Abstract
Recent years have witnessed an emergence of interest in understanding metabolic changes associated with immune responses, termed immunometabolism. As oxygen is central to all aerobic metabolism, hypoxia is now recognized to contribute fundamentally to inflammatory and immune responses. Studies from a number of groups have implicated a prominent role for oxygen metabolism and hypoxia in innate immunity of healthy tissue (physiologic hypoxia) and during active inflammation (inflammatory hypoxia). This inflammatory hypoxia emanates from a combination of recruited inflammatory cells (e.g., neutrophils, eosinophils, and monocytes), high rates of oxidative metabolism, and the activation of multiple oxygen-consuming enzymes during inflammation. These localized shifts toward hypoxia have identified a prominent role for the transcription factor hypoxia-inducible factor (HIF) in the regulation of innate immunity. Such studies have provided new and enlightening insight into our basic understanding of immune mechanisms, and extensions of these findings have identified potential therapeutic targets. In this review, we summarize recent literature around the topic of innate immunity and mucosal hypoxia with a focus on transcriptional responses mediated by HIF.
Keywords: granulocyte, monocyte, macrophage, dendritic cell, epithelia, inflammation, immunometabolism, prolyl hydroxylase
INTRODUCTION
The last decade has witnessed developing recognition of the importance of understanding the interface between metabolism and immune development and function, termed immunometabolism (1). Since O2 is a central component either directly or indirectly to all of tissue oxidative metabolism, it would stand to reason that a clear understanding of cell and tissue responses to the lack of O2 (hypoxia) may provide important insight into immune function. Studies detailing immunometabolism of inflammatory processes have identified profound shifts in tissue metabolism with active inflammation (2). These changes are strongly associated with increases in oxygen consumption and the generation of reactive oxygen intermediates as well as the localized depletion of nutrients. Such responses can result in profound tissue hypoxia (3). Some studies have shown that changes in tissue oxygenation correlate with the active recruitment of innate immune cells such as neutrophils [polymorphonuclear leukocytes (PMNs)], eosinophils, macrophages, and innate lymphoid cells (ILCs) (4). By contrast, the development of adaptive immunity is associated with high rates of local T and B cell proliferation that require different metabolic demands (5, 6). It is therefore important to understand the intersection of metabolism with active immunity in the local tissue environment.
Studies in mucosal tissue (i.e., tissues lined by an epithelium and exposed to the outside world) have provided important insight into the balance between metabolism and productive inflammation. The gastrointestinal tract, for example, is defined by a unique oxygenation profile, where the mucosa experiences fluctuations in blood perfusion at regular intervals (3). Even at baseline, epithelial cells lining the mucosa exist at a relatively low-oxygen-tension environment, previously defined as physiologic hypoxia (7). In older studies examining blood flow in the intestine, countercurrent oxygen exchange in the small intestine showed that oxygen from arterial blood supply along the crypt-villus axis diffuses to adjacent venules and results in graded hypoxia from the lumen to the serosa (8). Migrating leukocytes encounter this steep oxygen gradient during active inflammation (see below).
The transcription factor hypoxia-inducible factor 1α (HIF-1α) was cloned by the Semenza lab in 1993 (9) and is recognized as one of the central regulators of global metabolism in mammals. Original studies, based largely on analysis of the erythropoietin gene, revealed that HIF regulates the expression of target genes that enable adaptation to low-oxygen environments (10-13). Given the substantial shifts in metabolism and oxygen availability during inflammation, a number of studies have shown that stabilization of hypoxia-inducible factor (HIF) in low oxygen triggers the expression of genes that enhance the ability of epithelial cells to function effectively as a barrier (14-17).
With this background, here we review pertinent literature related to HIF signaling in innate immune responses, a timely review given the awarding of the 2019 Nobel Prize in Physiology or Medicine for the discovery of the HIF pathway and its O2-dependent regulation. Given the availability of a number of excellent reviews on the influence of HIF on adaptive immunity (18-20), this review focuses primarily on innate immunity in the mucosa.
WHY INVESTIGATE HYPOXIA IN INNATE IMMUNITY?
Because of its vital role as the final electron acceptor of the electron transport chain during mitochondrial oxidative phosphorylation, a constant and sufficient supply of molecular O2 is essential for the maintenance of cell, tissue, and organism viability in all respiring organisms. However, hypoxia, which arises when O2 demand exceeds supply, is a commonly encountered biological stress and can be associated with a large number of physiologic and pathophysiologic states ranging from ascent to high altitude and rigorous exercise to tissue ischemia and cancer (21). More recently, it has become clear that hypoxia is also a prominent microenvironmental feature during innate immunological activity at sites of inflammation. For example, tissue hypoxia has been documented in the inflamed colon (7, 22), esophagus (23), skin (24), and arthritic joint (25, 26). Furthermore, tissue hypoxia has been documented at sites of inflammation associated with pathogenic infection including Mycobacterium-infected granuloma (27), Leishmania-infected cutaneous wounds (28), and Pseudomonas-infected airways (29). Therefore, a significant amount of evidence now supports the concept that focal hypoxia is a common and prominent microenvironmental feature at sites where the innate immune system is activated and therefore should be considered as a potential microenvironmental influence on disease progression.
WHY ARE INFLAMED SITES HYPOXIC?
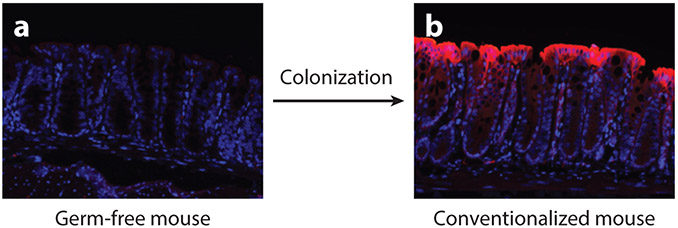
While at first glance the occurrence of hypoxia at sites of inflammation may appear paradoxical during the acute phase of inflammation (e.g., blood flow is known to increase—a cardinal feature of inflammation), in states of chronic inflammation multiple factors can contribute to the occurrence of hypoxia. Firstly, chronic inflammation is often associated with a significant degree of vascular damage and infarction (30). This is likely due to the activation of platelets (and associated thrombosis) and the production of reactive oxygen species, which can block and damage the delicate capillaries responsible for the delivery of blood to surface tissues. Secondly, inflammation is a metabolically expensive process, and levels of oxygen consumption in inflamed tissues is high in order to provide sufficient ATP for cells to support synthesis, processing, and release of the high levels of inflammation-associated proteins such as inflammatory enzymes, cytokines, and antibodies, all of which are highly induced during inflammation. Thirdly, some specialized cells of the innate immune system, most prominently infiltrating neutrophils and eosinophils, consume high levels of oxygen during the oxidative burst (22,23). This is driven by the highly induced activity of neutrophilic NADPH oxidase. Finally, at most body surfaces, including the gastrointestinal tract, lung, and skin, there is an intimate association between the microbiota and the underlying tissue. The low–partial O2 pressure (pO2) environment of the intestine, for example, provides a home for the growth of anaerobes and is likely acquired shortly after birth. Indeed, microbial growth and microbe-derived molecules likely deplete luminal mucosal O2 content during colonization. Studies in germ-free mice have shown that the measurable pO2 (e.g., visualized using pimonidazole adduct staining) in the intestine is significantly higher than in conventionalized mice (31) (Figure 1). Extensions of this work demonstrated that depletion of butyrate-producing microbiota increases mucosal oxygenation allowing for the aerobic expansion of Salmonella (32). Not surprisingly, similar methods (e.g., pimonidazole adduct localization) demonstrated that physiologic hypoxia is not a common feature of the normal healthy lung (33).
Figure 1.
The microbiome is a vital determinant of mucosal oxygen levels in the gastrointestinal tract. Pimonidazole staining (red), which detects areas of severe hypoxia (pO2 < 10 mm Hg) in tissues, demonstrates a lack of physiologic hypoxia in the large intestine of germ-free mice lacking a microbiome (a) compared to conventional mice with an intact microbiome (b).
Thus, cells undergoing immune activity at sites of high innate immune activity and inflammation, be they resident cells or leukocytes that have been recruited from the well-oxygenated circulation, are often subjected to environmental hypoxia. Importantly, it has now been clearly shown in multiple studies that exposure to hypoxia has a profound and complex impact upon immune cell function through the activation of HIF in a highly cell-type-specific manner (reviewed in 34). The impact of hypoxia and mechanisms of HIF stabilization on cells of the innate immune system is described in detail below.
MECHANISMS OF HIF STABILIZATION IN INNATE IMMUNITY
The three α isoforms of HIF (HIF-1α, HIF-2α, and HIF-3α), are Per-ARNT-Sim (PAS) members of the basic helix-loop-helix (bHLH) family of transcription factors and function as central regulators of tissue O2 metabolism (35). The stabilization of the α subunit in the cytoplasm depends on the O2-dependent degradation domain (ODD) and subsequent nuclear localization to form a functional heterodimeric complex with the common β subunit HIF-1β, also called the aryl hydrocarbon receptor nuclear translocator (ARNT) (36). When oxygen supply is sufficient, oxygen- and iron-dependent hydroxylation of two proline motifs within the ODD of the α subunit of HIF initiates von Hippel–Lindau tumor suppressor protein (pVHL)-dependent ubiquitylation and degradation by the proteasome (37). These hydroxylase responses are mediated by one or more of a family of three HIF prolyl hydroxylases (PHDs) (38). These PHDs were discovered in a search of Caenorhabditis elegans genome database for sequences that could encode members of the 2-oxoglutarate-dependent oxygenases (39). This search led to the egl-9 gene. egl-9 mutant worms constitutively expressed the C. elegans ortholog to mammalian HIF-1α, and use of the EGL-9 sequence resulted in the identification of three ubiquitously expressed EGL-9 orthologs in mammals, designated PHD1, PHD2, and PHD3, each of which hydroxylates HIF-α. Each of the PHD enzymes are encoded by different genes, and their expression shows some tissue specificity (38). It is also notable that these PHDs have been shown to hydroxylate substrates other than HIF-α sub-units, including proteins associated with innate immunity, such as NF-κB, MAPK6, FOXO3a, and p53 (40). The hypoxia response is further refined by the asparagine hydroxylase factor inhibiting HIF-1 (FIH-1) on the carboxy terminal transactivation domain of HIF-α, where hypoxia blocks the hydroxylation of Asp80, facilitating the recruitment of CBP/p300 (41).
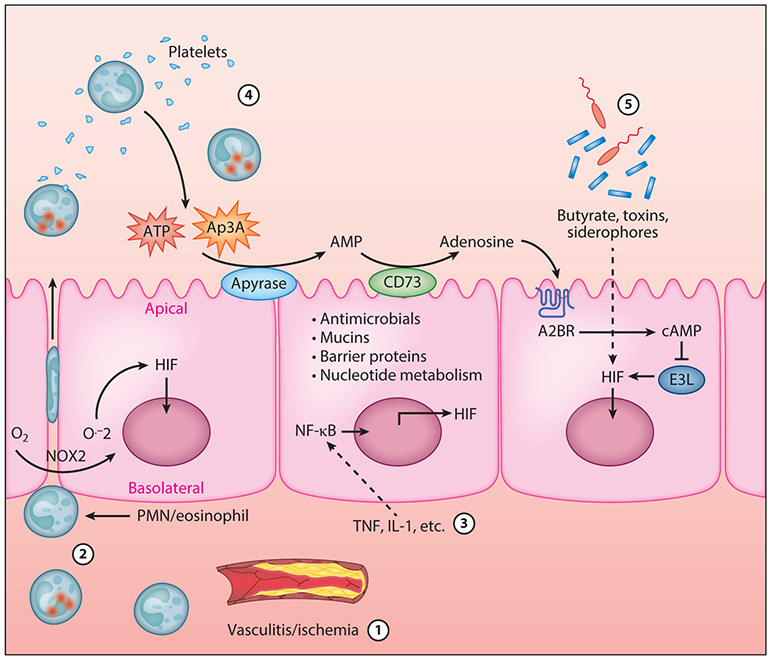
Inflammation-associated HIF stabilization can occur through at least five distinct mechanisms (Figure 2). The most direct and best understood involves diminished blood flow, such as occurs with ischemia and vasculitis (42). Hypoxia can also occur through rapid increases in oxygen consumption. For instance, some studies have shown that during PMN migration across colonic epithelial cells (22), oxygen becomes rapidly depleted within the tissue microenvironment. Such changes in local oxygen were attributable to gene expression changes within the epithelium and the consumption of local O2 by PMNs through the NADPH oxidase complex (NOX-2) (Figure 2). These studies revealed that O2 consumption by activated PMNs resulted in the stabilization of HIF within the epithelium. Utilizing murine models of colitis, it was shown that both the presence of PMNs as well as PMN-elicited hypoxia were necessary for mucosal protection during inflammation. Depletion of PMNs led to exacerbated tissue destruction during colitis (Figure 2).
Figure 2.
Mechanisms of HIF activation in the inflamed intestinal mucosa. HIF activation in the inflamed intestinal mucosa can occur as a result of at least five distinct mechanisms. (①) Decreased oxygen supply caused by vascular damage or ischemia leading to mucosal hypoxia. (②) Increased oxygen demand caused by high levels of oxygen consumption by transmigrating neutrophils and eosinophils leading to mucosal hypoxia. (③) Cytokine-mediated increases in HIF-1α transcription. (④) Extracellular purinergic signaling through PMNs and platelet-derived ATP and Ap3A. (⑤) Increased epithelial oxygen consumption or iron chelation induced by components of the microbiome. Abbreviation: PMN, polymorphonuclear leukocyte.
While most studies have focused on diminished oxygen supply as the key regulator of HIF activity, nonhypoxic HIF stabilization has also been described. For example, inflammatory cytokines such as IL-1, IL-6, and TNF are known to regulate the activity of HIF-1 in both normoxia and hypoxia (43) (Figure 2). Such regulation occurs through a variety of pathways, including increased HIF-1 transcript, protein stabilization, common signaling loops, and increases in HIF-DNA interactions (44, 45).
Adenosine is generated at high levels during inflammation and influences a plethora of immune responses. It is notable that a number of adenosine metabolic target genes are regulated by hypoxia and HIF (46). Recent work has implicated a role for adenosine in the inhibition of degradation and therefore stabilization of HIF-α. Indeed, previous studies had demonstrated a connection between HIF-α degradation and Cullin-2 (Cul-2) protein neddylation (47, 48). Analysis of the neddylation status of Cul-2 after adenosine receptor stimulation revealed that adenosine modulated Cul-2 neddylation and further influenced HIF-α protein stabilization and downstream targets. Regulated protein degradation is an essential part of cell signaling for many adaptive processes. The proteasomal degradation of HIF-α (Figure 2) is but one example of a rapid response by the cell to signal for growth, differentiation, apoptosis, or inflammation. The E3 SCF ubiquitin ligase responsible for HIF-α degradation is comprised of Elongin B/C, Cul-2, and the F-box domain of the vHL protein and is responsible for the polyubiquitination and subsequent degradation of HIF-α (49-51). The COP9 signalsome (CSN) must conjugate the small protein Nedd8 to Cul-2 in order for the E3 SCF to be active, and deneddylated Cul-2 therefore inhibits the ubiquitination of HIF-α (52). One mechanism of deneddylation occurs through the deneddylase-1 (DEN-1, also called SENP8) protein. DEN-1 is a Nedd8-specific protease that has isopeptidase activity capable of directly deneddylating Cullin targets (53, 54).
Finally, as shown in Figure 2, microbe-derived products found within the mucosa can also stabilize HIF through a number of diverse mechanisms. For example, multiple microbial toxins and their iron-binding siderophores (salmochelin, aerobactin, yersiniabactin) cause dose-dependent HIF-1 stabilization in endothelial and epithelial cells (55, 56). Likewise, microbial by-products of fermentation such as short-chain fatty acids can stabilize HIF and induce HIF-target genes through mechanisms that involve β-oxidation of butyrate and increases in oxidative phosphorylation–dependent epithelial O2 consumption (57). These studies have shown that butyrate, but not propionate or acetate, locally depletes O2 content to the extent that HIF is stabilized and transcriptionally active (31, 58). Finally, some studies have shown that the toxin liberated by Clostridium difficile induces epithelial HIF-1 though mechanisms that likely involve both HIF-1 transcription and posttranslational modifications (59).
In summary, a diverse set of mechanisms have been established to stabilize HIF at sites of inflammation and innate immune activity.
HIF ACTIVITY IN INNATE IMMUNE CELLS
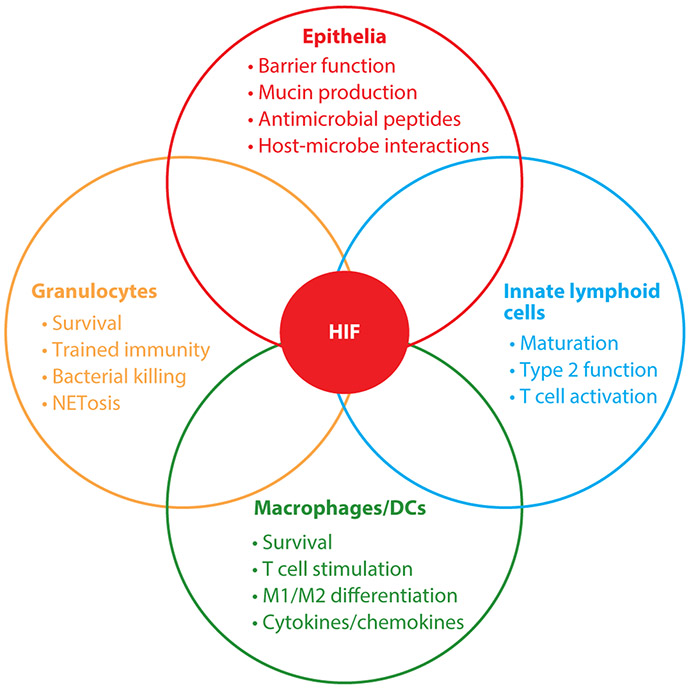
The HIF pathway is evolutionarily conserved across all multicellular organisms (60). Furthermore, the role of HIF in the regulation of immunity also appears to be evolutionarily conserved (61). For example, a role for HIF in regulating immune-related gene expression has been proposed for invertebrate species including insects (Drosophila melanogaster) and nematodes (C. elegans). Exposure of D. melanogaster to hypoxia results in the increased expression of a cohort of genes associated strongly with immunity (62). Similarly, for C. elegans, a number of studies have demonstrated a HIF-dependent protective effect of hypoxia against bacterial infection through the regulation of immune-related genes (63-65). Importantly, neither D. melanogaster nor C. elegans has an adaptive immune system, thereby underscoring the ancient relationship, in evolutionary terms, that exists between the hypoxic response and innate immunity. Individual components of the innate immune system provide specialized roles that function primarily to clear infection and promote inflammatory resolution. As described below, studies using pharmacologic and genetic loss and gain of HIF function implicate a surprisingly variable response in individual cell types (Figure 3).
Figure 3.
HIF mediates cell-type-specific influences on distinct populations of innate immune cells. When exposed to hypoxia, HIF becomes stabilized and activated in cells of the innate immune system. However, the cohorts of HIF-dependent target genes that are activated differ between cell types, which can lead to cell-specific consequences of HIF activation. Abbreviation: DC, dendritic cell.
Granulocytes
Numerous studies have examined the impact of tissue hypoxia and low tissue pO2 on myeloid cell function and the clearance of infections. HIF is essential in support of glycolytic metabolism of phagocytes, but it also regulates critical functions such as antimicrobial peptide (AMP) expression (e.g., serine proteases and cathelicidins) and phagocytosis/killing and enhances the lifespan of PMNs (66). Interestingly, HIF-1 and HIF-2 appear to support opposing roles in eosinophil function, especially chemotaxis (67). A consistent observation has been the differences in which innate and adaptive immune cells procure energy. Cells of myeloid lineages derive energy almost exclusively from glycolysis, whereas lymphocyte subtypes use a combination of glycolysis (especially Th17 cells) and oxidative phosphorylation (especially regulatory T cells) (2, 68). HIF-1 is essential for maintaining metabolic function within both the innate and adaptive immune systems (68).
Original studies from Peyssonnaux et al. (69) and Cramer et al. (70) revealed an important role for HIF-1 in innate immune function of myeloid phagocytes. With the use of conditional deletion approaches in mice, loss of Hif1a in myeloid populations showed a decreased capacity for bacterial killing. Conversely, targeting myeloid VHL (i.e., resulting in stabilized HIF) enhanced acute inflammatory responses. This issue was complicated by the fact that VHL has a number of substrates and may influence these responses indirectly.
Activated PMNs mold the tissue environment in fundamental ways, suggesting that acute inflammation triggers repair processes that involve HIF through direct and indirect mechanisms. As alluded to above, studies in the colon (22), lung (71), and skin (72, 73) have demonstrated that PMN recruitment and function elicit responses within the parenchyma that can be long-lasting and resolving. Similar results were recently shown in a murine model of eosinophilic esophagitis (23). Some studies with myeloid conditional deletion suggest that HIF-1 activation may be detrimental to wound healing (74), though the relative contribution of PMNs or other myeloid cell types remains to be determined.
Neutrophil extracellular traps (NETs) are matrices of chromatin that are studded with AMPs (75) that trap and kill pathogens (76). So-called NETosis is distinct from necrosis and apoptosis and functions as a safety net to control active infection (77). The formation of such NETs requires reactive oxygen species (78), PMN myeloperoxidase, and elastase (79). PMN HIF-1 was recently identified as a central component to the release of NETs (80), where it was shown that both genetic and pharmacologic inhibition of HIF-1α expression attenuated NET deployment. Likewise, inhibition of mTOR and HIF-1α inhibited NET-associated extracellular bacterial killing. It is therefore possible that PMN NETosis provides redundancy for insufficient bacterial killing by innate immune cells in a number of settings.
Most recently, the concept of trained immunity has provided a paradigm shift in our thinking about the functional roles of innate immune cells such as neutrophils, eosinophils, macrophages, and natural killer (NK) cells. Prior work suggested that these cells elicit rapid, nonspecific responses and retain no immune memory (81). In the last decade, multiple studies have revealed that innate immune cells can acquire long-term functional changes through metabolic reprogramming and epigenetic drift (82). Trained immunity has been demonstrated in both NK cells and monocytes; however, PMNs have been largely ignored due to their relatively short life span. It is notable that stabilization of HIF (83), particularly the HIF-2 isoform (84), promotes PMN longevity by delaying apoptosis. In addition, PMN half-life can be extended in tumors and during more chronic inflammation, both of which could result in a more important role for trained immunity in these settings.
Macrophages/Dendritic Cells
Macrophages and dendritic cells (DCs) are central components to innate immunity and provide the bridge to adaptive immunity. Since macrophages and DCs branch from granulocytes through myeloid development, targeting HIF in the myeloid compartment (e.g., LysM-Cre) deletes HIF in both macrophage and DC lineages. It is notable that targeted knockout of HIF-1 in myeloid cells does not impact the development of either PMNs or monocytes within the bone marrow (70).
In the mucosa, macrophages often encounter bacterial by-products such as lipopolysaccharide (LPS). It is now clear that macrophage polarization and function are significantly influenced by the stabilization of HIF-1. Studies dating back to the early 2000s have consistently demonstrated that HIF-1 is central to the polarization of macrophages along the M1 lineage (e.g., LPS stimulation) (85). These studies have provided important insight into immunometabolic regulatory circuits that reveal macrophage M1 polarization toward glycolytic and pentose phosphate metabolism (86). Our understanding of the mechanism(s) behind this important observation is only recently developed. Tannahill et al. (87), using LPS-stimulated macrophages and DCs, revealed that succinate, a TCA intermediate (and by-product of HIF hydroxylation), inhibits PHD enzymes necessary for HIF-α stabilization. While it is clear that succinate accumulates during hypoxia and ischemia, it is not known exactly how succinate inhibits PHD enzymes. By contrast, macrophage M2 polarization (e.g., via IL-4 stimulation) tends to favor oxidative phosphorylation with low to moderate glycolysis (86). Some studies have suggested that these differences result from selective HIF-2 over HIF-1 induction with M2 polarization, thereby limiting the capacity for glycolysis (88).
The functional impact of HIF on DCs is more recently studied than its impact on macrophages. The stabilization of HIF-1 in DCs does not appear to influence their development at baseline (89), though some work has suggested that LPS-stimulated costimulatory molecule expression may be decreased in hypoxia (90). The migratory capacity of DCs is most significantly influenced by hypoxia and HIF-1 stabilization. For example, hypoxia decreases some chemokine receptor expression (91) and slows DC migration to C-C chemokine receptor type 7 (CCR7) ligands (90). Additionally, Flück et al. (92) recently demonstrated that knockout of HIF-1α in the DC compartment results in significant attenuation of regulatory T cell development in a murine model of colitis. Finally, to complement this work, Liu et al. (93) recently showed that the long noncoding RNA lnc-Dpf3 restrains CCR7-mediated migration through a mechanism that involves inhibition of HIF-1α transcription and a loss of glycolytic capacity.
Innate Lymphoid Cells
ILCs are a relatively recently identified population of T cells that provide innate immunity at barrier surfaces. Unlike T cells and B cells, ILCs do not express antigen-specific receptors but rather provide rapid responses to pathogens and the ability to secrete large amounts of cytokines, and it is notable that the development of ILCs shares common transcriptional mechanisms with DCs (94). Our current understanding of ILCs suggests that they represent an arm of the innate immune system that battles intracellular microbes (i.e., type 1 immunity, called group 1 ILCs or ILC1 cells), helminths and allergic environmental molecules (ILC2 cells), and extracellular fungi and bacteria (ILC3 cells) (94). While ample evidence implicates HIF in T and B cell development and function (18-20), less is known about HIF and ILC function. One study showed that conditional deletion of the E3 ligase component VHL resulted in a selective and intrinsic defect in mature ILC2 function and a dysfunctional type 2 immune response (95). Peripheral ILC2 cells were diminished and paralleled loss of the IL-33 receptor ST2. The functional defects were associated with stabilization of HIF-1 and increased glycolysis; all of this could be rectified by inhibition of HIF-1. Additional work along this ILC-HIF axis provides a promising pathway for new insight into innate immunity.
Epithelial Cells
Epithelial cells provide a first line of defense to the outside world. It is more and more appreciated that epithelial cells constitute an integral part of innate immunity (96). In addition to their primary roles in ion transport and the formation of a selective barrier, epithelial cells play an active role in orchestrating immune responses and monitoring host-microbe interactions. The maintenance of a selectively permeable barrier is provided by interactions of organized transmembrane proteins found in domains of the plasma membrane (e.g., tight and adherens junctions) and between the extracellular matrix and the epithelial basement membrane (97). These complex membrane domains define the 3D features of mucosal tissues and establish the classic polarized nature of the plasma membrane (i.e., the so-called fence function of the epithelium) (98). HIF has been shown to contribute fundamentally to the epithelial barrier and the regulation of target genes within these pathways. Other recent reviews have summarized the influence of HIF stabilization on barrier formation and tight junction regulation (99-101). Here we focus on the role of HIF in epithelial innate immunity as it relates to direct epithelial-microbial interactions.
Mucosal tissues are classically defined to include a mucous layer. Mucins that form the mixture of glycoproteins at the epithelial surface are made and secreted by goblet cells. Goblet cells can secrete up to 10 distinct mucins, characterized as either surface-localized or gel-forming mucins (102). Under normal conditions, the mucosa is constituted by a sterile adherent mucous layer and a superficial layer that may be several times the depth of the epithelial layer (103, 104). HIF has been shown to regulate a number of the components that establish and maintain a healthy mucous gel layer. For example, Young et al. (105) revealed that the promoter of the mucin MUC5AC gene harbors evolutionarily conserved regions proximal to the mRNA-coding region that bind functional HIF-1α and SMAD4 binding domains. In the colon, mucin-3 (MUC3) and the mucin-binding protein intestinal trefoil factor (ITF, aka TTF3) are HIF-1α target genes (14, 106) shown to be important for epithelial protection as part of barrier function.
In addition to providing a selective coating to epithelial surfaces, mucous membranes also provide a reservoir for secreted factors, including AMPs (107). For instance, defensins are a family of cysteine-rich AMPs that associate with mucins and contribute to innate immunity through broad antimicrobial activity (108, 109). Human β-defensin-1 (hBD1) is secreted into the lumen by intestinal epithelia in a constitutive manner, whereas other defensin-like AMPs are released only in response to inflammatory mediators (110). Given the low baseline pO2 of the intestine (7), constitutive expression of hBD1 was demonstrated to depend on HIF signaling, where hBD1 expression correlated with other HIF-target genes in human tissue (111). It is also notable that defective expression of hBD1 is associated with mucosal disease such as inflammatory bowel disease (IBD) (112-114), dental infections (115), and Candida infections (116). HIF-1 also regulates AMP expression in keratinocytes (e.g., cathelicidin) (72), where selective deletion of Hif1a in keratinocytes increased susceptibility to group A Streptococcus infections. In this instance, administration of a HIF stabilizer enhanced the bactericidal capacity of keratinocytes (72, 117).
Epithelial autophagy responses are also an important part of mucosal innate immunity, especially in the clearance of bacteria (termed xenophagy) (118). Autophagy is a primitive, highly conserved cellular degradation process that facilitates cell survival during metabolic stress and in starvation (119). HIF-1 has been shown to regulate the expression of genes in the autophagy/xenophagy pathways (120, 121). Variants in a number of genes that regulate autophagy have emerged as risk alleles for diseases such as IBD and include autophagy-related 16-like 1 (ATG16L1) (122, 123) and immunity-related GTPase familyM(IRGM) (124, 125). Murine models have been revealing in this regard, where conditional deletion of Atg16l1 or Atg5 within the intestinal epithelial cell compartment impaired xenophagy and increased dissemination of Salmonella enterica Typhimurium to distal sites (126, 127). Moreover, germ-free mice infected with invasive Enterococcus sp., but not noninvasive Lactobacillus sp., demonstrated selective targeting of these organisms to autophagosomes (126). Thus, these studies provide an essential role for epithelial xenophagy as an innate immune component to pathogen clearance.
INNATE REGULATION OF DISTINCT ANATOMICAL BARRIERS BY HIF
A key aspect of innate immunity is the provision of anatomical barriers at surface tissues to prevent pathogen entry (128). The tissues that line the body’s surfaces, including the skin and oral, pulmonary, and intestinal mucosae are the primary ports of microbial pathogen entry (128). In health, these surface tissues provide an effective and dynamic barrier between the internal and external compartments through the provision of both a physical (structural) and chemical (secreted) barrier. Barrier constituents at different anatomical sites share a number of common features that relate to the provision of innate immunity (96). In the section above we summarize cell-specific HIF responses within the innate immune system. Below, we compare and contrast tissue-specific barrier responses and their contribution to HIF-controlled innate immunity.
Physical Barrier Function
All surface tissues provide physical barriers that prevent the free mixing of extracorporeal pathogens such as parasites, fungi, bacteria, and viruses as well as dead antigenic material into the inner compartments of the body (96, 128). However, different surface tissues provide a physical barrier function in distinct ways. For example, in the small and large intestines, a series of well-defined proteins [including zonula occludins-1 (ZO-1), occludin, E-cadherin, junctional adhesion molecule A (JAM-A) and the claudin family] associate to form physical junctions between the constituent cells of the intestinal epithelium to maintain a tight, simple columnar epithelial barrier (129). While effective in maintaining a physical barrier preventing the movement of luminal antigenic material into the mucosa, the intestinal epithelial barrier is also a dynamic one. This dynamic barrier allows the selective uptake of macromolecules, nutrients, and antigens as well as the bidirectional transport of ions and water in order to maintain fluidic and ionic homeostasis (130). The epithelial barrier found in the upper gastrointestinal tract tissue of the esophagus is structurally different, again comprising a stratified squamous nonkeratinized epithelium that plays a more protective rather than absorptive function (131).
Somewhat in contrast to the gastrointestinal mucosa, the physically stronger barrier provided by the epidermis of the skin is achieved by the presence of a stratified keratinized squamous epithelium consisting of five distinct layers termed the basal layer, the stratum spinosum, the stratum granulosum, the stratum lucidum, and the stratum corneum. This is a much thicker, denser, and consequently less permeable barrier designed to withstand the physical damage associated with day-to-day interactions with the macroenvironment (132, 133). The physical barrier of the epidermis is provided primarily by the stratum corneum as well as the presence of tight junctions between constituent epidermal epithelial cells. Important barrier function proteins in the skin include ZO-1, occludin, JAM-A, claudins, and cingulin as well as the keratin-bundling protein filaggrin (132).
Finally, the alveoli of the lungs present the most delicate of the epithelial barriers comprising predominantly squamous alveolar epithelial cells, which rarely encounter severe physical stress but play a vital role in providing efficient gas exchange between the atmosphere and the internal compartments of the body. The epithelial apical junctional complex is the primary contributor to barrier function in the airway and also consists of ZO proteins as well as occludin, JAM-A, and E-cadherin (134).
A number of studies have demonstrated that HIF plays an important role in the regulation of physical barrier function. In the gastrointestinal tract, HIF plays a key role as a positive regulator of intestinal epithelial barrier function (101, 135). This is likely through mechanisms that include the upregulation of the junctional protein claudin-1 and inhibition of intestinal epithelial cell apoptosis, which thereby enhances barrier function (136, 137). HIF-1-dependent upregulation of claudin-1 has also been shown to be prebarrier in the esophagus (23). HIF has also been demonstrated to be probarrier in airway epithelial cells (138-141). Less is known about the potential role of HIF in the regulation of the physical barrier provided by the skin; however, pharmacologic activation of HIF is protective in a model of atopic dermatitis where epidermal barrier function is compromised (142). In summary, all surface tissues of the body provide physical barrier function and as such are key players in providing innate immunity. However, due to the differing anatomical location and related required functions, the nature of these barriers differs greatly between different anatomical sites at both the cellular and molecular levels.
Chemical Barrier Function
A complementary way in which cells of surface tissues contribute to innate immune barriers is through the secretion of substances that either thwart the entry of pathogens and antigenic material into the body or alternatively contain inherent antimicrobial activity. This is generally referred to as the chemical innate immune barrier (143).
In terms of the intestine, a specialized subtype of epithelial cells termed goblet cells is responsible for the synthesis and release of mucus, which is secreted through the epithelium to act as lubricant layer protecting the epithelial surface against physical damage as the intestinal contents pass through the length of the gastrointestinal tract (144). Mucus is composed of a number of secreted mucin proteins and stabilizing proteins including ITF/TFF3 (145). Mucus also acts as an important addition to the epithelial barrier by producing a viscous layer through which potential pathogens need to transmigrate before having an opportunity to enter the body (144). A number of cells present within the intestinal mucosa including Paneth cells produce AMPs including α-defensins, lysosome C, secretory phospholipase 2, angiogenin 4, lectin, and cathelicidins (146, 147). AMPs are endogenously produced factors that have inherent antimicrobial properties and can also modulate innate immune cell function and therefore contribute to the chemical barrier of the innate immune system. Finally, intestinal epithelial cells possess electrogenic ion transport mechanisms that, while normally net absorptive, can become secretory in cases of infection in what has been termed the enteric tear, which reduces pathogen burden but runs the risk of dehydration and electrolyte loss (148).
In the lung, secreted factors that contribute to innate immunity include surfactant and lung-specific AMPs (147, 149). Surfactant is a lipoprotein complex that is secreted by type II pulmonary epithelial cells. As well as contributing to the ability of pulmonary tissue to stretch during breathing by reducing tissue surface tension, it contributes to innate immunity through the activity of surfactant proteins SP-A and SP-D, which can opsonize bacterial pathogens (149). Surfactant degradation is associated with increased risk of pulmonary infection. Like other surface tissues, pulmonary epithelial cells can also produce AMPs (147).
Secretions from the skin that contribute to innate immunity include the secretion of salt and water in the form of sweat, which creates a tear-like capacity to keep the skin clear of microbial pathogens. The human skin is also a rich source of AMPs including β-defensins and cathelicidins (149).
In summary, similar to the provision of the physical barrier, a number of common and tissue-specific secreted factors including mucins, salt, fluids, and AMPs contribute to the innate immunity provided by the human body’s surface tissues. Interestingly, the integrity and functions of the chemical barrier are also under the influence of the HIF pathway, with a range of studies demonstrating the HIF pathway in the regulation of genes including those responsible for mucus production and stability (MUC3 and ITF, respectively) and AMP production (14, 106, 111, 150).
OXYGEN GRADIENTS ACROSS SURFACE TISSUES
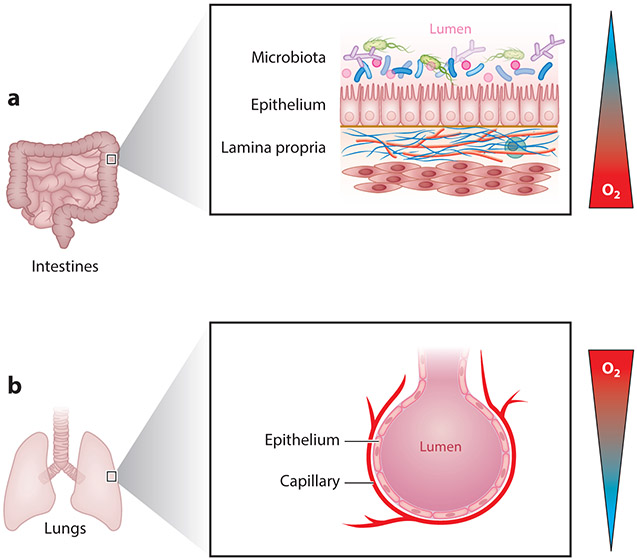
As a result of their anatomical location, different surface tissues vary greatly in terms of the oxygen gradients that they experience in health and disease. Probably the clearest example of this can be observed when comparing the pulmonary and intestinal mucosa (Figure 4). The air we breathe at sea level contains a pO2 of ~145 mm Hg (approximately 21% O2). Measurements in healthy lung alveoli revealed a pO2 of 100–110 mmHg (151). By contrast, the most luminal aspect of the healthy colon is maintained at a pO2 of <10 mm Hg (7, 152). The pO2 decreases along the crypt-villus axis from the intestinal submucosa to the lumen (Figure 1), which is home to trillions of anaerobic microbes. The intestine is supported by a rich underlying mucosal vasculature that supplies the high bioenergetics requirements of the intestinal epithelium as it maintains ion and fluid homeostasis, absorbs nutrients, and samples luminal antigens for oral tolerance. This highly oxygenated capillary bed is juxtaposed with the anoxic lumen of the gut, thereby providing a steep O2 gradient ranging from relatively high O2 levels in the subepithelial compartment to anaerobic conditions in the lumen. In contrast, pulmonary epithelial cells are exposed on the apical surface to a highly oxygenated lumen and a basolateral compartment designed to efficiently remove oxygen from the lung to the bloodstream. The pulmonary mucosa therefore normally experiences a steep oxygen gradient from the lumen to the submucosa, the opposite of that seen in the intestine. The skin experiences a different type of oxygen gradient, as it is exposed to atmospheric oxygen at the surface; however, the outer layer of dead cells is poorly oxygen permeable, and most of the oxygen present in the subdermal compartment is provided for by the mucosal capillary bed.
Figure 4.
Opposing mucosal oxygen gradients exist at distinct anatomical barriers. (a) In the gastrointestinal mucosa, a steep oxygen gradient exists from the well-oxygenated capillary bed to the anoxic lumen of the gut, rendering the epithelium in a state of physiologic hypoxia. (b) By contrast, in the alveolar compartment of the lung, a steep oxygen gradient exists from the highly oxygenated lumen of the airway to the subepithelial compartment, where oxygen is efficiently removed.
Therefore, depending on the anatomical positioning, surface tissues experience widely varying oxygen gradients even in the normal physiologic state. Furthermore, as described above, under conditions where innate immune activity is heavily increased, such as in chronic inflammation, these oxygen gradients can be significantly steeper, leading to cells being exposed to hypoxia with resultant alterations in function. A number of preclinical models have demonstrated that HIF loss-of-function is detrimental in inflammatory disease, implicating a protective role for activation of the HIF pathway in inflammatory disease of surface tissues. Likewise, HIF gain of function using genetic approaches (e.g., deletion of VHL) are protective (7), thus providing a rationale to develop therapies that promote the stabilization and transcriptional function of HIF.
THERAPEUTIC IMPLICATIONS OF HIF IN INNATE IMMUNITY
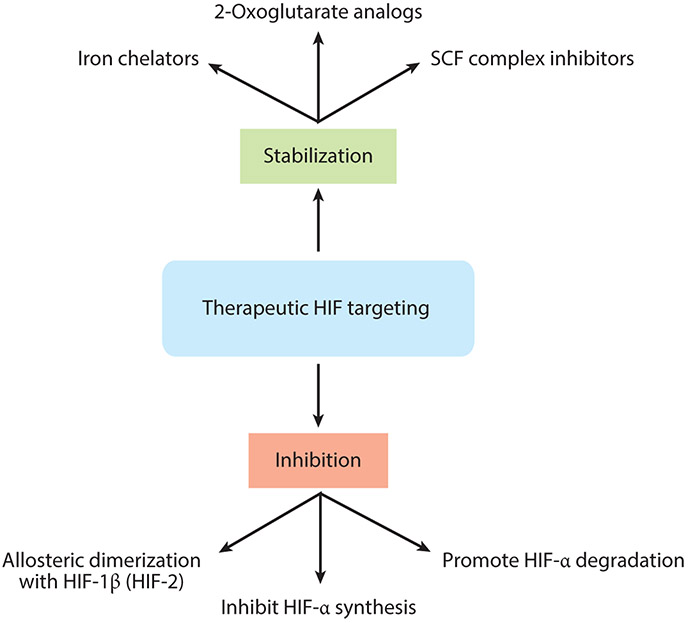
The positive impact of HIF-1α stabilization in restoring acute mucosal inflammatory conditions provides a unique therapeutic opportunity. As discussed above, inflammation-associated stabilization of HIF-1α results in induction of a number of protective molecules with important implications for inflammatory resolution. Thus, pharmacologic intervention directed at activating HIF-1α, stabilizing HIF-1α, or decreasing HIF-1α degradation offers significant potential benefit (Figure 5).
Figure 5.
Therapeutic intervention in the HIF pathway. A range of pharmacologic agents with distinct mechanisms of action have been developed to either inhibit or activate the HIF pathway.
A significant effort has been made to develop pharmacologic activators of HIF through inhibition of PHDs, otherwise called prolyl hydroxylase inhibitors (PHIs). The development of these molecules has focused on iron chelators and 2-oxoglutarate mimetics (Figure 5). These PHIs have undergone human studies in phase 1–3 clinical trials examining their influence on erythropoietin stimulation in patients with chronic renal anemia (reviewed in 153). These PHIs are generally well tolerated, and there are promising results using GSK-1278863 and AKB-6548, which produce an increase in circulating erythropoietin. Targeted delivery of such compounds may provide a therapeutic impact while limiting the potential for untoward systemic effects in healthy organs (142, 154).
It is notable that these molecules have been demonstrated to have a positive impact on the innate immune system. The beneficial aspects of PHIs in the kidney, lung, and liver have been examined in a number of preclinical models (reviewed in 155). Within the gastrointestinal tract, PHIs have been studied in acute models of mouse colitis and ileitis (Table 1). Systemic and local delivery of the nonspecific PHI dimethyloxalylglycine (DMOG), a competitive inhibitor of 2-oxoglutarate, showed positive results on clinical and histological end points in mice. For instance, intraperitoneal administration of DMOG during oral administration of dextran sodium sulfate led to improvement in weight, disease activity, and colitis compared to untreated mice (156). In the trinitrobenzenesulfonic acid (TNBS) model, intraperitoneal injection of the PHI FG-4497 led to increase in erythropoietin as well as attenuation of weight loss, preservation of mucosal architecture and colonic length, and improvement in intestinal permeability compared to untreated mice (157). In the chronic TNF-DARE ileitis mouse model, DMOG reduced the ileal inflammatory score, restored barrier function, and mechanistically reduced Fas-associated cell death domain proteins (158). Likewise, the targeted oral delivery of PHIs led to mucosal healing in TNBS colitis (159). These findings are likely related to the upregulation of barrier-enhancing genes and antiapoptotic responses of epithelial cells as well as alterations in NF-κB-related proinflammatory pathways. Finally, recent studies suggest a clinical impact of PHIs in allergic diseases including atopic dermatitis and eosinophilic esophagitis. In a chemically induced contact dermatitis mouse model, DMOG reduced neutrophil and eosinophil, but not lymphocyte, infiltration (142). Eosinophil infiltration was diminished in a mouse model of eosinophilic esophagitis following systemic treatment with DMOG as well as with transgenic expression of HIF-1α signaling (23).
Table 1.
PHD inhibitors in preclinical models
| PHI | Model | Year | Reference |
|---|---|---|---|
| DMOG | DSS colitis | 2008 | 156 |
| DSS colitis | 2011 | 166 | |
| Radiation injury | 2014 | 167 | |
| TNF-ΔARE ileitis | 2010 | 158 | |
| Clostridium difficile infection | 2010 | 59 | |
| Indomethacin-induced gastritis | 2011 | 168 | |
| Pseudomonas aeruginosa infection | 2013 | 169 | |
| Contact dermatitis | 2019 | 142 | |
| Oral Fusobacterium infection | 2019 | 170 | |
| Eosinophilic esophagitis | 2019 | 23 | |
| Periodontitis | 2018 | 171 | |
| FG-4497 | TNBS colitis | 2008 | 157 |
| EtOH-induced liver disease | 2019 | 172 | |
| AKB-4924 | TNBS colitis | 2014 | 159 |
| TNF-ΔARE ileitis | 2014 | 159 | |
| TRC160334 | TNBS/DSS colitis | 2014 | 173 |
Abbreviations: DMOG, dimethyloxalylglycine; DSS, dextran sodium sulfate; TNBS, trinitrobenzenesulfonic acid.
Pharmacological inhibition of cullin neddylation may be an alternative approach for stabilizing HIF (see Figure 5). The small molecular inhibitor MLN4924, a structural analog of adenosine monophosphate, functions by inhibiting the Nedd8-activating enzyme (NAE), thereby inhibiting the neddylation of cullin proteins necessary for HIF-α degradation (160). As such, MLN4924 stabilizes HIF in a manner similar to PHI. Some studies have suggested that MLN4924 activates HIF-1 and HIF-1 target genes in vitro and attenuates disease severity in a chemically induced colitis model in vivo (161). A pan-cullin inhibitor, such as MLN4924, may not provide the best approach, since higher doses resulted in inhibition of other cullins (e.g., Cul-1 necessary for NF-κB activation) that proved detrimental in murine colitis (162).
Opportunities also exist for the development of selective inhibitors that target HIF pathways. While no selective PHD-1, −2 or −3 inhibitors have come available, Chen et al. (163) identified an approach for the selective inhibition of HIF-2α that resulted in the development of PT2977 (164), currently in clinical trials for renal cell carcinoma. Since some evidence suggests that HIF-2α stabilization can exacerbate inflammation in the mucosa (165), it is intriguing to consider whether PT2977 alone, or in combination with a HIF-1α stabilizer, might prove an interesting approach in the treatment of mucosal disease.
CONCLUSION
In summary, innate immune responses within the mucosa are profoundly influenced by conditions of the local environment. The discrepancies in local pO2 within different mucosal environments determine shifts in tissue and cellular metabolism. Of particular interest in the last decade are metabolic shifts toward hypoxia and associated stabilization of HIF and HIF-target genes that drive innate immune gene expression patterns associated with cell migration, antimicrobial activity, tissue barrier function, wound healing, and autophagy. Studies in vitro and in vivo have provided new insights toward a better understanding of productive inflammatory responses and mechanisms that promote inflammatory resolution. Also relevant is the shift in tissue redox potential that mediates collateral tissue damage and end point organ function. A better understanding of the transcriptional programs, environmental clues, and integrated signaling that determine inflammatory resolution responses will go far to the development of new therapies for mucosal diseases where boosting innate immunity may be beneficial.
Footnotes
DISCLOSURE STATEMENT
C.T.T. is a member of the scientific advisory board of Akebia Therapeutics and has received paid consultancies.
LITERATURE CITED
- 1.Mathis D, Shoelson SE. 2011. Immunometabolism: an emerging frontier. Nat. Rev. Immunol 11:81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kominsky DJ, Campbell EL, Colgan SP. 2010. Metabolic shifts in immunity and inflammation. J. Immunol 184:4062–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colgan SP, Taylor CT. 2010. Hypoxia: an alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol 7:281–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. 1999. Macrophage responses to hypoxia: relevance to disease mechanisms. J. Leukoc. Biol 66:889–900 [DOI] [PubMed] [Google Scholar]
- 5.Fox CJ, Hammerman PS, Thompson CB. 2005. Fuel feeds function: energy metabolism and the T-cell response. Nat. Rev. Immunol 5:844–52 [DOI] [PubMed] [Google Scholar]
- 6.Sitkovsky M, Lukashev D. 2005. Regulation of immune cells by local-tissue oxygen tension: HIF1α and adenosine receptors. Nat. Rev. Immunol 5:712–21 [DOI] [PubMed] [Google Scholar]
- 7.Karhausen J, Furuta GT,Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. 2004. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Investig 114:1098–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepherd AP. 1982. Metabolic control of intestinal oxygenation and blood flow. Fed. Proc 41:2084–89 [PubMed] [Google Scholar]
- 9.Wang GL, Semenza GL. 1993. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. PNAS 90:4304–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semenza GL, Roth PH, Fang HM, Wang GL. 1994. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem 269:23757–63 [PubMed] [Google Scholar]
- 11.Wang GL, Jiang BH, Rue EA, Semenza GL. 1995. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular oxygen tension. PNAS 92:5510–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, et al. 1996. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol 16:4604–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, et al. 1998. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 12:149–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, et al. 2001. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Exp. Med 193:1027–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. 2002. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 62:3387–94 [PubMed] [Google Scholar]
- 16.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, et al. 2002. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Investig 110:993–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, et al. 2003. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J. Ex. Med 198:783–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burrows N, Maxwell PH. 2017. Hypoxia and B cells. Exp. Cell Res 356:197–203 [DOI] [PubMed] [Google Scholar]
- 19.Phan AT, Goldrath AW. 2015. Hypoxia-inducible factors regulate T cell metabolism and function. Mol. Immunol 68:527–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao JH, Barbi J, Pan F. 2015. Hypoxia-inducible factors in T lymphocyte differentiation and function. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol 309:C580–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor CT. 2008. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem. J 409:19–26 [DOI] [PubMed] [Google Scholar]
- 22.Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, et al. 2014. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40:66–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masterson JC, Biette KA, Hammer JA, Nguyen N, Capocelli KE, et al. 2019. Epithelial HIF-1α/claudin-1 axis regulates barrier dysfunction in eosinophilic esophagitis. J. Clin. Investig 130:126744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing D, Liu L, Marti GP, Zhang X, Reinblatt M, et al. 2011. Hypoxia and hypoxia-inducible factor in the burn wound. Wound Repair Regen. 19:205–13. 10.1111/j.1524-475X.2010.00656.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fearon U, Canavan M, Biniecka M, Veale DJ. 2016. Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat. Rev. Rheumatol 12:385–97 [DOI] [PubMed] [Google Scholar]
- 26.Ng CT, Biniecka M, Kennedy A, McCormick J, Fitzgerald O, et al. 2010. Synovial tissue hypoxia and inflammation in vivo. Ann. Rheum. Dis 69:1389–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aly S, Wagner K, Keller C, Malm S, Malzan A, et al. 2006. Oxygen status of lung granulomas in Mycobacterium tuberculosis-infected mice. J. Pathol 210:298–305 [DOI] [PubMed] [Google Scholar]
- 28.Araujo AP, Arrais-Silva WW, Giorgio S. 2012. Infection by Leishmania amazonensis in mice: a potential model for chronic hypoxia. Acta Histochem. 114:797–804 [DOI] [PubMed] [Google Scholar]
- 29.Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, et al. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig 109:317–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakefield AJ, Dhillon AP, Rowles PM, Sawyer AM, Pitilo RM, et al. 1989. Pathogenesis of Crohn’s disease: Multifocal gastrointestinal infarction. Lancet 2:1057–62 [DOI] [PubMed] [Google Scholar]
- 31.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, et al. 2015. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17:662–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivera-Chavez F, Zhang LF, Faber F, Lopez CA, Byndloss MX, et al. 2016. Depletion of butyrate-producing Clostridia from the gut microbiota drives an aerobic luminal expansion of Salmonella. Cell Host Microbe 19:443–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burman A, Kropski JA, Calvi CL, Serezani AP, Pascoalino BD, et al. 2018. Localized hypoxia links ER stress to lung fibrosis through induction of C/EBP homologous protein. JCI Insight. 3:e99543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor CT, Colgan SP. 2017. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat. Rev. Immunol 17:774–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semenza GL. 2011. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb. Symp. Quant. Biol 2011:22–40 [DOI] [PubMed] [Google Scholar]
- 36.Semenza GL. 2001. HIF-1, O2, and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107:1–3 [DOI] [PubMed] [Google Scholar]
- 37.Tanimoto K, Makino Y, Pereira T, Poellinger L. 2000. Mechanism of regulation of the hypoxia-inducible factor-1α by the von Hippel-Lindau tumor suppressor protein. EMBO J. 19:4298–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaelin WG Jr., Ratcliffe PJ. 2008. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30:393–402 [DOI] [PubMed] [Google Scholar]
- 39.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, et al. 2001. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43–54 [DOI] [PubMed] [Google Scholar]
- 40.Strowitzki MJ, Cummins EP, Taylor CT. 2019. Protein hydroxylation by hypoxia-inducible factor (HIF) hydroxylases: unique or ubiquitous? Cells 8:384–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lando D, Peet DJ, Whelan DA, Gorman JJ, Murray LW. 2002. Asparagine hydroxylation of the HIF transactivation domain: a hypoxic switch. Science 295:858–61 [DOI] [PubMed] [Google Scholar]
- 42.Eltzschig HK, Bratton DL, Colgan SP. 2014. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat. Rev. Drug Discov 13:852–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hellwig-Burgel T, Rutkowski K, Metzen E, Fandrey J, Jelkmann W. 1999. Interleukin-1β and tumor necrosis factor-α stimulate DNA binding of hypoxia-inducible factor-1. Blood 94:1561–7 [PubMed] [Google Scholar]
- 44.D’Ignazio L, Batie M, Rocha S. 2017. Hypoxia and inflammation in cancer, focus on HIF and NF-κB. Biomedicines 5:21–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’Ignazio L, Bandarra D, Rocha S. 2016. NF-κB and HIF crosstalk in immune responses. FEBS J. 283:413–24. 10.1111/febs.13578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colgan SP, Eltzschig HK. 2012. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu. Rev. Physiol 74:153–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neish AS, Gewirtz AT, Zeng H, Young AN, Hobert ME, et al. 2000. Prokaryotic regulation of epithelial responses by inhibition of IκB-α ubiquitination. Science 289:1560–63 [DOI] [PubMed] [Google Scholar]
- 48.Read MA, Brownell JE, Gladysheva TB, Hottelet M, Parent LA, et al. 2000. Nedd8 modification of cul-1 activates SCFβTrCP-dependent ubiquitination of IκBα. Mol. Cell. Biol 20:2326–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, et al. 1999. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–75 [DOI] [PubMed] [Google Scholar]
- 50.Wada H, Yeh ET, Kamitani T. 1999. Identification of NEDD8-conjugation site in human cullin-2. Biochem. Biophys. Res. Commun 257:100–5 [DOI] [PubMed] [Google Scholar]
- 51.Liakopoulos D, Busgen T, Brychzy A, Jentsch S, Pause A. 1999. Conjugation of the ubiquitin-like protein NEDD8 to cullin-2 is linked to von Hippel-Lindau tumor suppressor function. PNAS 96:5510–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sufan RI, Ohh M. 2006. Role of the NEDD8 modification of Cul2 in the sequential activation of ECV complex. Neoplasia 8:956–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mendoza HM, Shen LN, Botting C, Lewis A, Chen J, et al. 2003. NEDP1, a highly conserved cysteine protease that deNEDDylates Cullins. J. Biol. Chem 278:25637–3 [DOI] [PubMed] [Google Scholar]
- 54.Wu K, Yamoah K, Dolios G, Gan-Erdene T, Tan P, et al. 2003. DEN1 is a dual function protease capable of processing the C terminus of Nedd8 and deconjugating hyper-neddylated CUL1. J. Biol. Chem 278:28882–91 [DOI] [PubMed] [Google Scholar]
- 55.Hartmann H, Eltzschig HK, Wurz H, Hantke K, Rakin A, et al. 2008. Hypoxia-independent activation of HIF-1 by Enterobacteriaceae and their siderophores. Gastroenterology 134:756–67 [DOI] [PubMed] [Google Scholar]
- 56.Holden VI, Lenio S, Kuick R, Ramakrishnan SK, Shah YM, Bachman MA. 2014. Bacterial siderophores that evade or overwhelm lipocalin 2 induce hypoxia inducible factor 1α and proinflammatory cytokine secretion in cultured respiratory epithelial cells. Infect. Immun 82:3826–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamer KM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. 2008. The role of butyrate on colonic function. Aliment Pharmacol. Ther 27:104–19 [DOI] [PubMed] [Google Scholar]
- 58.Zheng L, Kelly CJ, Battista KD, Schaefer R, Lanis JM, et al. 2017. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of claudin-2. J. Immunol 199:2976–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirota SA, Fines K, Ng J, Traboulsi D, Lee J, et al. 2010. Hypoxia-inducible factor signaling provides protection in Clostridium difficile-induced intestinal injury. Gastroenterology 139:259–69.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor CT, McElwain JC. 2010. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology 25:272–79 [DOI] [PubMed] [Google Scholar]
- 61.Wang L, Cui S, Ma L, Kong L, Geng X. 2015. Current advances in the novel functions of hypoxia-inducible factor and prolyl hydroxylase in invertebrates. Insect Mol. Biol 24:634–48 [DOI] [PubMed] [Google Scholar]
- 62.Zhou D, Haddad GG. 2013. Genetic analysis of hypoxia tolerance and susceptibility in Drosophila and humans. Annu. Rev. Genom. Hum. Genet 14:25–43 [DOI] [PubMed] [Google Scholar]
- 63.Bellier A, Chen CS, Kao CY, Cinar HN, Aroian RV. 2009. Hypoxia and the hypoxic response pathway protect against pore-forming toxins in C. elegans. PLOS Pathog. 5:e1000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luhachack LG, Visvikis O, Wollenberg AC, Lacy-Hulbert A, Stuart LM, Irazoqui JE. 2012. EGL-9 controls C. elegans host defense specificity through prolyl hydroxylation-dependent and -independent HIF-1 pathways. PLOS Pathog. 8:e1002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shao Z, Zhang Y, Ye Q, Saldanha JN, Powell-Coffman JA. 2010. C. elegans SWAN-1 binds to EGL-9 and regulates HIF-1-mediated resistance to the bacterial pathogen Pseudomonas aeruginosa PAO1. PLOS Pathog. 6:e1001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eltzschig HK, Carmeliet P. 2011. Hypoxia and inflammation. N. Engl. J. Med 364:656–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crotty Alexander LE, Akong-Moore K, Feldstein S, Johansson P, Nguyen A, et al. 2013. Myeloid cell HIF-1α regulates asthma airway resistance and eosinophil function. J. Mol. Med 91:637–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palazon A, Goldrath A, Nizet V, Johnson RS. 2014. HIF transcription factors, inflammation, and immunity. Immunity 41:518–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, et al. 2005. HIF-1α expression regulates the bactericidal capacity of phagocytes. J. Clin. Investig 115:1806–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, et al. 2003. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112:645–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zemans RL, Briones N, Campbell M, McClendon J, Young SK, et al. 2011. Neutrophil transmigration triggers repair of the lung epithelium via β-catenin signaling. PNAS 108:15990–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peyssonnaux C, Boutin AT, Zinkernagel AS, Datta V, Nizet V, Johnson RS. 2008. Critical role of HIF-1α in keratinocyte defense against bacterial infection. J. Investig. Dermatol 128:1964–68 [DOI] [PubMed] [Google Scholar]
- 73.Leire E, Olson J, Isaacs H, Nizet V, Hollands A. 2013. Role of hypoxia inducible factor-1 in keratinocyte inflammatory response and neutrophil recruitment. J. Inflamm 10:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Owings RA, Boerma M, Wang J, Berbee M, Laderoute KR, et al. 2009. Selective deficiency of HIF-1α in myeloid cells influences secondary intention wound healing in mouse skin. In Vivo 23:879–84 [PubMed] [Google Scholar]
- 75.Jann NJ, Schmaler M, Kristian SA, Radek KA, Gallo RL, et al. 2009. Neutrophil antimicrobial defense against Staphylococcus aureus is mediated by phagolysosomal but not extracellular trap-associated cathelicidin. J. Leukoc. Biol 86:1159–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, et al. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–35 [DOI] [PubMed] [Google Scholar]
- 77.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. 2011. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 18:581–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, et al. 2007. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol 176:23l–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. 2010. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol 191:677–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McInturff AM, Cody MJ, Elliott EA, Glenn JW, Rowley JW, et al. 2012. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 α. Blood 120:3118–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Netea MG, Quintin J, van der Meer JW. 2011. Trained immunity: a memory for innate host defense. Cell Host Microbe 9:355–61 [DOI] [PubMed] [Google Scholar]
- 82.Mulder WJM, Ochando J, Joosten LAB, Fayad ZA, Netea MG. 2019. Therapeutic targeting of trained immunity. Nat. Rev. Drug Discov 18:553–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walmsley SR, Chilvers ER, Thompson AA, Vaughan K, Marriott HM, et al. 2011. Prolyl hydroxylase 3 (PHD3) is essential for hypoxic regulation of neutrophilic inflammation in humans and mice. J. Clin. Investig 121:1053–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thompson AA, Elks PM, Marriott HM, Eamsamarng S, Higgins KR, et al. 2014. Hypoxia-inducible factor 2α regulates key neutrophil functions in humans, mice, and zebrafish. Blood 123:366–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Domblides C, Lartigue L, Faustin B. 2018. Metabolic stress in the immune function of T cells, macrophages and dendritic cells. Cells 7(7):e7070068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haschemi A, Kosma P, Gille L, Evans CR, Burant CF, et al. 2012. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 15:813–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, et al. 2013. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496:238–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takeda N, O’Dea EL, Doedens A, Kim JW, Weidemann A, et al. 2010. Differential activation and antagonistic function of HIF-α isoforms in macrophages are essential for NO homeostasis. Genes Dev. 24:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elia AR, Cappello P, Puppo M, Fraone T, Vanni C, et al. 2008. Human dendritic cells differentiated in hypoxia down-modulate antigen uptake and change their chemokine expression profile. J. Leukoc. Biol 84:1472–82 [DOI] [PubMed] [Google Scholar]
- 90.Mancino A, Schioppa T, Larghi P, Pasqualini F, Nebuloni M, et al. 2008. Divergent effects of hypoxia on dendritic cell functions. Blood 112:3723–34 [DOI] [PubMed] [Google Scholar]
- 91.Ricciardi A, Elia AR, Cappello P, Puppo M, Vanni C, et al. 2008. Transcriptome of hypoxic immature dendritic cells: modulation of chemokine/receptor expression. Mol. Cancer Res 6:175–85 [DOI] [PubMed] [Google Scholar]
- 92.Flück K, Breves G, Fandrey J, Winning S. 2016. Hypoxia-inducible factor 1 in dendritic cells is crucial for the activation of protective regulatory T cells in murine colitis. Mucosal Immunol. 9:379–90 [DOI] [PubMed] [Google Scholar]
- 93.Liu J, Zhang X, Chen K, Cheng Y, Liu S, et al. 2019. CCR7 chemokine receptor-inducible lnc-Dpf3 restrains dendritic cell migration by inhibiting HIF-1α-mediated glycolysis. Immunity 50:600–15 [DOI] [PubMed] [Google Scholar]
- 94.Bagadia P, Huang X, Liu T, Murphy KM. 2019. Shared Transcriptional Control of Innate Lymphoid Cell and Dendritic Cell Development. Annu. Rev. Cell Dev. Biol 15:16–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Q, Li D, Zhang X, Wan Q, Zhang W, et al. 2018. E3 ligase VHL promotes group 2 innate lymphoid cell maturation and function via glycolysis inhibition and induction of interleukin-33 receptor. Immunity 48:258–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Allaire JM, Crowley SM, Law HT, Chang SY, Ko HJ, Vallance BA. 2018. The Intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 39:677–96 [DOI] [PubMed] [Google Scholar]
- 97.Koch S, Nusrat A. 2012. The life and death of epithelia during inflammation: lessons learned from the gut. Annu. Rev. Pathol 7:35–60 [DOI] [PubMed] [Google Scholar]
- 98.Diamond JM. 1977. Twenty-first Bowditch lecture. The epithelial junction: bridge, gate, and fence. Physiologist 20:8–10 [PubMed] [Google Scholar]
- 99.Cummins EP, Crean D. 2017. Hypoxia and inflammatory bowel disease. Microbes Infect. 19:210–21 [DOI] [PubMed] [Google Scholar]
- 100.Flück K, Fandrey J. 2016. Oxygen sensing in intestinal mucosal inflammation. Pflugers Arch. 468:77–84 [DOI] [PubMed] [Google Scholar]
- 101.Glover LE, Colgan SP. 2017. Epithelial barrier regulation by hypoxia-inducible factor. Ann. Am. Thorac. Soc 14:S233–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. 2008. Mucins in the mucosal barrier to infection. Mucosal Immunol. 1:183–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Atuma C, Strugala V, Allen A, Holm L. 2001. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol 280:G922–29 [DOI] [PubMed] [Google Scholar]
- 104.Strugala V, Allen A, Dettmar PW, Pearson JP. 2003. Colonic mucin: methods of measuring mucus thickness. Proc. Nutr. Soc 62:237–43 [DOI] [PubMed] [Google Scholar]
- 105.Young HW, Williams OW, Chandra D, Bellinghausen LK, Perez G, et al. 2007. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am. J. Respir. Cell Mol. Biol 37:273–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. 2006. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J. Cell Biochem 99:1616–27 [DOI] [PubMed] [Google Scholar]
- 107.Antoni L, Nuding S, Weller D, Gersemann M, Ott G, et al. 2013. Human colonic mucus is a reservoir for antimicrobial peptides. J. Crohns Colitis 7:e652–64 [DOI] [PubMed] [Google Scholar]
- 108.Ganz T 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol 3:710–20 [DOI] [PubMed] [Google Scholar]
- 109.Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. 2006. Human β-defensins. Cell Mol. Life Sci 63:1294–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zheng L, Kelly CJ, Colgan SP. 2015. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol 309:C350–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kelly CJ, Glover LE, Campbell EL, Kominsky DJ, Ehrentraut SF, et al. 2013. Fundamental role for HIF-1α in constitutive expression of human β defensin-1. Mucosal Immunol. 6:1110–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peyrin-Biroulet L, Beisner J, Wang G, Nuding S, Oommen ST, et al. 2010. Peroxisome proliferator-activated receptor gamma activation is required for maintenance of innate antimicrobial immunity in the colon. PNAS 107:8772–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kocsis AK, Lakatos PL, Somogyvari F, Fuszek P, Papp J, et al. 2008. Association of beta-defensin 1 single nucleotide polymorphisms with Crohn’s disease. Scand.J. Gastroenterol 43:299–307 [DOI] [PubMed] [Google Scholar]
- 114.Wehkamp J, Harder J, Weichenthal M, Mueller O, Herrlinger KR, et al. 2003. Inducible and constitutive beta-defensins are differentially expressed in Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis 9:215–23 [DOI] [PubMed] [Google Scholar]
- 115.Schaefer AS, Richter GM, Nothnagel M, Laine ML, Ruhling A, et al. 2010. A 3′ UTR transition within DEFB1 is associated with chronic and aggressive periodontitis. Genes Immun. 11:45–54 [DOI] [PubMed] [Google Scholar]
- 116.Jurevic RJ, Bai M, Chadwick RB, White TC, Dale BA. 2003. Single-nucleotide polymorphisms (SNPs) in human β-defensin 1: high-throughput SNP assays and association with Candida carriage in type I diabetics and nondiabetic controls. J. Clin. Microbiol 41:90–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Okumura CY, Hollands A, Tran DN, Olson J, Dahesh S, et al. 2012. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J. Mol. Med 90:1079–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Levine B 2005. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell 120:159–62 [DOI] [PubMed] [Google Scholar]
- 119.Levine B, Mizushima N, Virgin HW. 2011. Autophagy in immunity and inflammation. Nature 469:323–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, et al. 2009. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell. Biol 29:2570–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Majmundar AJ, Wong WJ, Simon MC. 2010. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40:294–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, et al. 2007. A genome-wide association scan of non-synonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet 39:207–11 [DOI] [PubMed] [Google Scholar]
- 123.Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, et al. 2007. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat. Genet 39:596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McCarroll SA, Huett A, Kuballa P, Chilewski SD, Landry A, et al. 2008. Deletion polymorphism upstream of IRGM associated with altered IRGM expression and Crohn’s disease. Nat. Genet 40:1107–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, et al. 2007. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat. Genet 39:830–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Benjamin JL, Sumpter R Jr., Levine B, Hooper LV. 2013. Intestinal epithelial autophagy is essential for host defense against invasive bacteria. Cell Host Microbe 13:723–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Conway KL, Kuballa P, Song JH, Patel KK, Castoreno AB, et al. 2013. Atg16l1 is required for autophagy in intestinal epithelial cells and protection of mice from Salmonella infection. Gastroenterology 145:1347–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brazil JC, Quiros M, Nusrat A, Parkos CA. 2019. Innate immune cell-epithelial crosstalk during wound repair. J. Clin. Investig 130:124618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Buckley A, Turner JR. 2018. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb. Perspect. Biol 10:a029314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Suzuki T 2013. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol. Life Sci 70:631–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blevins CH, Iyer PG, Vela MF, Katzka DA. 2018. The esophageal epithelial barrier in health and disease. Clin. Gastroenterol. Hepatol 16:608–17 [DOI] [PubMed] [Google Scholar]
- 132.Basler K, Bergmann S, Heisig M, Naegel A, Zorn-Kruppa M, Brandner JM. 2016. The role of tight junctions in skin barrier function and dermal absorption. J. Control. Release 242:105–18 [DOI] [PubMed] [Google Scholar]
- 133.Madison KC. 2003. Barrier function of the skin: “la raison d’être” of the epidermis. J. Invest. Dermatol 121:231–41 [DOI] [PubMed] [Google Scholar]
- 134.Georas SN, Rezaee F 2014. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J. Allergy Clin. Immunol 134:509–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Manresa MC, Taylor CT. 2017. Hypoxia inducible factor (HIF) hydroxylases as regulators of intestinal epithelial barrier function. Cell Mol. Gastroenterol. Hepatol 3:303–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saeedi BJ, Kao DJ, Kitzenberg DA, Dobrinskikh E, Schwisow KD, et al. 2015. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol. Biol. Cell 26:2252–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, et al. 2010. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology 2010:30. [DOI] [PubMed] [Google Scholar]
- 138.Jiang X, Tian W, Tu AB, Pasupneti S, Shuffle E, et al. 2019. Endothelial hypoxia-inducible factor-2α is required for the maintenance of airway microvasculature. Circulation 139:502–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McClendon J, Jansing NL, Redente EF, Gandjeva A, Ito Y, et al. 2017. Hypoxia-inducible factor 1α signaling promotes repair of the alveolar epithelium after acute lung injury. Am. J. Pathol 187:1772–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Olson N, Hristova M, Heintz NH, Lounsbury KM, van der Vliet A. 2011. Activation of hypoxia-inducible factor-1 protects airway epithelium against oxidant-induced barrier dysfunction. Am. J. Physiol. Lung. Cell Mol. Physiol 301:L993–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tojo K, Tamada N, Nagamine Y, Yazawa T, Ota S, Goto T. 2018. Enhancement of glycolysis by inhibition of oxygen-sensing prolyl hydroxylases protects alveolar epithelial cells from acute lung injury. FASEB J. 32:2258–68 [DOI] [PubMed] [Google Scholar]
- 142.Manresa MC, Smith L, Casals-Diaz L, Fagundes RR, Brown E, et al. 2019. Pharmacologic inhibition of hypoxia-inducible factor (HIF)-hydroxylases ameliorates allergic contact dermatitis. Allergy 74:753–66 [DOI] [PubMed] [Google Scholar]
- 143.Riera Romo M, Perez-Martinez D, Castillo Ferrer C. 2016. Innate immunity in vertebrates: an overview. Immunology 148:125–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Birchenough GM, Johansson ME, Gustafsson JK, Bergstrom JH, Hansson GC. 2015. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 8:712–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sands BE, Podolsky DK. 1996. The trefoil peptide family. Annu. Rev. Physiol 58:253–73 [DOI] [PubMed] [Google Scholar]
- 146.Ostaff MJ, Stange EF, Wehkamp J. 2013. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol. Med 5:1465–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang LJ, Gallo RL. 2016. Antimicrobial peptides. Curr Biol. 26:R14–19 [DOI] [PubMed] [Google Scholar]
- 148.Viswanathan VK, Hodges K, Hecht G. 2009. Enteric infection meets intestinal function: how bacterial pathogens cause diarrhoea. Nat. Rev. Microbiol 7:110–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Han S, Mallampalli RK. 2015. The role of surfactant in lung disease and host defense against pulmonary infections. Ann. Am. Thorac. Soc 12:765–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sakamoto K, Hashimoto N, Kondoh Y, Imaizumi K, Aoyama D, et al. 2012. Differential modulation of surfactant protein D under acute and persistent hypoxia in acute lung injury. Am. J. Physiol. Lung. Cell Mol. Physiol 303:L43–53 [DOI] [PubMed] [Google Scholar]
- 151.Schaible B, Schaffer K, Taylor CT. 2010. Hypoxia, innate immunity and infection in the lung. Respir. Physiol. Neurobiol 174:235–43. 10.1016/j.resp.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 152.Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, et al. 2014. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 18:1055–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Haase VH. 2017. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial. Int 21:S110–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tambuwala MM, Manresa MC, Cummins EP, Aversa V, Coulter IS, Taylor CT. 2015. Targeted delivery of the hydroxylase inhibitor DMOG provides enhanced efficacy with reduced systemic exposure in a murine model of colitis. J. Control. Release 217:221–27 [DOI] [PubMed] [Google Scholar]
- 155.Lee JW, Ko J, Ju C, Eltzschig HK. 2019. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med 51:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cummins EP, Seeballuck F, Keely SJ, Adangan NE, Callanan JJ, et al. 2008. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134:156–65 [DOI] [PubMed] [Google Scholar]
- 157.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. 2008. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134:145–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hindryckx P, De Vos M, Jacques P, Ferdinande L, Peeters H, et al. 2010. Hydroxylase inhibition abrogates TNF-α–induced intestinal epithelial damage by hypoxia-inducible factor-1–dependent repression of FADD. J. Immunol 185:6306–16 [DOI] [PubMed] [Google Scholar]
- 159.Keely S, Campbell EL, Baird AW, Hansbro PM, Shalwitz RA, et al. 2014. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol. 7:114–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, et al. 2009. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458:732–36 [DOI] [PubMed] [Google Scholar]
- 161.Curtis VE, Ehrentraut SF, Campbell EL, Glover LE, Bayless AJ, et al. 2015. Stabilization of HIF through inhibition of Cullin-2 neddylation is protective in mucosal inflammatory responses. FASEB J. 29:208–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ehrentraut SF, Curtis VE, Wang RX, Saeedi BJ, Ehrentraut H, et al. 2016. Perturbation of neddylation-dependent NF-κB responses in the intestinal epithelium drives apoptosis and inhibits resolution of mucosal inflammation. Mol. Biol. Cell 27:3687–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen W, Hill H, Christie A, Kim MS, Holloman E, et al. 2016. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539:112–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xu R, Wang K, Rizzi JP, Huang H, Grina JA, et al. 2019. 3-[(1S,2S,3R)-2,3-Difluoro-1-hydroxy-7-methylsulfonylindan-4-yl]oxy-5-fluorobenzonitrile (PT2977), a hypoxia-inducible factor 2α (HIF-2α) inhibitor for the treatment of clear cell renal cell carcinoma. J. Med. Chem 62:6876–93 [DOI] [PubMed] [Google Scholar]
- 165.Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, et al. 2013. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology 145:831–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hindryckx P, Laukens D, De Vos M. 2011. Boosting the hypoxia-induced adaptive response in inflammatory bowel disease: a novel concept of treatment. Inflamm. Bowel Dis 17:2019–22 [DOI] [PubMed] [Google Scholar]
- 167.Taniguchi CM, Miao YR, Diep AN, Wu C, Rankin EB, et al. 2014. PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2. Sci. Transl. Med 6:236–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marchbank T, Mahmood A, Harten S, Maxwell PH, Playford RJ. 2011. Dimethyloxalyglycine stimulates the early stages of gastrointestinal repair processes through VEGF-dependent mechanisms. Lab. Invest 91:1684–94. 10.1038/labinvest.2011.129 [DOI] [PubMed] [Google Scholar]
- 169.Schaible B, McClean S, Selfridge A, Broquet A, Asehnoune K, et al. 2013. Hypoxia modulates infection of epithelial cells by Pseudomonas aeruginosa. PLOS ONE 8:e56491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shang L, Kang W, Li S, Ge S. 2019. Prolyl hydroxylase inhibitor DMOG suppressed inflammatory cytokine production in human gingival fibroblasts stimulated with Fusobacterium nucleatum. Clin. Oral. Investig 23:3123–32 [DOI] [PubMed] [Google Scholar]
- 171.Hirai K, Furusho H, Hirota K, Sasaki H. 2018. Activation of hypoxia-inducible factor 1 attenuates periapical inflammation and bone loss. Int.J. Oral. Sci 10:12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Laitakari A, Ollonen T, Kietzmann T, Walkinshaw G, Mennerich D, et al. 2019. Systemic inactivation of hypoxia-inducible factor prolyl 4-hydroxylase 2 in mice protects from alcohol-induced fatty liver disease. Redox Biol. 22:101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gupta R, Chaudhary AR, Shah BN, Jadhav AV, Zambad SP, et al. 2014. Therapeutic treatment with a novel hypoxia-inducible factor hydroxylase inhibitor (TRC160334) ameliorates murine colitis. Clin. Exp. Gastroenterol 7:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]