value of ε280 = 1400, the average concentration of TEE on the Sepharose gel was 16 μmol/ml.
Subsequent steps utilizing the TEE-Sepharose in affinity purification of Helix pomatia sulfatase were carried out at 4°C. A 25 mL volume of the affinity gel was placed in a 2.5×10 cm column, and the gel was washed with 3×10 mL of Buffer A, followed by 3×20 mL washes with 10 mM meta-vanadate in Buffer A. A volume of 1 mL of crude Helix pomatia sulfatase (24 mg of protein) was added to 1 mL of 100 mM meta-vanadate in Buffer A. The mixture was loaded onto the affinity column and allowed to incubate with the affinity matrix at the top of the column for 30 min. The column was then eluted with 40 mL of Buffer A containing 10 mM meta-vanadate, and this was followed by elution with 100 mL of Buffer A containing 25 mM EDTA. The column was eluted at a flow rate of 0.6 mL/min, and fractions of 5 mL were collected.
Assay of Sulfatase and Glucuronidase Activity
Both sulfatase and glucuronidase activities were determined for all fractions. Sulfatase activity was measured at 37 °C as the rate of increase in concentration of p-nitrophenol (absorbance at 400 nm; ε400=8000) due to the enzyme-dependent hydrolysis of p-nitrophenyl sulfate. Glucuronidase activity in the elution fractions was determined at 37 °C by monitoring the hydrolysis of p-nitrophenyl glucuronide to p-nitrophenol. Fractions eluted from the affinity column may contain meta-vanadate, which will inhibit sulfatase catalytic activity. To chelate any residual meta-vanadate, 100 μl of an elution fraction was mixed with 100 μl of Buffer A containing 25 mM EDTA in a 1.5 ml spectrophotometer cuvette. A volume of 780 μl of 10 mM Mops containing 0.1 M KCl, pH 7.0 buffer (Buffer B) was added into the cuvette, and this was followed by the addition of 20 μl of either 10 mM p-nitrophenyl sulfate (p-NPS), or 10 mM p-nitrophenyl glucuronide (p-NPG) as appropriate for determining sulfatase activity or glucuronidase activity. The increase in absorbance at 400nm was monitored for 300 s against a blank cuvette containing only Buffer B. The concentrations of protein in the column fractions were determined using the modified Lowry protein assay,34 and the specific activity of the enzyme is reported as nmol of p-NP formed/min/mg of protein. Fractions that had high sulfatase activities but no glucuronidase activities were pooled and concentrated by centrifugal filtration. The highly purified sulfatase was stored at −80°C in 1 mL aliquots.
Assay for the Activity of the Purified Sulfatase with Selected PCB Sulfates
Ten PCB sulfate congeners (i.e., 2’-PCB 3 sulfate, 4’-PCB 9 sulfate, 4’- PCB 3 sulfate, 4’- PCB 26 sulfate, 4-PCB 52 sulfate, 4’-PCB 8 sulfate, 4’-PCB 12 sulfate, 4-PCB 11 sulfate , 4’-PCB 33 sulfate, and 4’-PCB 25 sulfate) were selected to verify the ability of the purified sulfatase to hydrolyze PCB sulfates with varying degrees of chlorination and substitution patterns in the biphenyl ring. The hydrolysis of these PCB sulfates catalyzed by the purified sulfatase was determined using an assay that takes advantage of the ability to readily quantitate the concentration of organic sulfates due to the formation of a paired-ion with methylene blue, extraction of the paired ion into chloroform, and subsequent absorbance at 651nm.35 In each assay, 40 μl of a 500 μM PCB sulfate solution was mixed with 340 μl of 200 mM sodium acetate buffer, pH 6.8 in disposable borosilicate glass tubes. Enzymatic reactions were carried out by placing the tubes in a 37°C water bath and initiating the reaction with 20 μl of purified sulfatase. Reactions were stopped at 10 min by adding 0.5 mL of methylene blue reagent (250 mg methylene blue, 50 g Na2SO4, 10 ml H2SO4 in a total of 1 L of aqueous solution), followed by addition of 2 mL of chloroform. Control assays (all reagents and enzyme were present, but the methylene blue reagent and chloroform were added before any incubation) were conducted alongside each incubated assay. Following addition of the methylene blue reagent and chloroform, the mixture was vortex-mixed and then centrifuged at 1000 × g to separate the phases. The chloroform layer was removed, and 50–100 mg of solid anhydrous MgSO4 was added to sequester any water in the sample. The absorbance of each chloroform layer was determined at 651nm, and the value for the sample without incubation was subtracted from the absorbance for the sample that was incubated for 10 min. The changes in concentrations of PCB sulfate in the reaction mixture were calculated based upon previous determinations that 10 nmol of an organic sulfate in a 0.4 ml assay carried through this procedure yields a value of 0.3 absorbance units at 651 nm.36, 37 These results on rates of hydrolysis were combined with the protein concentration to calculate the specific activities of the purified sulfatase for each PCB sulfate congener.
Extraction of PCB Sulfates from Serum Samples and Subsequent Hydrolysis to OH-PCBs
A commercially available pool of human serum was used for method development, and sample sizes of 2 g/tube (approximately 2 mL) were used for each assay. A mixture of surrogate standards was prepared using a 13C-labelled chlorinated biphenylol solution/mixture at a final concentration of 50 ng/mL in methanol. This mixture included 13C-4’-OH-PCB 12, 13C-4’-OH-PCB 29, 13C-4’-OH-PCB 61, 13C-4’-OH-PCB 120, 13C-4’-OH-PCB 159, 13C-4’-OH-PCB 172, and 13C-4-OH-PCB 187, and a total volume of 100 μl of surrogate standards (50 ng/mL in methanol) was spiked into each 2 g serum sample. Following the addition of 2 mL of 1% formic acid and 6 mL of acetonitrile, samples were thoroughly mixed and incubated at 4°C for 2h. Each sample was then subjected to centrifugation for 30-min at 3000 × g. The supernatant layers were transferred into new test tubes containing approximately 100 mg of solid NaCl plus approximately 300 mg of solid MgSO4. The samples were then vortexed for at least 20 s followed by centrifugation at 3000 × g for 30-min. The organic layer of each sample was transferred to a new test tube and evaporated under gentle nitrogen flow to a final volume of approximately 0.5 mL. To each of the concentrated samples, approximately 1.5 mL of 200 mM sodium acetate buffer, pH 6.8 was added and mixed on a vortex mixer. In a volume of 20 μL, a total of 12.6 enzyme units of purified sulfatase (an enzyme unit catalyzed the hydrolysis of 1 nmol of substrate per min) was added to half of the samples, and the other half of the samples received an equivalent volume of 200 mM sodium acetate buffer, pH 6.8 instead of purified sulfatase. All samples were vortexed, sealed, and then incubated in a shaking water bath at 37°C for 1h.
Extraction and Derivatization of OH-PCBs for GC-MS/MS Analysis
Methods for extraction, separation, derivatization, and cleanup of OH-PCBs resulting from extraction and/or hydrolysis of PCB sulfates were carried out using a modification of a previously published procedure.28–30 Following incubation of the serum extracts, 0.5 mL of 6 M HCl was added, and, after vortex mixing, 5 mL of 2-propanol and 5 mL of hexane: methyl-tert-butyl ether (MTBE) (1:1, v/v) were added. After 5-min inversion and 5-min centrifugation at 1000 × g, the top layers were transferred to new Pyrex test tubes containing 4 mL of aqueous KCl (1%, w/w). The bottom layers were re-extracted with 3 mL of hexane:MTBE (1:1), and the resulting organic layers were transferred to tubes containing the aqueous KCl solution. Test tubes with organic solvents and KCl were inverted for 3 min followed by centrifugation at 1000 × g for 5 min. The resulting organic layers were transferred to new clean test tubes. The aqueous layers were re-extracted with 4 mL hexane:MTBE (1:1), and the resulting organic layers were combined with previous extractions and concentrated under gentle nitrogen flow to approximately 0.5 mL. Hexane (4 mL) was added to each tube followed by vortex mixing. After the addition of 2 mL KOH-solution (0.5 M in 50% ethanol), 3 min inversion, and 3 min centrifugation at 1000 × g, the top layers were discarded. Bottom layers were re-extracted with 3 mL hexane followed by 3 min inversion and 3 min centrifugation at 1000 × g. Top layers were discarded. The bottom alkaline solutions in the tubes were acidified with 0.5 mL of 2 M HCl. Additional HCl was added if the pH value was not acidic. 4 mL of hexane:MTBE (9:1) was added to tubes, inverted for 3 min, centrifuged for 3 min at 1000 × g and the top layers were transferred to new test tubes. Bottom acidic layers were re-extracted with 3 mL of hexane: MTBE (9:1), and the resulting organic layers were transferred to the previous tubes. The combined hexane:MTBE extractions (7 mL) were concentrated under gentle nitrogen flow to approximately 0.5–1 mL, and 3 drops of methanol were added to each tube to increase solubility of the OH-PCBs and maximize derivatization efficiency in the subsequent reaction with diazomethane.
Derivatization of the resulting OH-PCBs to the corresponding methoxy-PCBs (MeO-PCBs) was accomplished by adding 0.5 mL of diazomethane in diethyl ether to each sample. After gentle mixing, tubes were kept at 4–8°C for at least 3 h. The samples were evaporated under gentle nitrogen flow until only a few drops were left on the bottom, and 4 mL hexane was added. Removal of lipids from each sample was then carried out by adding 2 mL of concentrated H2SO4 to each tube followed by vortex mixing and inverting the tube for 2 min. After 5 min of centrifugation at 1000 × g, the top layers were transferred to new test tubes, and the bottom layers were re-extracted with 3 mL of hexane, inverted for 2 min, and centrifuged for 5 min at 1000 × g. The top layers were combined with previous extractions and concentrated under gentle nitrogen flow to approximately 0.5 mL. Concentrated samples were eluted through a sulfuric acid-activated silica gel column with 10 mL dichloromethane (DCM). Eluted DCM fractions containing MeO-PCBs were evaporated to approximately 0.5 mL under gentle nitrogen flow followed by the addition of 3 mL hexane for solvent exchange. Samples in hexane were evaporated to approximately 0.5 mL, and then transferred into standard 2.0 mL glass autosampler vials. The bottom of each sample tube was rinsed with 0.5 mL hexane and then transferred into the autosampler vial. The contents of each autosampler vial was then spiked with 100 μl of internal standards (IS) mixture that contained PCB 204 (2,2′,3,4,4′,5,6,6′-octachlorobiphenyl) and deuterated-PCB 30 (2,4,6-trichlorinatedbiphenyl-d5), where each was present at a concentration of 100 ng/mL in hexane. Samples were either immediately analyzed by GC-MS/MS, or they were stored at −10°C prior to analysis.
Quality Control and Assessment
Each group of samples carried through the above procedure included one reference standard and three method blanks. The reference tube contained only surrogate standards to which 3 drops of methanol were added, and the contents were stored at −10°C until the derivatization step. The reference was derivatized and concentrated to a few drops, and this was followed by the addition of 0.5 mL of hexane. The contents were then frozen and stored before the spiking with 100 μl IS and analyzing by GC-MS/MS. The matrix for the method blanks was 1% (w/w) KCl in ddH2O. Method blanks were carried through the same procedure as serum samples, except that no sulfatase was added at the incubation step. The MeO-PCBs quantified in the reference were assumed to be the same percentage derivatization from spiked hydroxylated surrogate standards as in serum samples. The surrogate standard recoveries of 12 method blanks were the essential criteria for evaluating the reliability of manual operations as well as for calculating the limit of quantification (LOQ) values. Instrument blanks containing hexane were also analyzed before and after our calibration standard for each batch of samples. The recovery of a known concentration of PCB-sulfate spiked into a serum sample was assessed utilizing 4-PCB 11 sulfate, as this is a PCB sulfate that has been previously identified in human serum samples.
Analyte Quantification and Statistical Analysis
Prepared samples were analyzed with an Agilent J&W DB-1701 capillary column (30m × 0.25mm i.d., 0.25μm film thickness; Agilent Technologies, Santa Clara, CA) coupled with Agilent 7000D Triple Quadrupole (QqQ) MS system (GC-MS/MS). Calculation of the amount of each congener present was performed through the application of relative response factor and correction according to the percent recovery of surrogate standards on a per-sample basis, in which 13C-4’OH-PCB 29 was used for mono- to tetra- chlorinated congeners, 13C-4’-OH-PCB 61 was used for penta-chlorinated congeners, 13C-4’-OH-PCB 159 was used for hexa-chlorinated congeners, 13C-4’-OH-PCB 187 was used for hepta-chlorinated congeners, and 13C-4’-OH-PCB 172 was used for octa-chlorinated congeners. Congener-specific LOQ was determined as the 95% quantile from 12 method blanks. For those congeners where the concentration obtained from the serum sample was at or below the LOQ, it was reported as LOQ/2. Statistical comparison of results from a sulfatase-added sample vs the corresponding non-sulfatase-added sample was made by Student’s t-test (two samples assuming equal variance, α=0.05) for those congeners whose reported concentrations were above LOQ values. Conversion of the observed concentrations of MeO-PCBs (i.e., the end-products used for GC analysis) to the equivalent amount of parent PCB-sulfates was performed based on the molecular weight ratio. Initial serum concentrations of PCB sulfates were calculated based on the average recovery value of standard PCB 11 sulfate carried through the procedure and recorded as pg/g of fresh weight serum.
RESULTS AND DISCUSSION
Purification of Helix pomatia Sulfatase
The commercially available Helix pomatia sulfatase has significant β-glucuronidase activity. Since glucuronide conjugates are well known as potential metabolites of OH-PCBs,6, 13 any method to determine serum concentrations of PCB sulfates by differential analysis of OH-PCBs following enzymatic hydrolysis depends upon both the broad specificity of the sulfatase and the elimination of any contaminant glucuronidase activity.
One method that has been used to solve this problem of glucuronidase activity in a sulfatase preparation is the use of D-saccharic acid 1,4-lactone to inhibit glucuronidase activity.38 However, initial experiments indicated that the 20 mM concentration of D-saccharic acid 1,4-lactone necessary for complete inhibition of the glucuronidase activity led to problems in the reproducibility of subsequent extraction and analysis of the resulting OH-PCBs. Therefore, we sought an efficient purification method for the Helix pomatia sulfatase that removed all residual glucuronidase.
This purification of the sulfatase was achieved using a modification of a previously published affinity chromatographic procedure.33 This method takes advantage of the affinity of the sulfatase for a complex formed between vanadate and L-tyrosine ethyl ester that is immobilized on a Sepharose column. The sulfatase binds to the affinity column in the presence of vanadate, but the glucuronidase does not. Elution of the sulfatase is accomplished by a buffer containing EDTA without any vanadate. An elution profile for the affinity column in shown in Figure 1, and a summary of the purification is presented in Table 1. Fractions 11 through 17, which have high sulfatase activities without significant glucuronidase activity, were collected and concentrated. Sulfatase and glucuronidase activities in the concentrated enzyme were compared at 200 μM concentrations of the standard substrates, p-nitrophenyl sulfate and p-nitrophenyl glucuronide. As seen in Table 1, the residual activity of glucuronidase was less than 0.02 % of the sulfatase activity. Therefore, the single affinity column provided a 790-fold purification of the sulfatase with negligible residual glucuronidase activity. The purified sulfatase was stable when stored at −80°C, with 94% retention of its initial specific activity after 15 months.
Figure 1.

Elution of sulfatase and glucuronidase activities from the TEE-Sepharose affinity column. The fraction where the elution buffer was changed to one containing EDTA without vanadate (F8) is indicated. Column fractions were assayed for enzymatic hydrolysis of p-nitrophenyl sulfate or p-nitrophenyl glucuronide for arylsulfatase and glucuronidase, respectively.
Table 1.
Summary of the purification of Helix pomatia sulfatase
| Glucuronidase | Sulfatase | ||||||
|---|---|---|---|---|---|---|---|
| Stage | Protein (mg) | Protein Concentration (mg/ml) | Units/ml (nmol/min/ml) | Units/mg | Units/ml (nmol/min/ml) | Units/mg | Fold-Purification (Sulfatase) |
| Helix pomatia H-2 Crude Extract | 24.0 | 24.0 | 17.2 | 0.7 | 62.5 | 3.0 | l |
| Concentrated (Fractions 11–17) | 1.7 | 0.3 | 0.1 | 0.4 | 630 | 2100 | 790 |
Characterization of the Purified Sulfatase with Selected PCB Sulfates as Substrates
During the affinity purification, some of the sulfatase co-eluted with the glucuronidase, indicating that there may be a second sulfatase present that has a different affinity for the vanadate-TEE complex. This observation is consistent with a report that Helix pomatia contains two arylsulfatase subtypes.39 As a result, it was important to confirm that the purified sulfatase retained a broad specificity for PCB sulfates as substrates for hydrolysis. The purified sulfatase was evaluated with ten PCB sulfates that included mono-, di-, tri-, and tetra-chlorinated congeners. As shown in Figure 2, all ten PCB sulfates were good substrates for the purified sulfatase. This was an improvement in both activity and range of specificity from the sulfatase activity that was seen with these PCB sulfates when using the crude Helix pomatia preparation (See Supporting Information).
Figure 2.

Specific activities of purified Helix pomatia sulfatase in the hydrolysis of 10 selected PCB sulfate congeners. Values are the two replicates and mean.
Analysis of PCB Sulfates in Serum
Although PCB sulfates are known to have some hydrophobic characteristics,13 they are more polar than the corresponding OH-PCBs. Due to the negative charge on the PCB sulfates, however, they do not partition into the organic solvents such as hexane and methyl-tert-butyl ether (MTBE) that are commonly used to extract PCBs and OH-PCBs. For example, direct extractions using a 1:1 (v/v) mixture of hexane:MTBE yielded only 15±1% recoveries of PCB 11 sulfate added to human serum.27 A previous protocol for extraction of PCB sulfates from rat tissues employed treatment with acetonitrile and formic acid, and subsequent separation of aqueous and organic phases using NaCl and MgSO4, to extract PCB sulfates.40, 41 A modification of this procedure was coupled with HPLC analysis to provide the first detection and quantitation of 4-PCB 11 sulfate in human serum samples.27 However, the lack of a generally applicable method for determining a broad range of PCB sulfates in human serum at appropriate sensitivity has limited our understanding of the prevalence and importance of these metabolites.
This need for a more generally useful method with an expanded range of analytical standards was met by coupling an efficient extraction procedure with PCB sulfate-specific hydrolysis to yield OH-PCBs that could be identified and quantitated by GC-MS/MS Thus, the OH-PCBs that were products of the sulfatase-specific hydrolysis of PCB sulfates were converted to the corresponding methoxy-PCBs (MeO-PCBs) and analyzed by GC-MS/MS using a well-documented procedure.28–30
A mixture of commercially available surrogate standards of 13C-labeled OH-PCBs was also added to the serum samples and carried through the subsequent steps of extraction, incubation, derivatization, and analysis. Each analysis comprised two 2 g aliquots of serum. One aliquot was carried through the procedure, including a 1 h incubation with the purified sulfatase at 37°C in 0.2 M sodium acetate at pH 6.8, and the other aliquot was subjected to the same incubation conditions, but without sulfatase. Reference and method blanks were also carried through the procedure. The average recoveries of added surrogate standards are seen in Figure 3. We observed that the recovery of 13C-4’-OH-PCB 120 was consistently low, and, therefore, its recovery was not used as correction factor for calculating concentrations of congeners. The recoveries of other surrogate standards were used as correction factors in quantitation of OH-PCBs formed from hydrolysis of extracted PCB sulfates as summarized in Table 2.
Figure 3.
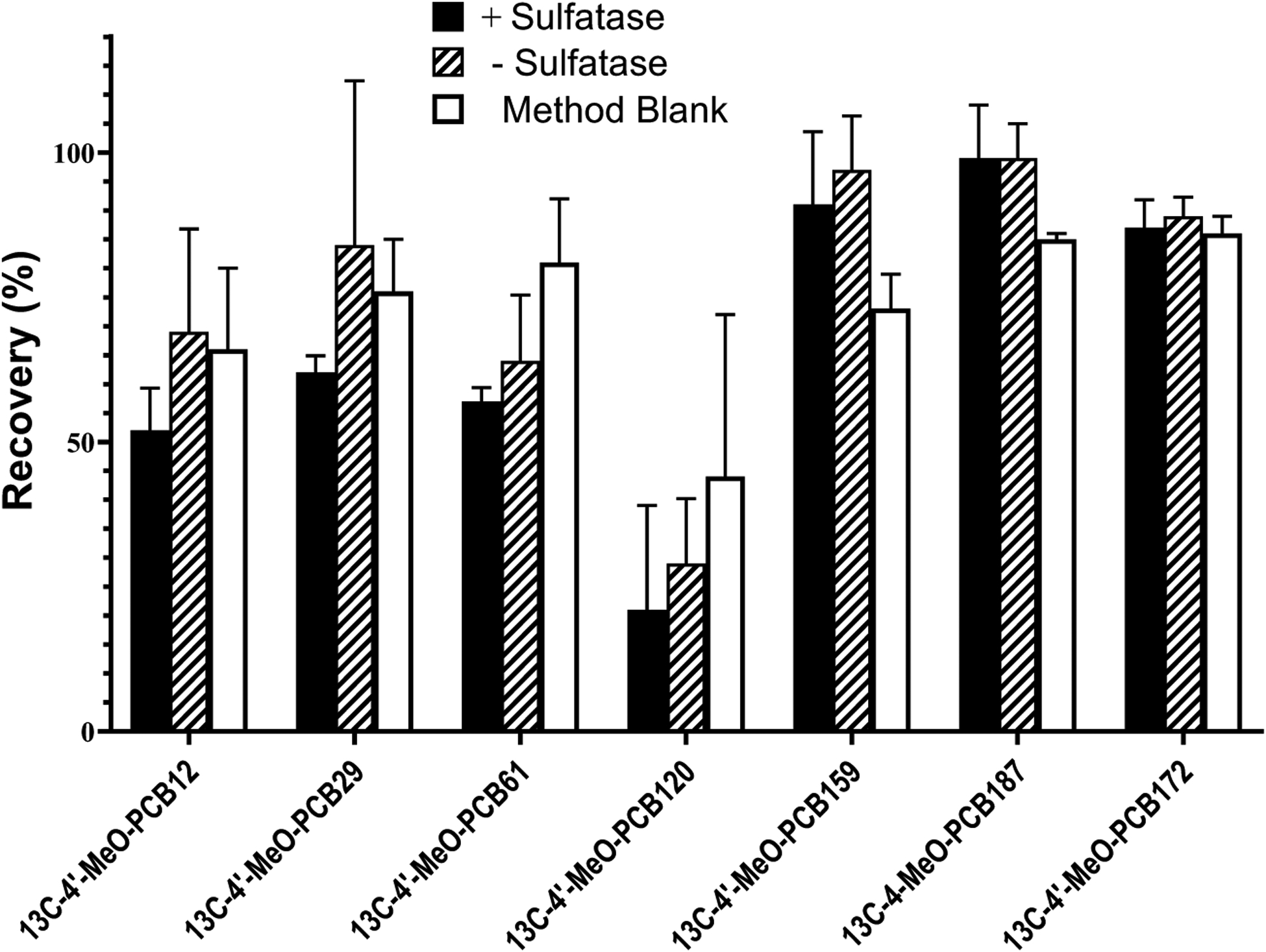
Recovery of each surrogate standard based on GC-MS/MS analysis of their MeO-derivatives in the +sulfatase group and the -sulfatase group. The corresponding method blank is also indicated. Values are the mean ± standard deviation of four determinations.
Table 2.
The number of chlorine atoms in the homologs of OH-PCBs and the corresponding surrogate standards utilized for analyses.
| OH-PCB Homologue | Surrogate Standard |
|---|---|
| 1, 2, 3, 4 | 13C-4’-MeO-PCB 29 |
| 5 | 13C-4’-MeO-PCB 61 |
| 6 | 13C-4’-MeO-PCB 159 |
| 7 | 13C-4’-MeO-PCB 187 |
| 8, 9 | 13C-4’-MeO-PCB 172 |
Since the essential concept of this method is to quantitate those OH-PCBs derived solely from sulfatase-catalyzed hydrolysis of PCB sulfates, it was important to distinguish between the OH-PCBs derived from the sulfate ester-specific hydrolysis and those OH-PCBs present in serum without sulfatase treatment. In our parallel experimental groups (i.e. sulfatase added versus no-sulfatase added), the observations of OH-PCBs in non-sulfatase-added groups could be derived from those intrinsic OH-PCBs that were extracted from serum. In addition, some OH-PCBs could also be derived from some non-specific hydrolysis of PCB sulfates during the extraction. Our expectation was to optimize the difference in OH-PCBs obtained from the two groups, because that difference would represent OH-PCBs derived from PCB sulfates. 4-PCB 11 sulfate was used as a model PCB sulfate to determine a value to be used as a correction for losses of PCB sulfates occurring throughout the analytical procedure. 4-PCB 11 sulfate was chosen primarily for two reasons. First, it is clear that PCB 11 is a major constituent of PCB profiles in both indoor and outdoor air11, it is found in blood samples28, and it is readily metabolized to its corresponding OH-PCB and PCB sulfate6. Secondly, 4-PCB 11 sulfate was the only PCB sulfate that had been previously identified in human serum samples27. These factors ensured that the model chosen was highly relevant to human exposure and metabolism, and it increased the likelihood that serum samples would contain at least some of the model PCB sulfate. A schematic representation of the analytical procedure for determining the recovery of synthetic 4-PCB 11 sulfate that is added to human serum samples is presented in Figure 4. Detailed calculations of 4-PCB 11 sulfate recovery are included in the Supporting Information. It should be noted that the percentages of 4-PCB 11 sulfate quantitated in this procedure are not standard recovery percentages, as they represent the difference between the concentration of the OH-PCB specifically formed from the sulfatase-catalyzed hydrolysis and the concentration of any OH-PCB either originally present or formed non-specifically during sample processing. In a total of 9 individual experiments with spiked 4-PCB 11 sulfate, these recovery differences were 45%, 41%, 47%, 43%, 59%, 37%, 55%, 50%, and 43% (Supporting Information, Figures S3 and S4). Since the distribution of values was not symmetric, the median, 42.9% ((Q1, Q3) = 42.9% (41.3%, 50.8%)), was chosen as a standard value for correction of recovery of PCB sulfates from serum samples. Additional supporting experiments were performed in 1% KCl (i.e., the matrix used for method blanks) with 8 replicates (4 with sulfatase and 4 without sulfatase), where a median recovery difference of 48.9% was observed based upon analysis following spiking with 10 ng of PCB 11 sulfate. This value was higher than the median of 42.9% seen with serum present, but it falls within the range of recovery differences derived from serum samples. This indicated that the presence of the serum did not significantly affect the value obtained for the recovery difference.
Figure 4.

Summary of the method for evaluating 4-PCB 11 sulfate recoveries in pooled human serum.
Identification of PCB Sulfate Congeners in the Pooled Human Serum
The previous finding that 4-PCB 11 sulfate was detected in the low nanomolar range in human serum samples suggested that other PCB sulfates may also be present, and that they could both indicate previous exposures to PCBs and contribute to toxicological responses.27 By coupling the expanded availability of OH-PCB standards with our specific hydrolysis of PCB sulfates, we had the ability to analyze for a total of 74 PCB sulfate congeners in human serum. As a result, we examined a pooled human serum sample for the presence of all PCB sulfates for which we had corresponding OH-PCB standards. Eight technical replicates were performed with the serum sample.
The experimental scheme of identification of PCB sulfates in the sample of human serum was essentially the same as that of the 4-PCB 11 sulfate recovery experiment but without spiking any known amount of PCB sulfate. Two corrections were included for analysis of PCB sulfate concentrations: one was based on the recovery of 4-PCB 11 sulfate, and the other was based on recoveries of surrogate standards. These corrections accounted for recovery of the losses of both PCB sulfates and OH-PCBs. Three method blanks were analyzed along with each batch for quality control, with a total of twelve method blanks collected for LOQ determinations. Surrogate recoveries of 13C labeled OH-PCB congeners were utilized for corrections of difference homologues with similar numbers of chlorine atoms (Figure 4 and Table 2). All observations that were below calculated LOQ values were reported as LOQ/2 in order to accommodate any situation where the sulfatase-added groups were above the LOQ, but the corresponding no-sulfatase added groups were lower than the LOQ. In addition, Student’s t-tests (two samples assuming equal variance) were performed on the values for each congener to determine if the difference between the treated and untreated groups was statistically significant (α=0.05 as criterion). This statistical selection further guaranteed that the final reported PCB sulfates detected in serum samples were reliable. The concentration of each PCB sulfate congener in the serum is seen in Table S4 (Supporting Information).
As summarized in Figure 5, our analysis revealed 10 PCB sulfates that were present at concentrations above the LOQ with statistical evidence to validate their significance. Mono-chlorinated congeners that were observed included 4-PCB 2 sulfate, 2’-PCB 3 sulfate, 4’-PCB 2 sulfate, and 4’-PCB 3 sulfate. 4-PCB 11 sulfate was the only di-chlorinated congener identified. 4’-PCB 25 sulfate and 4’-PCB 26 sulfate were the tri-chlorinated congeners identified, while those tetra-chlorinated congeners that were detected included 2’-PCB 61 sulfate and 4’-PCB 69 sulfate. One heptachlorinated congener, 4-PCB 187 sulfate, was detected. 4-PCB 2 sulfate was present in the highest concentration (3970 pg/g of serum) followed by 4-PCB 11 sulfate (1560 pg/g) and 2’-PCB 3 sulfate (1200 pg/g).
Figure 5.
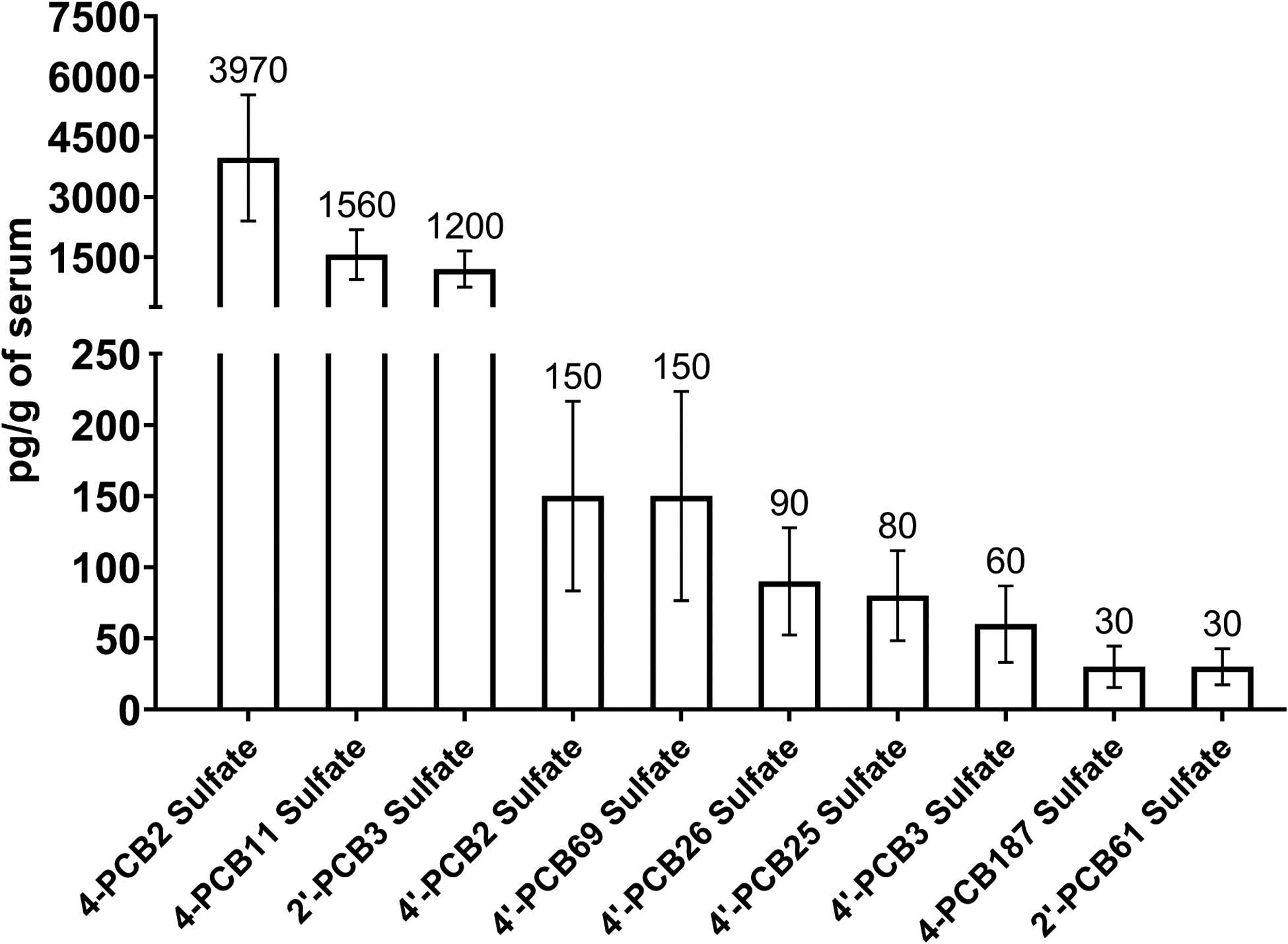
PCB sulfate congeners identified in the pooled human serum. A total of eight technical replicates were analyzed. Values are mean with population standard error.
Nine of the ten congeners of PCB sulfates that were detected contained lower numbers of chlorine atoms, and this is consistent with the known properties of PCB metabolism. That is, those PCBs with high numbers of chlorine atoms often accumulate in lipids and adipose, and generally undergo a more limited metabolic conversion to OH-PCBs, and subsequent conjugation.6, 8 Even when higher chlorinated OH-PCBs are formed, their lower water-solubility may limit their interaction with cytosolic sulfotransferases required for the formation of PCB sulfates. Moreover, any higher chlorinated OH-PCBs that are sulfated in the liver may be subject to biliary secretion, and the PCB sulfate may not accumulate in serum. Nevertheless, it was interesting to note the presence of one higher chlorinated PCB sulfate in the serum sample. Another factor that would determine the concentration of any individual PCB sulfate congener in serum is highlighted by recent cell culture studies indicating that some PCB sulfates may enter cells with the potential for subsequent hydrolysis and additional metabolic reactions.42 Finally, we cannot rule out the presence of low concentrations of additional PCB sulfates for which we currently do not have the corresponding OH-PCB standards. Other PCB sulfate congeners may also be present at concentrations below the LOQ for the current method.
While it is impossible to know the potential PCB exposures for individuals represented by the pooled serum sample that was analyzed as part of our method development, several observations can be made about the specific PCB sulfate congeners that were identified. For example, 4-PCB 11 sulfate is a metabolite of PCB 11, a commonly observed PCB in indoor and outdoor air.43, 44 Moreover, PCBs 2, 3, 25, 26, and 187 have been observed in samples of indoor air11, and these could be metabolic precursors of 4-PCB 2 sulfate and 4’-PCB 2 sulfate, 2’-PCB 3 sulfate and 4’-PCB 3 sulfate, 4’-PCB 25 sulfate, 4’-PCB 26 sulfate, and 4-PCB 187 sulfate, respectively, While 4’-PCB 26 sulfate could be derived from the metabolism of PCB 26 via 4’-OH-PCB 26 as an intermediate, it could also be derived from PCB 31 as a result of a CYP-catalyzed oxidation to an arene oxide followed by an NIH-shift to form 4’-OH-PCB 26 as precursor of 4’-PCB 26 sulfate. PCB 31 is commonly seen in urban air samples.45 Finally, 4’-OH-PCB 25, the metabolic precursor of the 4’-PCB 25 sulfate, has been shown to be a major metabolite of the commonly occurring PCB 28.46 It should be noted, however, that simple direct correlations between the concentration of a specific PCB sulfate and the serum concentration of its precursor PCB or OH-PCB are unlikely. Although our observations on potential sources of individual PCB sulfates in serum are useful in establishing relevance of both the new assay method and our experimental validation of the method in a sample of human serum, further studies will be necessary to determine the complex relationships between exposures to PCBs and the resulting serum concentrations of those PCBs as well as their metabolites such as OH-PCBs and PCB sulfates.
Therefore, we have developed a sensitive and specific method for the determination of PCB sulfates in human serum that yielded new information about the presence of PCB sulfates in humans. While the current method was developed with 74 OH-PCB standards, its usefulness can be readily expanded as more OH-PCB standards become available. Moreover, the overall analytical procedure using the affinity-purified Helix pomatia sulfatase may also be adaptable for use with other organo-sulfate compounds. Future studies will utilize this method for analysis of individual serum samples in order to better understand the toxicological effects and fates of PCB sulfate metabolites in humans.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Dr. Xueshu Li and Dr. Xianran He of the University of Iowa Superfund Synthesis Core for the synthesis and authentication of PCB derivatives, and the preparation of the diazomethane used in this research. The studies described were supported by the National Institutes of Health, National Institute of Environmental Health Sciences, through NIH P42 ES013661 and P30 ES005605. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
SUPPORTING INFORMATION
Additional details are provided on the rationale for purification of the sulfatase, comparison of substrate specificity for the crude and purified sulfatase, sources of methoxy-PCB standards, precursor and product masses for the methoxy-PCB derivatives, calculation and recovery of 4-PCB 11 sulfate from human serum, limit of quantification (LOQ) for PCB sulfate congeners, and data on the identification of PCB sulfate congeners in a pooled sample of human serum.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Ballschmiter K; Bacher R; Mennel A; Fischer R; Riehle U; Swerev M, The determination of chlorinated biphenyls, chlorinated dibenzodioxins, and chlorinated dibenzofurans by GC-MS. J. High Resolution Chromatography 1992, 15, 260–270. [Google Scholar]
- 2.Erickson MD; Kaley RG 2nd, Applications of polychlorinated biphenyls . Environ. Sci. Pollut. Res. Int 2011, 18, (2), 135–51. [DOI] [PubMed] [Google Scholar]
- 3.Erickson MD, Environmental PCB forensics: processes and issues. Environ. Sci. Pollut. Res. Int 2020, 27, (9), 8926–8937. [DOI] [PubMed] [Google Scholar]
- 4.ATSDR, Toxicological profile for polychlorinated biphenyls (PCBs). U.S. Department of Health and Human Services, Public Health Service 2000, https://www.atsdr.cdc.gov/toxprofiles/tp17.pdf, accessed 7/19/2020.
- 5.Dhakal K; Gadupudi GS; Lehmler HJ; Ludewig G; Duffel MW; Robertson LW, Sources and toxicities of phenolic polychlorinated biphenyls (OH-PCBs). Environ. Sci. Pollut. Res. Int 2018, 25, (17), 16277–16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimm FA; Hu D; Kania-Korwel I; Lehmler HJ; Ludewig G; Hornbuckle KC; Duffel MW; Bergman A; Robertson LW, Metabolism and metabolites of polychlorinated biphenyls. Crit. Rev. Toxicol 2015, 45, (3), 245–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson LW; Ludewig G, Polychlorinated Biphenyl (PCB) carcinogenicity with special emphasis on airborne PCBs. Gefahrst. Reinhalt. Luft 2011, 71, (1–2), 25–32. [PMC free article] [PubMed] [Google Scholar]
- 8.Safe SH, Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit. Rev. Toxicol 1994, 24, 87–149. [DOI] [PubMed] [Google Scholar]
- 9.Jahnke JC; Hornbuckle KC, PCB Emissions from Paint Colorants. Environ. Sci. Technol 2019, 53, (9), 5187–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herkert NJ; Jahnke JC; Hornbuckle KC, Emissions of Tetrachlorobiphenyls (PCBs 47, 51, and 68) from Polymer Resin on Kitchen Cabinets as a Non-Aroclor Source to Residential Air. Environ. Sci. Technol 2018, 52, (9), 5154–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marek RF; Thorne PS; Herkert NJ; Awad AM; Hornbuckle KC, Airborne PCBs and OH-PCBs Inside and Outside Urban and Rural U.S. Schools. Environ. Sci. Technol 2017, 51, (14), 7853–7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu D; Hornbuckle KC, Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ. Sci. Technol 2010, 44, (8), 2822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James MO, Polychlorinated biphenyls: Metabolism and metabolites. In PCBs: Recent Advances in Environmental Toxicology and Health Effects, Robertson LW; Hansen LG, Eds. The University Press of Kentucky: Lexington, KY, 2001; pp 35–46. [Google Scholar]
- 14.Grimm FA; Lehmler HJ; He X; Robertson LW; Duffel MW, Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ. Health Perspect 2013, 121, (6), 657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y; Apak TI; Lehmler HJ; Robertson LW; Duffel MW, Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1. Chem. Res. Toxicol 2006, 19, (11), 1420–5. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y; Smart JT; Song Y; Lehmler HJ; Robertson LW; Duffel MW, Structure-activity relationships for hydroxylated polychlorinated biphenyls as substrates and inhibitors of rat sulfotransferases and modification of these relationships by changes in thiol status. Drug Metab. Dispos 2009, 37, (5), 1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tampal N; Lehmler HJ; Espandiari P; Malmberg T; Robertson LW, Glucuronidation of hydroxylated polychlorinated biphenyls (PCBs). Chem. Res. Toxicol 2002, 15, 1259–1266. [DOI] [PubMed] [Google Scholar]
- 18.Pessah IN; Lein PJ; Seegal RF; Sagiv SK, Neurotoxicity of polychlorinated biphenyls and related organohalogens. Acta Neuropathol. 2019, 138, (3), 363–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinete N; Schettgen T; Bertram J; Kraus T, Occurrence and distribution of PCB metabolites in blood and their potential health effects in humans: a review. Environ. Sci. Pollut. Res. Int 2014, 21, (20), 11951–72. [DOI] [PubMed] [Google Scholar]
- 20.Gupta P; Thompson BL; Wahlang B; Jordan CT; Zach Hilt J; Hennig B; Dziubla T, The environmental pollutant, polychlorinated biphenyls, and cardiovascular disease: a potential target for antioxidant nanotherapeutics. Drug Deliv. Transl. Res 2018, 8, (3), 740–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karwacka A; Zamkowska D; Radwan M; Jurewicz J, Exposure to modern, widespread environmental endocrine disrupting chemicals and their effect on the reproductive potential of women: an overview of current epidemiological evidence. Hum. Fertil. (Camb) 2019, 22, (1), 2–25. [DOI] [PubMed] [Google Scholar]
- 22.Park HY; Park JS; Sovcikova E; Kocan A; Linderholm L; Bergman A; Trnovec T; Hertz-Picciotto I, Exposure to hydroxylated polychlorinated biphenyls (OH-PCBs) in the prenatal period and subsequent neurodevelopment in eastern Slovakia. Environ. Health Perspect 2009, 117, (10), 1600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimm FA; Lehmler HJ; He X; Robertson LW; Duffel MW, Modulating inhibitors of transthyretin fibrillogenesis via sulfation: polychlorinated biphenyl sulfates as models. Chemico-biol. Interact 2015, 228, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kester MH; Bulduk S; Tibboel D; Meinl W; Glatt H; Falany CN; Coughtrie MW; Bergman A; Safe SH; Kuiper GG; Schuur AG; Brouwer A; Visser TJ, Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs Endocrinology 2000, 141, (5), 1897–1900. [DOI] [PubMed] [Google Scholar]
- 25.Parker VS; Squirewell EJ; Lehmler HJ; Robertson LW; Duffel MW, Hydroxylated and sulfated metabolites of commonly occurring airborne polychlorinated biphenyls inhibit human steroid sulfotransferases SULT1E1 and SULT2A1. Environ. Toxicol. Pharmacol 2018, 58, 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez EA; Li X; Lehmler HJ; Robertson LW; Duffel MW, Sulfation of Lower Chlorinated Polychlorinated Biphenyls Increases Their Affinity for the Major Drug-Binding Sites of Human Serum Albumin. Environ. Sci. Technol 2016, 50, (10), 5320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimm FA; Lehmler HJ; Koh WX; DeWall J; Teesch LM; Hornbuckle KC; Thorne PS; Robertson LW; Duffel MW, Identification of a sulfate metabolite of PCB 11 in human serum. Environ. Int 2017, 98, 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marek RF; Thorne PS; DeWall J; Hornbuckle KC, Variability in PCB and OH-PCB serum levels in children and their mothers in urban and rural U.S. communities. Environ. Sci. Technol 2014, 48, (22), 13459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marek RF; Thorne PS; Wang K; Dewall J; Hornbuckle KC, PCBs and OH-PCBs in serum from children and mothers in urban and rural U.S. communities. Environ. Sci. Technol 2013, 47, (7), 3353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh WX; Hornbuckle KC; Wang K; Thorne PS, Serum polychlorinated biphenyls and their hydroxylated metabolites are associated with demographic and behavioral factors in children and mothers. Environ. Int 2016, 94, 538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X; Holland EB; Feng W; Zheng J; Dong Y; Pessah IN; Duffel MW; Robertson LW; Lehmler HJ, Authentication of synthetic environmental contaminants and their (bio)transformation products in toxicology: polychlorinated biphenyls as an example. Environ. Sci. Pollut. Res. Int 2018, 25, (17), 16508–16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X; Parkin S; Duffel MW; Robertson LW; Lehmler HJ, An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environ. Int 2010, 36, (8), 843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skorey KI; Johnson NA; Huyer G; Gresser MJ, A two-component affinity chromatography purification of Helix pomatia arylsulfatase by tyrosine vanadate. Protein Expr. Purif 1999, 15, (2), 178–87. [DOI] [PubMed] [Google Scholar]
- 34.Bensadoun A; Weinstein D, Assay of proteins in the presence of interfering materials. Anal. Biochem 1976, 70, 241–250. [DOI] [PubMed] [Google Scholar]
- 35.Roy AB, Arylsulfatases: colorimetric and fluorometric assays. Methods Enzymol. 1987, 143, 207–17. [DOI] [PubMed] [Google Scholar]
- 36.Sekura RD; Duffel MW; Jakoby WB, Aryl sulfotransferases. Methods Enzymol. 1981, 77, 197–206. [DOI] [PubMed] [Google Scholar]
- 37.Sheng JJ; Sharma V; Duffel MW, Measurement of aryl and alcohol sulfotransferase activity. Curr. Protocols Toxicol 2001, Chapter 4, Unit4 5. [DOI] [PubMed] [Google Scholar]
- 38.Thohan S; Zurich MC; Chung H; Weiner M; Kane AS; Rosen GM, Tissue slices revisited: evaluation and development of a short-term incubation for integrated drug metabolism. Drug Metab. Dispos 2001, 29, 1337–1342. [PubMed] [Google Scholar]
- 39.Roy AB; Williams EA, The Sulfatase of Helix-Pomatia - Purification and Kinetic-Properties. Comp. Biochem. Physiol. B-Biochem. Mol. Biol 1989, 93, (2), 229–237. [Google Scholar]
- 40.Dhakal K; He X; Lehmler HJ; Teesch LM; Duffel MW; Robertson LW, Identification of sulfated metabolites of 4-chlorobiphenyl (PCB3) in the serum and urine of male rats. Chem. Res. Toxicol 2012, 25, (12), 2796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grimm FA; He X; Teesch LM; Lehmler HJ; Robertson LW; Duffel MW, Tissue Distribution, Metabolism, and Excretion of 3,3’-Dichloro-4’-sulfooxy-biphenyl in the Rat. Environ. Sci. Technol 2015, 49, (13), 8087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez EA; Vanle BC; Doorn JA; Lehmler H-J; Robertson LW; Duffel MW, Hydroxylated and sulfated metabolites of commonly observed airborne polychlorinated biphenyls display selective uptake and toxicity in N27, SH-SY5Y, and HepG2 cells. Environ. Toxicol. Pharmacol 2018, 62, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ampleman MD; Martinez A; DeWall J; Rawn DF; Hornbuckle KC; Thorne PS, Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ. Sci. Technol 2015, 49, (2), 1156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu D; Martinez A; Hornbuckle KC, Discovery of non-aroclor PCB (3,3’-dichlorobiphenyl) in Chicago air. Environ Sci Technol 2008, 42, (21), 7873–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu D; Lehmler HJ; Martinez A; Wang K; Hornbuckle KC, Atmospheric PCB congeners across Chicago. Atmos. Environ 2010, 44, (12), 1550–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quinete N; Esser A; Kraus T; Schettgen T, PCB 28 metabolites elimination kinetics in human plasma on a real case scenario: Study of hydroxylated polychlorinated biphenyl (OH-PCB) metabolites of PCB 28 in a highly exposed German Cohort. Toxicol. Lett 2017, 276, 100–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


