Abstract
Rationale: Obesity is characterized by elevated pleural pressure (Ppl) and worsening atelectasis during mechanical ventilation in patients with acute respiratory distress syndrome (ARDS).
Objectives: To determine the effects of a lung recruitment maneuver (LRM) in the presence of elevated Ppl on hemodynamics, left and right ventricular pressure, and pulmonary vascular resistance. We hypothesized that elevated Ppl protects the cardiovascular system against high airway pressure and prevents lung overdistension.
Methods: First, an interventional crossover trial in adult subjects with ARDS and a body mass index ≥ 35 kg/m2 (n = 21) was performed to explore the hemodynamic consequences of the LRM. Second, cardiovascular function was studied during low and high positive end-expiratory pressure (PEEP) in a model of swine with ARDS and high Ppl (n = 9) versus healthy swine with normal Ppl (n = 6).
Measurements and Main Results: Subjects with ARDS and obesity (body mass index = 57 ± 12 kg/m2) after LRM required an increase in PEEP of 8 (95% confidence interval [95% CI], 7–10) cm H2O above traditional ARDS Network settings to improve lung function, oxygenation and / matching, without impairment of hemodynamics or right heart function. ARDS swine with high Ppl demonstrated unchanged transmural left ventricular pressure and systemic blood pressure after the LRM protocol. Pulmonary arterial hypertension decreased (8 [95% CI, 13–4] mm Hg), as did vascular resistance (1.5 [95% CI, 2.2–0.9] Wood units) and transmural right ventricular pressure (10 [95% CI, 15–6] mm Hg) during exhalation. LRM and PEEP decreased pulmonary vascular resistance and normalized the / ratio.
Conclusions: High airway pressure is required to recruit lung atelectasis in patients with ARDS and class III obesity but causes minimal overdistension. In addition, patients with ARDS and class III obesity hemodynamically tolerate LRM with high airway pressure.
Clinical trial registered with www.clinicaltrials.gov (NCT 02503241).
Keywords: obesity, acute respiratory distress syndrome, mechanical ventilation, intrathoracic pressure, hemodynamics
At a Glance Commentary
Scientific Knowledge on the Subject
Obesity is characterized by high pleural pressure (Ppl) causing atelectasis and hypoxia. A lung recruitment maneuver (LRM) with high airway pressure improves oxygenation and respiratory mechanics when compared with the Acute Respiratory Distress Syndrome (ARDS) Network positive end-expiratory pressure and FiO2 tables in patients with ARDS and obesity. However, excessively high airway pressure has been shown to cause lung injury and hemodynamic instability. The effects on the cardiovascular system of high airway pressure in the presence of high Ppl in patients with ARDS and obesity have not been fully elucidated.
What This Study Adds to the Field
In a crossover study in patients with ARDS and class III obesity, an LRM improved respiratory mechanics, recruited atelectasis, and prevented overdistension while maintaining hemodynamic stability. In a swine model of ARDS and high Ppl, high pulmonary vascular resistance was reduced after LRM, whereas left ventricular pressures remained unaltered despite high airway pressure. Taken together, these results suggest that, in the presence of recruitable lungs and class III obesity, high Ppl protects against the potential negative effects of high airway pressure on the pulmonary and cardiovascular systems.
In acute respiratory distress syndrome (ARDS), mechanical ventilation should be titrated to avoid ventilator-induced lung injury and to minimize the adverse effects of positive pressure ventilation on cardiovascular function (1–5). Although ARDS is primarily a pulmonary disease, patients with ARDS develop multiorgan failure as a consequence of hemodynamic impairment (6–8). Recent clinical trials and animal studies showed that inappropriately set mechanical ventilation in ARDS leads to pulmonary edema, heart failure, and cardiac arrest (9–11).
Patients with class III obesity (body mass index [BMI] ≥ 40 kg/m2) have been historically excluded from randomized clinical trials investigating mechanical ventilation strategies for ARDS (12–14). The prevalence of adults with obesity in the United States is around 40% (approximately 100 million people), 8% of whom have class III obesity (15). In obesity, the upward displacement of the diaphragm caused by increased abdominal load leads to loss of lung volume (16, 17). Both physiologic and retrospective studies in patients with obesity have demonstrated the importance of a lung recruitment maneuver (LRM) (18, 19). In a retrospective study by Florio and colleagues (20), an LRM in patients with ARDS and obesity improved survival, and with increased airway pressure, vasoactive agents were weaned more quickly. Contrary to commonly held hemodynamic concerns regarding high airway pressure in patients without obesity, we have observed that an LRM and high values of positive end-expiratory pressure (PEEP) in patients with ARDS and class III obesity does not lead to hemodynamic compromise, reverses atelectasis, and improves oxygenation without lung parenchymal overdistension (18, 21).
Pleural pressure (Ppl) can be considered a surrogate for intrathoracic pressure. The difference between heart-chamber pressure and Ppl is known as transmural pressure (Ptm), whereas the difference between airway pressure and Ppl is known as transpulmonary pressure (Pl). In patients without obesity, Ppl is relatively low or negative (−5 to 5 cm H2O) (22), and an excessive increase in airway pressure will increase Pl, leading to lung overdistension, and will increase Ptm, leading to increased right ventricular afterload and decreased left ventricular preload.
In patients with obesity, Ppl is high (up to 25–30 cm H2O) (17). We hypothesized that recruitment of the lungs and airway pressure titrated according to best respiratory compliance are well tolerated hemodynamically because high Ppl protects the transmission of elevated airway pressure on the heart and great vessels and prevents lung overdistension (23). We designed a two-part study to investigate the impact of an LRM and high PEEP on cardiac function and on pulmonary and systemic hemodynamics in patients with ARDS and obesity. In the first part, we conducted an interventional crossover trial in adult patients with ARDS and obesity to describe the hemodynamic consequences of an LRM. During similar PEEPs, a secondary analysis of regional lung mechanics and overdistension was performed comparing our study patients (with ARDS and obesity) to patients from the ART (Alveolar Recruitment for ARDS Trial) (with ARDS and without obesity) (9). In the second part, we used a swine model of ARDS and increased abdominal load (24) to observe how reversing atelectasis with high airway pressures affects cardiovascular function.
Some of these results have been previously reported in the form of an abstract (25).
Methods
Clinical Study: Preparation, Procedures, and Measurements
Patients admitted to the ICU with a diagnosis of ARDS and a BMI ≥ 35 kg/m2 who required positive-pressure ventilation were enrolled. The monitoring procedures were similar to those previously described (18): 1) esophageal manometry was used to estimate Pl (26), 2) electrical impedance tomography (EIT) was used to measure the regional distribution of ventilation (27, 28) and lung perfusion (27, 29) (see Figure E1A in the online supplement), and 3) transthoracic echocardiography (TTE) was used to measure tricuspid annular plane systolic excursion (TAPSE) and tricuspid systolic excursion velocity (S’) as surrogates for right ventricular function (30).
Clinical Study: Ventilator Interventions
The prospective interventional study had two steps (see Figure E2A): 1) mechanical ventilation guided by the ARDS Network (ARDSnet) (31) and 2) a sequence of procedures (LRM → decremental PEEP trial → second LRM → optimal PEEP) to recruit lung atelectasis (LungRECRUITED) (32).
During the first 4 hours after study procedures, fluid intake, urinary output, and the vasoactive–inotropic score (VIS) were monitored. Ventilation-free days, mortality, and hospital and ICU length of stay were followed for 28 days.
ARDS Patients without Obesity
We obtained data from the five patients monitored with EIT (enrolled in the ART trial at the University of São Paulo) to describe the differential effect of LRM on lung regions in patients with ARDS without and with obesity. Overdistension and collapse were compared, and regional pressure–volume (P–V) curves were built for dependent and nondependent lung regions.
Statistical Analysis of the Clinical Study
The primary aim was to assess the cardiovascular response to an LRM in patients with ARDS and obesity.
Continuous variables are expressed as the mean ± SD or median (interquartile range). The differences between the two phases (mechanical ventilation guided by the ARDS Network and LungRECRUITED) were compared by using the Student’s t test or Wilcoxon signed-rank test for paired samples, depending on data distribution. Categorical variables are expressed as the count (n) and proportion (percentage).
Finally, we performed a descriptive comparison of the response of lung mechanics to recruitment using EIT data from both our study and ART trial subjects. For detailed methods, see the online supplement.
Experimental Study: Preparation, Procedures, and Measurements
Swine were instrumented and monitored in a manner similar to that applied to the patients (esophageal manometry and EIT) (33). In addition, pressure catheters were placed in each heart ventricle to directly measure right ventricular systolic pressure (RVSP), left ventricular systolic pressure (LVSP), right ventricular diastolic pressure (RVDP), and left ventricular diastolic pressure (LVDP). The Ptm was calculated for the right and left ventricles during systole and diastole under expiratory and inspiratory pauses (11).
ARDS Induced in Swine with High Ppl
The swine model combined two previously described models: one of ARDS (33–35) and one of obesity (21).
ARDS was induced by lung lavage and injurious ventilation. Obesity was simulated by application of external abdominal weights, which, by increasing the abdominal loading, increased Ppl (see Figure E1B). The study protocol had two phases: 1) low airway pressure to promote alveolar derecruitment (LungCOLLAPSED) and 2)LungRECRUITED (32) (see Figure E2C).
Healthy Swine with Normal Ppl
After completing our study on swine with ARDS and high Ppl, six swine without induced ARDS and with normal Ppl were studied as a control group. We used the same PEEPs (median values) administered to the intervention group (see Figure E2B): 1) low airway pressure ventilation with PEEP = 7 cm H2O and 2) an LRM followed by PEEP = 19 cm H2O.
Statistical Analysis of the Swine Study
The primary aim of the swine study was to assess the cardiovascular response to an LRM in swine with ARDS and high Ppl, as well as in the control group.
Continuous variables are expressed as the mean ± SD. The differences between the two phases (LungCOLLAPSED vs. LungRECRUITED and low airway pressure ventilation with PEEP = 7 cm H2O vs. an LRM followed by PEEP = 19 cm H2O) were compared using the Student’s t test. For detailed methods, see the online supplement.
Results
Clinical Study
From April 2016 to July 2017, 21 subjects consented, with two subjects not meeting criteria for ARDS (36). A total of 19 subjects with ARDS and obesity (BMI of 57 ± 12 kg/m2, lowest BMI = 40 kg/m2) completed the study. At the time of enrollment, 12 subjects (63%) had already required vasoactive drugs (Table 1).
Table 1.
Patient Characteristics and Clinical Outcomes
| Age, yr | 52 ± 14 |
| M | 8 (42) |
| BMI, kg/m2 | 57 ± 12 |
| Underlying cause of ARDS | |
| Septic shock | 10 (53) |
| Due to pneumonia | 5 |
| Due to skin infection | 5 |
| Pneumonia | 4 (21) |
| Trauma and/or postsurgery status | 5 (26) |
| Vasoactive drugs at study onset | 12 (63) |
| APACHE II score | 22 ± 8 |
| Creatinine, mg/dl | 2.3 ± 1.3 |
| Reintubation | 4 (21) |
| Tracheostomy | 5 (26) |
| Pneumothorax | 0 (0) |
| Ventilator free, d | 16 ± 11 |
| ICU LOS, d | 12 ± 6 |
| Hospital LOS, d | 22 ± 11 |
| 28-d mortality | 3 (16) |
Definition of abbreviations: APACHE II = Acute Physiologic and Chronic Health Evaluation II; ARDS = acute respiratory distress syndrome; BMI = body mass index; LOS = length of stay.
N = 19 patients. Data are presented as the mean ± SD, n, or n (%).
The LungRECRUITED Approach Homogenized Ventilation of the Lungs by Reducing Lung Collapse and Avoiding Overdistension
The LungRECRUITED approach successfully opened previously closed lung parenchyma and promoted more homogeneous ventilation, with a reduction in lung collapse of 31% without causing additional overdistension. There was an improvement in respiratory system compliance (Crs) with a decrease in driving pressure after LungRECRUITED. The PaO2/FiO2 ratio increased by 129 mm Hg at LungRECRUITED (Table 2).
Table 2.
Clinical Study: Respiratory Mechanics, Oxygenation, Collapse and Overdistension, and Regional Crs
| LungARDSnet [Mean ± SD or Median (IQR)] | LungRECRUITED [Mean ± SD or Median (IQR)] | Difference (CI)* | P Value | |
|---|---|---|---|---|
| Vt, ml/IBW | 6.3 ± 0.9 | 6.3 ± 0.8 | 0.1 (-0.1 to 0.2) | 0.23 |
| RR, bpm | 25.1 ± 6.0 | 25.2 ± 6.0 | 0.1 (-0.1 to 0.3) | 0.33 |
| PEEP, cm H2O | 13 ± 1 | 21 ± 3 | 8 (7 to 10) | <0.01 |
| PesEXP, cm H2O | 17.1 ± 4.5 | 19.5 ± 5.1 | 2.4 (1.3 to 3.4) | <0.01 |
| PlE, cm H2O | −4.3 ± 4.2 | 1.4 ± 3.6 | 5.7 (4.3 to 7.2) | <0.01 |
| Plateau pressure, cm H2O | 25.6 ± 3.9 | 30.4 ± 4.5 | 4.8 (3.4 to 6.3) | <0.05 |
| DP, cm H2O | 13 ± 4 | 9 ± 2 | −4 (-6 to −2) | <0.01 |
| Crs, ml/cm H2O | 33 (24 to 41) | 41 (31 to 51) | 11 (5 to 14) | <0.01 |
| PaO2/FiO2 | 179 ± 108 | 308 ± 90 | 129 (64 to 194) | <0.01 |
| Collapse determined by EIT, % (n = 18) | 38 ± 11 | 7 ± 6 | −31 (-36 to −25) | <0.01 |
| Overdistension determined by EIT, % (n = 18) | 7 ± 7 | 9 ± 6 | 2 (-2 to 6) | 0.33 |
| Regional Crs, ΔZ/cm H2O (n = 10) | ||||
| ROI-1 | 8.9 ± 4.7 | 7.3 ± 2.8 | −1.7 (-3.6 to 0.2) | 0.07 |
| ROI-2 | 19.1 ± 8.1 | 25.5 ± 8.4 | 6.3 (4.3 to 8.4) | <0.01 |
| ROI-3 | 5.1 ± 2.8 | 10 ± 3.2 | 4.9 (2.9 to 6.9) | <0.01 |
Definition of abbreviations: bpm = beats per minute; CI = confidence interval; Crs = respiratory system compliance; ΔZ = delta impedance; DP = driving pressure; EIT = electrical impedance tomography; IBW = ideal body weight; IQR = interquartile range; LungARDSnet = mechanical ventilation guided by the Acute Respiratory Distress Syndrome Network; LungRECRUITED = a sequence of procedures (lung recruitment maneuver → decremental PEEP trial → second lung recruitment maneuver → optimal PEEP) to recruit lung atelectasis; PEEP = positive end-expiratory pressure; PesEXP = end-expiratory pleural pressure; PlE = end-expiratory transpulmonary pressure; ROI = region of interest; RR = respiratory rate.
N = 19 patients.
A median of differences and 98.08% CI are shown for Crs; mean differences and 95% CIs are shown for other categories.
Compared with Patients with ARDS without Obesity, an LRM in Patients with ARDS and Class III Obesity Led to Less Overdistension
At similar PEEP values, overdistension was higher for patients with ARDS and without obesity, whereas lung collapse was higher with ARDS and obesity (Figures 1A and 1B). To characterize regional overdistension and collapse, P–V curves were built for region of interest 1 (ROI-1), the nondependent ROI, and the dependent ROI (ROI-3). In patients with ARDS and class III obesity, the P–V shape was linear for ROI-1, suggesting an absence of overdistension up to a pressure of 25 cm H2O, and showed exponential positive growth for ROI-3, suggesting poor compliance of the atelectatic lung (Figures 1C and 1D). In patients with ARDS without obesity, the P–V curve showed exponential negative decay in ROI-1, suggesting overdistension in this nondependent region, and in ROI-3, the P–V curve assumed a linear shape, suggesting a highly heterogenous lung (Figures 1E and 1F).
Figure 1.
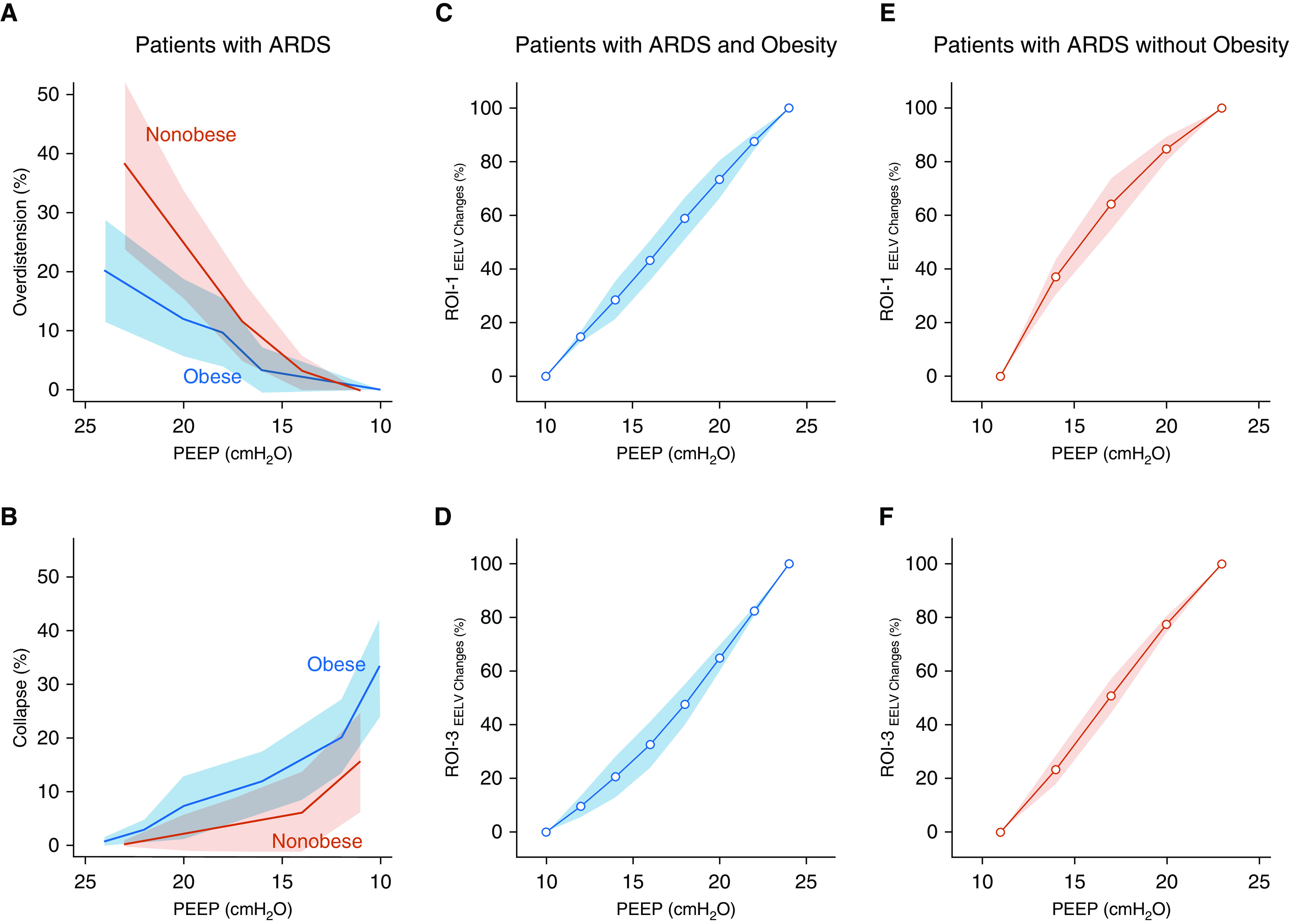
Clinical study of patients with acute respiratory distress syndrome (ARDS) and class III obesity versus patients with ARDS without obesity. Overdistension and collapse during a similar sequence of positive end-expiratory pressure (PEEP) and regional pressure–volume (P–V) curves for the most nondependent and the most dependent regions of interest (ROIs) are shown. (A) Overdistension and (B) collapse measured by using electrical impedance tomography in patients with ARDS and class III obesity versus patients with ARDS without obesity are shown. A mixed linear model was used for overdistension (P = 0.002 for interaction) and collapse (P < 0.001 for interaction), and for similar PEEP, overdistension was higher in patients with ARDS and without obesity and collapse was higher in patients with ARDS and class III obesity. Regional P–V curves were built for the most non–gravity-dependent ROI (ROI-1) and the most dependent ROI (ROI-3) (see Figure E1). The regional variations in EELV were calculated by using electrical impedance tomography for each PEEP (see online supplement). ROI-1 are shown in (C) patients with ARDS and class III obesity and in (E) patients with ARDS without obesity. Of note, for similar PEEP values, the P–V curve shape was different: in patients with ARDS and class III obesity, it was linear, and in patients with ARDS and without obesity, it showed positive exponential growth (mixed linear model, P = 0.002 for interaction). ROI-3 are shown in (D) patients with ARDS and class III obesity and in (F) patients with ARDS without obesity. Again, for similar PEEPs, P–V curve shapes were different. In patient with ARDS and class III obesity, the curve showed exponential negative decay, whereas in patients with ARDS without obesity, it was linear (mixed linear model, P = 0.001 for interaction). Data are presented as the mean ± SD (confidence interval). EELV = end-expiratory lung volume.
LungRECRUITED Did Not Affect Right Heart Function, Vasoactive Medication Requirement, or Net Fluid Balance
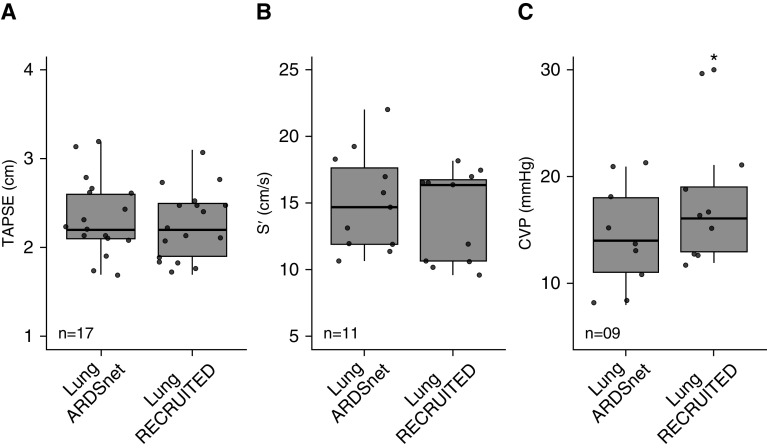
TAPSE and S’ remained unchanged after LungRECRUITED, suggesting that right heart function was not impaired with higher airway pressures (Figure 2).
Figure 2.
Clinical study. Transthoracic echocardiography in patients with acute respiratory distress syndrome (ARDS) and class III obesity. The right heart function was evaluated for each study phase: mechanical ventilation guided by the ARDS Network (LungARDSnet) and a sequence of procedures (lung recruitment maneuver → decremental positive end-expiratory pressure trial → second lung recruitment maneuver → optimal positive end-expiratory pressure) to recruit lung atelectasis (LungRECRUITED). (A) TAPSE was measured in 17 patients, and there was no significant difference between phases. (B) S′ was measured in 11 patients, and there was no significant difference between phases. (C) CVP was measure in nine patients for each study phase. Data are presented as the mean ± SD. *LungARDSnet versus LungRECRUITED: P = 0.03; mean of differences, 3; 95% confidence interval, 0.4–5.6. CVP = central venous pressure; S′ = tricuspid systolic excursion velocity; TAPSE = tricuspid annular plane systolic excursion.
The mean arterial pressure (MAP) and heart rate remained unchanged during the study procedure and at 4 hours after the procedure. Among the 12 subjects requiring inotropic and vasoactive agents before study enrollment, the VIS remained unchanged during the study procedure and at 4 hours after the procedure. The seven subjects who did not require vasoactive support before enrollment never required such medication during the study procedure or at 4 hours after the procedure. No subject required a fluid bolus at LungRECRUITED. The net fluid balance at 4 hours after the procedure was negligible. These physiologic and clinical data show that LungRECRUITED was well tolerated hemodynamically (Table 3).
Table 3.
Clinical Study: Vasopressors, Hemodynamics, and Fluid in Patients with ARDS and Obesity
| LungARDSnet (PEEP = 13 cm H2O) | LungRECRUITED (PEEP = 21 cm H2O) | Hemodynamic Assessment after LungRECRUITED |
||
|---|---|---|---|---|
| 2 h | 4 h | |||
| VIS (n = 12) | 12 (6–26) | 12 (6–27) | 13 (4–22) | 12 (3–20) |
| Total fluids, ml | — | — | 301 ± 235 | 586 ± 234 |
| Total urinary output, ml | — | — | 110 (85–270) | 230 (160–755) |
| Total fluid balance, ml | — | — | 100 ± 345 | 146 ± 517 |
| HR, bpm | 89 ± 21 | 86 ± 22 | 86 ± 19 | 88 ± 22 |
| MAP, mm Hg | 75 ± 7 | 80 ± 12 | 77 ± 14 | 77 ± 10 |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; bpm = beats per minute; HR = heart rate; LungARDSnet = mechanical ventilation guided by the Acute Respiratory Distress Syndrome Network; LungRECRUITED = a sequence of procedures (lung recruitment maneuver → decremental PEEP trial → second lung recruitment maneuver → optimal PEEP) to recruit lung atelectasis; MAP = mean arterial pressure; PEEP = positive end-expiratory pressure; VIS = vasoactive–inotropic score.
N = 19 patients. Data presented as the mean ± SD or median (interquartile range).
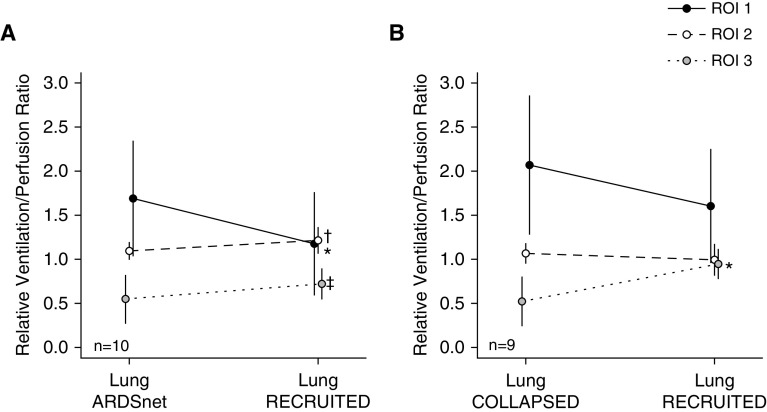
LungRECRUITED Improved Relative / Matching and Regional Crs
In the setting of alveolar recruitment in the gravitationally dependent region of the lung, the relative / ratio in ROI-2 and ROI-3 increased after LungRECRUITED. However, the relative / ratio of the anterior portion of the lung, ROI-1, decreased after LungRECRUITED (Figure 3A). The regional Crs increased in ROI-2 and ROI-3 and remained unchanged in ROI-1, correlating with these changes in ventilation (Table 2).
Figure 3.
Clinical and swine studies. Relative / ratio per region of interest (ROI) in patients with acute respiratory distress syndrome (ARDS) and class III obesity and swine with induced ARDS and high pleural pressure. Relative / ratio in 3 ROIs (see Figure E1 for ROI selection). (A) Patients with ARDS and class III obesity (10 patients underwent the / test with electrical impedance tomography). The ROIs changed the ratio toward a more balanced matching from LungARDSnet to LungRECRUITED. *ROI-1: P = 0.0195; median of differences, −0.36; 97.85% confidence interval (CI), −1.73 to 0.02. †ROI-2: P = 0.0195; median of differences, 0.11; 97.85% CI, −0.05 to 0.26. ‡ROI-3: P = 0.0195; median of differences, 0.15; 97.85% CI, 0.03 to 0.36. (B) Swine with induced ARDS and high pleural pressure (9 swine underwent the / test with electrical impedance tomography). *ROI-3: P = 0.0056; mean of differences, 0.4; 95% CI, 0.2 to 0.7. Data are presented as the mean ± SD. LungARDSnet = mechanical ventilation guided by the ARDS Network; LungCOLLAPSED = low airway pressure to promote alveolar derecruitment; LungRECRUITED = a sequence of procedures (lung recruitment maneuver → decremental positive end-expiratory pressure trial → second lung recruitment maneuver → optimal positive end-expiratory pressure) to recruit lung atelectasis.
Clinical Outcomes
Among the 19 patients of the study cohort, 9 (47%) failed extubation, 5 (26%) were tracheostomized, and no pneumothorax was observed. The 28-day mortality rate was 16% (3 patients), despite an elevated Acute Physiology and Chronic Health Evaluation II score (estimated mortality of 30–40%) (37) (Table 1).
Experimental Study
We performed our study on the experimental model of ARDS with high Ppl on nine swine between October and November 2017. A study of a control group of six swine without induced ARDS and normal Ppl was performed between January and February 2020. (Major findings on the control group are presented in Tables 4, E1, and E2.)
Table 4.
Swine Study
| Healthy Swine with Normal Ppl | LungPEEP7 | LungPEEP19 | Mean Difference (95% CI) | P Value |
|---|---|---|---|---|
| Right ventricle, mm Hg (n = 6) | ||||
| RVSP | 27 ± 6 | 33 ± 6 | 6 (2 to 10) | 0.02 |
| Transmural RVSP | 22 ± 6 | 23 ± 6 | 1 (−3 to 6) | 0.39 |
| RVDP | 8 ± 4 | 15 ± 5 | 7 (4 to 10) | <0.01 |
| Transmural RVDP | 2.7 ± 4.3 | 5.4 ± 5.0 | 2.7 (−0.7 to 6.1) | 0.09 |
| Left ventricle, mm Hg (n = 6) | ||||
| LVSP | 104 ± 9 | 78 ± 12 | −26 (−42 to −10) | <0.01 |
| Transmural LVSP | 98 ± 8 | 68 ± 12 | −30 (−47 to −14) | <0.01 |
| LVDP | 9 ± 5 | 15 ± 6 | 6 (3 to 8) | <0.01 |
| Transmural LVDP | 3.9 ± 4.9 | 4.7 ± 7.8 | 0.8 (−1.9 to 3.6) | 0.49 |
| ARDS Swine with High Ppl | LungCOLLAPSED | LungRECRUITED | Mean Difference (95% CI) | P Value |
|---|---|---|---|---|
| Right ventricle, mm Hg (n = 9) | ||||
| RVSP | 50 ± 8 | 43 ± 3 | −7 (−12 to −3) | <0.01 |
| Transmural RVSP | 42 ± 8 | 32 ± 3 | −10 (−15 to −6) | <0.01 |
| RVDP | 14 ± 3 | 14 ± 1 | 0 (−2 to 3) | 0.67 |
| Transmural RVDP | 5.3 ± 3.4 | 2.8 ± 1.7 | −2.6 (−4.5 to −0.6) | 0.02 |
| Left ventricle, mm Hg (n = 8) | ||||
| LVSP | 139 ± 19 | 135 ± 14 | −4 (−18 to 6) | 0.37 |
| Transmural LVSP | 130 ± 19 | 123 ± 14 | −7 (−17 to 3) | 0.14 |
| LVDP | 26 ± 11 | 25 ± 9 | −1 (−3 to 1) | 0.37 |
| Transmural LVDP | 17 ± 11 | 13 ± 9 | −4 (−7 to −1) | 0.02 |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; CI = confidence interval; LungCOLLAPSED = low airway pressure to promote alveolar derecruitment; LungPEEP7 = low airway pressure ventilation with a PEEP of 7 cm H2O; LungPEEP19 = lung recruitment maneuver followed by a PEEP of 19 cm H2O; LungRECRUITED = a sequence of procedures (lung recruitment maneuver → decremental PEEP trial → second lung recruitment maneuver → optimal PEEP) to recruit lung atelectasis; LVDP = left ventricular diastolic pressure at expiration; LVSP = left ventricular systolic pressure at expiration; PEEP = positive end-expiratory pressure; Ppl = pleural pressure; RVDP = right ventricular diastolic pressure at expiration; RVSP = right ventricular systolic pressure at expiration.
Data are presented as the mean ± SD or the mean difference (95% CI). The swine study measured ventricular pressures and respective transmural pressures at two PEEP values during an expiratory pause in healthy swine with normal pleural pressure and in swine with induced ARDS with high pleural pressure.
Swine Study of ARDS with High Ppl and Pulmonary Hypertension
After the abdominal loading, esophageal pressure increased an average of 5.3 cm H2O (95% confidence interval, 4.6–5.9; P < 0.01). As expected (4), pulmonary hypertension developed after ARDS onset, with a mean pulmonary arterial pressure (mPAP) of 40 ± 10 mm Hg and a pulmonary vascular resistance (PVR) of 5.5 ± 2.1 Wood units.
The PaO2/FiO2 ratio dropped from 488 ± 34 mm Hg before injury to 72 ± 32 mm Hg after injury.
Swine Lungs with ARDS and High Ppl Are Highly Recruitable
The LungRECRUITED strategy recruited lung parenchyma with a positive Pl throughout the entire respiratory cycle (Figure 4B). Recruitment was also demonstrated by a reduction in the collapse fraction from LungCOLLAPSED to LungRECRUITED, together with a significant decrease in the shunt fraction of 25% and a PaO2/FiO2 ratio increase of 125 mm Hg. In addition, there was an improvement in Crs at LungRECRUITED, with a decrease in driving pressure of 6 cm H2O (see Table E1).
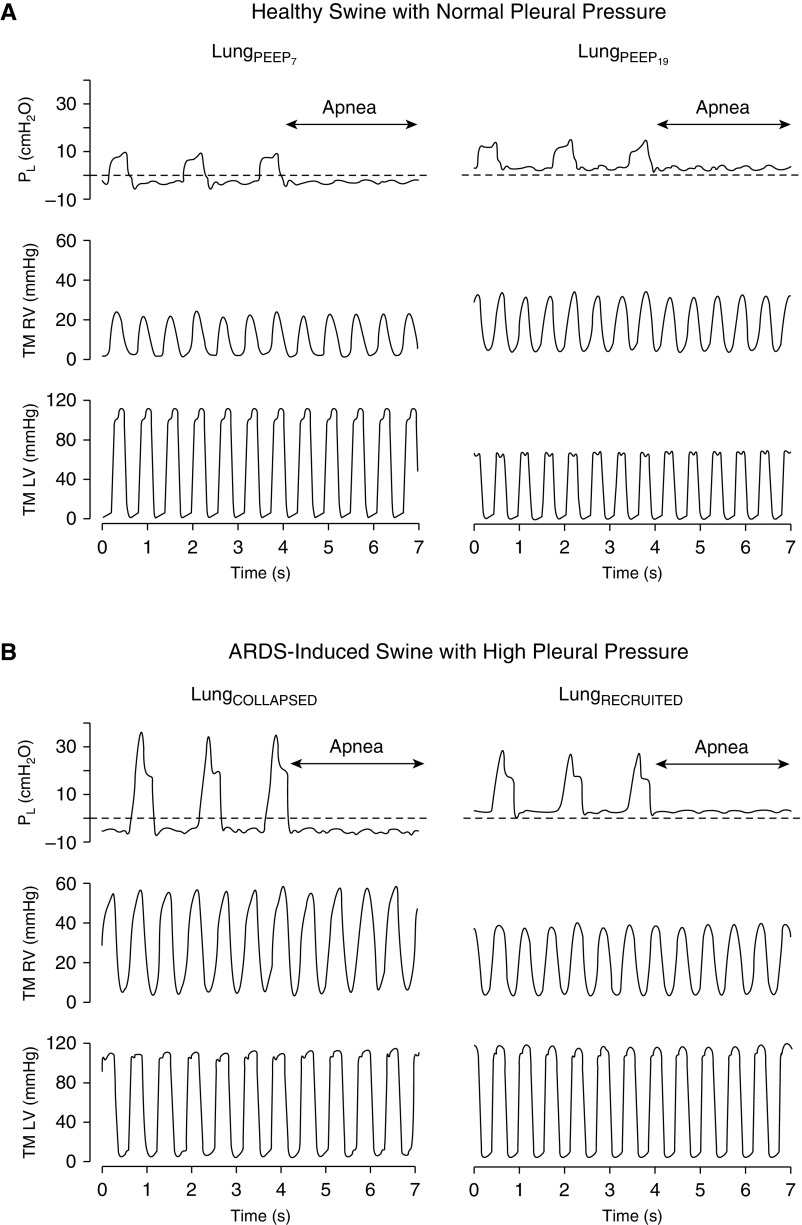
Figure 4.
Swine study. Effect of different numbers of transpulmonary pressure (Pl; during respiratory cycles and during an expiratory pause) on the transmural (TM) right ventricular (RV) and left ventricular (LV) pressure in one healthy swine with normal pleural pressure and in one swine with induced acute respiratory distress syndrome (ARDS) with high pleural pressure due to increased abdominal loading. (A) Healthy swine with normal pleural pressure. Pl, TM RV pressure, and TM LV pressure at low-airway-pressure ventilation with positive end-expiratory pressure (PEEP) = 7 cm H2O (LungPEEP7) and after a lung recruitment maneuver followed by PEEP = 19 cm H2O (LungPEEP19) are shown. TM RV pressure remained unaltered, and TM LV pressure showed a significant drop during the increase in Pl from LungPEEP7 to LungPEEP19 (black arrow). (B) Swine with induced ARDS with high pleural pressure due to increased abdominal loading. Pl, TM RV pressure, and TM LV pressure at LungCOLLAPSED and LungRECRUITED are shown. TM RV pressure expressively dropped (black arrow), and TM LV pressure did not change with the increase in Pl. LungCOLLAPSED = low airway pressure to promote alveolar derecruitment; LungRECRUITED = a sequence of procedures (lung recruitment maneuver → decremental PEEP trial → second lung recruitment maneuver → optimal PEEP) to recruit lung atelectasis.
The LungRECRUITED Strategy Decreased PVR and Did Not Alter Systemic Vascular Resistance or Systemic Perfusion in Swine
After the LungRECRUITED strategy, with an average PEEP increase of 11 cm H2O, the elevated baseline value of PVR decreased by 1.5 Wood units with a decrease of mPAP. The MAP was unchanged throughout the study protocol, and systemic vascular resistance (SVR) was also unaltered. The requirements for inotropes and vasopressors were minimal and did not change between LungCOLLAPSED and LungRECRUITED. Despite a 0.7-L/min decrease in from LungCOLLAPSED to LungRECRUITED, the mixed venous oxygen saturation improved significantly, suggesting adequate systemic perfusion (see Table E2).
The LungRECRUITED Approach Did Not Alter Baseline Physiologic Transmural RVDP, Decreased Elevated RVSP, and Did Not Impair Left Ventricular Function
Despite elevated RVDP numbers, transmural RVDP was not abnormally elevated at either LungCOLLAPSED or LungRECRUITED because of the persistent elevation in Ppl. However, the transmural RVDP decreased after LungRECRUITED. Because of an increased PVR at LungCOLLAPSED, RVSP was elevated. After LungRECRUITED, the reduction of PVR translated to a reduction of the transmural RVSP by 10 mm Hg (Figure 4 and Table 4).
The stable left ventricular pressures mirrored the unaltered SVR and MAP after LungRECRUITED. The LVSP was unaffected between LungCOLLAPSED and LungRECRUITED. Similarly, the transmural LVDP did not change after LungRECRUITED. These findings are consistent with preserved and unaltered left ventricular function from LungCOLLAPSED to LungRECRUITED (Figure 4 and Table 4).
The increased airway pressure at LungRECRUITED resulted in an increase in central venous pressure (CVP) of 2 mm Hg, without significant changes in the transmural CVP. The same trend applied for the wedge pressure: absolute values increased without changes in the transmural wedge pressure (see Table E2).
In contrast, in the control group with normal Ppl, MAP, LVSP, and transmural LVSP were reduced significantly by increasing PEEP from 7 to 19 cm H2O. Despite a slight increase in RVSP and RVDP after the LRM protocol in the control subjects, the corresponding transmural RVSP and transmural RVDP did not change (Table 4).
Improved Homogeneity of Ventilation with LungRECRUITED Improved / Matching
In the setting of significant recruitment of the gravitationally dependent lung after LungRECRUITED, the ratio of / in ROI-3 almost doubled (0.5 ± 0.3 at LungCOLLAPSED to 0.9 ± 0.2 at LungRECRUITED). However, the ratio of / in the initially overventilated ROI-1 demonstrated a trend toward a reduction of the / ratio (2.1 ± 0.8 at LungCOLLAPSED to 1.6 ± 0.6 at LungRECRUITED). The ratio of / of the ROI-2 remained mostly unchanged (1.1 ± 0.1 at LungCOLLAPSED vs. 0.9 ± 0.2 at LungRECRUITED) (Figure 3B).
Discussion
In this study of ARDS with class III obesity, we hypothesized that recruiting the lung and applying PEEP titrated to the best Crs would be hemodynamically tolerated in the setting of elevated Ppl. Our hypothesis is based on the novel concept that obesity protects the cardiovascular system against high ventilator pressures and prevents nondependent lung overdistension with elevated Ppl. Prior studies investigating heart–lung interactions in ARDS (as well as in non-ARDS settings) did not investigate the influence of high Ppl (38–41). We conducted a two-part study, both of critically ill patients with ARDS and obesity and of a swine model.
In our clinical investigation, we observed that LungRECRUITED was hemodynamically well tolerated in critically ill patients and improved lung mechanics. In contrast to using an LRM in patients with ARDS with recruitable lungs and without obesity (from the ART trial), LRM in patients with ARDS and class III obesity caused minimal lung overdistension during similar PEEP settings (Figure 1).
In our swine model of ARDS with elevated Ppl, we determined that LungRECRUITED did not lower left ventricular pressure (as it did in healthy control subjects) and decreased PVR.
In both settings of ARDS with high Ppl (clinical and experimental), the LungRECRUITED strategy significantly improved pulmonary mechanics without significant overdistension and improved oxygenation without compromising hemodynamics.
It should be emphasized that in our swine model of high Ppl, increasing Ppl was associated with an increase in CVP, wedge pressure, and both RVDP and LVDP (see Tables 4 and E2). This can be explained by considering the entire thorax as a single space: the upward pressure on the diaphragm by the abdominal loading will transmit force across each of the thorax’s subcompartments, including the heart and great vessels. CVP, wedge pressure, and ventricular diastolic pressure represent the vascular subcompartments at resting state and best depict the transmission of increased Ppl. However, when Ptm values were calculated (which account for Ppl values), transmural RVDP and transmural LVDP were not elevated.
In addition to the perturbations on resting pressures within the cardiovascular system caused by the high Ppl, we also increased PVR through the ARDS model (4). As is depicted in Figures 3 and 4 and Tables E1 and E2, atelectasis caused by ventilation with negative Ppl in ARDS induced significant / mismatch with a large shunt fraction; severe hypoxemia; and, ultimately, increased PVR with pulmonary hypertension and elevated RVSP. When the lung parenchyma was recruited (as suggested by a 40% decrease in EIT-observed atelectasis) and optimal Crs was achieved (as suggested by a 6–cm H2O decrease in driving pressure), the following physiologic improvements occurred: normalization of the / ratio with decreased shunt fraction, a greater-than-threefold increase in the PaO2/FiO2 ratio, and a substantial drop in PVR, mPAP, and RVSP.
The relationship between lung volumes and PVR has been well elucidated (42–44). The further lung volume is from a patient’s ideal functional residual capacity, the greater the PVR. Thus, either increases or decreases in lung volume affect PVR and, as a result, right ventricular pressure and right heart function. As expected, our findings demonstrated that LungCOLLAPSED was associated with atelectasis (i.e., lower than ideal lung volume) and high PVR. Only after LungRECRUITED did PVR, mPAP, and RVSP decrease. Although we did not record cardiac chamber pressures in the clinical portion of the study, right heart function was undisturbed by LungRECRUITED, as indicated by TTE findings.
With respect to left heart function and the systemic circulation, an average increase in PEEP of 11 cm H2O after LungRECRUITED did not reduce LVSP or lead to an increase in SVR. Had the large increase in PEEP decreased venous return to the heart, we would have expected these changes to occur. In both the swine and clinical studies, there was no increase in the need for vasoactive medication or fluid administration after LungRECRUITED.
Although it was expected that high Ppl would warrant high airway pressure to recruit collapsed lung, it was surprising that this ventilation strategy (i.e., LungRECRUITED) (45, 46) substantially improved right heart pressures and did not cause hemodynamic instability. We believe that two major components led to these findings. First, the lungs in our swine model and in our clinical study were safely recruitable, and the LungRECRUITED strategy significantly improved lung function. Second, as mentioned above, the increased Ppl in class III obesity protected the cardiovascular system against what otherwise would have been dangerously high airway pressure and prevented large swings in intrathoracic pressure. Had the lungs in our study not been recruitable, our intervention with high airway pressure could have further injured the lungs (1, 46–48). Had the Ppl not been elevated, we would have expected to observe hemodynamic collapse (49).
Our findings stand in contrast to those in the lean patients presented here from the ART trial, in whom similar airway pressure values led to significant overdistension in the nondependent lung regions (despite an improvement in overall Crs). Class III obesity prevents the overdistension from occurring with a high Ppl. In our subgroup analysis of five patients from the ART trial, overdistension of nondependent lung begins at a PEEP of 10–15 cm H2O. Clinically, we suggest on the basis of the findings of our study that an LRM in patients with class III obesity is safe to perform when regional compliance can be monitored.
Study Limitations
This study has limitations. First, our study population comprised patients with class III obesity, not covering completely the obesity spectrum. Second, during the clinical study, the subjects were not monitored with pulmonary arterial catheters because their placement is no longer part of routine intensive care (50). Nonetheless, we performed TTE at predetermined times (before and after ventilator procedures) and assessed MAP, heart rate, VIS, and net fluid balance, which provide useful clinical information to follow hemodynamics and cardiac function. In the experimental study, the porcine model with elevated Ppl is a highly recruitable model, although it may not perfectly mimic the gradual onset and distribution seen in an individual with obesity. However, the findings of previous studies using computed tomography, EIT, respiratory system mechanics, and oxygenation were consistent with the observed changes owing to an increased Ppl (18, 19, 21). The aim of the animal study was to compare the hemodynamic response to ventilation in a high-Ppl animal model with that in a low-Ppl model at the same PEEP values.
Conclusions
Using an LRM with high airway pressure in patients with ARDS has shown in some patients to be detrimental to hemodynamic stability. High Ppl due to obesity further contributes to atelectasis, worsening shunt fraction and oxygenation in patients with ARDS. In a crossover study in patients with ARDS and class III obesity, an LRM improved respiratory function and prevented lung overdistension while maintaining hemodynamic stability. In a swine model of ARDS and high Ppl, PVR was reduced after an LRM, whereas left ventricular pressures remained unaltered at high airway pressure. Taken together, these results suggest that the presence of recruitable lungs and obesity with high Ppl protects from the potential negative effects of high airway pressure on the cardiovascular system.
Supplementary Material
Acknowledgments
Lung Rescue Team Investigators: Adriana Sayuri Hirota, Daniela Davis Madureira Iope, Carolina Eimi Kajiyama, Andrea Fonseca, Otilia Batista, Silvia Cristina Leopoldino, Carlo Valsecchi, Erick Leon, Kathryn Hibbert, Charles C. Hardin, Kim Connelly, Daniel Fisher, Grant Michael Larson, Emanuele Vassena, Raffaele Di Fenza, Stefano Gianni, Bijan Safaee Fakhr, Jeanine Wiener-Kronish, and Brian Kavanagh (in memoriam).
Footnotes
Supported by the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital, Boston, Massachusetts, and by the Laboratório de Investigação Médica Nº 9, Faculdade de Medicina, Universidade de São Paulo, São Paulo, São Paulo, Brazil.
Author Contributions: Conception and design: R.D.S.S., M.T.D., M.B.P.A., R.M.K., and L.B. Acquisition, analysis, and interpretation of the data: R.D.S.S., M.T.D., J.F., F.M., G.F., L.G.G., S.G., C.C.A.M., O.P.S.R., M.B., R.P., D.A.I., A.B., K.S., A.S., E.A.B., M.B.P.A., R.M.K., and L.B. Drafting of the work: R.D.S.S., M.T.D., M.B.P.A., R.M.K., and L.B. Critical revision of the paper: J.F., F.M., G.F., L.G.G., S.G., C.C.A.M., O.P.S.R., M.B., R.P., D.A.I., A.B., K.S., A.S., E.A.B., and M.B.P.A. Final approval of the version to be published: R.D.S.S., M.T.D., J.F., F.M., G.F., L.G.G., S.G., C.C.A.M., O.P.S.R., M.B., R.P., D.A.I., A.B., K.S., A.S., E.A.B., M.B.P.A., R.M.K., and L.B. Agreement to be accountable for all aspects of the work: R.D.S.S., M.T.D., and L.B.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201909-1687OC on September 2, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the Lung Rescue Team Investigators, Adriana Sayuri Hirota, Daniela Davis Madureira Iope, Carolina Eimi Kajiyama, Andrea Fonseca, Otilia Batista, Silvia Cristina Leopoldino, Carlo Valsecchi, Erick Leon, Kathryn Hibbert, Charles C. Hardin, Kim Connelly, Daniel Fisher, Grant Michael Larson, Emanuele Vassena, Raffaele Di Fenza, Stefano Gianni, Bijan Safaee Fakhr, Jeanine Wiener-Kronish, and Brian Kavanagh
References
- 1.Kolobow T, Moretti MP, Fumagalli R, Mascheroni D, Prato P, Chen V, et al. Severe impairment in lung function induced by high peak airway pressure during mechanical ventilation: an experimental study. Am Rev Respir Dis. 1987;135:312–315. doi: 10.1164/arrd.1987.135.2.312. [DOI] [PubMed] [Google Scholar]
- 2.Pinsky MR. Heart-lung interactions during positive-pressure ventilation. New Horiz. 1994;2:443–456. [PubMed] [Google Scholar]
- 3.Mekontso Dessap A, Boissier F, Charron C, Bégot E, Repessé X, Legras A, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med. 2016;42:862–870. doi: 10.1007/s00134-015-4141-2. [DOI] [PubMed] [Google Scholar]
- 4.Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med. 1977;296:476–480. doi: 10.1056/NEJM197703032960903. [DOI] [PubMed] [Google Scholar]
- 5.Dreyfuss D, Basset G, Soler P, Saumon G. Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis. 1985;132:880–884. doi: 10.1164/arrd.1985.132.4.880. [DOI] [PubMed] [Google Scholar]
- 6.Del Sorbo L, Slutsky AS. Acute respiratory distress syndrome and multiple organ failure. Curr Opin Crit Care. 2011;17:1–6. doi: 10.1097/MCC.0b013e3283427295. [DOI] [PubMed] [Google Scholar]
- 7.Bull TM, Clark B, McFann K, Moss M National Institutes of Health/National Heart, Lung, and Blood Institute ARDS Network. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2010;182:1123–1128. doi: 10.1164/rccm.201002-0250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slutsky AS, Tremblay LN. Multiple system organ failure: is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157:1721–1725. doi: 10.1164/ajrccm.157.6.9709092. [DOI] [PubMed] [Google Scholar]
- 9.Cavalcanti AB, Suzumura ÉA, Laranjeira LN, Paisani DM, Damiani LP, Guimarães HP, et al. Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katira BH, Kuebler WM, Kavanagh BP. Inspiratory preload obliteration may injure lungs via cyclical “on-off” vascular flow. Intensive Care Med. 2018;44:1521–1523. doi: 10.1007/s00134-017-5024-5. [DOI] [PubMed] [Google Scholar]
- 11.Katira BH, Giesinger RE, Engelberts D, Zabini D, Kornecki A, Otulakowski G, et al. Adverse heart-lung interactions in ventilator-induced lung injury. Am J Respir Crit Care Med. 2017;196:1411–1421. doi: 10.1164/rccm.201611-2268OC. [DOI] [PubMed] [Google Scholar]
- 12.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 13.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Expiratory Pressure (Express) Study Group. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 14.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299:637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 15.Fryar C, Carrol M, Ogden C.Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960-1962 through 2015-2016 Atlanta GA: Centers for Disease Control and Prevention; 2018[accessed 2020 Dec 2]. Available from: https://www.cdc.gov/nchs/data/hestat/obesity_adult_15_16/obesity_adult_15_16.htm [Google Scholar]
- 16.Sharp JT, Henry JP, Sweany SK, Meadows WR, Pietras RJ. Effects of mass loading the respiratory system in man. J Appl Physiol. 1964;19:959–966. doi: 10.1152/jappl.1964.19.5.959. [DOI] [PubMed] [Google Scholar]
- 17.Behazin N, Jones SB, Cohen RI, Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol (1985) 2010;108:212–218. doi: 10.1152/japplphysiol.91356.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fumagalli J, Santiago RRS, Teggia Droghi M, Zhang C, Fintelmann FJ, Troschel FM, et al. Lung Rescue Team Investigators. Lung recruitment in obese patients with acute respiratory distress syndrome. Anesthesiology. 2019;130:791–803. doi: 10.1097/ALN.0000000000002638. [DOI] [PubMed] [Google Scholar]
- 19.Pirrone M, Fisher D, Chipman D, Imber DA, Corona J, Mietto C, et al. Recruitment maneuvers and positive end-expiratory pressure titration in morbidly obese ICU patients. Crit Care Med. 2016;44:300–307. doi: 10.1097/CCM.0000000000001387. [DOI] [PubMed] [Google Scholar]
- 20.Florio G, Ferrari M, Bittner EA, De Santis Santiago R, Pirrone M, Fumagalli J, et al. Investigators of the Lung Rescue Team. A lung rescue team improves survival in obesity with acute respiratory distress syndrome. Crit Care. 2020;24:4. doi: 10.1186/s13054-019-2709-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fumagalli J, Berra L, Zhang C, Pirrone M, Santiago RRS, Gomes S, et al. Transpulmonary pressure describes lung morphology during decremental positive end-expiratory pressure trials in obesity. Crit Care Med. 2017;45:1374–1381. doi: 10.1097/CCM.0000000000002460. [DOI] [PubMed] [Google Scholar]
- 22.Washko GR, O’Donnell CR, Loring SH. Volume-related and volume-independent effects of posture on esophageal and transpulmonary pressures in healthy subjects. J Appl Physiol (1985) 2006;100:753–758. doi: 10.1152/japplphysiol.00697.2005. [DOI] [PubMed] [Google Scholar]
- 23.Grasso S, Terragni P, Birocco A, Urbino R, Del Sorbo L, Filippini C, et al. ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med. 2012;38:395–403. doi: 10.1007/s00134-012-2490-7. [DOI] [PubMed] [Google Scholar]
- 24.Staffieri F, Stripoli T, De Monte V, Crovace A, Sacchi M, De Michele M, et al. Physiological effects of an open lung ventilatory strategy titrated on elastance-derived end-inspiratory transpulmonary pressure: study in a pig model. Crit Care Med. 2012;40:2124–2131. doi: 10.1097/CCM.0b013e31824e1b65. [DOI] [PubMed] [Google Scholar]
- 25.Departments of Intensive Care and Emergency Medicine of Erasme University Hospital; Université Libre de Bruxelles; Belgian Society of Intensive Care Medicine. 38th International Symposium on Intensive Care and Emergency Medicine: Brussels, Belgium. 20-23 March 2018. Crit Care. 2018;22:82. doi: 10.1186/s13054-018-1973-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talmor D, Sarge T, Malhotra A, O’Donnell CR, Ritz R, Lisbon A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359:2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachmann MC, Morais C, Bugedo G, Bruhn A, Morales A, Borges JB, et al. Electrical impedance tomography in acute respiratory distress syndrome. Crit Care. 2018;22:263. doi: 10.1186/s13054-018-2195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Victorino JA, Borges JB, Okamoto VN, Matos GF, Tucci MR, Caramez MP, et al. Imbalances in regional lung ventilation: a validation study on electrical impedance tomography. Am J Respir Crit Care Med. 2004;169:791–800. doi: 10.1164/rccm.200301-133OC. [DOI] [PubMed] [Google Scholar]
- 29.Borges JB, Suarez-Sipmann F, Bohm SH, Tusman G, Melo A, Maripuu E, et al. Regional lung perfusion estimated by electrical impedance tomography in a piglet model of lung collapse. J Appl Physiol (1985) 2012;112:225–236. doi: 10.1152/japplphysiol.01090.2010. [DOI] [PubMed] [Google Scholar]
- 30.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713, quiz 786–788. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 31.NIH–NHLBI ARDS Clinical Network Mechanical ventilation protocol summary Boston, MA: Massachusetts General Hospital Biostatistics Center; 2008[accessed 2020 Dec 2]. Available from: http://www.ardsnet.org/files/ventilator_protocol_2008-07.pdf. [Google Scholar]
- 32.Lachmann B.Open lung in ARDS Minerva Anestesiol 200268637–642.[Discussion, pp. 640, 643.] [PubMed] [Google Scholar]
- 33.Yoshida T, Torsani V, Gomes S, De Santis RR, Beraldo MA, Costa EL, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med. 2013;188:1420–1427. doi: 10.1164/rccm.201303-0539OC. [DOI] [PubMed] [Google Scholar]
- 34.Lachmann B, Robertson B, Vogel J. In vivo lung lavage as an experimental model of the respiratory distress syndrome. Acta Anaesthesiol Scand. 1980;24:231–236. doi: 10.1111/j.1399-6576.1980.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 35.Gomes S, Belmino R, Hirota A, Costa E, Barbeiro D, Tucci M, et al. A new experimental model of the acute lung injury [abstract] Am J Respir Crit Care Med. 2009;179:A3568. [Google Scholar]
- 36.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 37.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 38.Lansdorp B, Hofhuizen C, van Lavieren M, van Swieten H, Lemson J, van Putten MJ, et al. Mechanical ventilation-induced intrathoracic pressure distribution and heart-lung interactions. Crit Care Med. 2014;42:1983–1990. doi: 10.1097/CCM.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 39.Vieillard-Baron A, Matthay M, Teboul JL, Bein T, Schultz M, Magder S, et al. Experts’ opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med. 2016;42:739–749. doi: 10.1007/s00134-016-4326-3. [DOI] [PubMed] [Google Scholar]
- 40.Mahmood SS, Pinsky MR. Heart-lung interactions during mechanical ventilation: the basics. Ann Transl Med. 2018;6:349. doi: 10.21037/atm.2018.04.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lemaire F, Teboul JL, Cinotti L, Giotto G, Abrouk F, Steg G, et al. Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology. 1988;69:171–179. doi: 10.1097/00000542-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Simmons DH, Linde LM, Miller JH, O’Reilly RJO. Relation between lung volume and pulmonary vascular resistance. Circ Res. 1961;9:465–471. [Google Scholar]
- 43.Thomas LJ, Jr, Griffo ZJ, Roos A. Effect of negative-pressure inflation of the lung on pulmonary vascular resistance. J Appl Physiol. 1961;16:451–456. doi: 10.1152/jappl.1961.16.3.451. [DOI] [PubMed] [Google Scholar]
- 44.Biondi JW, Schulman DS, Soufer R, Matthay RA, Hines RL, Kay HR, et al. The effect of incremental positive end-expiratory pressure on right ventricular hemodynamics and ejection fraction. Anesth Analg. 1988;67:144–151. [PubMed] [Google Scholar]
- 45.Slutsky AS. Lung injury caused by mechanical ventilation. Chest. 1999;116:9S–15S. doi: 10.1378/chest.116.suppl_1.9s-a. [DOI] [PubMed] [Google Scholar]
- 46.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 47.Dreyfuss D, Saumon G. Role of tidal volume, FRC, and end-inspiratory volume in the development of pulmonary edema following mechanical ventilation. Am Rev Respir Dis. 1993;148:1194–1203. doi: 10.1164/ajrccm/148.5.1194. [DOI] [PubMed] [Google Scholar]
- 48.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema: respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 49.Katira BH, Engelberts D, Otulakowski G, Giesinger RE, Yoshida T, Post M, et al. Abrupt deflation after sustained inflation causes lung injury. Am J Respir Crit Care Med. 2018;198:1165–1176. doi: 10.1164/rccm.201801-0178OC. [DOI] [PubMed] [Google Scholar]
- 50.Ikuta K, Wang Y, Robinson A, Ahmad T, Krumholz HM, Desai NR. National trends in use and outcomes of pulmonary artery catheters among Medicare beneficiaries, 1999-2013. JAMA Cardiol. 2017;2:908–913. doi: 10.1001/jamacardio.2017.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.