This study describes the detection, mitigation, and analysis of a large cluster of SARS-CoV-2 infections in an acute care hospital with mature infection control policies and discusses insights that may inform additional measures to protect patients and staff.
Visual Abstract. SARS-CoV-2 Cluster in an Acute Care Hospital.
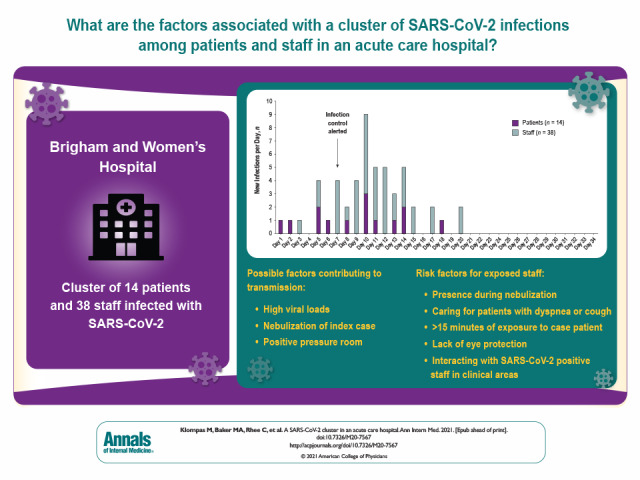
This study describes the detection, mitigation, and analysis of a large cluster of SARS-CoV-2 infections in an acute care hospital with mature infection control policies and discusses insights that may inform additional measures to protect patients and staff.
Abstract
Background:
Little is known about clusters of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in acute care hospitals.
Objective:
To describe the detection, mitigation, and analysis of a large cluster of SARS-CoV-2 infections in an acute care hospital with mature infection control policies.
Design:
Descriptive study.
Setting:
Brigham and Women's Hospital, Boston, Massachusetts.
Participants:
Patients and staff with cluster-related SARS-CoV-2 infections.
Intervention:
Close contacts of infected patients and staff were identified and tested every 3 days, patients on affected units were preemptively isolated and repeatedly tested, affected units were cleaned, room ventilation was measured, and specimens were sent for whole-genome sequencing. A case–control study was done to compare clinical interactions, personal protective equipment use, and breakroom and workroom practices in SARS-CoV-2–positive versus negative staff.
Measurements:
Description of the cluster, mitigation activities, and risk factor analysis.
Results:
Fourteen patients and 38 staff members were included in the cluster per whole-genome sequencing and epidemiologic associations. The index case was a symptomatic patient in whom isolation was discontinued after 2 negative results on nasopharyngeal polymerase chain reaction testing. The patient subsequently infected multiple roommates and staff, who then infected others. Seven of 52 (13%) secondary infections were detected only on second or subsequent tests. Eight of 9 (89%) patients who shared rooms with potentially contagious patients became infected. Potential contributing factors included high viral loads, nebulization, and positive pressure in the index patient's room. Risk factors for transmission to staff included presence during nebulization, caring for patients with dyspnea or cough, lack of eye protection, at least 15 minutes of exposure to case patients, and interactions with SARS-CoV-2–positive staff in clinical areas. Whole-genome sequencing confirmed that 2 staff members were infected despite wearing surgical masks and eye protection.
Limitation:
Findings may not be generalizable.
Conclusion:
SARS-CoV-2 clusters can occur in hospitals despite robust infection control policies. Insights from this cluster may inform additional measures to protect patients and staff.
Primary Funding Source:
None.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic continues to cause high case counts, hospitalizations, and deaths worldwide. Clusters have been reported in many different settings, but relatively few have been reported in acute care hospitals; indeed, some studies suggest that hospital-acquired SARS-CoV-2 may be rare in facilities with robust infection prevention and control programs (1–5). We report on a cluster of 52 SARS-CoV-2 infections involving 14 patients and 38 staff members in a large academic medical center that occurred despite mature infection prevention and control policies.
Methods
Description of Hospital and Baseline Infection Control Measures
Brigham and Women's Hospital is an 803-bed academic referral center associated with Harvard Medical School in Boston, Massachusetts. Between February and May 2020, the hospital implemented multiple infection control policies, including mandatory daily attestations of health by all employees, universal masking of all employees, eye protection for all encounters with unmasked patients (and later for all clinical encounters), encouraging inpatients to wear masks whenever providers enter their rooms, visitor restrictions, screening all patients on admission and daily thereafter for symptoms of coronavirus disease 2019 (COVID-19), nasopharyngeal polymerase chain reaction (PCR) testing of all patients on admission, use of N95 respirators or powered air-purifying respirators when caring for patients with suspected or confirmed COVID-19 (along with eye protection, gowns, and gloves), cohorting patients with confirmed COVID-19 into COVID care wards with negative pressure airflow rooms, free PCR testing for all symptomatic employees, and paid leave for all employees with confirmed COVID-19. These policies were associated with a very low rate of nosocomial infections during the first 7 months of the pandemic (2).
Cluster Detection
On 20 September 2020, the Infection Control Department was notified about an inpatient on the general medical service who tested positive for SARS-CoV-2 on hospital day 4 despite a negative admission test result. The patient was retested because of new fever. The following morning, a nurse working on the general medical service tested positive for SARS-CoV-2, and 2 medical residents reported new symptoms concerning for COVID-19. The nurse noted that her symptoms began 4 days earlier and that she had worked the 2 days before symptom onset.
The Infection Control Department identified patient and staff contacts of the infected patient and nurse for testing. Testing was initially limited to patients who received care from the infected nurse in the 2 days before her symptoms began, patients who shared a room with an infected patient, and employees who had face-to-face contact within 6 feet of an infected employee or patient for at least 15 minutes during which either party was not wearing a mask (providers wearing a mask and eye protection were initially excluded).
Testing identified 4 additional SARS-CoV-2–positive inpatients located on 3 hospital units, an environmental services worker assigned to 1 of the affected units, and a physician who had consulted on 1 of the newly identified SARS-CoV-2–positive inpatients 1 week earlier. Over the next 2 days, 3 additional inpatients, an outpatient who had been hospitalized on 1 of the affected units 1 week earlier, 4 physicians, 3 nurses, and a patient care assistant also tested positive for SARS-CoV-2. All patients were located on 1 of 3 units, and all staff members either were affiliated with an affected unit or had seen a case patient on 1 of the affected units. The patients were associated with 6 different clinical teams (4 general medical teams, a pulmonary transplant team, and a neurology team), all of whom were also managing patients in other areas of the hospital.
Cluster Response
The hospital's incident command structure was activated to mobilize resources and coordinate the cluster response. Given the number and rate of positive test results among employees and patients, mounting evidence that transmission had been taking place in the hospital for at least a week, and the breadth of medical teams involved (each with other patients throughout the hospital), testing indications were expanded. All staff members affiliated with the 3 affected units as well as all employees who spent at least 15 minutes face-to-face with any patient or staff member on an affected unit from 14 September onward, regardless of personal protective equipment use, were asked to undergo testing every 3 days. In addition, all patients on the affected units and all patients on medical teams throughout the hospital were preemptively placed on enhanced respiratory isolation (N95 respirator or powered air-purifying respirator, eye protection, gloves, and gowns for all encounters), and clinical teams began to test all hospitalized patients every 3 days. The hospital established a high-volume onsite anterior nares testing center open to any hospital employee concerned about possible infection, although testing was required only for contacts of confirmed cases and employees associated with affected units. Potentially exposed patients who had been discharged were contacted and referred for testing.
Infections were deemed potentially cluster-related if they occurred in a patient or staff member who spent at least 15 minutes interacting with staff or patients on 1 of the 3 cluster units or if they were direct contacts of potentially cluster-related SARS-CoV-2–positive staff or patients during their contagious periods (from 2 days before symptom onset—or 2 days before their positive test result for asymptomatic persons—until they were furloughed, discharged, or placed on enhanced respiratory isolation). Whenever possible, specimens from patients and staff with potentially cluster-related infections were sent to the Massachusetts Department of Public Health for whole-genome sequencing to confirm or refute their association with the cluster (see the Appendix, available at Annals.org, for a description of sequencing methods). If specimens from patients or staff with epidemiologic associations could not be retrieved or successfully amplified, they were presumed to be cluster-related. Staff and patients with epidemiologic associations but whole-genome sequences more than 2 single-nucleotide polymorphisms (SNPs) apart were deemed to not be cluster-related.
SARS-CoV-2–positive patients were moved to a dedicated COVID care unit. Additional measures included enhanced cleaning of all affected units; detailed chart reviews of affected patients to look for potential exposures outside the hospital and factors potentially contributing to spread in the hospital; electronic health record traces to identify all locations where infected patients received care and all staff who may have interacted with them; and occupational health interviews with all COVID-19–positive employees, using a structured tool to identify date of symptom onset, work history in the 2 days before symptoms, use of personal protective equipment, exposures to case patients in the preceding 14 days, and interactions with employees within 6 feet without masks (for example, eating together or sharing workrooms). Air changes were measured in all rooms on affected units, and the airflow pattern from the room of 1 patient associated with a disproportionate number of infections was assessed.
A case–control study was developed to compare work locations, care activities, personal protective equipment use, breakroom and workroom use patterns, and potential COVID-19 exposures outside the hospital in SARS-CoV-2–positive versus negative employees. The survey was distributed via REDCap (www.project-redcap.org) to all employees associated with cluster units who were tested for SARS-CoV-2. A copy of the survey is provided in the Supplement (available at Annals.org). Each responding employee with a cluster-related infection (as defined earlier) was matched to 4 SARS-CoV-2–negative employees on the basis of role group, test date, and work location. Exposure prevalence ratios for cases versus controls were calculated using the Mantel–Haenszel method to account for matching. Wald-type 95% CIs were calculated using the SE estimator proposed by Greenland and Robins for sparse data (6). Calculations were performed using R (r-project.org).
The cluster response was conducted under the auspices of hospital operations, but the decision to summarize and publish the cluster analysis was approved by the Mass General Brigham Institutional Review Board.
Role of the Funding Source
This study received no external funding.
Results
Fifteen patients and 42 employees met epidemiologic criteria for potentially cluster-related SARS-CoV-2 infection. After whole-genome sequencing, 14 patients and 38 employees were included in the cluster. One staff member was briefly hospitalized, and 6 of 14 (43%) patients and 13 of 38 (34%) staff members were asymptomatic. The daily counts of new patient and staff infections are shown in Figure 1. A map of patients' locations, positive staff members, and their interconnections is shown in Figure 2.
Figure 1. Epidemic curve showing the count of new patient and staff cases per day (by date of symptom onset or test date, whichever was earlier).
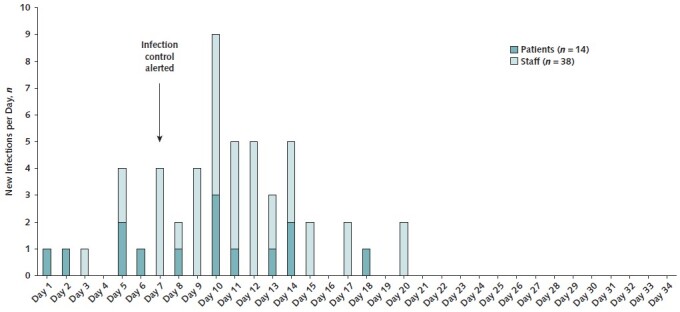
Figure 2. Cluster map depicting locations, role groups, medical teams, and interconnections among infected staff members and patients.
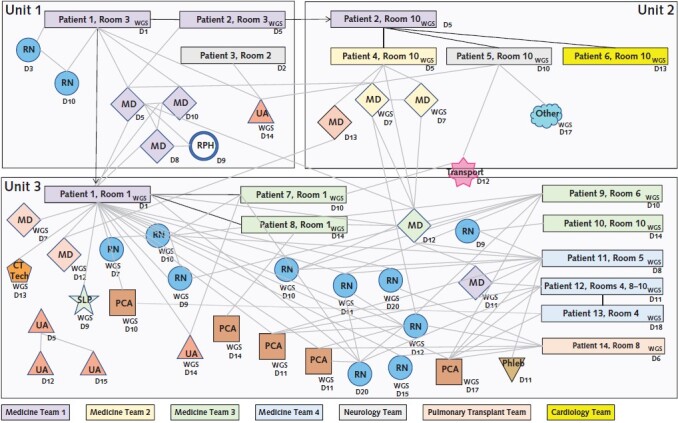
The colors of rectangles (patients) and diamonds (physicians) refer to their medical team affiliation (general medicine teams 1 to 4, neurology, pulmonary transplant, and cardiology). Nurses and other staff members are not affiliated with a specific team but rather with a specific inpatient unit. “WGS” indicates whole-genome sequencing confirmation of cluster association. Patients and staff without the “WGS” indicator were associated with the cluster on epidemiologic grounds. Solid lines between patient rectangles indicate that the 2 patients shared a room. CT Tech = computed tomography radiology technician; Dx = day of positive SARS-CoV-2 test result or symptom onset (whichever occurred earlier) relative to the start of the cluster; MD = medical doctor; PCA = patient care assistant; Phleb = phlebotomist; RN = registered nurse; RPH = registered pharmacist; SLP = speech and language pathology technician; UA = unit associate (environmental services worker).
Index Case and Subsequent Transmission
The index case appeared to be a patient with a history of chronic lung disease admitted for an elective procedure. The patient was dyspneic and tachycardic on arrival, so surgery was deferred. She was placed on enhanced respiratory isolation and tested for SARS-CoV-2. Isolation was discontinued after 2 negative results on nasopharyngeal PCRs obtained 12 hours apart. The patient's symptoms were attributed instead to exacerbation of chronic obstructive pulmonary disease. Treatment was initiated with oxygen by nasal cannula, prednisone, nebulized saline, and nebulized ipratropium bromide. The patient initially improved but then became progressively more dyspneic. Respiratory support was escalated to high-flow oxygen by nasal cannula on hospital day 11, the same day she was ultimately retested and diagnosed with COVID-19. Staff noted that the patient was frequently coughing, did not tolerate a mask, and had indistinct speech that led many providers to come near to understand her.
The index patient likely infected multiple staff on her initial unit as well as a patient who shared a room with her for 2 days. Her roommate was transferred to a second unit, where she shared a semiprivate room with 3 successive patients, each of whom became infected, along with multiple additional health care workers. None of these additional patients received nebulizers or underwent aerosol-generating procedures. The index patient was transferred to a third hospital unit on hospital day 3 and remained there until diagnosed with COVID-19 on hospital day 11. While on the third unit, 2 successive roommates of the index patient were infected, as well as 6 additional patients elsewhere on the unit (2 of the additionally infected patients shared a room with one another; 3 were in private rooms; and the sixth shared a room with 2 other patients for less than a day each, neither of whom was infected). All told, 9 patient pairs shared a room during which 1 patient was potentially contagious, and 8 of them led to transmission (median duration together, 28 hours [range, 18 to 132 hours]; distance between beds, 7 feet from midline to midline). In 3 of these instances, we were unable to identify an infected staff intermediary. Of the 33 patients admitted to the third unit during the 8 days the index patient was present, 8 became infected.
Staff Tracing and Testing
A total of 385 staff members were flagged as possible direct contacts of infected patients and/or employees through a combination of employee interviews and electronic health record system audits. An additional 1072 staff members were flagged by electronic health record audits and staffing assignments as having possibly spent at least 15 minutes interacting with staff or patients on 1 of the 3 cluster units. Of these, 1202 of 1457 were tested (testing was required for direct contacts of infected patients and strongly encouraged for staff affiliated with cluster units; staff who denied spending ≥15 minutes interacting with staff or patients on cluster units were permitted to opt out of testing). Eleven of 385 direct contacts of case patients and 27 of 1072 staff associated with cluster units tested positive for SARS-CoV-2.
Whole-Genome Sequencing and Serial Testing
Specimens were available for whole-genome sequencing from 14 of 15 patients and 31 of 42 employees who met epidemiologic criteria for potentially cluster-related infections. Sequencing was successful for 41 of the 45 available specimens. Of these, 36 had 0 to 2 SNP differences with at least 1 other specimen, and the remaining 5 had 10 to 30 SNP differences from all other strains and were thus deemed to be unrelated to the cluster. The 5 noncluster specimens were from 3 environmental services workers; 1 patient care assistant who worked on 1 of the cluster units; and 1 patient who received care from 2 presymptomatic cluster-related physicians, was discharged, and developed symptoms of SARS-CoV-2 infection 15 days later. Polymerase chain reaction cycle thresholds were less than 20 for 22 of the 44 (50%) cluster-related patients and staff for whom cycle thresholds were available. Seven of 52 (13%) cluster-related staff and patients tested positive only on their second, third, or fourth test (median of 8 days from initial negative result [range, 5 to 12 days]).
Assessment of Room Ventilation and Pressures
The rooms of all affected patients had at least 6 air changes per hour. The room that housed the index patient on unit 3 had 11 air changes per hour but was discovered to have positive pressure relative to the nursing station immediately outside the room. The room was not designed to be a protected environment. Tracer gas studies using sulfur hexafluoride confirmed airflow from the index patient's room to the nursing station (steady-state sulfur hexafluoride concentrations were 35 ppm in the patient's room, 11 ppm at the nursing station, and 0.5 ppm in all other rooms that housed case patients). Average humidity on cluster units was 34% (range, 20% to 45%).
Case–Control Study
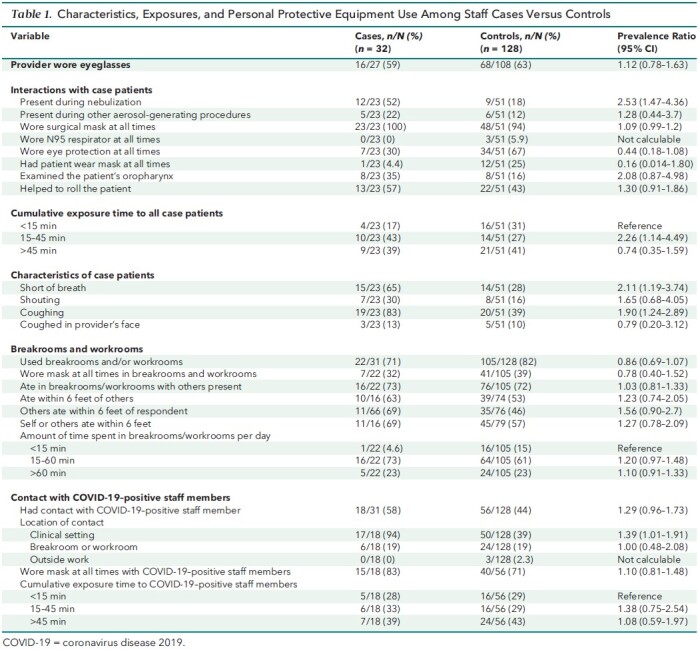
The results of the case–control study are summarized in Table 1. Responses were available from 32 of 38 employees with cluster-related infections and 552 of 1160 uninfected but exposed employees. The 32 cases included 8 physicians, 12 nurses, 4 patient care assistants, 4 environmental services workers, and 4 others. Infected staff members were more likely to report having been present while case patients received nebulizers (52% vs. 18%; prevalence ratio, 2.53 [95% CI, 1.47 to 4.36]), to have cared for patients who were short of breath (65% vs. 28%; prevalence ratio, 2.11 [CI, 1.19 to 3.74]) or coughing (83% vs. 39%; prevalence ratio, 1.90 [CI, 1.24 to 2.89]), to have interacted with SARS-CoV-2–positive staff members in clinical areas (94% vs. 39%; prevalence ratio, 1.39 [CI, 1.01 to 1.91]), and to have spent more time exposed to case patients (15 to 45 vs. <15 minutes, 43% vs. 27%; prevalence ratio, 2.26 [CI, 1.14 to 4.49]). Conversely, infected staff members were less likely to have worn eye protection (30% vs. 67%; prevalence ratio, 0.44 [CI, 0.18 to 1.08]). There were no differences between case and control employees' use of breakrooms and workrooms, amount of time spent in breakrooms and workrooms, or eating within 6 feet of others.
Table 1. Characteristics, Exposures, and Personal Protective Equipment Use Among Staff Cases Versus Controls.
Transmission Outside Cluster Units
Isolating the particular circumstances associated with most transmissions was difficult because cases on cluster units interacted with many different infected staff and/or patients on many different occasions. However, there were 2 instances in which the circumstances of transmission were more clearly defined because employees only interacted with a single case patient and only did so outside the cluster units (and hence were not exposed to nebulized air from the index patient and did not interact with any potentially infected staff members or other patients). The first was a radiology technician who performed computed tomography on the index patient 3 days after she was hospitalized. The technician reported that the encounter lasted 10 minutes; that he wore a surgical mask, eye protection, and gloves; and that the patient wore a surgical mask. He developed symptomatic COVID-19 twelve days later. The second transmission was to a speech and language therapy technician who assisted in performing a video swallow study on the index patient 5 days after she was hospitalized. The technician reported that the procedure took approximately 45 minutes, during which the patient was unmasked for approximately 10 to 15 minutes. The technician wore a surgical mask, a face shield, and gloves. The technician developed symptomatic COVID-19 six days later. There were no SNP differences between the index patient and either the radiology technician or the video swallow technician. These 2 staff members worked in different areas of the hospital and denied interacting with one another or with other case patients or staff from the cluster units.
Serial Testing of Patients Outside Cluster Units
Serial testing of inpatients outside the 3 known cluster units identified 4 asymptomatic patients with positive SARS-CoV-2 test results despite negative admission test results: 1 was negative on repeated testing and likely had a false-positive result, 2 had prior histories of COVID-19 and high cycle thresholds and thus the positive results probably reflected noninfectious residua of prior infections, and 1 likely had acute infection due to a household exposure immediately before admission (18 to 27 SNP differences from cluster strains). None were deemed to be cluster-related.
Discussion
We document a large cluster of SARS-CoV-2 infections among staff and patients in an acute care hospital despite a mature infection control program that included policies on universal screening and testing of all patients on admission, universal masking of providers, daily employee attestations of health, adequate supplies of personal protective equipment, and cohorting of SARS-CoV-2–positive patients in specialized units. The introduction, spread, and containment of this cluster provide several insights into SARS-CoV-2 transmission and possible strategies to enhance infection control programs to prevent similar events (Table 2).
Table 2. Summary of Cluster-Related Observations and Potential Strategies to Further Mitigate Risk for SARS-CoV-2 Transmission to Patients and Staff in Health Care Facilities Located in Communities With High Rates of New SARS-CoV-2 Infections.
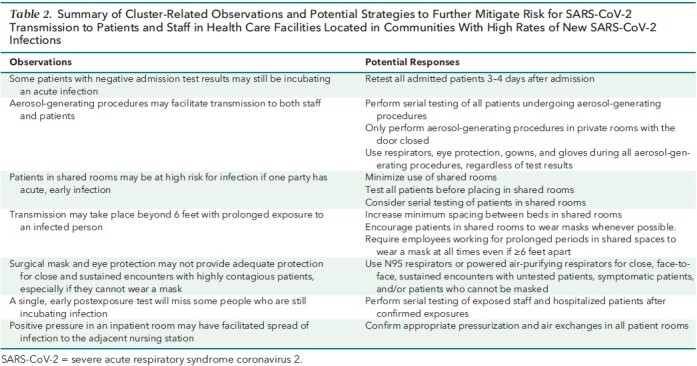
The virus was likely introduced into the facility by a symptomatic patient who tested negative twice on admission but in retrospect was contagious from at least hospital day 3 and infected staff and patients for at least a week before detection. This underscores the imperfect sensitivity of admission testing; false-negative results may be due to poor sample quality, heterogeneous distribution of virus across the respiratory tract, and testing during the incubation period when viral burden is low and possibly undetectable (7–9). Possible strategies to mitigate the limited sensitivity of admission testing include obtaining more than 1 specimen from higher-risk patients, routinely retesting all patients several days after admission, and obtaining lower respiratory tract specimens in patients with signs of lower respiratory tract infection (8, 9).
Several factors may have facilitated large numbers of secondary infections in this cluster. The index patient was early in the course of infection, was symptomatic with both dyspnea and cough, received frequent nebulizers, and was placed in a positive-pressure room. Viral loads and contagiousness tend to be highest early in infection, immediately before and after symptom onset (10–12). Patients incubating infection on admission may therefore pose a particularly high threat of causing nosocomial infections as they evolve into acute disease after admission (13). By contrast, most patients admitted to the hospital with confirmed COVID-19 have already been symptomatic for several days and may therefore be at a less contagious phase of the disease (14, 15). Patients also vary in their infectiousness; a disproportionate number of staff and patient infections were associated with a limited subset of patients (Figure 2), a finding echoed in contact tracing studies (16, 17).
Nebulizers may have played a role in facilitating transmission to staff and patients in the patient's room and possibly immediately outside her room. Many investigators have documented associations between nebulizers and transmission of respiratory pathogens (18–22). Some authorities consider nebulizers to be aerosol-generating procedures, but others do not because nebulizers are designed to aerosolize medications rather than respiratory secretions (23). It is conceivable, however, that aerosols generated by nebulizers may entrain exhaled respiratory particles and thus facilitate their spread. Alternatively, nebulizers may simply be a proxy for the severity of patients' respiratory symptoms (24). Nonetheless, our cluster cannot be attributed solely to aerosol-generating procedures because more than half of infected patients and staff had no association with the index patient and were not exposed to aerosol-generating procedures.
We documented multiple sets of infected roommates. Although it is possible that virus was transferred between roommates by infected staff, in several instances we were unable to identify an infected staff intermediary. Transmission to roommates may have occurred via respiratory emissions despite a curtain between patients and average separation of more than 6 feet. This bespeaks the possible role of aerosol transmission, particularly with prolonged exposure in confined spaces without masks (20). Other possible explanations include shared bathrooms and sinks, interactions between patients at less than 6 feet, or occult SARS-CoV-2–positive staff intermediaries.
Lapses in use of personal protective equipment may also have played a role in transmission. Infected staff members used eye protection and masked their patients less frequently than uninfected staff members. The Centers for Disease Control and Prevention (CDC) has stressed the importance of masking patients for source control, and other investigators have reported that eye protection may prevent infections (25–28). Similarly, a minority of staff members wore masks at all times in breakrooms and workrooms, and many ate within 6 feet of one another. However, we were not able to demonstrate meaningful differences in these practices between infected and uninfected staff.
We identified at least 2 patient-to-staff transmissions via whole-genome sequencing that occurred despite staff wearing both masks and face shields and in the absence of aerosol-generating procedures. The infected staff members worked outside the cluster units and did not interact with other infected staff members or patients. These infections despite use of surgical masks and face shields may have been due to short-range aerosol transmission in the context of a highly contagious symptomatic patient early in the disease course. Other possible explanations include fomite transmission or self-contamination during removal of personal protective equipment. Surgical masks reduce but do not eliminate aerosol and viral exposure and may therefore offer incomplete protection for providers who need to provide sustained, close-range care to COVID-19–positive patients (24, 29–33). This raises the question of whether wearing N95 respirators could prevent additional health care worker infections during sustained, near-range interactions with selected patients in high-incidence settings (such as untested patients, symptomatic patients, or patients unable to wear masks) (26). This merits testing.
Limitations of our study include the many interconnections among infected staff, between staff and patients, and among patients in an array of settings (patients' rooms, clinicians' workrooms, and staff breakrooms), which make it difficult to isolate the interactions and factors that led to transmission. We were able to obtain whole-genome sequences on most but not all potentially cluster-related infections and so may have misattributed some infections to the cluster. Staff members' responses to the case–control survey may have been colored by recall and favorability biases, leading to either underestimation or overestimation of adherence to infection control practices. The patterns of transmission we observed may be particular to this cluster, our institution, the patient population, or local practices and may not be generalizable to other patients and settings.
In conclusion, we documented a large nosocomial cluster potentially attributable to a missed case despite admission testing. Lessons learned include the limitations of admission testing, variability between patients in transmissibility, the high risk for roommate-to-roommate transmissions in the setting of occult acute infection, the potential value of serial testing to identify infections incubating on admission, opportunities to improve adherence to eye protection and masking of patients, the possible limitations of surgical masks and face shields to protect providers with near-range exposure to symptomatic patients, and the value of whole-genome sequencing to help define and contain hospital clusters.
Supplementary Material
Appendix: Supplementary Methods
Sequencing Laboratory Methods
Total nucleic acid from respiratory specimens was extracted using the Roche MagNA Pure 96 DNA and Viral NA Small Volume Pack. Presence and abundance estimates of SARS-CoV-2 RNA were evaluated by the CDC 2019-Novel Coronavirus Real-Time RT-PCR Diagnostic Panel (34). Tiled, whole-genome amplicon sequencing was performed using an adapted ARTIC V3 SARS-CoV-2 protocol (35) and a common protocol developed by a collaborative group of state public health laboratories (36, 37) and the CDC (38). Modifications include 1) increase the initial extracted RNA input from 10 μL to 15 μL, and 2) increase working stock of the primer working stocks (for both pool A and pool B) from 10 μM to 15 μM. Briefly, for each sample, 15 μL of extracted RNA was prepared for sequencing. By reverse transcription to complementary DNA, samples were then processed and amplified using 2 highly multiplex PCR v3 primer reactions. The samples were combined after PCR tiling, screened, and quantified for Illumina DNA Prep. The Illumina DNA Prep kit consists of a bead-based transposome complex tagging genomic DNA that, through limited-cycle PCR, tags the DNA with adapter sequences (39). Prepared libraries were sequenced on the Illumina MiSeq sequencer.
Bioinformatics Analysis Methods
The Cecret pipeline (https://github.com/UPHL-BioNGS/Cecret) was used, with minor modifications for our local environment, to generate consensus genomes for each sample. In brief, reads that had undergone quality control (FastQC v0.11.8 [40]; SeqyClean v1.10.09 [41]) were aligned (BWA v0.7.17 [42]) to a reference SARS-CoV-2 genome (MN908947.3), and a consensus genome was called for each sample (iVar v1.2.2 [43]). To ensure accuracy of results, we only considered highly complete (≥95% coverage) genomes in downstream analyses. We aligned these sequences (MAFFT v7.450 [44]) and computed pairwise distances between sample genomes. Resultant SNP distances were discussed within the context of epidemiologic linkage to rule in or rule out individuals from this particular cluster.
Footnotes
This article was published at Annals.org on 9 February 2021
References
- 1. Baker MA , Fiumara K , Rhee C , et al; CDC Prevention Epicenters Program. Low risk of COVID-19 among patients exposed to infected healthcare workers. Clin Infect Dis. 2020. [PMID: ] doi: 10.1093/cid/ciaa1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rhee C , Baker M , Vaidya V , et al; CDC Prevention Epicenters Program. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA Netw Open. 2020;3:e2020498. [PMID: ] doi: 10.1001/jamanetworkopen.2020.20498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng VCC , Wong SC , Chuang VWM , et al. Absence of nosocomial transmission of coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in the prepandemic phase in Hong Kong. Am J Infect Control. 2020;48:890-6. [PMID: ] doi: 10.1016/j.ajic.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rickman HM , Rampling T , Shaw K , et al. Nosocomial transmission of COVID-19: a retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clin Infect Dis. 2020. [PMID: ] doi: 10.1093/cid/ciaa816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dimcheff DE , Schildhouse RJ , Hausman MS , et al. Seroprevalence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection among Veterans Affairs healthcare system employees suggests higher risk of infection when exposed to SARS-CoV-2 outside the work environment. Infect Control Hosp Epidemiol. 2020:1-7. [PMID: ] doi: 10.1017/ice.2020.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greenland S , Robins JM . Estimation of a common effect parameter from sparse follow-up data. Biometrics. 1985;41:55-68. [PMID: ] [PubMed] [Google Scholar]
- 7. Ai T , Yang Z , Hou H , et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32-E40. [PMID: ] doi: 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Someren Gréve F, Juffermans NP, Bos LDJ, et al.. Respiratory viruses in invasively ventilated critically ill patients—a prospective multicenter observational study. Crit Care Med. 2018;46:29-36. [PMID: ] doi: 10.1097/CCM.0000000000002752 [DOI] [PubMed] [Google Scholar]
- 9. Mohammadi A , Esmaeilzadeh E , Li Y , et al. SARS-CoV-2 detection in different respiratory sites: a systematic review and meta-analysis. EBioMedicine. 2020;59:102903. [PMID: ] doi: 10.1016/j.ebiom.2020.102903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Salvatore PP , Dawson P , Wadhwa A , et al. Epidemiological correlates of PCR cycle threshold values in the detection of SARS-CoV-2. Clin Infect Dis. 2020. [PMID: ] doi: 10.1093/cid/ciaa1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng HY , Jian SW , Liu DP , et al; Taiwan COVID-19 Outbreak Investigation Team. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180:1156-63. [PMID: ] doi: 10.1001/jamainternmed.2020.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He X , Lau EHY , Wu P , et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672-5. [PMID: ] doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi T , Trannel A , Holley SA , et al. COVID-19 serial testing among hospitalized patients in a midwest tertiary medical center, July–September 2020. Clin Infect Dis. 2020. [PMID: ] doi: 10.1093/cid/ciaa1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Basile K , McPhie K , Carter I , et al. Cell-based culture of SARS-CoV-2 informs infectivity and safe de-isolation assessments during COVID-19. Clin Infect Dis. 2020. [PMID: ] doi: 10.1093/cid/ciaa1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rhee C , Kanjilal S , Baker M , et al. Duration of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectivity: when is it safe to discontinue isolation. Clin Infect Dis. 2020. [PMID: ] doi: 10.1093/cid/ciaa1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laxminarayan R , Wahl B , Dudala SR , et al. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science. 2020;370:691-7. [PMID: ] doi: 10.1126/science.abd7672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adam DC , Wu P , Wong JY , et al. Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong. Nat Med. 2020;26:1714-9. [PMID: ] doi: 10.1038/s41591-020-1092-0 [DOI] [PubMed] [Google Scholar]
- 18. Jones AM , Govan JR , Doherty CJ , et al. Identification of airborne dissemination of epidemic multiresistant strains of Pseudomonas aeruginosa at a CF centre during a cross infection outbreak. Thorax. 2003;58:525-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loeb M , McGeer A , Henry B , et al. SARS among critical care nurses, Toronto. Emerg Infect Dis. 2004;10:251-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu IT , Wong TW , Chiu YL , et al. Temporal-spatial analysis of severe acute respiratory syndrome among hospital inpatients. Clin Infect Dis. 2005;40:1237-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heinzerling A , Stuckey MJ , Scheuer T , et al. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient - Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69:472-6. [PMID: ] doi: 10.15585/mmwr.mm6915e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cummings DAT , Radonovich LJ , Gorse GJ , et al. Risk factors for healthcare personnel infection with endemic coronaviruses (HKU1, OC43, NL63, 229E): results from the Respiratory Protection Effectiveness Clinical Trial (ResPECT). Clin Infect Dis. 2020. [PMID: ] doi: 10.1093/cid/ciaa900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tran K , Cimon K , Severn M , et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7:e35797. [PMID: ] doi: 10.1371/journal.pone.0035797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klompas M , Baker M , Rhee C . What is an aerosol-generating procedure. JAMA Surg. 2020. [PMID: ] doi: 10.1001/jamasurg.2020.6643 [DOI] [PubMed] [Google Scholar]
- 25. Raboud J , Shigayeva A , McGeer A , et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One. 2010;5:e10717. [PMID: ] doi: 10.1371/journal.pone.0010717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chu DK , Akl EA , Duda S , et al; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-87. [PMID: ] doi: 10.1016/S0140-6736(20)31142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zeng W , Wang X , Li J , et al. Association of daily wear of eyeglasses with susceptibility to coronavirus disease 2019 infection. JAMA Ophthalmol. 2020. [PMID: ] doi: 10.1001/jamaophthalmol.2020.3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bhaskar ME , Arun S . SARS-CoV-2 infection among community health workers in India before and after use of face shields. JAMA. 2020;324:1348-9. [PMID: ] doi: 10.1001/jama.2020.15586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leung NHL , Chu DKW , Shiu EYC , et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676-80. [PMID: ] doi: 10.1038/s41591-020-0843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clapp PW , Sickbert-Bennett EE , Samet JM , et al; US Centers for Disease Control and Prevention Epicenters Program. Evaluation of cloth masks and modified procedure masks as personal protective equipment for the public during the COVID-19 pandemic. JAMA Intern Med. 2020. [PMID: ] doi: 10.1001/jamainternmed.2020.8168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sickbert-Bennett EE , Samet JM , Clapp PW , et al. Filtration efficiency of hospital face mask alternatives available for use during the COVID-19 pandemic. JAMA Intern Med. 2020. [PMID: ] doi: 10.1001/jamainternmed.2020.4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan JF , Yuan S , Zhang AJ , et al. Surgical mask partition reduces the risk of noncontact transmission in a golden Syrian hamster model for coronavirus disease 2019 (COVID-19). Clin Infect Dis. 2020;71:2139-49. [PMID: ] doi: 10.1093/cid/ciaa644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ueki H , Furusawa Y , Iwatsuki-Horimoto K , et al. Effectiveness of face masks in preventing airborne transmission of SARS-CoV-2. m. Sphere. 2020;5. [PMID: ] doi: 10.1128/mSphere.00637-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel: Instructions for Use. 2[WEB_ONLY]020. Accessed at www.fda.gov/media/134922/download on 14 January 2021.
- 35. ARTIC Network. SARS-CoV-[WEB_ONLY]2. Accessed at https://artic.network/ncov-2019 on 14 January 2021.
- 36. Sevinsky J, Nassiri A, Young E, et al. SARS-CoV-2 sequencing on Illumina MiSeq using ARTIC protocol: part 1 – tiling PCR V.[WEB_ONLY]1. Accessed at www.protocols.io/view/sars-cov-2-sequencing-on-illumina-miseq-using-arti-bfefjjbn on 14 January 2[WEB_ONLY]021. doi:10.17504/protocols.io.bfefjjbn
- 37. Sevinsky J, Nassiri A, Blankenship H, et al. SARS-CoV-2 sequencing on Illumina MiSeq using ARTIC protocol: part 2 – Illumina DNA Flex protocol V.[WEB_ONLY]1. Accessed at www.protocols.io/view/sars-cov-2-sequencing-on-illumina-miseq-using-arti-bffyjjpw on 14 January 2[WEB_ONLY]021. doi:10.17504/protocols.io.bffyjjpw
- 38.CDCgov/SARS-CoV-2_Sequencing. Accessed at https://github.com/CDCgov/SARS-CoV-2_Sequencing on 14 January 2021.
- 39. Illumina. Illumina DNA Prep: Reference Guide. 2[WEB_ONLY]020. Accessed at https://sapac.support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/illumina_prep/illumina-dna-prep-reference-guide-1000000025416-09.pdf on 14 January 2021.
- 40. Babraham Bioinformatics. FastQC. Accessed at www.bioinformatics.babraham.ac.uk/projects/fastqc on 14 January 2021.
- 41. Zhbannikov IY, Hunter SS, Foster JA, et al. SeqyClean: a pipeline for high-throughput sequence data preprocessing. In: ACM-BCB ‘17: Proceedings of the 8th ACM International Conference on Bioinformatics, Computational Biology, and Health Informatics, Boston, Massachusetts, August 2[WEB_ONLY]017. Association for Computing Machinery; 2017:407-[WEB_ONLY]16. doi:10.1145/3107411.3107446
- 42. Li H , Durbin R . Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754-60. [PMID: ] doi: 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grubaugh ND , Gangavarapu K , Quick J , et al. An amplicon-based sequencing framework for accurately measuring intrahost virus diversity using PrimalSeq and iVar. Genome Biol. 2019;20:8. [PMID: ] doi: 10.1186/s13059-018-1618-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Katoh K, Standley DM. MAFFT: iterative refinement and additional methods. In: Russell DJ, ed. Multiple Sequence Alignment Methods. Springer; 2014:131-[WEB_ONLY]46. doi:10.1007/978-1-62703-646-7_8 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


