Abstract
Objectives: To describe a prospective cohort of patients with rheumatoid arthritis associated with interstitial lung disease (RA-ILD) and identify risk factors associated with disease progression and mortality in this cohort. Patients and methods: We performed a multicenter, prospective, observational study of patients with RA-ILD receiving disease-modifying antirheumatic drugs (DMARDs) between 2015 and 2020. The patients were assessed using high-resolution computed tomography and pulmonary function tests at baseline and at 60 months. The main endpoint was “Progression to ILD at the end of follow-up” in terms of the following outcomes: (1) improvement (i.e., improvement in forced vital capacity (FVC) ≥10% or diffusing capacity of the lungs for carbon monoxide (DLCO) ≥15% and absence of radiological progression); (2) nonprogression (stabilization or improvement in FVC ≤10% or diffusing capacity of the lungs for carbon monoxide (DLCO) <15% and absence of radiological progression); (3) progression (worsening of FVC >10% or DLCO >15% and radiological progression); or (4) death. We recorded demographic and clinical characteristics, lung function, and the incidence of adverse events. A Cox regression analysis was performed to identify factors associated with the worsening of ILD. Results: After 60 months, lung disease had stabilized in 66 patients (56.9%), improved in 9 (7.8%), and worsened in 23 (19.8%). Eighteen patients (15.5%) died, with a mean survival of 71.8 (1.9) months after diagnosis of ILD. The Cox multivariate analysis revealed the independent predictors of worsening of RA-ILD to be usual interstitial pneumonia (hazard ratio (HR), 2.6 (95%CI, 1.0–6.7)), FVC <80% (HR, 3.8 (95%CI, 1.5–6.7)), anticitrullinated protein antibody titers (HR, 2.8 (95%CI, 1.1–6.8)), smoking (HR, 2.5 (95%CI, 1.1–6.2)), and treatment with abatacept, tocilizumab, or rituximab (HR, 0.4 (95%CI, 0.2–0.8)). During follow-up, 79 patients (68%) experienced an adverse event, mostly infection (61%). Infection was fatal in 10/18 patients (55.5%) during follow-up. Conclusions: Lung function is stable in most patients with RA-ILD receiving treatment with disease-modifying anti-rheumatic drugs (DMARDs), although one-third worsened or died. Identifying factors associated with worsening in RA-ILD is important for clinical management.
Keywords: rheumatoid arthritis, interstitial lung disease, biologics, non-anti-TNF biologics
1. Introduction
Interstitial lung disease (ILD) is the most frequent pulmonary manifestation in rheumatoid arthritis (RA), with an incidence of 4 to 4.5/1000 patient-years. The prevalence of RA-ILD varies according to the detection methods used and cohort studied. Although clinically evident ILD occurs in approximately 10% of RA patients, recent studies using high-resolution computed tomography (HRCT) reported a prevalence of 27–67%, with a large percentage of asymptomatic patients. In addition, ILD leads to increased morbidity and mortality and is currently the second most common cause of death in patients with RA after cardiovascular disease [1].
Several studies have attempted to identify factors that help predict poorer prognosis and/or greater mortality in patients with RA-ILD. Those associated with poorer prognosis include advanced age [2,3,4,5,6,7], male sex [3,5,8,9], and factors related to RA itself, such as greater disease duration, autoantibody levels, and poorer control of inflammation [5,10]. Other factors associated with more marked disease progression and mortality in RA-ILD include reduced forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLCO) [11,12] and the radiological pattern of usual interstitial pneumonia (UIP) in high-resolution computed tomography (HRCT) [7,10,13,14,15,16,17,18,19,20].
Despite numerous advances in immunosuppressive therapy, antifibrotic agents, and disease-modifying antirheumatic drugs (DMARDs), data on the effectiveness and safety of these treatments for this challenging condition remain scarce. While some immunosuppressants, such as mycophenolate mofetil, azathioprine, and cyclophosphamide have proven beneficial in ILD associated with systemic autoimmune disease, including RA, these drugs are of little use for joint involvement [21,22,23]. Similarly, antifibrotic agents such as nintedanib could be beneficial only with respect to lung involvement in patients with RA-ILD, as shown in the INBUILD trial [24]. Whereas older studies associated methotrexate with ILD, more recent papers indicate that methotrexate does not appear to be associated with a greater risk [25,26]. Evidence for other conventional synthetic DMARDs (csDMARDs) is rarer, although one meta-analysis did not find a greater frequency of respiratory adverse events with leflunomide [27]. As for biologic DMARDs (bDMARDs), available evidence, which is based mainly on cross-sectional or retrospective studies, suggests that rituximab, abatacept, and tocilizumab could prove safe for treatment of RA-ILD [28,29,30,31,32], whereas tumor necrosis factor inhibitors (anti-TNF) have been associated with a risk of lung impairment [27]. Our group found that non-anti-TNF bDMARDs were associated with poorer short-term progression of lung disease in a prospective cohort of 70 patients with RA-ILD [33]. Given the scarcity of data on the long-term efficacy and safety of these treatments in patients with RA-ILD, the objectives of our study were to describe a prospective cohort of patients with rheumatoid arthritis associated with interstitial lung disease (RA-ILD) and identify risk factors that could help predict prognosis and mortality in these patients in the medium term.
2. Patients and Methods
2.1. Design
We performed a multicenter observational prospective study of a prevalent cohort of patients with RA-ILD from 6 teaching hospitals in Andalusia, Spain. Recruitment ran from March 2015 to December 2020. The study was approved by the Research Ethics Committee of Hospital Regional Universitario de Málaga (HRUM) (Code 1719-N-15). All of the patients provided their written informed consent before participating in the study.
2.2. Study Population
We consecutively recruited adults with RA classified as per the 2010 criteria of the ACR/EULAR [34] and ILD confirmed by means of pulmonary function testing (PFT) and HRCT or lung biopsy. Time since diagnosis of ILD differed when patients were included in the study, and all patients had been receiving a DMARD. We excluded patients who were pregnant and patients with inflammatory or rheumatic diseases other than RA (except secondary Sjögren syndrome), infection, primary pulmonary hypertension, congestive heart failure, and known exposure to fibrosing environmental agents.
2.3. Protocol
Selected patients were seen by a rheumatologist who followed a pre-established protocol for clinical and laboratory data collection at the inclusion date (v0), 24 months (v24) [33], and 60 months (v60). PFT and HRCT were performed at v0, v24, v60, and at any other visit if the patients showed symptoms of respiratory impairment or at the attending physician’s discretion. All HRCT scans were performed using an axial 1.5-mm or 2-mm slice at intervals of 1 cm along the thorax. Images were reconstructed using a high spatial frequency algorithm, with an acquisition of 20–25 slices per patient. In order to homogenize the interpretation of findings, the radiological evaluation was centralized at HRUM and performed blind and independently by 2 experts in pulmonary radiology (María Carmen Aguilar-Hurtado and María Isabel Padin-Martín). Radiological progression was defined as a ≥20% increase in the presence and extension of ground-glass opacities, reticulation, honeycombing, diminished attenuation, centrilobular nodules, other nodules, emphysema, or consolidation compared with the HRCT scan at v0. Discrepancies in the reports were resolved by agreement. Data were collected at v0, v24, and v60 or the last lung and joint assessment if the patient did not reach v60. Data on adverse events were collected systematically at each visit by asking the patient about possible adverse events and infections they had. Adverse events were recorded in the medical history at each visit.
2.4. Working Definitions and Endpoints
The main endpoint was a composite endpoint, namely, “Progression to ILD at the end of follow-up” in terms of the following outcomes: (1) improvement (i.e., improvement in FVC ≥10% or DLCO ≥15% and absence of radiological progression); (2) nonprogression (stabilization or improvement in FVC ≤10% or DLCO <15% and absence of radiological progression); (3) progression (worsening of FVC >10% or DLCO >15% and radiological progression); or (4) death [30]. Radiological progression was defined as a ≥20% increase in the presence and extension of ground-glass opacities, reticulation, honeycombing, diminished attenuation, centrilobular nodules, other nodules, emphysema, or consolidation compared with the HRCT scan at v0.
The different patterns of ILD were defined based on the lung biopsy or HRCT according to the standard criteria of the American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias [35], as follows: (1) nonspecific interstitial pneumonia (NSIP); (2) usual interstitial pneumonia (UIP); and (3) other (bronchiolitis obliterans, organizing pneumonia, lymphocytic interstitial pneumonitis, and mixed patterns). PFT included spirometry, whose results were expressed as percent predicted and adjusted for age, sex, and height. FVC was considered abnormal when <80% predicted. DLCO was evaluated using the single-breath method (DLCO-SB), and a value <80% was considered abnormal.
Other variables included the duration of joint symptoms, diagnostic delay, smoking history (current or past), body mass index (weight/height squared), Sjögren syndrome, and osteoporosis. Joint involvement was evaluated based on the DAS28 (28-joint Disease Activity Score) and its components [36], acute-phase reactants, and physical functioning (Health Assessment Questionnaire) [37]. We also collected variables associated with severity, as follows: rheumatoid factor (reference value, 20 U/mL; high titers, >60 U/mL); anticyclic citrullinated protein antibody (ACPA) (reference value, 10 U/mL, high values >340 U/mL); presence of at least 1 radiological erosion. We recorded treatment with csDMARDs, bDMARDs, immunosuppressants, and antifibrotic agents during follow-up before we also recorded the mean number of corticosteroids used during follow-up. Adverse effects were classified as mild and severe: mild adverse effects were easily tolerated signs and symptoms that do not require medical intervention or treatment; severe adverse events were those that required intervention, resulted in death, were life-threatening, required hospitalization or prolonged an existing hospitalization, led to a congenital abnormality, or led to significant disability [38].
2.5. Statistical Analysis
A descriptive analysis of the main variables was performed. Qualitative variables were expressed as whole numbers and percentages; quantitative variables were expressed as mean and standard deviation (SD) or as the median and interquartile range (IQR) depending on the normality of their distribution, as assessed using the Kolmogorov–Smirnov test. The bivariate analysis was performed using the paired t test or Wilcoxon test, as applicable, between v0 and the end of follow-up. The Kaplan–Meier and log-rank tests were used to estimate the survival of patients with RA-ILD and to compare survival between patients with the UIP and NSIP patterns. Survival time was measured from v0 until the end of follow-up (v60) or progression/death and type of censoring done was right censoring. Cox regression analysis was used to identify prognostic factors for the time to progression or death using univariate and multivariate models (forward stepwise). All variables reaching a p value of <0.10 were included in the Cox multivariate model. The incidence rates for total, severe, and mild adverse effects were also analyzed. The analysis was carried out using Rcommander.
3. Results
3.1. Baseline Clinical Characteristics
From March 2015 until December 2020, we prospectively followed up 116 patients with RA-ILD treated with DMARDs for a mean (SD) of 49.1 (14.4) months, that is, 454.9 patient-years. The main baseline characteristics are shown in Table 1. Patients were aged around 70 years, with an even distribution between the sexes. Half of the patients had been smokers or were smokers at inclusion. Almost all patients had long-term erosive and seropositive joint disease and, upon entering the study, they had had ILD for a mean of 2.2 years.
Table 1.
Baseline characteristics of 116 with RA-ILD treated with DMARDs.
| Variable | Total = 116 |
|---|---|
| Epidemiological characteristics | |
| Female sex, n (%) | 63 (54.3) |
| Caucasian race, n (%) | 113 (97.4) |
| Age, years, mean (SD) | 68.3 (9.9) |
| Clinical and analytical characteristics | |
| Current smoker | – |
| Nonsmoker, n (%) | 57 (49.1) |
| Smoker, n (%) | 23 (19.8) |
| Exsmoker, n (%) | 36 (31.0) |
| Body mass index, mean (SD) | 27.8 (4.1) |
| Time since diagnosis of RA, months, median (25%–75%) | 148.5 (71.5–217.8) |
| Diagnostic delay, months, median (25%–75%) | 8.5 (4.9–16.8) |
| Time since diagnosis of ILD, months, median (25%–75%) | 27.5 (9.8–60.0) |
| Positive rheumatoid factor (>10), n (%) | 111 (95.7) |
| Positive ACPA titer (>20), n (%) | 100 (86.2) |
| High ACPA titer (>340), n (%) | 48 (41.4) |
| Erosive disease, n (%) | 76 (65.5) |
| Sjögren syndrome, n (%) | 18 (15.5) |
| Osteoporosis, n (%) | 51 (44.0) |
| Treatment | |
| Synthetic DMARD | 100 (86.2) |
| Methotrexate, n (%) | 51 (44.0) |
| Leflunomide, n (%) | 30 (25.9) |
| Sulfasalazine, n (%) | 9 (7.8) |
| Hydroxychloroquine, n (%) | 21 (18.1) |
| Biologic DMARD | 50 (43.1) |
| Infliximab, n (%) | 1 (0.9) |
| Etanercept, n (%) | 6 (5.2) |
| Adalimumab, n (%) | 3 (2.6) |
| Golimumab, n (%) | 3 (2.6) |
| Certolizumab, n (%) | 3 (2.6) |
| Tocilizumab, n (%) | 6 (5.2) |
| Abatacept, n (%) | 15 (12.9) |
| Rituximab, n (%) | 13 (11.2) |
| Immunosuppressants | 11 (9.5) |
| Mycophenolate, n (%) | 7 (6.0) |
| Azathioprine, n (%) | 4 (3.4) |
| Antifibrotic agents, nintedanib, n (%) | 1 (0.9) |
| Baseline corticosteroids, n (%) | 69 (60.0) |
| Dose of baseline corticosteroids, median (25%–75%) | 5.0 (0.0–7.5) |
SD: standard deviation; RA: rheumatoid arthritis; ILD: interstitial lung disease; ACPA: anticyclic citrullinated protein antibody; DMARD: disease-modifying antirheumatic drug.
At v0, all patients were taking a DMARD: most were receiving a csDMARD and almost half were receiving a bDMARD. Fifty-nine patients (50.9%) were receiving a csDMARD in monotherapy, 36 (31%) were receiving a combination of a csDMARD and a bDMARD, 9 (7.8%) were receiving monotherapy with a bDMARD, 6 (5.1%) were receiving a combination of a csDMARD and an immunosuppressant, and a further 5 (4.3%) were taking a bDMARD with an immunosuppressant. Only one patient (0.9%) was receiving a csDMARD combined with nintedanib. The different DMARDs prescribed at v0 are shown in Table 1. More than half of the patients were taking corticosteroids. Eighty-four patients (72.4%) had received at least one csDMARD before v0, 29 (25%) had taken a bDMARD for a median (25%–75%) of 26 months (12.0–39.0), and 8 (6.8%) had received an immunosuppressant (Supplementary Table S1).
The most frequent radiological pattern was UIP in 71/116 patients (61.2%), followed by NSIP in 32/116 patients (27.6%), fibrotic NSIP in 9/116 patients (7.8), and other types of ILD in 4/116 patients (3.4%). UIP was confirmed by histopathology in 4 patients. Table 2 shows the differences in baseline characteristics between the UIP and NSIP patterns. Patients with the UIP pattern were more frequently male (p = 0.003) with positive ACPA titers (p = 0.020) and erosive disease (p = 0.023) than patients with NSIP. There was no difference between the groups in the remaining baseline clinical characteristics or in the treatment received. Summary statistics for all continuous variables are shown in (Supplementary Table S2).
Table 2.
Comparison of baseline characteristics between patients with RA-ILD according to radiological pattern (UIP and NSIP).
| Variable | UIP, n = 71 | NSIP, n = 41 | p Value |
|---|---|---|---|
| Epidemiological characteristics | |||
| Female sex, n (%) | 31 (43.7) | 30 (73.2) | 0.003 |
| Caucasian, n (%) | 68 (95.8) | 41 (100) | 0.182 |
| Age, years, mean (SD) | 68.9 (9.4) | 68.0 (10.9) | 0.639 |
| Clinical and analytical characteristics | |||
| Current smoker | 0.815 | ||
| Nonsmoker, n (%) | 32 (45.1) | 21 (51.2) | |
| Smoker, n (%) | 13 (18.3) | 7 (17.1) | |
| Exsmoker, n (%) | 26 (36.6) | 13 (31.7) | |
| Body mass index, mean (SD) | 28.1 (4.3) | 27.5 (4.1) | 0.578 |
| Time since diagnosis of RA, months, median (25%–75%) | 146.1 (69.2–227.9) | 167.7 (87.5–224.2) | 0.987 |
| Diagnostic delay, months, median (25%–75%) | 10.9 (4.9–18.4) | 7.0 (4.9–15.5) | 0.395 |
| Time since diagnosis of ILD, months, mean (SD) | 23.8 (9.6–59.9) | 36.4 (11.3–67.9) | 0.337 |
| Positive rheumatoid factor (>10), n (%) | 69 (97.2) | 38 (92.7) | 0.267 |
| ACPA titer (>20), n (%) | 65 (91.5) | 31 (75.6) | 0.020 |
| Erosive disease, n (%) | 53 (74.6) | 22 (53.7) | 0.023 |
| Sjögren syndrome, n (%) | 11 (15.5) | 7 (17.1) | 0.826 |
| Osteoporosis, n (%) | 32 (45.1) | 17 (41.5) | 0.711 |
| Treatment | |||
| Synthetic DMARD | 60 (84.5) | 37 (90.2) | 0.390 |
| Methotrexate, n (%) | 28 (39.4) | 22 (50.7) | 0.145 |
| Leflunomide, n (%) | 20 (28.2) | 9 (22.0) | 0.469 |
| Sulfasalazine, n (%) | 7 (9.9) | 1 (2.4) | 0.142 |
| Hydroxychloroquine, n (%) | 14 (19.7) | 7 (17.1) | 0.730 |
| Biologic DMARD | 30 (42.3) | 19 (46.3) | 0.674 |
| Infliximab, n (%) | 1 (1.4) | 0 (0.0) | 0.445 |
| Etanercept, n (%) | 3 (4.2) | 3 (4.2) | 0.485 |
| Adalimumab, n (%) | 1 (1.4) | 2 (4.9) | 0.273 |
| Golimumab, n (%) | 2 (2.8) | 1 (2.4) | 0.905 |
| Certolizumab, n (%) | 2 (2.8) | 1 (2.4) | 0.905 |
| Tocilizumab, n (%) | 4 (4.2) | 1 (2.4) | 0.324 |
| Abatacept, n (%) | 9 (12.7) | 6 (14.6) | 0.769 |
| Rituximab, n (%) | 9 (12.7) | 4 (9.8) | 0.642 |
| Immunosuppressants | 7 (9.9) | 4 (9.8) | 0.986 |
| Mycophenolate, n (%) | 5 (7.0) | 2 (4.9) | 0.649 |
| Azathioprine, n (%) | 2 (2.8) | 2 (4.9) | 0.571 |
| Antifibrotic agents, nintedanib, n (%) | 1 (0.9) | 0 (0.0) | 0.045 |
| Corticosteroids at baseline, n (%) | 42 (59.1) | 22 (50.7) | 0.800 |
| Dose of corticosteroids at baseline, median (25%–75%) | 5.0 (0.0–6.0) | 5.0 (0.0–7.5) | 0.140 |
RA: rheumatoid arthritis; ILD: interstitial lung disease; DMARD: disease-modifying antirheumatic drug; SD: standard deviation; ACPA, anticyclic citrullinated protein antibody.
3.2. Course of ILD after 60 Months of Follow-Up
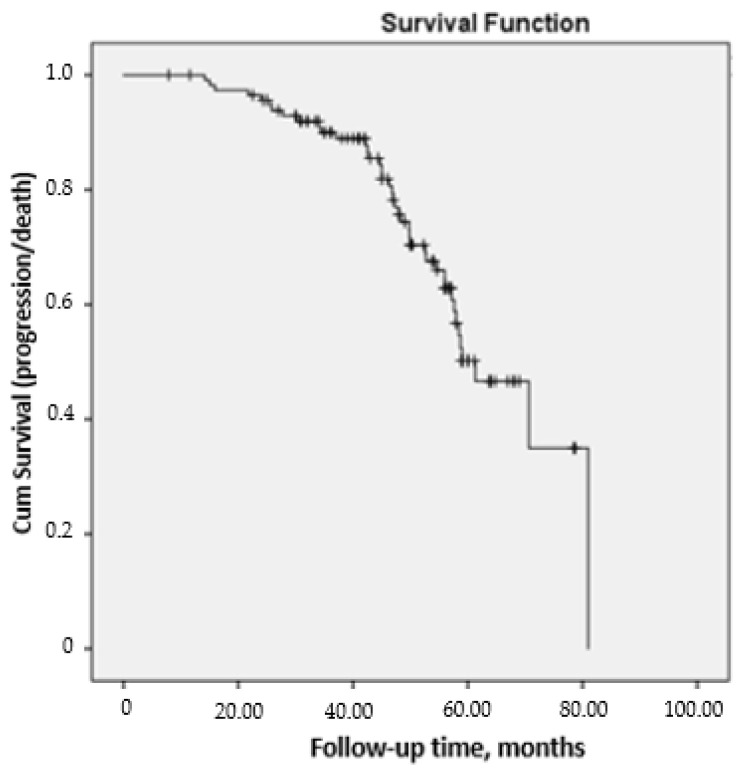
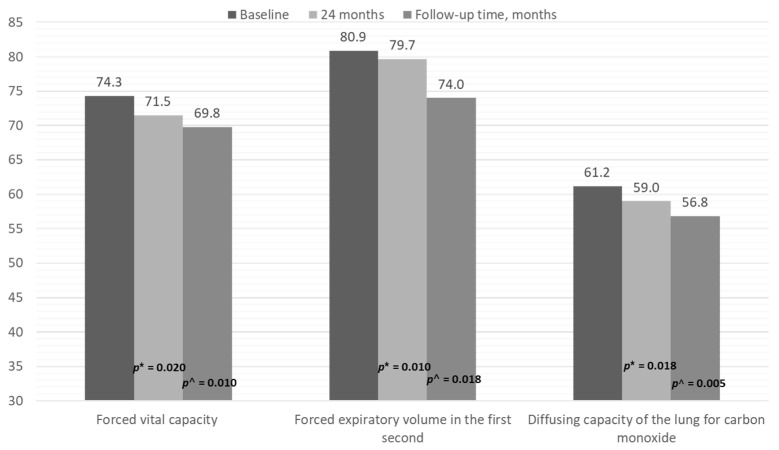
After 60 months of follow-up, a total of 98 patients (84.5%) remained in the study, 23 (19.8%) had progressed, and 18 (15.5%) had died, with a mean survival of 71.8 (1.9) months (Figure 1). As shown in Table 3, all mean PFT values were significantly worse at the end of follow-up. Figure 2 shows the progressive decrease in FVC, FEV1, and DLCO at 24 months and at the end of follow-up. HRCT showed that disease had progressed in 32/116 patients (27%), whereas 23/116 (20%) fulfilled the criteria for ILD and 18 (15%) died.
Figure 1.
Survival curve was measured from v0 until the end of follow-up (v60) or progression/death in 116 patients at risk with RA-ILD under treatment with DMARDs.
Table 3.
Progress of symptoms and lung function at the end of follow-up of 116 patients with RA-ILS taking DMARDs.
| Variable | Baseline | End of Follow-Up | p Value |
|---|---|---|---|
| Duration of follow-up, mean (SD) | – | 49.1 (14.4) | – |
| Respiratory function | |||
| Oxygen saturation, mean (SD) | 96.1 (2.2) | 95.0 (3.1) | 0.018 |
| Pulmonary function testing | |||
| FVC, mean (SD) | 74.3 (17.3) | 69.8 (23.4) | 0.010 |
| FVC <80%, n (%) | 69 (59.5) | 79 (68.1) | 0.012 |
| FVC ≥80%, n (%) | 47 (40.5) | 37 (31.9) | |
| FEV1, mean (SD) | 80.9 (20.5) | 74.0 (21.1) | 0.018 |
| DLCO-SB, mean (SD) | 61.2 (16.2) | 56.8 (18.5) | 0.005 |
| HRCT | |||
| Radiological pattern | 0.720 | ||
| UIP, n (%) | 71 (61.2) | 74 (63.7) | |
| NSIP, n (%) | 32 (27.6) | 31 (26.7) | |
| Fibrotic NSIP, n (%) | 9 (7.8) | 7 (6.0) | |
| Other, n (%) | 4 (3.4) | 4 (3.4) | |
| Course | |||
| Progression, n (%) | – | 32 (27.6) | |
| Stabilization, n (%) | – | 77 (66.4) | |
| Improvement, n (%) | – | 7 (6.0) | |
| Progression of lung disease (total) * | |||
| Improvement, n (%) | – | 9 (7.8) | |
| Stabilization, n (%) | – | 66 (56.9) | |
| Worsening, n (%) | – | 23 (19.8) | |
| Death, n (%) | – | 18 (15.5) | |
| Inflammatory activity | |||
| DAS28, median (25%–75%) | 2.8 (2.3–4.0) | 3.0 (3.0–5.2) | 0.627 |
| C-reactive protein, median (25%–75%) | 5.3 (2.9–13.0) | 8.0 (2.0–22.0) | 0.320 |
| ESR, median (25%–75%) | 21.0 (9.7–36.5) | 20.0 (8.0–29.0) | 0.136 |
| HAQ, median (25%–75%) | 1.0 (0.2–1.8) | 1.1 (0.6–1.9) | 0.484 |
RA: rheumatoid arthritis; ILD: interstitial lung disease; DMARD: disease-modifying antirheumatic drug; SD: standard deviation; FVC: forced vital capacity; FEV1: forced expiratory volume in the first second; DLCO: diffusing capacity of the lung for carbon monoxide; UIP: usual interstitial pneumonia; NSIP: nonspecific interstitial pneumonia; HRCT: high-resolution computed tomography; DAS28: 28-joint Disease Activity Score; ESR: erythrocyte sedimentation rate; HAQ: Health Assessment Questionnaire; * Progression of lung disease (total): taking into account HRCT and pulmonary function testing (FVC and DLCO).
Figure 2.
Progression of pulmonary function test results at the end of follow-up in patients with RA and ILD receiving DMARDs. * p-value for the comparison between 24 months and baseline; ^ p-value for the comparison between end of follow-up and baseline.
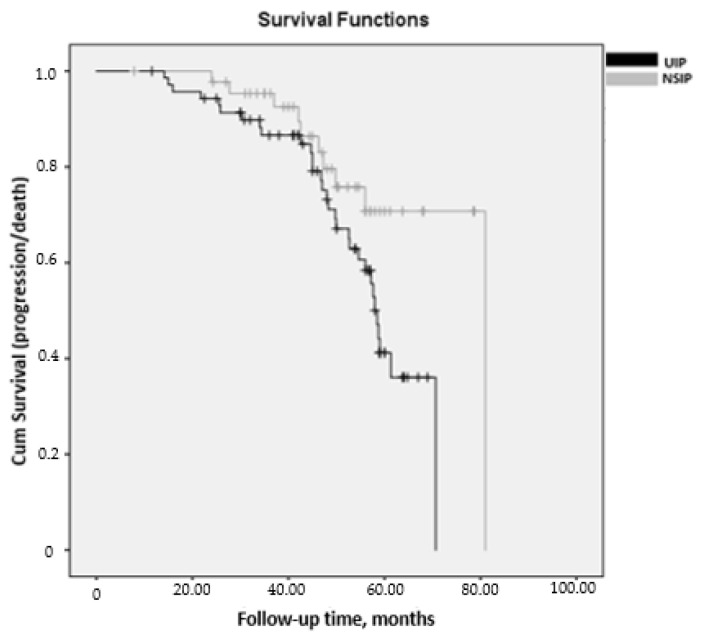
Table 4 shows the comparison between the UIP and NSIP patterns in the course of lung disease in patients with RA-ILD. The disease worsened more markedly in patients with the UIP pattern than in those with the NSIP pattern in terms of PFT values at the end of follow-up, especially in FVC (mean (SD) = 67.6 (22.6) vs. 78.6 (25.4); p = 0.037), with an incidence rate for progression (95% CI) of 0.11 (0.07–0.15) patient-years in those with UIP and 0.05 (0.02–0.09) patient-years in those with NSIP. Similarly, HRCT revealed a higher percentage of patients with disease progression (p = 0.002), overall progression of lung disease (p = 0.032), and mortality (p = 0.032). Survival was greater in patients with NSIP than in those with UIP (mean (95% CI), 70.0 months (63.5–76.5) vs. 55.7 months (51.6–59.8); p = 0.033, log-rank) (Figure 3).
Table 4.
Comparison of progression of symptoms and lung disease in patients with RA-ILD according to radiological pattern (UIP or NSIP).
| Variable | UIP | NSIP | p Value |
|---|---|---|---|
| Duration of follow-up, mean (SD) | 46.8 (14.5) | 47.5 (14.9) | 0.808 |
| Pulmonary function testing | |||
| Last FVC, mean (SD) | 67.6 (22.0) | 78.6 (25.4) | 0.037 |
| Last FEV1<, mean (SD) | 72.1 (21.9) | 78.2 (20.7) | 0.225 |
| Last DLCO, mean (SD) | 66.9 (18.5) | 70.1 (16.7) | 0.235 |
| HRCT | |||
| Course | 0.002 | ||
| Progression, n (%) | 27 (38.0) | 9 (22.0) | |
| Stabilization, n (%) | 44 (62.0) | 26 (63.4) | |
| Improvement, n (%) | 0 (0.0) | 6 (14.6) | |
| Progression of lung disease (total) * | 0.032 | ||
| Improvement, n (%) | 2 (2.8) | 6 (14.6) | |
| Stabilization, n (%) | 38 (53.5) | 25 (61.0) | |
| Worsening, n (%) | 16 (22.5) | 7 (17.1) | |
| Death, n (%) | 15 (21.1) | 3 (7.3) | |
| Inflammatory activity | |||
| Last DAS28, mean (SD) | 3.3 (1.1) | 3.2 (1.4) | 0.428 |
| Last C-reactive protein, mean (SD) | 18.5 (16.9) | 8.6 (7.3) | 0.037 |
| Last ESR, mean (SD) | 26.1 (17.4) | 23.2 (18.2) | 0.823 |
| Last HAQ, mean (SD) | 1.5 (0.7) | 1.2 (0.7) | 0.341 |
RA: rheumatoid arthritis; ILD: interstitial lung disease; DMARD: disease-modifying antirheumatic drug; SD: standard deviation; FVC: forced vital capacity; FEV1: forced expiratory volume in the first second; DLCO: diffusing capacity of the lung for carbon monoxide; UIP: usual interstitial pneumonia; NSIP: nonspecific interstitial pneumonia; HRCT: high-resolution computed tomography; DAS28: 28-joint Disease Activity Score; ESR: erythrocyte sedimentation rate; HAQ: Health Assessment Questionnaire; * Progression of lung disease (total): taking into account HRCT and pulmonary function testing (FVC and DLCO).
Figure 3.
Kaplan–Meier survival curves measured from v0 until the end of follow-up (v60) or progression/death, stratified by radiological pattern in 116 patients at risk with RA-ILD. Survival was greater in patients with NSIP than in those with UIP (mean (95% CI), 70.0 months (63.5–76.5) vs. 55.7 months (51.6–59.8); p = 0.033, log-rank).
With respect to joint involvement, patients remained stable in the last evaluation. At the end of follow-up, 52 patients were taking monotherapy with csDMARDs, 30 were taking combination therapy, 6 were taking monotherapy with bDMARDs, 5 were taking a csDMARD and an immunosuppressant, 4 were taking a bDMARD and an immunosuppressant, and 1 was taking an antifibrotic agent and mycophenolate. Twelve csDMARDs were modified (10 because of inefficacy and 2 because of adverse effects), and several bDMARDs were suspended for the following reasons: anti-TNF owing to progression of lung disease, 5 patients; tocilizumab owing to adverse effects, 2 patients; abatacept owing to ineffective treatment of joint involvement, 2 patients; and rituximab owing to adverse effects, 2 patients. Four patients started abatacept, 4 rituximab, and 1 started mycophenolate with an antifibrotic.
3.3. Factors Associated with Progression and Mortality of ILD after 60 Months of Follow-Up
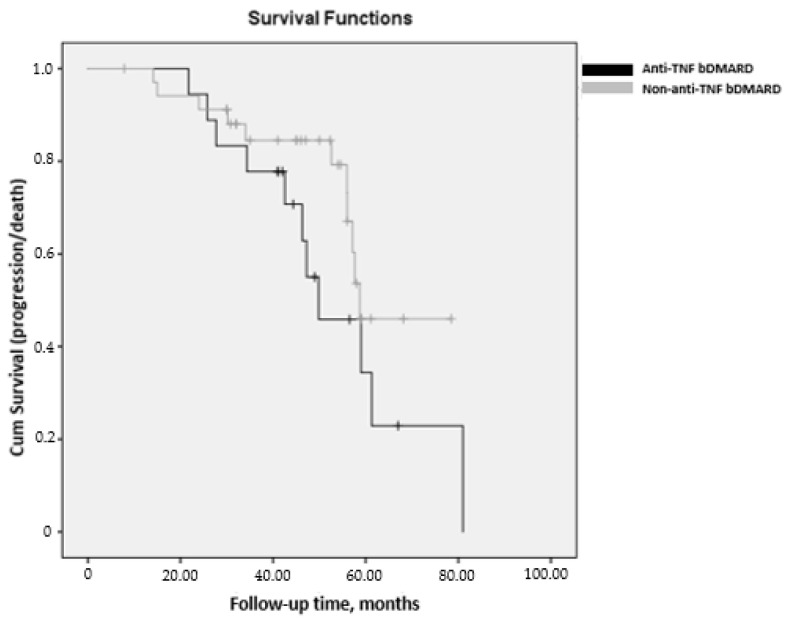
Table 5 shows the results of the Cox multivariate analysis (DV: progression or death), with a total of 116 patients with RA-ILD over a mean (SD) follow-up period of 49.1 (14.4) months. The event “progression” or “death” was recorded in 41/116 patients. The parameters assessed at baseline are included in the multivariate models. The multivariate analysis identified treatment with a non-anti-TNF bDMARD (i.e., abatacept, rituximab, or tocilizumab) as being associated with a 50% reduced risk of progression of ILD in patients with RA, whereas smoking, UIP radiological pattern, ACPA at high titers (>340), and FVC <80% at the initiation of follow-up were associated with a higher probability of progression of lung disease (Table 5). Survival was greater in patients with non–anti-TNF bDMARD than in those with anti-TNF bDMARD (mean (95%CI), 62.7 months (57.3–68.1) vs. 54.5 months (43.8–65.2); p = 0.190, log-rank) (Figure 4). The comparison of patients with RA-ILD according to bDMARD (anti-TNF or non-anti-TNF) is shown in Supplementary Table S3.
Table 5.
Multivariate analysis of progression and mortality of lung disease in patients with RA-ILD taking DMARDs.
| Variable | Univariate HR (95% CI) | Multivariate HR (95% CI) | p Value |
|---|---|---|---|
| Age, years | 1.930 (0.98–1.07) | ||
| Male sex | 1.041 (0.28–2.23) | ||
| History of smoking | 2.204 (1.01–4.85) | 2.543 (1.03–6.24) | 0.042 |
| Radiological pattern, UIP | 2.712 (1.82–7.11) | 2.661 (1.04–6.77) | 0.040 |
| Rheumatoid factor, titer | 1.001 (1.00–1.01) | ||
| High ACPA (>340) | 2.556 (1.17–5.58) | 2.810 (1.17–6.75) | 0.021 |
| Baseline FVC <80 | 2.517 (1.10–5.75) | 3.840 (1.50–6.70) | 0.003 |
| Baseline DLCO-SB <80 | 2.800 (0.90–8.10) | ||
| Corticosteroids | 1.603 (0.71–3.57) | ||
| DMARDs | 0.662 (0.22–1.93) | ||
| Immunosuppressants | 0.661 (0.16–2.64) | ||
| Anti-TNF | 2.692 (1.02–7.87) | ||
| Non–anti-TNF | 0.618 (0.36–0.89) | 0.472 (0.25–0.86) | 0.014 |
R2 = 0.316. RA: rheumatoid arthritis; ILD: interstitial lung disease; DMARD: disease-modifying antirheumatic drug; HR: hazard ratio. Independent variables: sex, age, history of smoking, radiological pattern (UIP/NSIP), baseline FVC, baseline DLCO-SB, tumor necrosis factor inhibitors (anti-TNF) treatment (infliximab, adalimumab, etanercept, golimumab, certolizumab), non-anti-TNF (rituximab, abatacept, tocilizumab), csDMARDs (methotrexate, leflunomide, hydroxychloroquine, sulfasalazine), immunosuppressants (azathioprine, mycophenolate), corticosteroids.
Figure 4.
Kaplan–Meier survival curves measured from v0 until the end of follow-up (v60) or progression/death, stratified by bDMARD in 116 patients at risk with RA-ILD. Survival was greater for patients taking non–anti-TNF bDMARDs than for those taking anti-TNF bDMARDs (mean (95% CI), 62.7 months (57.3–68.1) vs. 54.5 months (43.8–65.2); p = 0.190, log-rank).
3.4. Adverse Events
The main adverse events are shown in Table 6. During follow-up, 79 patients (68.1%) had 88 adverse events, which were mainly mild (41.4%). Infection was the most common, affecting 71/116 patients (61%), especially respiratory infection (54.3%). The most severe events included eight (7.7%) that were not associated with infection: three tumors and five patients with rapid progression of ILD who died.
Table 6.
Adverse effects in patients with RA-ILD taking DMARDs.
| Variable | Sample = 116 |
|---|---|
| Adverse effects, n (%) | 79 (68.1) |
| Mild adverse effects, n (%) | 48 (41.4) |
| Severe adverse effects, n (%) | 40 (34.4) |
| Incidence of adverse effects (patient-years) | 0.17 |
| Incidence of mild adverse effects (patient-years) | 0.10 |
| Incidence of severe adverse effects (patient-years) | 0.08 |
| Infection, n (%) | 71 (61.2) |
| Respiratory infection, n (%) | 63 (54.3) |
| Other infections, n (%) | 13 (11.2) |
| Cold sore, n (%) | 2 (1.7) |
| Dental infection, n (%) | 1 (0.8) |
| Cutaneous infection, n (%) | 3 (2.5) |
| Urinary infection, n (%) | 7 (6.0) |
| Incidence of infection (patient-years) | 0.15 |
| Incidence of respiratory infection (patient-years) | 0.13 |
| Incidence of other infections (patient-years) | 0.02 |
| Mortality | 18 (15.5) |
| Incidence of mortality (patient-years) | 0.03 |
RA: rheumatoid arthritis; ILD: interstitial lung disease. Patient-years: incidence per patient-years during observation time in the study.
Supplementary Table S4 shows the follow-up times, treatment administered, and cause of death for the 18 (15.5%) patients who died. In addition, of the patients who died, only two discontinued DMARDs permanently: one with methotrexate and another with leflunomide owing to severe lung infection.
4. Discussion
We prospectively evaluated 116 patients with RA-ILD receiving treatment with various DMARDs after 60 months of follow-up. While PFT values fell slowly and progressively during follow-up, slightly more than one-third of the patients progressed poorly (20% experienced progression of lung disease and 15% died). According to many studies, most patients’ condition stabilizes or progresses slowly, although some patients progress quickly and die [33,39,40,41,42].
Previous studies have tried to identify factors associated with more marked progression and death in RA-ILD and specifically examine the effect of DMARDs on disease progression. In this sense, we did not find csDMARDs to be associated with a more pronounced progression of lung disease or death after 60 months of follow-up. Recent studies show that while methotrexate can lead to hypersensitivity pneumonitis during the first months of treatment [43], it was not associated with a greater risk of RA-ILD than in patients who do not take methotrexate [25]. Similarly, the results of a meta-analysis point to a lower risk of respiratory adverse events with leflunomide than with methotrexate or placebo [44]. Furthermore, bDMARDs have also been reported to trigger ILD, worsen existing ILD, and increase susceptibility to infection [45]. Our Cox multivariate analysis adjusted for follow-up time showed that non–anti-TNF bDMARDs were associated with a lower risk of progression of lung disease and mortality. In this sense, our group previously found that non-anti-TNF bDMARDs were associated with reduced progression of lung disease in patients with RA-ILD in the short term in a prospective cohort of 70 patients [33], although we observed that despite the increase in follow-up time and number of patients included, this association remains unchanged. We do not know whether these biologics have an intrinsic effect on RA-ILD, although studies published in the last few years point to the stabilization of lung disease with non-anti-TNF bDMARDs. Fernández-Díaz et al. [31] recently reported that lung disease remained stable or improved in 80% of patients with RA-ILD treated with abatacept. Abatacept has shown a more favorable effect on lung involvement in patients with RA-ILD than anti-TNF agents, as in the study by Nakashita et al. [46]. As for rituximab, we found that two of 17 patients who had been taking rituximab during follow-up died. While this drug is usually introduced in more severely ill patients and there may be an indication bias, rituximab was not associated with greater mortality or disease progression in these patients. Narvaez et al. [28] recently reported that rituximab can prove effective as rescue therapy in up to 80% of patients with progressive RA-ILD and major impairment of lung function. In the case of tocilizumab, some authors have reported isolated case reports of progression of RA-ILD [47,48], whereas others reported stabilization [32].
The multivariate analysis showed that some clinical–epidemiological factors such as smoking and high ACPA titers, as well as pulmonary factors such as the UIP radiological pattern and baseline FVC <80%, were associated with poorer pulmonary outcomes at the end of follow-up. A retrospective registry study of 290 patients with RA showed that smoking doubled the risk of ILD and that most patients had positive ACPA titers [40]. The lung damage caused by tobacco smoke and other harmful substances stimulates the citrullination of proteins associated with a break in immune tolerance and more severe RA [49]. As for PFT parameters, we found that the UIP radiological pattern was associated with a 2.5-fold greater risk of progression or death than the NSIP pattern. Furthermore, we were able to verify that the progression of lung disease was observed both in PFT and HRCT and that mortality was greater with lower survival than in patients with NSIP. This observation is consistent with findings from other studies, which show that patients with RA and the UIP pattern have an almost 3-fold greater risk of progression [50,51] and an almost 3-fold greater risk of dying than patients with the NSIP pattern [11]. However, it is noteworthy that only two patients in our study were treated with nintedanib, even though more than half had UIP despite the encouraging data on treatment of pulmonary fibrosis in the recent IMBUILD study [24], thus indicating that insufficient time had passed for the results to be translated to clinical practice in the same way as in idiopathic pulmonary fibrosis.
The present study has both limitations and strengths. First, patients with RA-ILD are prevalent cases that were already treated at initiation of follow-up, thus hampering interpretation of the effect of each drug during the natural course of ILD. The major drawback of including prevalent cases is that patients with stable ILD can be mixed with patients who have early-onset, progressive disease. However, the main objective of this prospective cohort study was to evaluate the course of lung and joint involvement in different types of patients with RA-ILD and treated under conditions of daily clinical practice. One of the strengths of this study is its 60-month prospective evaluation of patients with RA-ILD based on a comparison of various treatment strategies and clinical characteristics. Furthermore, there is no standard definition of progression of ILD, with some disparity between the criteria applied in the different studies we analyzed. However, we evaluated a concept of progression used in other studies of RA-ILD, that is, based on both PFT and HRCT parameters. Similarly, we evaluated the progression of each of these functional tests independently. Compared with other RA cohorts, the mean age of patients in our study was relatively high. This may be due to the fact that older age and late onset of the disease have been associated with an increased risk of RA-ILD [49,50]. However, the possibility of differences resulting from the inclusion of older patients should be considered. In relation to AEs, it may be that the definition used in our study of AEs, used in clinical trials (GCP guidelines), is responsible for finding lower rates of AEs compared to other prospective studies. The cox regression analysis and the analysis in two groups of bDMARDS were not adjusted based on matching using the propensity score or some multivariate method, so this may lead to a selection bias. Lastly, as this was a multicenter study, there could be differences in the evaluation of lung disease. In order to palliate this discrepancy, HRCT was centralized, thus enabling us to take advantage of the fact that radiology findings could be accessed remotely. In addition, thanks to our prospective follow-up, there were no missing data.
In conclusion, lung function stabilized, and inflammatory activity remained well controlled after 60 months of follow-up in more than half of patients with RA-ILD in treatment with various DMARDs. However, one-third of patients progressed quickly and died. csDMARDs were not associated with a significant risk of progression of lung disease. Among bDMARDs, anti-TNF agents were associated with risk of progression, while the non-anti-TNF bDMARDs are associated with a reduced risk of progression of lung disease. The main factors associated with progression of lung disease and death were smoking, high ACPA titers, lower FVC at baseline, and the UIP pattern. Identifying patients at greater risk of progression of lung disease will enable closer follow-up and more specific and earlier treatment.
Acknowledgments
To the Spanish Rheumatology Society (SER) for the translation of the manuscript and the Andalusian Network for Research in Rheumatic Diseases (RAIER).
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/10/4/874/s1, Table S1: Previous treatment in patients with RA-ILD, Table S2: Summary statistics for all continuous variables in 116 patients with RA-ILD, Table S3: Comparison of patients with RA-ILD according to bDMARD (anti-TNF or non-anti-TNF) at the beginning of the observation period, Table S4: Characteristics of patients with RA-ILD taking DMARDs who died.
Author Contributions
N.M.-V. participated in the design of the study, carried out patient recruitment and statistical analysis, and drafted the manuscript. N.M.-V., M.R.-G., C.M.R.-B. and S.M.-A. assisted with patients. They were major contributors in writing the manuscript as well as analyzing and interpreting the patient data. M.C.A.-H., F.G.J.-N. and M.I.P.-M. collected radiology data. E.F. collected pneumology data. I.A.-O., L.P.-A., R.O.-C., F.J.G.-N., I.U.-G., M.L.V.-F., R.R.-R. and B.P.L. assisted with patients. A.F.-N.: contributed to writing the manuscript as well as analyzing and interpreting the patient data. All authors have read and agreed to the published version of the manuscript.
Funding
Grant for Medical Researchers of the “Fundación Española de Reumatología” 2019.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Research Ethics Committee of Hospital Regional Univer-sitario de Málaga (HRUM) (Code 1719-N-15).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Robles-Pérez A., Luburich P., Bolivar S., Dorca J., Nolla J.M., Molina-Molina M., Narváez J. A prospective study of lung disease in a cohort of early rheumatoid arthritis patients. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-72768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim D., Cho S.-K., Choi C.-B., Choe J.-Y., Chung W.T., Hong S.-J., Jun J.-B., Jung Y.O., Kim T.-H., Kim T.-J., et al. Impact of interstitial lung disease on mortality of patients with rheumatoid arthritis. Rheumatol. Int. 2017;37:1735–1745. doi: 10.1007/s00296-017-3781-7. [DOI] [PubMed] [Google Scholar]
- 3.Hyldgaard C., Hilberg O., Pedersen A.B., Ulrichsen S.P., Løkke A., Bendstrup E., Ellingsen T. A population-based cohort study of rheu-matoid arthritis-associated interstitial lung disease: Comorbidity and mortality. Ann. Rheum. Dis. 2017;76:1700–1706. doi: 10.1136/annrheumdis-2017-211138. [DOI] [PubMed] [Google Scholar]
- 4.Koduri G., Norton S., Young A., Cox N., Davies P., Devlin J., Dixey J., Gough A., Prouse P., Winfield J., et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: Results from an inception cohort. Rheumatology. 2010;49:1483–1489. doi: 10.1093/rheumatology/keq035. [DOI] [PubMed] [Google Scholar]
- 5.Kakutani T., Hashimoto A., Tominaga A., Kodama K., Nogi S., Tsuno H., Ogihara H., Nunokawa T., Komiya A., Furukawa H., et al. Related factors, increased mortality and causes of death in patients with rheumatoid arthritis-associated interstitial lung disease. Mod. Rheumatol. 2019;30:458–464. doi: 10.1080/14397595.2019.1621462. [DOI] [PubMed] [Google Scholar]
- 6.Solomon J.J., Ryu J., Tazelaar H., Myers J.L., Tuder R., Cool C., Swigris J.J., Brown K.K. Fibrosing interstitial pneumonia predicts survival in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD). In Proceedings of the American Thoracic Society 2011 International Conference, Denver, CO, USA, 13–18 May 2011. Respir. Med. 2011;107:1247–1252. doi: 10.1016/j.rmed.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Yang J.A., Lee J.S., Park J.K., Lee E.Y., Song Y.W. Clinical characteristics associated with occurrence and poor prognosis of interstitial lung disease in rheumatoid arthritis. Korean J. Intern. Med. 2019;34:434–441. doi: 10.3904/kjim.2016.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim E.J., Elicker B.M., Maldonado F., Webb W.R., Ryu J.H., Van Uden J.H., Lee J.S., King T.E., Collard H.R. Usual interstitial pneumonia in rheumatoid arthri-tis-associated interstitial lung disease. Eur. Respir. J. 2010;35:1322–1328. doi: 10.1183/09031936.00092309. [DOI] [PubMed] [Google Scholar]
- 9.Kim H.C., Choi K.H., Jacob J., Song J.W. Prognostic role of blood KL-6 in rheumatoid arthritis—Associated interstitial lung disease. PLoS ONE. 2020;15:e0229997. doi: 10.1371/journal.pone.0229997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamora-Legoff J.A., Krause M.L., Crowson C.S., Ryu J.H., Matteson E.L. Patterns of interstitial lung disease and mortality in rheumatoid arthritis. Rheumatology. 2017;56:344–350. doi: 10.1093/rheumatology/kew391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh N., Varghese J., England B.R., Solomon J.J., Michaud K., Mikuls T.R., Healy H.S., Kimpston E.M., Schweizer M.L. Impact of the pattern of interstitial lung disease on mortality in rheumatoid arthritis: A systematic literature review and meta-analysis. Semin. Arthritis Rheum. 2019;49:358–365. doi: 10.1016/j.semarthrit.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Kelly C.A., Saravanan V., Nisar M., Arthanari S., Woodhead F.A., Price-Forbes A.N., Dawson J., Sathi N., Ahmad Y., Koduri G., et al. Rheumatoid arthritis-related interstitial lung disease: Associations, prognostic factors and physiological and radiological characteristics—A large multicentre UK study. Rheumatology. 2014;53:1676–1682. doi: 10.1093/rheumatology/keu165. [DOI] [PubMed] [Google Scholar]
- 13.Fu Q., Wang L., Li L., Li Y., Liu R., Zheng Y. Risk factors for progression and prognosis of rheumatoid arthritis-associated inter-stitial lung disease: Single center study with a large sample of Chinese population. Clin. Rheumatol. 2019;38:1109–1116. doi: 10.1007/s10067-018-4382-x. [DOI] [PubMed] [Google Scholar]
- 14.Hozumi H., Nakamura Y., Johkoh T., Sumikawa H., Colby T.V., Kono M., Hashimoto D., Enomoto N., Fujisawa T., Inui N., et al. Acute exacerbation in rheumatoid arthri-tis-associated interstitial lung disease: A retrospective case control study. BMJ Open. 2013;3:e003132. doi: 10.1136/bmjopen-2013-003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob J., Song J.W., Yoon H.-Y., Cross G., Barnett J., Woo W.L., Adams F., Kokosi M., Devaraj A., Renzoni E., et al. Prevalence and Effects of Emphysema in Never-Smokers with Rheumatoid Arthritis Interstitial Lung Disease. EBioMedicine. 2018;28:303–310. doi: 10.1016/j.ebiom.2018.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H.-K., Kim D.S., Yoo B., Seo J.B., Rho J.-Y., Colby T.V., Kitaichi M. Histopathologic pattern and clinical features of rheumatoid arthri-tis-associated interstitial lung disease. Chest. 2005;127:2019–2027. doi: 10.1378/chest.127.6.2019. [DOI] [PubMed] [Google Scholar]
- 17.Nurmi H.M., Purokivi M.K., Kärkkäinen M.S., Kettunen H.-P., Selander T.A., Kaarteenaho R.L. Variable course of disease of rheu-matoid arthritis-associated usual interstitial pneumonia compared to other subtypes. BMC Pulm. Med. 2016;16:107. doi: 10.1186/s12890-016-0269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rojas-Serrano J., Herrera-Bringas D., Pérez-Román D.I., Pérez-Dorame R., Mateos-Toledo H., Mejía M. Rheumatoid arthri-tis-related interstitial lung disease (RA-ILD): Methotrexate and the severity of lung disease are associated to prognosis. Clin. Rheumatol. 2017;36:1493–1500. doi: 10.1007/s10067-017-3707-5. [DOI] [PubMed] [Google Scholar]
- 19.Solomon J.J., Chung J.H., Cosgrove G.P., Demoruelle M.K., Fernandez-Perez E.R., Fischer A., Frankel S.K., Hobbs S.B., Huie T.J., Ketzer J., et al. Predictors of mortality in rheu-matoid arthritis-associated interstitial lung disease. Eur. Respir. J. 2016;47:588–596. doi: 10.1183/13993003.00357-2015. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchiya Y., Takayanagi N., Sugiura H., Miyahara Y., Tokunaga D., Kawabata Y., Sugita Y. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur. Respir. J. 2010;37:1411–1417. doi: 10.1183/09031936.00019210. [DOI] [PubMed] [Google Scholar]
- 21.Saketkoo L.A., Espinoza L.R. Rheumatoid arthritis interstitial lung disease: Mycophenolate mofetil as an antifibrotic and dis-ease-modifying antirheumatic drug. Arch. Intern. Med. 2008;168:1718–1719. doi: 10.1001/archinte.168.15.1718. [DOI] [PubMed] [Google Scholar]
- 22.Oldham J.M., Lee C., Valenzi E., Witt L.J., Adegunsoye A., Hsu S., Chen L., Montner S., Chung J.H., Noth I., et al. Azathioprine response in patients with fibrotic connective tissue disease-associated interstitial lung disease. Respir. Med. 2016;121:117–122. doi: 10.1016/j.rmed.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes H., Holland A.E., Westall G.P., Goh N.S., Glaspole I.N. Cyclophosphamide for connective tissue disease-associated interstitial lung disease. Cochrane Database Syst. Rev. 2018;1:010908. doi: 10.1002/14651858.CD010908.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flaherty K.R., Wells A.U., Cottin V., Devaraj A., Walsh S.L., Inoue Y., Richeldi L., Kolb M., Tetzlaff K., Stowasser S., et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019;381:1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 25.Ibfelt E.H., Jacobsen R.K., Kopp T.I., Cordtz R.L., Jakobsen A.S., Seersholm N., Shaker S.B., Dreyer L. Methotrexate and risk of interstitial lung disease and respiratory failure in rheumatoid arthritis: A nationwide population-based study. Rheumatology. 2021;60:346–352. doi: 10.1093/rheumatology/keaa327. [DOI] [PubMed] [Google Scholar]
- 26.Kiely P., Busby A.D., Nikiphorou E., Sullivan K.A., Walsh D., Creamer P., Dixey J., Young A. Is incident rheumatoid arthritis interstitial lung disease associated with methotrexate treatment? Results from a multivariate analysis in the ERAS and ERAN inception cohorts. BMJ Open. 2019;9:e028466. doi: 10.1136/bmjopen-2018-028466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cubero C.C., Carmona E.C., Casasempere P.V. Systematic Review of the Impact of Drugs on Diffuse Interstitial Lung Disease Associated with Rheumatoid. Arthritis Reumatol. Clin. 2020 doi: 10.1016/j.reumae.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Narváez J., Robles-Pérez A., Molina-Molina M., Vicens-Zygmunt V., Luburich P., Yañez M.A., Alegre J.J., Nolla J.M. Real-world clinical effec-tiveness of rituximab rescue therapy in patients with progressive rheumatoid arthritis-related interstitial lung disease. Semin. Arthritis Rheum. 2020;50:902–910. doi: 10.1016/j.semarthrit.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Md Yusof M.Y., Kabia A., Darby M., Lettieri G., Beirne P., Vital E.M., Dass S., Emery P. Effect of rituximab on the progression of rheumatoid arthritis-related interstitial lung disease: 10 years’ experience at a single centre. Rheumatology. 2017;8:1348–1357. doi: 10.1093/rheumatology/kex072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernández-Díaz C., Loricera J., Castañeda S., López-Mejías R., Ojeda-García C., Olivé A., Rodríguez-Muguruza S., Carreira P.E., Pérez-Sandoval T., Retuerto M., et al. Abatacept in patients with rheumatoid arthritis and interstitial lung disease: A national multicenter study of 63 patients. Semin. Arthritis Rheum. 2018;48:22–27. doi: 10.1016/j.semarthrit.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Fernández-Díaz C., Castañeda S., Melero-González R.B., Ortiz-Sanjuán F., Juan-Mas A., Carrasco-Cubero C., Casafont-Solé I., Olivé A., Rodríguez-Muguruza S., Almodóvar-González R., et al. Abatacept in interstitial lung disease associated with rheumatoid arthritis: National multicenter study of 263 patients. Rheumatology. 2020;59:3906–3916. doi: 10.1093/rheumatology/keaa621. [DOI] [PubMed] [Google Scholar]
- 32.Manfredi A., Cassone G., Furini F., Gremese E., Venerito V., Atzeni F., Arrigoni E., Della Casa G., Cerri S., Govoni M., et al. Tocilizumab therapy in rheumatoid arthritis with interstitial lung disease: A multicenter retrospective study. Intern. Med. J. 2019;50:1085–1090. doi: 10.1111/imj.14670. [DOI] [PubMed] [Google Scholar]
- 33.Mena-Vázquez N., Godoy-Navarrete F.J., Manrique-Arija S., Aguilar-Hurtado M.C., Romero-Barco C.M., Ureña-Garnica I., Espildora F., Añón-Oñate I., Pérez-Albaladejo L., Gomez-Cano C., et al. Non-anti-TNF biologic agents are associated with slower worsening of interstitial lung disease secondary to rheumatoid ar-thritis. Clin. Rheumatol. 2020;40:133–142. doi: 10.1007/s10067-020-05227-9. [DOI] [PubMed] [Google Scholar]
- 34.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O., III, Birnbaum N.S., Burmester G.R., Bykerk V.P., Cohen M.D., et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 35.Travis W.D., Costabel U., Hansell D.M., King T.E., Jr., Lynch D.A., Nicholson A.G., Ryerson C.J., Ryu J.H., Selman M., Wells A.U., et al. An official American Thoracic Socie-ty/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic inter-stitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaujoux-Viala C., Mouterde G., Baillet A., Claudepierre P., Fautrel B., Le Loët X., Maillefert J.-F. Evaluating disease activity in rheumatoid arthritis: Which composite index is best? A systematic literature analysis of studies comparing the psychometric properties of the DAS, DAS28, SDAI and CDAI. Jt. Bone Spine. 2012;79:149–155. doi: 10.1016/j.jbspin.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Maska L., Anderson J., Michaud K. Measures of functional status and quality of life in rheumatoid arthritis: Health Assessment Questionnaire Disability Index (HAQ), Modified Health Assessment Questionnaire (MHAQ), Multidimensional Health Assessment Questionnaire (MDHAQ), Health Assessment. Arthritis Rheum. 2011;63:S4–S13. doi: 10.1002/acr.20620. [DOI] [PubMed] [Google Scholar]
- 38.Agencia Española de Medicamentos y Productos Sanitarios Información para las notificaciones de sospechas de reacciones adversas a medicamentos por parte de profesionales sanitarios. [(accessed on 20 January 2021)]; Available online: https://www.aemps.gob.es/vigilancia/medicamentosUsoHumano/SEFVH/NRA-SEFV-H/notificaSospechas-RAM-profSanitarios.htm#NSRAPSqueRAM2015.
- 39.Spagnolo P., Lee J.S., Sverzellati N., Rossi G., Cottin V. The Lung in Rheumatoid Arthritis: Focus on Interstitial Lung Disease. Arthritis Rheumatol. 2018;70:1544–1554. doi: 10.1002/art.40574. [DOI] [PubMed] [Google Scholar]
- 40.Kelly C.A., Nisar M., Arthanari S., Carty S., Woodhead F.A., Price-Forbes A., Middleton D., Dempsey O., Miller D., Basu N., et al. Rheumatoid arthritis related interstitial lung disease—Improving outcomes over 25 years: A large multicentre UK study. Rheumatology. 2020 doi: 10.1093/rheumatology/keaa577. [DOI] [PubMed] [Google Scholar]
- 41.Farquhar H., Vassallo R., Edwards A.L., Matteson E.L. Pulmonary Complications of Rheumatoid Arthritis. Semin. Respir. Crit. Care Med. 2019;40:194–207. doi: 10.1055/s-0039-1683995. [DOI] [PubMed] [Google Scholar]
- 42.Nannini C., Ryu J.H., Matteson E.L. Lung disease in rheumatoid arthritis. Curr. Opin. Rheumatol. 2015;20:340–346. doi: 10.1097/BOR.0b013e3282f798ed. [DOI] [PubMed] [Google Scholar]
- 43.Conway R., Low C., Coughlan R.J., O’Donnell M.J., Carey J.J. Methotrexate and lung disease in rheumatoid arthritis: A meta-analysis of randomized controlled trials. Arthritis Rheumatol. 2015;66:803–812. doi: 10.1002/art.38322. [DOI] [PubMed] [Google Scholar]
- 44.Conway R., Low C., Coughlan R.J., O’Donnell M.J., Carey J.J. Leflunomide Use and Risk of Lung Disease in Rheumatoid Arthritis: A Systematic Literature Review and Metaanalysis of Randomized Controlled Trials. J. Rheumatol. 2016;43:855–860. doi: 10.3899/jrheum.150674. [DOI] [PubMed] [Google Scholar]
- 45.Perez-Alvarez R., Perez-De-Lis M., Diaz-Lagares C., Pego-Reigosa J.M., Retamozo S., Bove A., Brito-Zeron P., Bosch X., Ramos-Casals M. Interstitial Lung Disease Induced or Exacerbated by TNF-Targeted Therapies: Analysis of 122 Cases. Semin. Arthritis Rheum. 2011;41:256–264. doi: 10.1016/j.semarthrit.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Nakashita T., Ando K., Takahashi K., Motojima S. Possible effect of abatacept on the progression of interstitial lung disease in rheumatoid arthritis patients. Respir. Investig. 2016;54:376–379. doi: 10.1016/j.resinv.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Kawashiri S.-Y., Kawakami A., Sakamoto N., Ishimatsu Y., Eguchi K. A fatal case of acute exacerbation of interstitial lung disease in a patient with rheumatoid arthritis during treatment with tocilizumab. Rheumatol. Int. 2012;32:4023–4026. doi: 10.1007/s00296-010-1525-z. [DOI] [PubMed] [Google Scholar]
- 48.Wendling D., Vidon C., Godfrin-Valnet M., Rival G., Guillot X., Prati C. Exacerbation of combined pulmonary fibrosis and em-physema syndrome during tocilizumab therapy for rheumatoid arthritis. Jt. Bone Spine. 2013;80:670–671. doi: 10.1016/j.jbspin.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 49.Mori S., Koga Y., Sugimoto M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir. Med. 2012;106:1591–1599. doi: 10.1016/j.rmed.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Li L., Liu R., Zhang Y., Zhou J., Li Y., Xu Y., Gao S., Zheng Y. A retrospective study on the predictive implications of clinical characteristics and therapeutic management in patients with rheumatoid arthritis-associated interstitial lung disease. Clin. Rheumatol. 2019;39:1457–1470. doi: 10.1007/s10067-019-04846-1. [DOI] [PubMed] [Google Scholar]
- 51.Zamora-Legoff J.A., Krause M.L., Crowson C.S., Ryu J.H., Matteson E.L. Progressive Decline of Lung Function in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Arthritis Rheumatol. 2017;69:542–549. doi: 10.1002/art.39971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data presented in this study are available on request from the corresponding author.