Abstract
Transactive response DNA-binding protein of 43 kilodaltons (TDP-43) is a 414 amino acid peptide that under physiologic conditions localizes to the nucleus and participates in the regulation of RNA metabolism through two RNA recognition motifs (RRM1 and RRM2). In neurodegenerative diseases, TDP-43 may become hyperphosphorylated, ubiquitinated, and aggregate into cytoplasmic inclusions. TDP-43 is now well-characterized as a pathologic protein of amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP). Additionally, a common TDP-43 proteinopathy arising outside of the context of ALS and FTLD-TDP has been recently described, termed “limbic predominant age-related TDP-43 encephalopathy (LATE).” In the current study, two novel mouse-derived monoclonal antibodies, 2G11 and 2H1, raised against an epitope within the RRM2 domain of TDP-43 (residues 198-216), were characterized for specificity and immunohistochemical application in human brain from cases of Alzheimer’s disease (AD), Lewy Body Disease (LBD), amyotrophic lateral sclerosis (ALS), and frontotemporal lobe degeneration with TDP-43 inclusions (FTLD-TDP). Immunoblot analysis of these antibodies in HEK293T cells revealed efficient detection of intact human TDP-43 protein, and in N2A cells showed no reactivity for mouse TDP-43. Immunohistochemically applied to formalin-fixed paraffin-embedded tissues, 2G11 and 2H1 robustly identified the classic inclusions of ALS and FTLD-TDP, and efficaciously provided a diagnosis of LATE in cases of AD and LBD. These novel antibodies label aberrant intracytoplasmic protein inclusions without relying on hyperphosphorylated epitopes, and provide elegant discrimination between TDP-43 and tau neurofibrillary tangles within neurodegenerative comorbidity.
Keywords: TDP-43, LATE, FTLD-TDP, ALS, immunohistochemistry, neuropathology
Introduction
The importance of transactive response DNA-binding protein of 43 kilodaltons (TDP-43) in neurodegenerative diseases became clear upon the discovery that aberrant, cytoplasmic inclusions of the protein comprise the pathologic substrate of most cases of sporadic amyotrophic lateral sclerosis (ALS) [1]. Similarly, the protein deposits of frontotemporal lobar degeneration with ubiquitinated inclusions (FTLD-U), whose inclusions were previously characterized only by their non-specific association to ubiquitin, were found to be composed of aberrant cytoplasmic TDP-43, ultimately warranting a taxonomic change to “frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP”) [1]. TDP-43 was initially discovered as a protein capable of binding the transactive response DNA element of the human immunodeficiency virus (HIV) [2]. In its physiologic state, the TDP-43 protein primarily localizes to the cell nucleus, reflective of its function related to regulation of RNA transcription, splicing, and translation via RNA and DNA interactions through its RNA recognition motif 1 (RRM1) and RNA recognition motif 2 (RRM2) domains [3,4]. In mouse models, TDP-43 has been shown to be expressed throughout the primitive neuroepithelium during embryonic development, and within cerebral neocortical, hippocampal, cerebellar, and spinal cord structures in adult tissue [5]. TDP-43 exhibits a high degree of conservation between species [6], with approximately 96% of amino acid residue concordance with mouse (Mus musculus) protein ( https://www.ncbi.nlm.nih.gov/protein/Q13148.1 and https://www.ncbi.nlm.nih.gov/protein/NP_663531.1) [6].
As the study of TDP-43’s contribution to neurodegeneration continued, it became increasingly recognized that TDP-43 inclusions occur outside of the classical distribution associated with ALS and FTLD-TDP. TDP-43 proteinopathy outside of the ALS and FTLD spectrum has been ascribed to evolving terminologies, which have included “hippocampal sclerosis of aging” and “cerebral age-related TDP-43 and sclerosis (CARTS)” [7,8]. In a recent attempt to discretely articulate TDP-43 proteinopathy outside of ALS and FTLD, the term “Limbic-Predominant Age-Related TDP-43 encephalopathy” (LATE) was proposed [9]. LATE describes an amnestic dementia syndrome arising in elderly populations that may occur in isolation or (more commonly) co-morbidly with other neurodegenerative diseases. Neuropathologically, LATE is defined and staged by virtue of the presence of intracytoplasmic deposits of TDP-43 within specific, reproducible areas of the neuraxis; early stages originate inclusions within medial limbic structures, while advanced stages show inclusions in cerebral neocortex. Although previous iterations of staging schemes for non-ALS/FTLD TDP-43 proteinopathy provided for up to five stages of disease stratification [10,11], the current working group criteria integrate the staging of LATE neuropathologic change (LATE-NC) into stage 1, 2, and 3 disease based upon progressive involvement of amygdala, hippocampus, and midfrontal neocortex, respectively [9].
ALS, FTLD-TDP, and LATE have each been described as capable of producing a neurodegenerative condition independently. However, it is increasingly recognized that TDP-43 proteinopathies frequently occur in the context of comorbidity with other neurodegenerative diseases [12], especially LATE [13,14]. As such, sensitive and specific tools for detecting aberrant deposits of TDP-43 protein are needed in order to effectively detect and stage disease, as well as clarify relationships between proteinopathies. The neuropathological evaluation of these diseases occurs in human post-mortem material and is most commonly predicated upon study of formalin-fixed, paraffin embedded (FFPE) tissue. Within this tissue medium, immunohistochemistry represents a versatile and widely available testing modality. In this study, we describe the generation and characterization of two novel mouse-derived monoclonal antibodies with elegant specificity for aberrant human TDP-43 inclusions within FFPE tissue of TDP-43 proteinopathies.
Materials and Methods
Mice
All animal experimental procedures were performed according to University of Florida Institutional Animal Care and Use Committee regulatory policies. Mice were housed in a stable environment with a 12-hour light/dark cycle and access to food and water ad libitum. BALB/c mice were procured from the Jackson Laboratory (Bar Harbor, MA).
Commercial Antibodies
The rabbit polyclonal total TDP-43 antibody (ProteinTech, catalogue number: 10782-2-AP) was utilized for western blotting, as well as a comparative benchmark for immunohistochemistry. The mouse monoclonal anti-actin C4 antibody (ThermoFisher Scientific) was utilized for western blotting as a loading control.
Production of Monoclonal Antibodies
A synthetic peptide (GenScript, Piscataway, NJ) containing residues 198-216 of human TDP-43 (CTEDMTEDELREFFSQYGD) was conjugated to maleimide-activated mariculture keyhold limpet hemocyanin (mcKLH; Thermo Scientific, Waltham, MA). Following conjugation, the peptide was used to immunize BALB/c mice. Six weeks post the initial immunization, the mice were sacrificed and the spleens harvested for hybridoma fusion as described [15]. Positive clones were screened for reactivity to the synthetic peptide using an established ELISA procedure [15].
Western blot detection of TDP-43 in cultured cells
HEK293T and Neuro 2A cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) and 100 U/ml penicillin/100 μg/ml streptomycin at 37°C and 5% CO2. For detection of endogenous TDP-43, cells were plated into 6 cm2 wells to 30-40% confluency and maintained for two days, after which time total cell lysate was harvested in 400 μL of sample buffer (10 mM Tris, pH 6.8, 1 mM EDTA, 40 mM DTT, 0.005% Bromophenol Blue, 0.0025% Pyronin Yellow, 1% SDS, 10% sucrose). For transient transfection of TDP-43, HEK293T cells were similarly plated into 6 cm2 wells to 30-40% confluency and transfected with Lipofectamine 2000 reagents (Thermo Fisher Scientific) according to the manufacturer’s instructions; total cell lysate was harvested 48 hours after transfection. The mammalian expression vector utilized for transfection contained TDP-43;mCherry cloned into the pEF.BOS vector [16]. Following harvest of total cell lysate, samples were heated to 100°C for 10 minutes prior to SDS-PAGE on 10% polyacrylamide gels (~8 μg of lysate per well) after which electrophoretic transfer onto 0.45 μm nitrocellulose membranes was performed. Membranes were incubated with blocking solution (5% milk in TBS) for 1 hour, and primary antibodies were diluted in blocking solution for overnight incubation at 4°C. Membranes were washed with TBS and incubated with goat anti-mouse or anti-rabbit secondary antibodies conjugated to horseradish peroxidase (Jackson Immuno Research Labs, Westgrove, PA) diluted in block solution for 1 hour at room temperature. Immunoreactivity was assessed using Western Lightning-Plus ECL reagents (PerkinElmer, Waltham, MA) followed by chemiluminescence imaging (PXi, Syngene, Frederick, MD).
Autopsy case material
In accordance with protocols approved by the Institutional Review Board, human brain tissue was obtained through the University of Florida Neuromedicine Human Brain and Tissue Bank (Table 1). Diagnoses of ADNC, LBD, FTLD, and LATE were made according to current parameters established by (respectively) the National Institute of Aging-Alzheimer’s Association [17], the Dementia with Lewy bodies Consortium [18], the Consortium for Frontotemporal Lobar Degeneration [19], and the LATE working group [9]. TDP-43 pathology in ALS was assessed by published criteria [20]. Five AD cases were studied: all exhibited a high degree (A3, B3, C3) of ADNC, two of which had co-morbid neocortical (case ID: X2) and amygdala-predominant (case ID: X3) variants of LBD. Five LBD cases were studied: four cases of “neocortical (diffuse)” LBD (case ID’s: Y1, Y2, Y3, and Y5) and one case of “limbic (transitional)” LBD (case ID: Y4). Of these LBD cases, four demonstrated co-morbid ADNC (case ID’s: Y1, Y2, Y4, and Y5), and one exhibited findings consistent with globular glial tauopathy (GGT; case ID: Y3). Two cases of FTLD-TDP were evaluated, including a case of FTLD-TDP type A (case ID: Z1) and FTLD-TDP type B (case ID: Z2). ALS tissues were obtained from the Mayo Clinic Alzheimer's Disease Research Center, including two cases of C9orf72-expansion ALS (case ID’s: Z3, Z4), and one case of FTLD-MND (case ID: Z5). One additional case of sporadic ALS and one case AD with high ADNC and LATE-NC stage 2 were recruited from the University of Florida Neuromedicine Human Brain and Tissue Bank (case ID’s: Z6 and Z7).
Table 1.
Patient demographics of cases studied. AD: Alzheimer’s disease; ADNC: Alzheimer’s disease neuropathologic change; LBD: Lewy body disease; MSA: GGT: globular glial tauopathy; LATE-NC: limbic-predominant age-related TDP-43 encephalopathy- neuropathologic change; ALS: amyotrophic lateral sclerosis; FTLD: frontotemporal lobar degeneration; MND: motor neuron disease; PMI: post-mortem interval (hours).
| Sex | Age at Death | PMI | Primary Diagnosis | Co-Pathology (if present) |
Brain Area Studied | Braak Stage | Thal Phase | CERAD Score |
|
|---|---|---|---|---|---|---|---|---|---|
| AD | |||||||||
| X1 | Male | 64 | 6 | ADNC, high | LATE-NC, stage 2 | Amygdala, hippocampus, midfrontal neocortex | VI | 5 | 3 |
| X2 | Male | 80 | 21 | ADNC, high | LBD (neocortical) LATE, stage 2 | Amygdala, hippocampus, midfrontal neocortex | VI | 4 | 3 |
| X3 | Male | 64 | 3 | ADNC, high | LBD (amygdala-predominant) | Amygdala, hippocampus, midfrontal neocortex | V | 5 | 3 |
| X4 | Male | 78 | 20 | ADNC, high | LATE-NC, stage 1 | Amygdala, hippocampus, midfrontal neocortex | V | 4 | 3 |
| X5 | Male | 83 | 7 | ADNC, high | Amygdala, hippocampus, midfrontal neocortex | VI | 4 | 3 | |
| LBD | |||||||||
| Y1 | Male | 62 | 10 | LBD (neocortical) | ADNC, low | Amygdala, hippocampus, midfrontal neocortex | II | 1 | 1 |
| Y2 | Female | 88 | 2 | LBD (neocortical) | ADNC, intermediate LATE-NC, stage 2 | Amygdala, hippocampus, midfrontal neocortex | III | 4 | 3 |
| Y3 | Male | 80 | 4 | LBD (neocortical) | GGT | Amygdala, hippocampus, midfrontal neocortex | 0 | 1 | 1 |
| Y4 | Male | 80 | 10 | LBD (transitional) | ADNC, intermediate LATE-NC, stage 2 | Amygdala, hippocampus, midfrontal neocortex | III | 3 | 2 |
| Y5 | Male | 68 | 39 | LBD (neocortical) | ADNC, intermediate LATE-NC, stage 2 | Amygdala, hippocampus, midfrontal neocortex | V | 3 | 3 |
| FTLD/ALS | |||||||||
| Z1 | Male | 59 | 16 | FTLD-TDP, type A | Hippocampus | I | 0 | 0 | |
| Z2 | Female | 58 | 23 | FTLD-TDP, type B | Hippocampus | I | 0 | 0 | |
| Z3 | Female | 62 | 38 | ALS C9orf72 | Hippocampus | II | 0 | 0 | |
| Z4 | Male | 63 | 16 | ALS C9orf72 | Hippocampus | I | 0 | 0 | |
| Z5 | Male | 59 | 8 | FTLD-MND | Hippocampus | II | 0 | 0 | |
| Z6 | Female | 75 | 15 | ALS | ADNC int. | Spinal Cord | III | 3 | 1 |
| Z7 | Female | 87 | 4 | ADNC high | LATE, stage 2, hippocampal sclerosis | Amygdala | V | 5 | 3 |
Immunohistochemical staining in neuropathologic human tissue
Novel TDP-43 antibodies 2G11 and 2H1 were applied to LATE-NC staging sites (amygdala, hippocampus, midfrontal neocortex) across five cases of AD and five cases of LBD [9], and onto a section of medial temporal lobe in three cases of FTLD and two cases of ALS. Upon confirmation of the presence of numerous inclusions, the amygdala of one case of neocortical type LBD (Y3) was utilized specifically to examine the effect of formic acid treatment on antigen retrieval efficacy. One case of ADNC high with LATE-NC stage 2 was utilized for an immunohistochemical dilution study, and another case of sporadic ALS was utilized to examine for the presence of spinal motor neurons inclusions.
Xylene immersion was utilized to deparaffinize the formalin-fixed, paraffin-embedded tissue sections, which were then sequentially rehydrated via graded ethanol solutions (100%, 90%, 70%). Heat-induced epitope retrieval (HIER) via steam bath in a 0.05% Tween-20 solution for 60 minutes was applied to all tissue sections. Additional sections of amygdala of case Y3 were subjected to a twenty-minute immersion in 70% formic acid after HIER. Endogenous tissue peroxidase activity in all sections was quenched via twenty-minute immersion in a solution of 1.5% hydrogen peroxide, 0.005% Triton-X-100, and phosphate-buffered saline (PBS), with subsequent water rinse. Blocking of sections was conducted with 2% FBS/0.1 M Tris (pH 7.6), with subsequent overnight incubation of sections with primary antibody at 4 °C. After washing sections with 0.1 M Tris (pH 7.6), biotinylated goat anti-mouse IgG secondary antibody or biotinylated goat anti-rabbit IgG secondary antibody diluted in 2% FBS/0.1 M Tris (pH 7.6) was applied to tissue for 1 h (secondary antibody concentrations: 1:3000). Sections were then washed with 0.1 M Tris (pH 7.6) and incubated with streptavidin-conjugated HRP (VECTASTAIN ABC kit; Vector Laboratories, Burlingame, CA) diluted in 2% FBS/ 0.1 M Tris, pH 7.6 for 1 h. Sections were again washed with 0.1 M (pH 7.6) and were developed via exposure to 3, 3′-diaminobenzidine (DAB kit; KPL, Gaithersburg, MD), with elapsed time to inclusion visualization annotated for each run. DAB development was halted by immersing sections in water. Sections were immersed in Mayer’s hematoxylin for counter-staining (Sigma Aldrich, St. Louis, MO). Counter-stained sections were ultimately dehydrated via an ascending ethanol series (70%, 90%, 100%) and xylenes, and were cover-slipped using cytoseal (Thermo Scientific, Waltham, MA).
In all AD and LBD tissues, the immunohistochemical profile of 2G11 (dilution of primary antibody 1:10,000) and 2H1 (1:10,000) were then compared to that of a commercially available TDP-43 antibody (1:1000). In a subset of FTLD and ALS tissues (Z1-Z5), only novel antibodies were applied to hippocampus at a concentration of 1:10,000. In one ALS case (Z6), novel antibodies (1:10,000) and commercially available antibody (1:1000) were applied to a section of lumbar spinal cord. Only novel antibodies were applied to one ADNC high case with LATE-NC stage 2 (Z7) to perform a dilution study on a section of amygdala at four antibody concentrations (1:1000, 1:25000, 1:5000, and 1:10,000). The presence or absence of immunoreactive inclusions as elucidated by each antibody was independently assessed and annotated by two observers (JTL, SP) utilizing an Olympus BX41 microscope (Olympus Optical, Tokyo, Japan) and a Zeiss Axioscope AX10 microscope (Jena, Germany) (respectively), with concordant interpretations independently reached upon thirteen of fifteen cases. Minor interpretative discordance in two cases was discussed, with diagnostic agreement reached between observers.
Results
Comparison of TDP-43 detection in cultured cells using new monoclonal antibodies
To generate novel monoclonal antibodies uniquely specific for the RRM2 domain of TDP-43, a 19 amino acid epitope (residues 198-216) was targeted (Fig. 1A). This region harbors only 68% overlap between mouse and human TDP-43, while the remainder of the amino acid sequence outside of this epitope region is 97% conserved across TDP-43 from both species (Fig. 1B). Multiple hybridoma clones against this epitope were generated, and antibodies capable of detecting the synthetic TDP-43 198-216 peptide through ELISA assay were further screened for their ability to detect endogenous TDP-43 in a human cell line (HEK293T cells) and a murine neuronal cell line (Neuro 2A cells). Clones 2G11 and 2H1 were equivalent in their specific detection of human TDP-43, as each antibody detected only a single ~43 kDa band corresponding to TDP-43 in HEK293T cells (Fig. 2A). These antibodies did not detect mouse TDP-43, which was present in the Neuro 2A cells as evidenced by its detection by the commercial polyclonal TDP-43 antibody. The specificity of the 2G11 and 2H1 antibodies was further confirmed using HEK293T cells overexpressing human TDP-43 tagged with mCherry; compared with non-transfected cells, an intense band equivalent in size to that of TDP-43 and mCherry (~70 kDa) is apparent, with the polyclonal TDP-43 antibody demonstrating similar results (Fig. 2B). These results demonstrate that the monoclonal antibodies 2G11 and 2H1 efficiently detect full length human TDP-43.
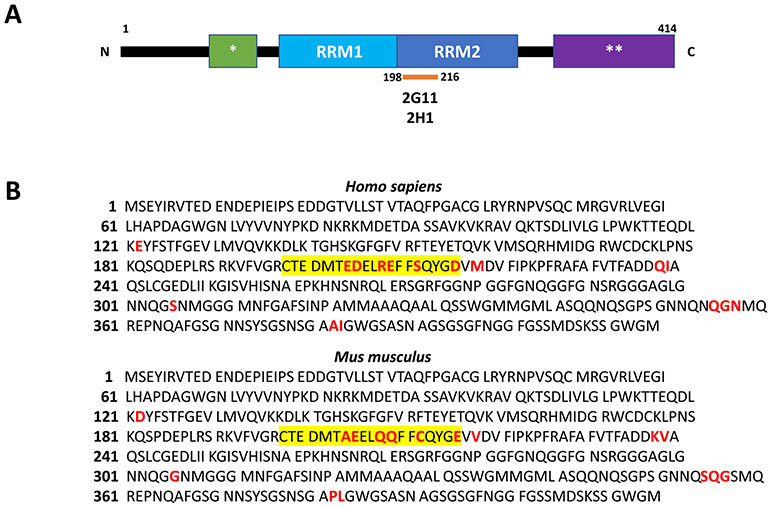
Figure 1. Epitope localization of antibodies 2G11 and 2H1 for human TDP-43.
(A): Schematic diagram of human TDP-43 with the location of the epitopes (residues 198-216) for antibodies 2G11 and 2H1 within RNA-recognition motif 2. (B): Comparison of the amino acid sequence for human and murine TDP-43. The epitope comprised of residues 198-216 (highlighted yellow) confers specificity for human protein, with 6 of the 18 residues differing from murine TDP-43 (highlighted red). RRM1: RNA-recognition motif 1; RRM2: RNA-recognition motif 2; *Nuclear localization signal; **C-terminal glycine-rich domain.
Figure 2. Immunoblot analyses showing specificity of TDP-43 antibodies 2G11 and 2H1.
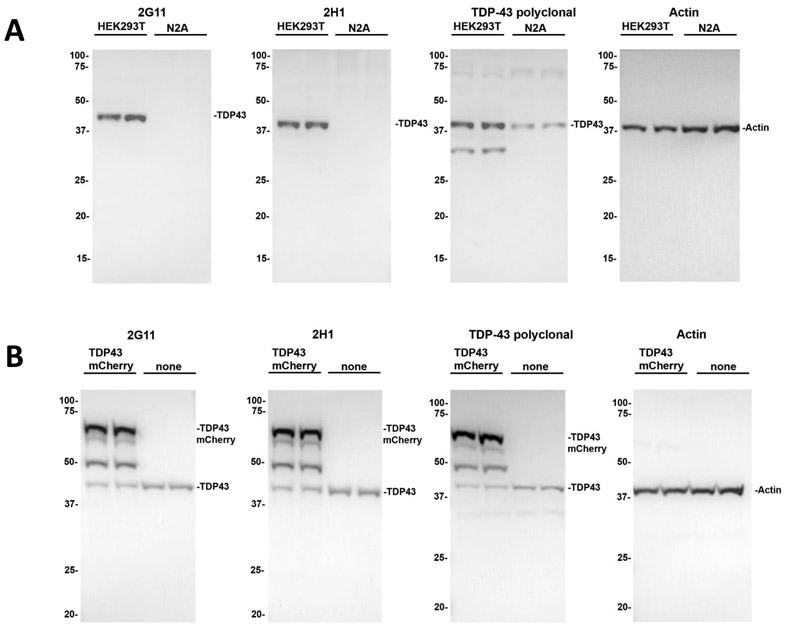
(A): Western blot analyses demonstrating specificity of antibodies 2H1 and 2G11 for endogenous human TDP-43. In human HEK293T cells, both clones revealed specificity for human protein superior to a rabbit-derived polyclonal TDP-43 antibody. In murine Neuro 2A (N2A) cells, they showed no reactivity with endogenous mouse TDP-43. (B): Specificity of 2H1 and 2G11 antibodies for human TDP-43 is further demonstrated by detection of overexpressed mCherry-tagged human TDP-43 in HEK293T cells as described in “Materials and Methods”. The mobilities of molecular mass markers are indicated on the left.
Performance of TDP-43 antibodies in detecting LATE inclusions
In five cases of AD and five cases of LBD, 2G11 and 2H1 detected the diagnostic inclusions of LATE within six cases, including five cases of stage 2 disease (X1, X2, Y2, Y4, Y5), and one case of stage 1 disease (X4). As detected by these novel antibodies, LATE inclusions in the amygdala were intraneuronal, cytoplasmic, and morphologically compact to globular (Fig. 3B, 3C). In stage 2 cases, inclusions in the hippocampal subiculum tended to show morphologic variability, often located in close apposition to non-reactive neurofibrillary tangles (NFT). Despite close approximation of LATE inclusions to NFT, 2G11 and 2H1 showed high specificity for pathologic TDP-43, allowing for reliable discrimination between these two inclusion types (Fig. 3E, 3F). The sensitivity and specificity of these novel antibodies for LATE inclusions was comparable to that of commercial TDP-43, including a paucity of pathologic TDP-43 inclusions in sections of midfrontal neocortex cortex (Fig. 3 H-J). Only a modest increase in cytoplasmic inclusions was noted of commercial total anti-TDP-43 polyclonal antibody, 2G11, and 2H1 after addition of formic acid to antigen retrieval in representative sections (Supplemental Fig. 1). In the amygdala of a case of AD with LATE (Z7), a dilution study was performed with 2H1 and 2G11. At higher concentrations of primary antibody (1:1,000 or 1:2,500, Fig. 4A-B, E-F), both neurons and glia showed nuclear reactivity. At lower concentrations of primary antibody (1:5,000 or 1:10,000, Fig. 4C-D, G-H), only very weak neuronal or glial nuclear reactivity was observed, but with preserved strong labelling within pathological cytoplasmic neuronal inclusions.
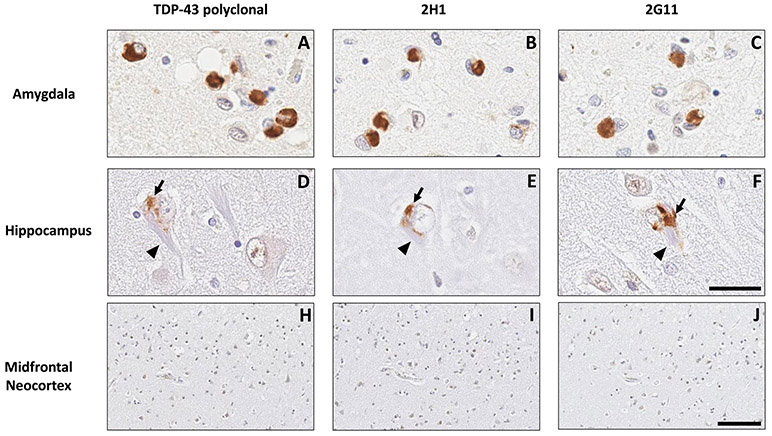
Figure 3. Antibodies 2G11 and 2H1 effectively detect the neuronal cytoplasmic inclusions of LATE-NC.
In a fashion comparable to commercial total anti-TDP-43 antibody , antibodies 2H1 and 2G11 revealed neuronal cytoplasmic inclusions in the amygdala (A-C) and hippocampus (D-F) of a case of stage 2 LATE-NC, with no inclusions identified in midfrontal neocortex (H-J). Similarly to the commercial antibody, these novel antibodies showed a high fidelity for pathologic TDP-43 protein, detecting neuronal cytoplasmic inclusions (arrows) and allowing for their discrimination from closely-apposed neurofibrillary tangles (arrowheads). Scale bars: A-F: 30 um; H-J: 100 um.
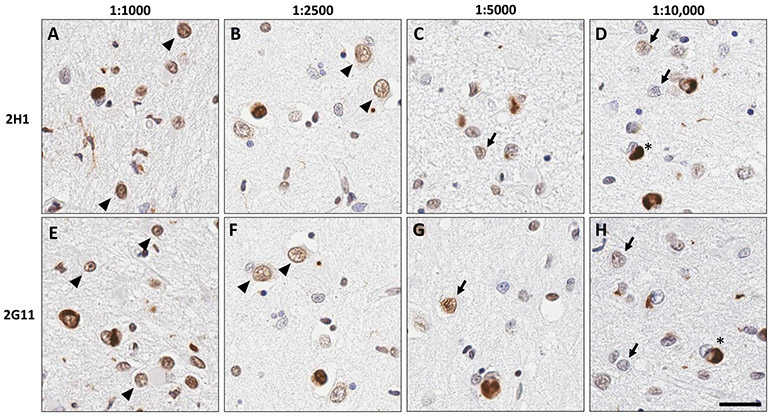
Figure 4. Dilution studies of 2G11 and 2H1 indicate high sensitivity for TDP-43 protein in FFPE tissue.
A dilution series of antibodies 2G11 and 2H1 reveals both antibodies to exhibit a concentration-dependent ability to highlight physiologic nuclear reactivity. On application of primary antibody at the higher concentrations of 1:1000 and 1:2500 (A-B, E-F), nuclear reactivity in neurons and glia (arrowheads) is observed. At lower concentrations of primary antibodies such as 1:5000 and 1:10,000 (C-D, G-H), reduced to minimal nuclear reactivity is noted (arrows), with preserved strong reactivity within aberrant cytoplasmic inclusions (asterisks). Scale bar: 25 um.
Performance of TDP-43 antibodies in detecting FTLD and ALS inclusions
Across cases of FTLD-TDP and ALS (Z1, Z2, Z3, Z4, Z5), novel antibodies 2G11 and 2H1 effectively detected diagnostic TDP-43 cytoplasmic inclusions within sections of medial temporal lobe. Neurons of the hippocampal dentate gyrus demonstrated compact cytoplasmic inclusions across these cases, albeit in varying numbers (Fig. 5A, 5B, 5E, 5F). In addition to scattered dentate gyrus neuronal inclusions, a case of FTLD-TDP type B (Z2) showed neuronal intracytoplasmic inclusions and neuropil threads in the inferior temporal neocortex (Fig. 5C, 5D). In a case of ALS (Z6), commercial anti-TDP-43 polyclonal antibody (Fig. 6A-D) and novel TDP-43 monoclonal antibodies (Fig. 6E-L) were applied to sections of lumbar spinal cord, in which the new antibodies effectively revealed skein-like cytoplasmic inclusions within motor neurons characteristic of this disease.
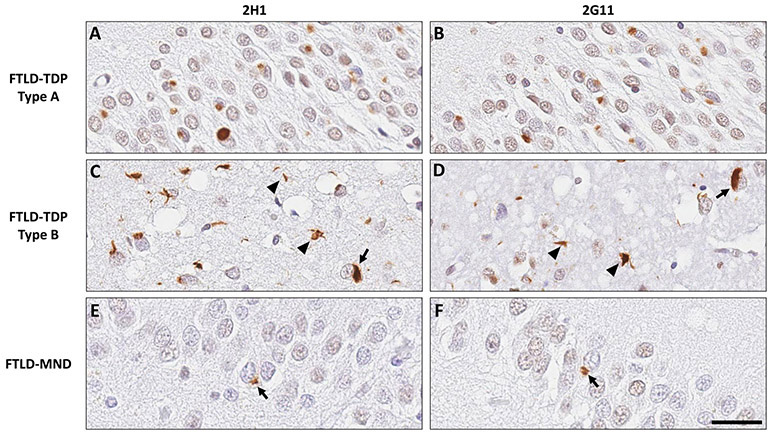
Figure 5. Antibodies 2G11 and 2H1 robustly detect cerebral neuronal TDP-43 cytoplasmic inclusions in FTLD-MND and FTLD-TDP.
In sections of medial temporal lobe, both novel TDP-43 antibodies elucidated abundant cytoplasmic inclusions within neurons of the hippocampal dentate gyrus in a case of FTLD-TDP type A (A-B), neuronal inclusions (arrows) and neuropil threads (arrowheads) in FTLD-TDP type B (C-D), and neuronal cytoplasmic inclusions (arrows) within hippocampal dentate gyrus in FTLD-MND (E-F). Scale bar: 30 um.
Figure 6. Antibodies 2G11 and 2H1 efficaciously detect neuronal cytoplasmic inclusions characteristic of ALS within spinal motor neurons.
Along with commercial total anti-TDP-43 polyclonal antibody (A-D), both 2H1 (E-H) and 2G11 (I-L) are able to detect the cytoplasmic skein-like inclusions within spinal motor neurons representing the classic TDP-43 inclusions of ALS. Scale bar: 25 um.
Discussion
This work describes the generation of 2G11 and 2H1, two novel antibodies for human TDP-43 protein, with subsequent examination of their efficacy as diagnostic tools in TDP-43 proteinopathies. Raised against residues 198-216 of human TDP-43, these antibodies target the TDP-43 RRM2 domain, a highly functional region within TDP-43 involved in nucleic acid binding (Fig. 1A). While TDP-43 shows a high degree (96%) of conservation between human and mouse species, it is notable that residues 198-216 show only 68% concordance between species (Fig. 1B). As such, 2G11 and 2H1 sport an observably high specificity for human TDP-43, as indicated by Western blot characterization. By comparison, the commercial rabbit-derived polyclonal TDP-43 antibody that served as an immunohistochemical benchmark, itself predicated on an N-terminal epitope, exhibited detection of mouse TDP-43 protein within murine neuronal cell lines (Fig. 2A). Other TDP-43 antibodies available include those with specificity for hyperphosphorylated sites in the C-terminal region unique to pathologic forms of the TDP-43 protein, the best-characterized of which occur on amino acids Ser409 and Ser410 (Ser409/410) [21,22]. Amid the field of commercially available antibodies, 2G11 and 2H1 present novel human-specific additions that target a non-hyperphosphorylated epitope, and present an effective profile of immunohistochemical reactivity when applied to formalin-fixed, paraffin-embedded tissue of TDP-43 proteinopathies.
These novel antibodies successfully detect the inclusions of ALS and FTLD-TDP. Both 2G11 and 2H1 were also able to detect the classic skein-like inclusions of ALS found within spinal motor neurons. Additionally, 2G11 and 2H1 were applied to sections of medial temporal lobe across cases of ALS and FTLD-TDP, as this location represents an overlap region in which diagnostic inclusions may be found in both disease types. Both 2G11 and 2H1 reliably detected compact intracytoplasmic inclusions within the neurons of the hippocampal dentate gyrus across cases. Furthermore, in a case of FTLD-TDP type B, both antibodies highlighted globular neuronal cytoplasmic inclusions and dystrophic neurites within the inferior temporal neocortex.
Of equal importance, novel antibodies 2G11 and 2H1 served as effective tools for the diagnosis of LATE. In five cases of AD, these antibodies provided for the identification of LATE-NC in three cases, and in five cases of LBD, elucidated LATE-NC in three cases (Table 1). These findings were concordant with those yielded by the commercial TDP-43 polyclonal antibody, with novel antibodies detecting inclusions in the same areas as the commercial antibody and providing the same LATE stage upon application of working group consensus criteria. From this examination, several points are to be made in favor of these novel antibodies. While many commercial TDP-43 antibodies targeting non-hyperphosphorylated epitopes often yield reactivity within the nuclei of neurons and glia due to detection of physiologic forms of TDP-43, 2G11 and 2H1 exhibited a concentration-dependent ability to preferentially highlight pathologic cytoplasmic inclusions with only minimal nuclear reactivity when used at low concentrations, yielding a staining profile reminiscent to that typically observed of antibodies targeting C-terminal region hyperphosphorylated epitopes, such as pSer409/410. These non-phosphorylation specific antibodies achieved results comparable to hyperphosphorylation-specific formulations likely secondary to their high sensitivity for their epitope, as evidenced by the fact that their immunohistochemical performance occurred most optimally at markedly dilute primary concentrations (1:10,000). At increasing concentrations of primary antibody (1:2,500 to 1:1,000), progressively stronger nuclear reactivity in neurons and glia was noted. Conversely, progressively more dilute concentrations of primary antibody (1:5,000 to 1:10,000) produced consistently reduced to minimal staining of nuclei, nevertheless with retained strong reactivity for the aberrant cytoplasmic inclusions of interest. Interestingly, specificity for the RRM2 domain itself may confer selectivity for pathologic inclusions, as the current findings are in line with results yielded by previous characterization of an RRM2-specific monoclonal TDP-43 antibody [23]. As discussed by Kwong et. al, possible explanations for the apparent selectivity of RRM2-specific antibodies for aberrant cytoplasmic inclusions may relate to RRM2’s ability to self-dimerize into thermally stable dimeric structures [24] for which cytoplasmic inclusions may hypothetically be enriched, or other amyloidogenic differences that RRM2 may adopt between the protein’s aberrant state within cytoplasmic inclusions and its physiologic nuclear state [25]. Ultimately, 2G11 and 2H1 provided for a sharp resolution of pathologic cytoplasmic TDP-43 inclusions without reliance on hyperphosphorylated epitopes.
Of additional merit, 2G11 and 2H1 showed an excellent specificity for TDP-43 protein within the context of neurodegenerative co-morbidity. These novel antibodies allowed for the elegant discrimination of TDP-43 cytoplasmic deposits from the tau NFT of ADNC, despite the often-intimate approximation of these inclusions (Fig. 3). The co-localization of TDP-43 and tau inclusions within the same neuron has been well-documented, with confocal microscopy and double-label immunohistochemistry revealing close apposition between the two inclusion types [26-28]. Indeed, there is growing evidence supporting the concept that TDP-43 and tau proteinopathy share many overlapping genetic and upstream risk factors for initiation and progression [29,30]. Given the common diathesis from which these proteins may potentially arise, and the shared geography they commonly occupy, the availability of antibodies that reliably discriminate between TDP-43 and its kindred proteinopathies will be critical in clarifying disease relationships.
As an additional item of consideration, both 2G11 and 2H1 exhibited the ability to detect a considerable morphologic and topographic heterogeneity of TDP-43 inclusions across TDP-43 proteinopathies. It has been shown that TDP-43 proteinopathy, from ALS and FTLD-TDP to LATE, produces a variety of truncated forms of pathologic protein, including 20-25 kDa species [3]. Immunohistochemistry provides a powerful but kaleidoscopic lens for the evaluation of neurodegenerative protein deposits, as different antibody iterations for the same protein may elucidate apparently differing protein amounts and forms, depending on their given epitope specificity. Previous characterization of a panel of novel antibodies for α-synuclein showed subtle variations in protein deposit number, types, and distributions when studies across cases of MSA [31]. Future work may utilize 2G11 and 2H1 help further characterize topography of disease across TDP-43 proteinopathies.
Conclusion
This work presents two novel mouse-derived monoclonal antibodies for human TDP-43, 2G11 and 2H1. These antibodies are specific for residues 198-216, which comprise a functionally significant epitope situated in the RRM2 domain. On immunohistochemical application to formalin-fixed, paraffin-embedded tissue, these antibodies show excellent sensitivity for human protein manifesting as selectively strong reactivity for aberrant intracytoplasmic protein inclusions at low concentrations of primary antibody, and provide for elegant morphologic discrimination between TDP-43 and tau NFT. These antibodies will aid in diagnostic and research efforts within the context of TDP-43 proteinopathies.
Supplementary Material
Supplemental figure 1. Effect of formic acid antigen retrieval on the performance of 2G11 and 2H1 antibodies in immunohistochemistry. In a similar fashion to commercial total anti-TDP-43 polyclonal antibody (A, D), 2H1 (B, E) and 2G11 (C, F) showed an appreciable but overall modest increase in detectable cytoplasmic inclusions upon application of formic acid to antigen retrieval (D-F) compared just HIER treatment (A-C). HIER: heat-induced epitope retrieval; FA: formic acid. Scale bar: 50 um.
Highlights.
Novel monoclonal antibodies 2G11 and 2H1 targeting TDP-43 residues 198-216 are highly sensitive for the human protein
These antibodies effectively identify TDP-43 inclusions across neurodegenerative diseases including ALS, FTLD-TDP and LATE
2G11 and 2H1 distinctively reveal pathological TDP-43 inclusions
Acknowledgements
These studies were supported by grants NS089622, AG047266 and AG062677 from the National Institutes of Health. ZAS was supported by a fellowship F30AG063446 from the National Institutes of Health. Tissue samples were supplied by the University of Florida Neuromedicine Human Brain and Tissue Bank and the Mayo Clinic Alzheimer's Disease Research Center.
Footnotes
Conflicts of interest: The authors have no duality or conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM-Y, Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis., Science. 314 (2006) 130–3. 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- [2].Ou S-HH, Wu F, Harrich D, García-Martínez LF, Gaynor RB, Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs., J. Virol 69 (1995) 3584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee EB, Lee VM-Y, Trojanowski JQ, Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration, Nat. Rev. Neurosci 13 (2012) 38–50. 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morera AA, Ahmed NS, Schwartz JC, TDP-43 regulates transcription at protein-coding genes and Alu retrotransposons, Biochim. Biophys. Acta - Gene Regul. Mech 1862 (2019) 194434. 10.1016/j.bbagrm.2019.194434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sephton CF, Good SK, Atkin S, Dewey CM, Mayer P, Herz J, Yu G, TDP-43 is a developmentally regulated protein essential for early embryonic development., J. Biol. Chem 285 (2010) 6826–34. 10.1074/jbc.M109.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang H-Y, Wang I-F, Bose J, Shen C-KJ, Structural diversity and functional implications of the eukaryotic TDP gene family, Genomics. 83 (2004) 130–139. 10.1016/S0888-7543(03)00214-3. [DOI] [PubMed] [Google Scholar]
- [7].Nelson PT, Smith CD, Abner EL, Wilfred BJ, Wang W-X, Neltner JH, Baker M, Fardo DW, Kryscio RJ, Scheff SW, Jicha GA, Jellinger KA, Van Eldik LJ, Schmitt FA, Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease, Acta Neuropathol. 126 (2013) 161–177. 10.1007/s00401-013-1154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nelson PT, Trojanowski JQ, Abner EL, Al-Janabi OM, Jicha GA, Schmitt FA, Smith CD, Fardo DW, Wang WX, Kryscio RJ, Neltner JH, Kukull WA, Cykowski MD, Van Eldik LJ, Ighodaro ET, “New old pathologies”: Ad, part, and cerebral age-related TDP-43 with sclerosis (CARTS), J. Neuropathol. Exp. Neurol 75 (2016) 482–498. 10.1093/jnen/nlw033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, Rademakers R, Alafuzoff I, Attems J, Brayne C, Coyle-Gilchrist ITS, Chui HC, Fardo DW, Flanagan ME, Halliday G, Hokkanen SRK, Hunter S, Jicha GA, Katsumata Y, Kawas CH, Keene CD, Kovacs GG, Kukull WA, Levey AI, Makkinejad N, Montine TJ, Murayama S, Murray ME, Nag S, Rissman RA, Seeley WW, Sperling RA, White CL III, Yu L, Schneider JA, Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report, Brain. 142 (2019) 1503–1527. 10.1093/brain/awz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, Petersen RC, Dickson DW, Staging TDP-43 pathology in Alzheimer’s disease, Acta Neuropathol. 127 (2014) 441–450. 10.1007/s00401-013-1211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA, Schneider JA, TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer’s disease, Acta Neuropathol. Commun. 6 (2018) 33. 10.1186/s40478-018-0531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Spires-Jones TL, Attems J, Thal DR, Interactions of pathological proteins in neurodegenerative diseases., Acta Neuropathol. 134 (2017) 187–205. 10.1007/s00401-017-1709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Besser LM, Teylan MA, Nelson PT, Limbic Predominant Age-Related TDP-43 Encephalopathy (LATE): Clinical and Neuropathological Associations, J. Neuropathol. Exp. Neurol 79 (2020) 305–313. 10.1093/jnen/nlz126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Katsumata Y, Fardo DW, Kukull WA, Nelson PT, Dichotomous scoring of TDP-43 proteinopathy from specific brain regions in 27 academic research centers: associations with Alzheimer’s disease and cerebrovascular disease pathologies, Acta Neuropathol. Commun 6 (2018) 142. 10.1186/s40478-018-0641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Strang KH, Goodwin MS, Riffe C, Moore BD, Chakrabarty P, Levites Y, Golde TE, Giasson BI, Generation and characterization of new monoclonal antibodies targeting the PHF1 and AT8 epitopes on human tau, Acta Neuropathol. Commun 5 (2017) 58. 10.1186/s40478-017-0458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Iradi MCG, Triplett JC, Thomas JD, Davila R, Crown AM, Brown H, Lewis J, Swanson MS, Xu G, Rodriguez-Lebron E, Borchelt DR, Characterization of gene regulation and protein interaction networks for Matrin 3 encoding mutations linked to amyotrophic lateral sclerosis and myopathy, Sci. Rep 8 (2018) 4049. 10.1038/s41598-018-21371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, V Vinters H, Hyman BT, National Institute on Aging, Alzheimer’s Association, National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach., Acta Neuropathol. 123 (2012) 1–11. 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McKeith IG, Boeve BF, DIckson DW, Halliday G, Taylor J-PP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K, Diagnosis and management of dementia with Lewy bodies, 2017. Neurology 89 (2017) 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cairns NJ, Bigio EH, Mackenzie IRA, Neumann M, Lee VM-Y, Hatanpaa KJ, White CL, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DMA, Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration, Acta Neuropathol. 114 (2007) 5–22. 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, Kwong L, Lee EB, Elman L, McCluskey L, Fang L, Feldengut S, Ludolph AC, Lee VM-Y, Braak H, Trojanowski JQ, Stages of pTDP-43 pathology in amyotrophic lateral sclerosis, Ann. Neurol 74 (2013) 20–38. 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H, Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis, Ann. Neurol 64 (2008) 60–70. 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Inukai Y, Nonaka T, Arai T, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle FE, Akiyama H, Hisanaga S, Hasegawa M, Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS, FEBS Lett. 582 (2008) 2899–2904. 10.1016/j.febslet.2008.07.027. [DOI] [PubMed] [Google Scholar]
- [23].Kwong LK, Irwin DJ, Walker AK, Xu Y, Riddle DM, Trojanowski JQ, Lee VMY, Novel monoclonal antibodies to normal and pathologically altered human TDP-43 proteins., Acta Neuropathol. Commun 2 (2014) 33. 10.1186/2051-5960-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kuo P-H, Doudeva LG, Wang Y-T, Shen C-KJ, Yuan HS, Structural insights into TDP-43 in nucleic-acid binding and domain interactions, Nucleic Acids Res. 37 (2009) 1799–1808. 10.1093/nar/gkp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Guenther EL, Ge P, Trinh H, Sawaya MR, Cascio D, Boyer DR, Gonen T, Zhou ZH, Eisenberg DS, Atomic-level evidence for packing and positional amyloid polymorphism by segment from TDP-43 RRM2, Nat. Struct. Mol. Biol 25 (2018) 311–319. 10.1038/s41594-018-0045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nelson PT, Abner EL, Patel E, Anderson S, Wilcock DM, Kryscio RJ, Van Eldik LJ, Jicha GA, Gal Z, Nelson RS, Nelson BG, Gal J, Azam MT, Fardo DW, Cykowski MD, The Amygdala as a Locus of Pathologic Misfolding in Neurodegenerative Diseases, J. Neuropathol. Exp. Neurol 77 (2018) 2–20. 10.1093/jnen/nlx099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Kosaka K, Arai H, Concurrence of TDP-43, tau and α-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies, Brain Res. (2007). 284–294. 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- [28].Smith VD, Bachstetter AD, Ighodaro E, Roberts K, Abner EL, Fardo DW, Nelson PT, Overlapping but distinct TDP-43 and tau pathologic patterns in aged hippocampi, Brain Pathol. 28 (2018) 264–273. 10.1111/bpa.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chornenkyy Y, Fardo DW, Nelson PT, Tau and TDP-43 proteinopathies: kindred pathologic cascades and genetic pleiotropy, Lab. Investig 99 (2019) 993–1007. 10.1038/s41374-019-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gu J, Chen F, Iqbal K, Gong C-X, Wang X, Liu F, Transactive response DNA-binding protein 43 (TDP-43) regulates alternative splicing of tau exon 10: Implications for the pathogenesis of tauopathies, J. Biol. Chem 292 (2017) 10600–10612. 10.1074/jbc.M117.783498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dhillon J-KS, Trejo-Lopez JA, Riffe C, McFarland NR, Hiser WM, Giasson BI, Yachnis AT, Dissecting α-synuclein inclusion pathology diversity in multiple system atrophy: implications for the prion-like transmission hypothesis, Lab. Investig 99 (2019) 982–992. 10.1038/s41374-019-0198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. Effect of formic acid antigen retrieval on the performance of 2G11 and 2H1 antibodies in immunohistochemistry. In a similar fashion to commercial total anti-TDP-43 polyclonal antibody (A, D), 2H1 (B, E) and 2G11 (C, F) showed an appreciable but overall modest increase in detectable cytoplasmic inclusions upon application of formic acid to antigen retrieval (D-F) compared just HIER treatment (A-C). HIER: heat-induced epitope retrieval; FA: formic acid. Scale bar: 50 um.