Abstract
Rationale: Considerable morbidity and disease progression in people with cystic fibrosis (CF) result from pulmonary exacerbations (PExs). PEx guidelines note insufficient evidence to recommend for or against the concomitant use of inhaled and intravenous antibiotics.
Objectives: We hypothesize that the addition of inhaled antibiotics for PEx therapy is associated with improvements in lung function and a longer time to next PEx compared with standard intravenous antibiotics alone.
Methods: We performed a retrospective cohort study using the CF Foundation Patient Registry-Pediatric Health Information System linked dataset. People with CF were included if they were hospitalized for PEx between 2006 and 2016 and 6 to 21 years of age. Lung function outcomes were assessed by linear mixed effect modeling and generalized estimating equations. The time to next PEx was assessed by Cox proportional hazards regression. To estimate independent causal effects while accounting for indication bias and other confounders, inverse probabilities of treatment weights were calculated based on covariates believed to influence the likelihood of inhaled antibiotic use during PEx treatment.
Results: A total of 3,253 children and adolescents contributed 9,040 PEx events for analysis. Inhaled antibiotics were used in 23% of PEx events but were not associated with better pre- to post-PEx percent predicted forced expiratory volume in 1 second responses (mean difference, −1.11%; 95% confidence interval [CI], −1.83 to −0.38; P = 0.003), higher odds of returning to lung function baseline (odds ratio, 0.94; 95% CI, 0.82 to 1.07; P = 0.34), or longer time to next PEx (hazard ratio, 1.05; 95% CI, 0.99 to 1.12; P = 0.098).
Conclusions: The addition of inhaled antibiotics to standard intravenous antibiotic PEx treatment was not associated with improved lung function outcomes or a longer time to next PEx.
Keywords: cystic fibrosis, antibiotics, epidemiology, pulmonary exacerbations
Considerable morbidity and disease progression in people with cystic fibrosis (CF) results from pulmonary exacerbations (PExs) (1–3). Treatment for severe PEx events typically consists of the initiation of intravenous antibiotics together with aggressive airway clearance. Although 2009 CF Foundation PEx management guidelines exist, most of the statements are consensus rather than evidence based (4). In particular, evidence is lacking on the concomitant use of inhaled and intravenous antibiotics for PEx treatment, as these guidelines note insufficient evidence exists to recommend for or against this practice.
Chronic inhaled antibiotic treatment of endobronchial Pseudomonas aeruginosa (P. aeruginosa) infection in people with CF is very common (5) and has been associated with fewer PEx events and improved lung function (6–8). In contrast, inhaled antibiotic use during PEx treatment is less common but not inconsequential, occurring in approximately one in four PEx events (9–11). There is an argument to be made that adding topical antibiotic administration to systemic antibiotic administration may increase airway drug levels and overall drug exposure, which could be beneficial when treating less-susceptible bacterial strains. However, mucus plugging and airway obstruction are frequently present during a PEx, which may limit drug delivery of the inhaled antibiotic. In addition, a Cochrane review found only two trials in the 1980s evaluating the use of inhaled antibiotics for PEx treatment and found no differences in clinical outcomes between the inhaled and noninhaled antibiotic groups (12). Additional concerns related to the concomitant administration of inhaled and intravenous antibiotics include the potential to increase the risk of unwanted antibiotic side effects, such as acute kidney injury, ototoxicity, and vestibular toxicity.
Using the CF Foundation Patient Registry (5) (CFFPR)-Pediatric Health Information System (13) (PHIS) (Children’s Hospital Association) linked dataset (14), we studied the effectiveness of the addition of inhaled antibiotics to intravenous antibiotic PEx treatment in children and adolescents with CF. We hypothesize that the addition of inhaled antibiotics to standard intravenous antibiotics during PEx treatment is associated with improved lung function outcomes and a reduced hazard of subsequent PEx requiring intravenous antibiotics compared with intravenous antibiotic treatment alone. Studies rigorously evaluating the role of antibiotics in clinical practice are critical for validating and refining the use of these drugs. Our goal is to better inform clinicians regarding optimal antimicrobial treatment strategies for in-hospital PEx management.
Methods
Study Design
This retrospective cohort study used the CFFPR-PHIS linked dataset that includes data from 2005 to 2016 on 10,660 children and adolescents with CF from 45 large free-standing U.S. children’s hospitals (14). The CFFPR and PHIS databases were linked at the individual level using indirect identifiers in a stepwise, deterministic linkage approach. We sought to determine whether the addition of inhaled antibiotics to standard intravenous antibiotic therapy (defined as the use of two i.v. antipseudomonal antibiotics) for PEx treatment led to 1) a greater change (pre– to post–PEx treatment) in percent predicted forced expiratory volume in 1 second (ppFEV1), 2) a greater proportion of people with CF recovering to 90% or more of baseline ppFEV1 (defined as lung function baseline), and 3) a longer time to next PEx requiring intravenous antibiotics (time to next PEx) compared with intravenous antibiotics alone.
Pre–PEx treatment lung function was defined as the lowest ppFEV1 recorded within 30 days before admission up to the first in-hospital ppFEV1 measurement. Post–PEx treatment lung function was defined as the last in-hospital ppFEV1 measurement or the earliest ppFEV1 measurement recorded after the hospital stay up to day 30, whichever occurred first. The baseline ppFEV1 was the highest ppFEV1 recorded within 6 months before the study PEx. To evaluate for return to lung function baseline, the best ppFEV1 within 3 months after the study PEx was compared with the baseline ppFEV1 (15). The time to next PEx was defined as the time between the study PEx discharge date and the subsequent PEx admission date. In the event that no subsequent PEx occurred, individuals were censored at the last CFFPR encounter date. P. aeruginosa infection was defined using modified Leeds criteria (16) (see online supplement for details).
The CFFPR provided clinical and demographic information, including lung function, respiratory microbiology, and additional covariates, whereas in-hospital medication information (i.e., inhaled antibiotic use), age at admission, and length of stay were found in PHIS. Exact/close match and PHIS-only encounters were included for analysis, whereas CFFPR-only encounters were excluded because in-hospital inhaled antibiotic exposure could not be ascertained (see online supplement for additional details) (17). Additional study definitions are available in the online supplement. This study was approved by the Seattle Children’s Hospital institutional review board (STUDY00000549).
Inclusion and Exclusion Criteria
People with CF were included if they were hospitalized for a PEx at one or more time points between 2006 and 2016, if they were between 6 and 21 years old on hospital discharge, and if on admission, they had a drop in baseline ppFEV1 of 5% or more. Exclusion criteria included Burkholderia cepacia species or non–tuberculous mycobacteria respiratory culture positivity in the 12 months before a study PEx, intensive care unit stay during the study PEx, and PEx events requiring intravenous antibiotics within 3 months preceding the study PEx. PEx events requiring intravenous antibiotics within 3 months preceding the study PEx were excluded to ensure that an individual had an opportunity to return to their lung function baseline before having a study PEx included in the analysis. We also excluded PExs with length of stay less than 5 or more than 21 days. Less than 5 days was selected to remove both PExs in which antibiotics were initiated in-hospital but completed at home (home i.v. antibiotic treatments) and PExs in which dramatic improvement was seen over the first 2 to 3 days likely because of airway clearance rather than antibiotic exposure; PEx events with more than 21 days were excluded to avoid including less representative PEx events. The unit of analysis was the PEx.
Analytic Methods
Cohort baseline demographic and clinical characteristics were summarized using descriptive statistics. Pre- to post-PEx change in ppFEV1 was regressed on inhaled antibiotic exposure (yes/no) using a linear mixed-effect model that contained random intercept terms for study participant and study site. Return to lung function baseline for each study PEx was regressed on inhaled antibiotic exposure using a generalized linear effect model with generalized estimating equations for parameter estimation and specified binary outcome. For each study PEx, the time to next PEx was regressed on inhaled antibiotic exposure using a conditional Cox proportional hazard regression model in which the baseline hazard was stratified by study PEx number, and multiple study PEx events within a patient were accounted for with robust standards errors. A full list of model covariates is available in the online supplement.
Because of concerns for indication bias (i.e., people with CF who received inhaled antibiotics during a PEx would have more severe disease), we used stabilized inverse probability of treatment weighting (IPTW) (model details are available in the online supplement). Models that were IPTW adjusted contained length of stay as a covariate.
Sensitivity analyses are described in the online supplement.
Results
Primary Analyses
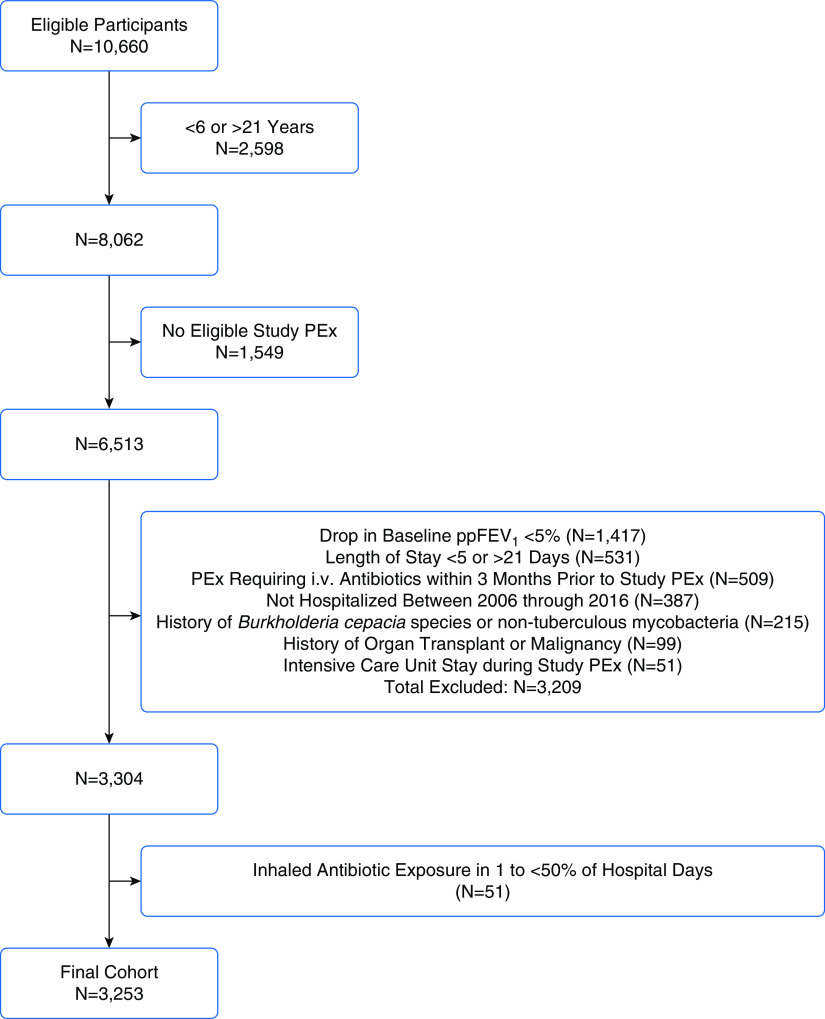
After inclusion/exclusion criteria were applied, 3,253 children and adolescents with 9,040 PEx events were included in the analysis (Figure 1). Among the study PEx events, 2,110 (23%) had inhaled antibiotic exposure in 50% or more hospital days. Tobramycin (83%) was the most commonly prescribed inhaled antibiotic, followed by colistimethate (9%), aztreonam (4%), and amikacin (1%). The proportion of PExs treated with inhaled antibiotics varied widely among the hospitals included in the dataset (mean, 23%; range, 1–70%) (Figure E1 in the online supplement). Median age at admission was 12.8 years (interquartile range [IQR], 9–16.2 years), 55% were female, and 70% had recorded inhaled antibiotic use in the prior 12 months (Table 1). The median length of stay was 12 days (IQR, 9–14 days) and 11 days (IQR, 8–14 days) among PEx with and without inhaled antibiotic use, respectively.
Figure 1.
Flow diagram illustrating cohort selection. i.v. = intravenous; PEx = pulmonary exacerbation; ppFEV1 = percent predicted forced expiratory volume in 1 second.
Table 1.
Cohort characteristics at time of first study PEx
| Variable | Study Participants (N = 3,253) | Inhaled Antibiotic Exposed (n = 678) | Inhaled Antibiotic Nonexposed (n = 2,575) |
|---|---|---|---|
| Demographic variables | |||
| Age, yr, median (IQR) | 12.8 (9.0–16.2) | 13.4 (9.7–16.6) | 12.6 (8.9–16.0) |
| Sex, F, n (%) | 1,774 (55) | 374 (55) | 1,400 (54) |
| Non-Hispanic white, n (%) | 2,535 (78) | 485 (72) | 2,050 (80) |
| Hispanic white, n (%) | 430 (13) | 128 (19) | 302 (12) |
| Other ethnicity, n (%) | 288 (9) | 65 (10) | 223 (9) |
| One class I–III mutation, n (%) | 94 (3) | 17 (3) | 77 (3) |
| Two class I–III mutations, n (%) | 2,647 (81) | 548 (81) | 2,099 (82) |
| Two class IV–V mutations, n (%) | 5 (0) | 0 (0) | 5 (<1) |
| Other genotype, n (%) | 438 (13) | 99 (15) | 339 (13) |
| Missing genotype, n (%) | 69 (2) | 14 (2) | 55 (2) |
| Public insurance, n (%) | 1,929 (59) | 426 (63) | 1,503 (58) |
| Private insurance, n (%) | 1,256 (39) | 233 (34) | 1,023 (40) |
| Other insurance, n (%) | 68 (2) | 19 (3) | 49 (2) |
| Clinical variables | |||
| Baseline ppFEV1*, median (IQR) | 89.0 (74.4–100.8) | 83.8 (69.7–96.3) | 90.2 (75.8–102.1) |
| CF-related diabetes, n (%) | 654 (20) | 166 (24) | 488 (19) |
| Chronic Pa infection†, n (%) | 1,175 (36) | 337 (50) | 838 (33) |
| Intermittent Pa infection‡, n (%) | 620 (19) | 128 (19) | 492 (19) |
| Other/No Pa infection, n (%) | 1,458 (45) | 213 (31) | 1,245 (48) |
| MDR-Pa infection, n (%) | 239 (7) | 80 (12) | 159 (6) |
| MRSA infection, n (%) | 1,224 (38) | 286 (42) | 938 (36) |
| Number of PEx events, n (%) | — | — | — |
| Requiring i.v. antibiotics in prior 12 mo, n (%) | — | — | — |
| — | — | — | |
| 0 | 1,731 (53) | 304 (45) | 1,427 (55) |
| 1 | 971 (30) | 230 (34) | 741 (29) |
| ≥2 | 551 (17) | 144 (21) | 407 (16) |
| Medication, n (%) | |||
| Pancreatic enzymes | 3,152 (97) | 664 (98) | 2,488 (97) |
| Chronic inhaled antibiotic use in prior 12 mo | 2,291 (70) | 613 (90) | 1,678 (65) |
| Azithromycin (during PEx) | 1,480 (45) | 384 (57) | 1,096 (43) |
| Corticosteroid (during PEx) | 824 (25) | 168 (25) | 656 (25) |
| CFTR modulator (during PEx) | 76 (2) | 9 (1) | 67 (3) |
Definition of abbreviations: CF = cystic fibrosis; CFTR = cystic fibrosis transmembrane receptor; i.v. = intravenous; IQR = interquartile range; MDR = multidrug resistant; MRSA = methicillin-resistant Staphylococcus aureus; Pa = Pseudomonas aeruginosa; PEx = pulmonary exacerbation; ppFEV1 = percent predicted forced expiratory volume in 1 second.
Defined as the highest ppFEV1 recorded within 6 months before a study PEx.
Defined as ≥50% of respiratory cultures Pa positive over the prior 12 months.
Defined as at least one but <50% of respiratory cultures Pa positive over the prior 12 months.
The median baseline ppFEV1 was 80.5% (IQR, 65.8–92.4%) before PEx events with inhaled antibiotic exposure and 86.6% (IQR, 71.2–99.3%) before PEx events not treated with an inhaled antibiotic (Figure E2). The median change from baseline to pre–PEx treatment ppFEV1 was −15.1% (IQR, −10.5% to −22.8%) among PEx events treated with inhaled antibiotics compared with −16.3% (IQR, −10.7% to −24.3%) among PEx events not treated with inhaled antibiotics.
The median pre–PEx treatment to post–PEx treatment change in ppFEV1 was 13.8% (IQR, 7.1–21.5%) among PEx events with inhaled antibiotic exposure compared with 13.8% (IQR, 7.2–22.2%) among PEx events without inhaled antibiotic exposure (Figure 2). Among all study PEx events, 76% recovered to 90% or more of lung function baseline within 3 months. Fifty three percent of post-PEx ppFEV1 measurements were obtained on the same day as discharge.
Figure 2.
Pre– to post–pulmonary exacerbation treatment change in percent predicted forced expiratory volume in 1 second by inhaled antibiotic exposure. PEx = pulmonary exacerbation; ppFEV1 = percent predicted forced expiratory volume in 1 second.
Results of regression and proportional hazards models are presented in Table 2. In the unadjusted analysis, inhaled antibiotic exposure during a PEx was not associated with a significant difference in the pre– to post–PEx treatment ppFEV1 (−0.66%; 95% confidence interval [CI], −1.38 to 0.07; P = 0.077) or higher odds of returning to lung function baseline (odds ratio [OR], 1.09; 95% CI, 0.96 to 1.24; P = 0.16) compared with PEx without inhaled antibiotic exposure. After applying IPTW to control for time-dependent confounding by indication, inhaled antibiotic exposure was associated with a significantly lower difference in pre– to post–PEx treatment ppFEV1 (−1.11%; 95% CI, −1.83 to −0.38; P = 0.003) and was not associated with increased odds of returning to lung function baseline (OR, 0.94; 95% CI, 0.82–1.07; P = 0.34). Inhaled antibiotic exposure was not associated with time to next PEx in either the non–propensity-adjusted (hazard ratio [HR], 1.01; 95% CI, 0.95–1.07; P = 0.72) or IPTW-adjusted analyses (HR, 1.05; 95% CI, 0.99–1.12; P = 0.098).
Table 2.
Mean difference in pre– to post–PEx treatment ppFEV1, return to ≥90% of baseline ppFEV1, and time to next PEx requiring i.v. antibiotics (IPTW unadjusted and adjusted) in inhaled antibiotic-exposed patients
| IPTW Unadjusted |
IPTW Adjusted |
|||
|---|---|---|---|---|
| Results | P Value | Results | P Value | |
| Change in ppFEV1, estimate (95% CI) | −0.66% (−1.38 to 0.07) | 0.077 | −1.11% (−1.83 to −0.38) | 0.003 |
| Return to ≥90% of baseline ppFEV1, odds ratio (95% CI) | 1.09 (0.96 to 1.24) | 0.16 | 0.94 (0.82 to 1.07) | 0.34 |
| Time to next PEx hazard ratio (95% CI) | 1.01 (0.95 to 1.07) | 0.72 | 1.05 (0.99 to 1.12) | 0.098 |
Definition of abbreviations: CI = confidence interval; IPTW = inverse probability of treatment weighting; i.v. = intravenous; PEx = pulmonary exacerbation; ppFEV1 = percent predicted forced expiratory volume in 1 second.
Sensitivity Analyses
With respect to different inhaled and intravenous antibiotic combinations, 65% of the study PEx events (5,373) had two intravenous antibiotics and no inhaled antibiotic exposure, whereas 17% (1,375) of the study PEx events had two intravenous antibiotics and one inhaled antibiotic, and 7% (602) had one intravenous antibiotic and one inhaled antibiotic exposure (Table 3). In multivariate models, when compared with the reference group (two i.v. antibiotics and no inhaled antibiotic exposure), two intravenous antibiotics with one inhaled antibiotic was not associated with an increased difference in pre– to post–PEx treatment ppFEV1, higher odds of returning to lung function baseline, or a longer time to next PEx (Table 4). Intravenous β-lactams were used in the clear majority (88%) of PEx events treated with one intravenous antibiotic in combination with one inhaled antibiotic. When compared with the reference group, there was no significance difference in pre– to post–PEx treatment ppFEV1 (−0.30%; 95% CI, −1.45 to 0.86; P = 0.61) or odds of returning to lung function baseline (OR 0.98; 95% CI 0.78–1.22; P = 0.84) associated with exposure to one intravenous and one inhaled antibiotic; however, there was a shorter time to next PEx (HR, 1.11; 95% CI, 1.01–1.23; P = 0.04).
Table 3.
Antipseudomonal inhaled and i.v. antibiotic regimens among study PEx
| Antibiotic | Two i.v. and Zero Inhaled (n = 5,373 PEx [65%]) | Two i.v. and One Inhaled (n = 1,375 PEx [17%]) | One i.v. and One Inhaled (n = 602 PEx [7%]) | One i.v. and Zero Inhaled (n = 961 PEx [12%]) |
|---|---|---|---|---|
| i.v. aminoglycoside* | 5,134 (96) | 1,230 (89) | 41 (7) | 169 (18) |
| i.v. β-lactam† | 5,270 (98) | 1,360 (99) | 527 (88) | 693 (72) |
| i.v. fluoroquinolone‡ | 546 (10) | 240 (17) | 34 (6) | 99 (10) |
| Inhaled tobramycin | N/A | 1,164 (85) | 528 (88) | N/A |
| Inhaled colistin | N/A | 157 (11) | 71 (12) | N/A |
| Inhaled aztreonam | N/A | 76 (6) | 16 (3) | N/A |
| Inhaled amikacin | N/A | 15 (1) | 5 (1) | N/A |
Definition of abbreviations: i.v. = intravenous; N/A = not applicable; PEx = pulmonary exacerbation.
Data are presented as n (%).
i.v. aminoglycosides include amikacin, gentamicin, kanamycin, and tobramycin.
†i.v. β-lactams include piperacillin-tazobactam, ticarcillin-clavulanate, ceftazidime, cefepime, doripenem, imipenem-cilastatin, meropenem, aztreonam, colistin, and polymixin B.
‡i.v. Fluoroquinolones include ciprofloxacin, gatifloxacin, levofloxacin, and moxifloxacin.
Table 4.
IPTW-adjusted mean difference in pre– to post–PEx treatment ppFEV1, return to ≥90% of baseline ppFEV1, and time to next PEx requiring i.v. antibiotics, by number of i.v. and inhaled antipseudomonal antibiotics
| Antibiotic Combination | Change in ppFEV1 Estimate [% (95% CI)] | P Value | Return to ≥90% of Baseline ppFEV1 [Odds Ratio (95% CI)] | P Value | Time to Next PEx [Hazard Ratio (95% CI)] | P Value |
|---|---|---|---|---|---|---|
| Two i.v. and one inhaled | −1.16 (−2.05 to −0.28) | 0.01 | 0.92 (0.79 to 1.08) | 0.30 | 1.04 (0.96 to 1.12) | 0.31 |
| One i.v. and one inhaled | −0.30 (−1.45 to 0.86) | 0.61 | 0.98 (0.78 to 1.22) | 0.84 | 1.11 (1.01 to 1.23) | 0.04 |
| One i.v. and no inhaled | −0.33 (−1.24 to 0.57) | 0.47 | 0.89 (0.75 to 1.06) | 0.20 | 1.08 (0.99 to 1.17) | 0.072 |
Definition of abbreviations: CI = confidence interval; IPTW = inverse probability of treatment weighting; i.v. = intravenous; PEx = pulmonary exacerbation; ppFEV1 = percent predicted forced expiratory volume in 1 second.
Antibiotic category reference (two i.v.; no inhaled).
We were concerned that P. aeruginosa status would predict inhaled antibiotic use for PEx treatment; to more rigorously characterize this relationship, in addition to our primary analysis that used modified Leed’s criteria, we performed a sensitivity analysis (8,662 study PEx among 3,159 children and adolescents) evaluating an alternate P. aeruginosa definition that included an additional P. aeruginosa status (persistent P. aeruginosa, which was defined as 100% of respiratory cultures P. aeruginosa positive in the prior 12 mo) and required three or more respiratory cultures over the prior 12 months. Similar to the primary results, inhaled antibiotic exposure was associated with a mildly reduced difference in pre– to post–PEx treatment ppFEV1 (−1.03%; 95% CI, −1.76 to −0.29; P = 0.006), no increased odds of returning to lung function baseline (OR, 0.96; 95% CI, 0.84–1.09; P = 0.51), or a longer time to next PEx (HR, 1.06; 95% CI, 1.00–1.13]; P = 0.067) (Table E1).
We were concerned that inhaled aztreonam use might be underreported in PHIS because this drug is less likely to be on hospital formularies than other antibiotics. For this reason, to avoid a potential misclassification bias, an additional sensitivity analysis was performed that excluded participants with documented inhaled aztreonam use in the two most recent encounters before a study PEx. Removal of these participants (10% of the noninhaled antibiotic-exposed PEx events) did not appreciably affect any of the analyses (mean difference in pre– to post–PEx treatment ppFEV1, −1.25%; 95% CI, −2.0 to −0.5; P = 0.001), return to lung function baseline (OR, 0.94; 95% CI, 0.83–1.08; P = 0.40), or time to next PEx (HR, 1.1; 95% CI, 0.99–1.23; P = 0.063) (Table E2).
Recognizing that 16% of hospital encounters in the CFFPR-PHIS linked dataset occurred at non-PHIS hospitals, we performed a sensitivity analysis using the addition of CFFPR-only hospital encounters to improve the sensitivity of the time to next PEx analysis. Results from this analysis similarly found that inhaled antibiotic exposure was not associated with a longer time to next PEx (HR, 1.06; 95% CI, 0.99–1.13; P = 0.084).
Discussion
Given the dearth of existing evidence regarding the efficacy of inhaled antibiotics as adjunctive therapy to intravenous antibiotics during inpatient PEx treatment, we took advantage of the recently created CFFPR-PHIS linked dataset to answer this question. Contrary to our hypothesis, we were unable to demonstrate that the addition of inhaled antibiotics to standard two intravenous antibiotic therapy for in-hospital pediatric PEx treatment was associated with either improved lung function outcomes or delayed time to subsequent PEx. A small but statistically significant decrease was found in the mean difference in pre– to post–PEx treatment ppFEV1 for PEx events with inhaled antibiotic use. We were concerned that people with CF who received inhaled antibiotics would differ from people who did not because of disease severity (i.e., sicker people and/or those with P. aeruginosa would be more likely to receive inhaled antibiotics during a PEx); as evidence of this concern, children and adolescents in our cohort who received inhaled antibiotics during a PEx had a lower median baseline ppFEV1 (83.8% vs. 90.2%). For this reason, IPTW was used to minimize indication bias (18). This approach attempts to mimic a randomized controlled trial in that the covariates of interest are distributed equally between exposed and unexposed participants.
Results from our IPTW analyses indicate that inhaled antibiotic use during a PEx was associated with less improvement of ppFEV1 (mean difference in improvement, −1.11%), no increased odds of returning to lung function baseline, and no increased time to next PEx compared with intravenous antibiotic treatment alone. The reduced difference in pre– to post–PEx treatment lung function we identified as associated with inhaled antibiotics may indicate residual indication bias even after covariate adjustment with conventional regression and IPTW analyses. Although statistically significant, it is unlikely to be clinically significant. It is also unlikely that addressing any residual indication bias would reverse this association.
A 2018 Cochrane review sought to determine whether PEx treatment with inhaled antibiotics improves CF-related outcomes (12). Only two trials (both from the 1980s and each with fewer than 100 participants) were found comparing inhaled/intravenous antibiotic use to intravenous antibiotic use alone for PEx treatment. In both studies, no differences were seen between treatment arms with respect to clinical outcomes or lung function from admission to discharge (19, 20); however, P. aeruginosa sputum density (19) and colony counts (20) were lower in the inhaled antibiotic arms compared with the intravenous-alone arms. In both trials, there were no serious adverse events or antibiotic toxicities reported. Our study found similar results, although we were unable to assess for antibiotic toxicities because of CFFPR and PHIS data limitations. One plausible reason for the lack of additional benefit of inhaled antibiotics for acute PEx treatment relates to airway obstruction seen in the airways of acutely ill people with CF. Mucus plugging can cause lung perfusion defects, which have been detected on magnetic resonance imaging in children with PEx events (21), suggesting decreased airway drug exposure in the acute setting. In addition, the presence of mucus plugging and airway obstruction might make it more difficult for inhaled antibiotics to reach infected airways and provide clinical benefit.
In a sensitivity analysis, we created four antibiotic category groups (reference group, two intravenous antibiotics and zero inhaled antibiotics) to determine whether certain combinations of intravenous and inhaled antibiotics would be most beneficial. In IPTW-adjusted models, the use of two intravenous antibiotics and one inhaled antibiotic had a lower difference in pre– to post–PEx treatment ppFEV1 (mean difference, −1.16%) when compared with the reference group, but there were no differences between groups with respect to return to lung function baseline or time to next PEx. Interestingly, a similar change in ppFEV1 was seen between the reference group and the use of one intravenous and one inhaled antibiotic with respect to lung function outcomes. Among PEx treated with one intravenous and one inhaled antibiotic, intravenous β-lactams were most frequently used (88%) in combination with inhaled tobramycin (88%), whereas intravenous aminoglycosides (when only one i.v. antibiotic was used) were only used in 7% of PEx events. These results suggest that clinicians are substituting inhaled tobramycin for intravenous tobramycin, perhaps to avoid ototoxicity or nephrotoxicity from repeat intravenous aminoglycoside exposure with comparable lung function outcomes.
Although our analyses did not illustrate a clinical benefit of inhaled antibiotics for PEx treatment, it is possible specific populations of people with CF (e.g., people with P. aeruginosa that is highly resistant to tobramycin or people with chronic kidney disease) might benefit from the substitution of inhaled for intravenous antibiotics to avoid antibiotic toxicity. Interestingly, current CF Foundation PEx guidelines note that insufficient evidence exists to recommend one versus two intravenous antibiotics to treat P. aeruginosa infection (4), although we previously showed that in 95% of PEx events, clinicians at U.S. pediatric CF centers use two intravenous antibiotics for PEx treatment of P. aeruginosa infection (11). In this current study, 12% of all PEx events were treated with one intravenous antibiotic alone, and no differences were seen in lung function outcomes or time to next PEx in this group when compared with two intravenous antibiotics alone. A 2016 Cochrane review evaluating single versus combination intravenous antipseudomonal antibiotic therapy for people with CF found no differences between therapies with respect to lung function, although the authors ultimately concluded that enough evidence did not exist to adequately compare single therapy with combination therapy (22). In the interest of antimicrobial stewardship, which places emphasis on optimizing antibiotic choice, dose, route of administration, and duration (23), more research is needed to determine the optimal number of intravenous antibiotics to treat PEx events with or without P. aeruginosa infection to not only improve clinical outcomes but also to minimize risks of unwanted antibiotic exposure, including the development of antibiotic resistance, hearing loss, and kidney injury.
Although repeated exposure to intravenous antibiotics (e.g., vancomycin and i.v. aminoglycosides) has clearly been associated with chronic kidney disease, hearing loss, and vestibulotoxicity (24–26), less is known about possible toxicities with repeated inhaled antibiotic exposure for PEx treatment or in combination with intravenous antibiotic use. Maintenance inhaled antibiotics are recommended for the treatment of people with CF and chronic P. aeruginosa infection (27). An early inhaled tobramycin pharmacokinetic study in people with CF showed high airway drug concentrations with relatively low serum concentrations (28), indicating minimal systemic inhaled antibiotic absorption. In clinical practice, however, adverse events, such as increased dyspnea, chest pain, and wheezing, have been reported during and after inhaled antibiotic administration in a minority of people with CF (29). In addition, small studies and case reports have suggested a potential for adverse side effects from inhaled antibiotic use, including acute kidney injury and additional toxicities in the setting of known renal insufficiency, such as the development of toxic serum trough concentrations, sensorineural hearing loss, and vestibulotoxicity (30–34). Our study results, in combination with these reports, provide a possible rationale for limiting the concomitant use of inhaled and intravenous antibiotics for in-hospital PEx treatment.
Strengths of this study include the large number of PEx events available for analysis as well as the inclusion of 45 large, free-standing U.S. children’s hospitals from across the country, increasing generalizability. In addition, results from the primary and sensitivity analyses all consistently fail to illustrate clinical benefits of inhaled antibiotic use for PEx treatment, thus strengthening the overall validity of the study conclusions.
Importantly, this study has several limitations. The most important is the potential for indication bias; although we attempted to minimize this bias using propensity score matching (IPTW), we are unable to account for unmeasured confounders, including the reasons why people with CF were or were not prescribed inhaled antibiotics for PEx treatment or whether people with CF received oral antibiotics before admission. All registry-based studies are prone to misclassification, and it is possible that CFFPR-recorded medication exposures (e.g., previous outpatient inhaled antibiotic use) might be misclassified, although the large cohort size likely limits this effect. In addition, although PHIS data accurately record medications billed during a hospitalization, there is no way to know if the medications were administered; however, a recent study comparing PHIS medication billing data with aggregate electronic medical record data noted an extremely high correlation (r2 = 0.98) (35), suggesting that PHIS medication data accurately reflect medication administration. Our aztreonam-restricted sensitivity analysis illustrated that inhaled aztreonam may be poorly captured in PHIS, but results from this analysis did not differ from the primary analysis conclusions. We excluded home intravenous antibiotic PEx treatment because we could not determine whether inhaled antibiotics were concomitantly prescribed, and we also excluded people with ppFEV1 drops of less than 5%, which might have affected generalizability. Finally, we were not able to include adults in our analyses because the PHIS dataset only includes pediatric hospitals.
In conclusion, this study, which used propensity score matching to address indication bias, found that inhaled antibiotic use for in-hospital pediatric CF PEx treatment was not associated with a higher change in ppFEV1, higher odds of returning to lung function baseline, or a longer time to next PEx. Additional prospective studies are needed not only to further determine whether inhaled antibiotics are beneficial in PEx treatment but also to address additional antibiotic-related PEx concerns, including the use of broad versus narrow spectrum antibiotics and the optimal number of antibiotics needed for PEx treatment.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the Cystic Fibrosis Foundation for the use of Cystic Fibrosis Foundation Patient Registry data to conduct the study. They also thank the patients, care providers, and clinic coordinators at cystic fibrosis centers throughout the United States for their contributions to the Cystic Fibrosis Foundation Patient Registry as well as members of the Cystic Fibrosis Foundation Patient Registry-Pediatric Health Information System working group for their input related to study design.
Footnotes
Supported by the Thrasher Research Fund Early Career Award Program (Principal Investigator: [J.D.C.]); Seattle Children’s Hospital Center for Clinical and Translational Research Clinical Research Scholars Program (Principal Investigator: [J.D.C.]); COGEN18Y7 Cystic Fibrosis Statistical Experience and Network Award from the Cystic Fibrosis Foundation (Principal Investigator: [J.D.C.]).
Author Contributions: J.D.C. conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. A.V.F. and F.O. conceptualized and designed the study, conducted the analyses, and critically reviewed and revised the manuscript. L.R.H., M.P.K., M.N., D.P.N., M.R., D.R.V., and R.L.G. participated in study design and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr. 2006;148:259–264. doi: 10.1016/j.jpeds.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Goss CH, Burns JL. Exacerbations in cystic fibrosis: 1. Epidemiology and pathogenesis. Thorax. 2007;62:360–367. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waters V, Stanojevic S, Atenafu EG, Lu A, Yau Y, Tullis E, et al. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J. 2012;40:61–66. doi: 10.1183/09031936.00159111. [DOI] [PubMed] [Google Scholar]
- 4.Flume PA, Mogayzel PJ, Jr, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, et al. Clinical Practice Guidelines for Pulmonary Therapies Committee. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 5.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The cystic fibrosis foundation patient registry: design and methods of a National Observational Disease Registry. Ann Am Thorac Soc. 2016;13:1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 6.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, et al. Cystic Fibrosis Inhaled Tobramycin Study Group. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 7.Ratjen F, Munck A, Kho P, Angyalosi G ELITE Study Group. Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: the ELITE trial. Thorax. 2010;65:286–291. doi: 10.1136/thx.2009.121657. [DOI] [PubMed] [Google Scholar]
- 8.Taccetti G, Campana S, Festini F, Mascherini M, Döring G. Early eradication therapy against Pseudomonas aeruginosa in cystic fibrosis patients. Eur Respir J. 2005;26:458–461. doi: 10.1183/09031936.05.00009605. [DOI] [PubMed] [Google Scholar]
- 9.Moskowitz SM, Silva SJ, Mayer-Hamblett N, Pasta DJ, Mink DR, Mabie JA, et al. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis (ESCF) Shifting patterns of inhaled antibiotic use in cystic fibrosis. Pediatr Pulmonol. 2008;43:874–881. doi: 10.1002/ppul.20873. [DOI] [PubMed] [Google Scholar]
- 10.Wagener JS, Rasouliyan L, VanDevanter DR, Pasta DJ, Regelmann WE, Morgan WJ, et al. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 2013;48:666–673. doi: 10.1002/ppul.22652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cogen JD, Oron AP, Gibson RL, Hoffman LR, Kronman MP, Ong T, et al. Characterization of inpatient cystic fibrosis pulmonary exacerbations. Pediatrics. 2017;139:e20162642. doi: 10.1542/peds.2016-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith S, Rowbotham NJ, Charbek E. Inhaled antibiotics for pulmonary exacerbations in cystic fibrosis. Cochrane Database Syst Rev. 2018;10:CD008319. doi: 10.1002/14651858.CD008319.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Children’s Hospital Association. Lenexa, KS: Children’s Hospital Association; Pediatric Health Information Systems database (PHIS) Available from: https://www.childrenshospitals.org/Programs-and-Services/Data-Analytics-and-Research/Pediatric-Analytic-Solutions/Pediatric-Health-Information-System. [Google Scholar]
- 14.Cogen JD, Hall M, Loeffler DR, Gove N, Onchiri F, Sawicki GS, et al. Linkage of the CF foundation patient registry with the pediatric health information system database. Pediatr Pulmonol. 2019;54:721–728. doi: 10.1002/ppul.24272. [DOI] [PubMed] [Google Scholar]
- 15.Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182:627–632. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee TWR, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2:29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 17.Cogen JD, Faino AV, Onchiri F, Hall M, Fink AK. Evaluation of hospitalization data for the CFFPR-PHIS linked data set. Pediatr Pulmonol. 2020;55:30–32. doi: 10.1002/ppul.24527. [DOI] [PubMed] [Google Scholar]
- 18.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephens D, Garey N, Isles A, Levison H, Gold R. Efficacy of inhaled tobramycin in the treatment of pulmonary exacerbations in children with cystic fibrosis. Pediatr Infect Dis. 1983;2:209–211. doi: 10.1097/00006454-198305000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Schaad UB, Wedgwood-Krucko J, Suter S, Kraemer R. Efficacy of inhaled amikacin as adjunct to intravenous combination therapy (ceftazidime and amikacin) in cystic fibrosis. J Pediatr. 1987;111:599–605. doi: 10.1016/s0022-3476(87)80130-0. [DOI] [PubMed] [Google Scholar]
- 21.Wielpütz MO, Puderbach M, Kopp-Schneider A, Stahl M, Fritzsching E, Sommerburg O, et al. Magnetic resonance imaging detects changes in structure and perfusion, and response to therapy in early cystic fibrosis lung disease. Am J Respir Crit Care Med. 2014;189:956–965. doi: 10.1164/rccm.201309-1659OC. [DOI] [PubMed] [Google Scholar]
- 22.Elphick HE, Scott A. Single versus combination intravenous anti-pseudomonal antibiotic therapy for people with cystic fibrosis. Cochrane Database Syst Rev. 2016;12:CD002007. doi: 10.1002/14651858.CD002007.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18:638–656. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prayle A, Smyth AR. Aminoglycoside use in cystic fibrosis: therapeutic strategies and toxicity. Curr Opin Pulm Med. 2010;16:604–610. doi: 10.1097/MCP.0b013e32833eebfd. [DOI] [PubMed] [Google Scholar]
- 25.Al-Aloul M, Miller H, Alapati S, Stockton PA, Ledson MJ, Walshaw MJ. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatr Pulmonol. 2005;39:15–20. doi: 10.1002/ppul.20138. [DOI] [PubMed] [Google Scholar]
- 26.Garinis AC, Cross CP, Srikanth P, Carroll K, Feeney MP, Keefe DH, et al. The cumulative effects of intravenous antibiotic treatments on hearing in patients with cystic fibrosis. J Cyst Fibros. 2017;16:401–409. doi: 10.1016/j.jcf.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mogayzel PJ, Jr, Naureckas ET, Robinson KA, Brady C, Guill M, Lahiri T, et al. Cystic Fibrosis Foundation Pulmonary Clinical Practice Guidelines Committee. Cystic fibrosis foundation pulmonary guideline: pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Ann Am Thorac Soc. 2014;11:1640–1650. doi: 10.1513/AnnalsATS.201404-166OC. [DOI] [PubMed] [Google Scholar]
- 28.Geller DE, Pitlick WH, Nardella PA, Tracewell WG, Ramsey BW. Pharmacokinetics and bioavailability of aerosolized tobramycin in cystic fibrosis. Chest. 2002;122:219–226. doi: 10.1378/chest.122.1.219. [DOI] [PubMed] [Google Scholar]
- 29.Barker AF, Couch L, Fiel SB, Gotfried MH, Ilowite J, Meyer KC, et al. Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. Am J Respir. Crit Care Med. 2000;162:481–485. doi: 10.1164/ajrccm.162.2.9910086. [DOI] [PubMed] [Google Scholar]
- 30.Florescu MC, Lyden E, Murphy PJ, Florescu DF, Fillaus J. Long-term effect of chronic intravenous and inhaled nephrotoxic antibiotic treatment on the renal function of patients with cystic fibrosis. Hemodial Int. 2012;16:414–419. doi: 10.1111/j.1542-4758.2012.00675.x. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann IM, Rubin BK, Iskandar SS, Schechter MS, Nagaraj SK, Bitzan MM. Acute renal failure in cystic fibrosis: association with inhaled tobramycin therapy. Pediatr Pulmonol. 2002;34:375–377. doi: 10.1002/ppul.10185. [DOI] [PubMed] [Google Scholar]
- 32.Kahler DA, Schowengerdt KO, Fricker FJ, Mansfield M, Visner GA, Faro A. Toxic serum trough concentrations after administration of nebulized tobramycin. Pharmacotherapy. 2003;23:543–545. doi: 10.1592/phco.23.4.543.32122. [DOI] [PubMed] [Google Scholar]
- 33.Patatanian L. Inhaled tobramycin-associated hearing loss in an adolescent with renal failure. Pediatr Infect Dis J. 2006;25:276–278. doi: 10.1097/01.inf.0000202126.44544.41. [DOI] [PubMed] [Google Scholar]
- 34.Edson RS, Brey RH, McDonald TJ, Terrell CL, McCarthy JT, Thibert JM. Vestibular toxicity due to inhaled tobramycin in a patient with renal insufficiency. Mayo Clin Proc. 2004;79:1185–1191. doi: 10.4065/79.9.1185. [DOI] [PubMed] [Google Scholar]
- 35.Courter JD, Parker SK, Thurm C, Kronman MP, Weissman SJ, Shah SS, et al. Accuracy of administrative data for antimicrobial administration in hospitalized children. J Pediatric Infect Dis Soc. 2018;7:261–263. doi: 10.1093/jpids/pix064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.