Abstract
Rationale: Socioeconomic factors are associated with worse disease severity at presentation in sarcoidosis, but the relative importance of socioeconomic variables on morbidity and disease burden has not been fully elucidated.
Objectives: To determine the association between income and sarcoidosis outcomes after controlling for socioeconomic and disease-related factors.
Methods: Using the Sarcoidosis Advanced Registry for Cures database, we analyzed data from 2,318 patients with sarcoidosis in the United States to determine the effect of income and other variables on outcomes. We divided comorbidities arising after diagnosis into those likely related to steroid use and those likely related to sarcoidosis. We assessed the development of health-related, functional, and socioeconomic outcomes following the diagnosis of sarcoidosis.
Measurements and Main Results: In multivariate analysis, low-income patients had significantly higher rates of new sarcoidosis-related comorbidities (<$35,000, odds ratio [OR], 2.4 [1.7–3.3]; $35,000–84,999, OR, 1.4 [1.1–1.9]; and ≥$85,000 [reference (Ref)]) and new steroid-related comorbidities (<$35,000, OR, 1.3 [0.9–2.0]; $35,000–84,999, OR, 1.5 [1.1–2.1]; and ≥$85,000 [Ref]), had lower health-related quality of life as assessed by the Sarcoidosis Health Questionnaire (P < 0.001), and experienced more impact on family finances (<$35,000, OR, 7.9 [4.9–12.7]; $35,000–84,999, OR, 2.7 [1.9–3.9]; and ≥$85,000 [Ref]). The use of supplemental oxygen, need for assistive devices, and job loss were more common in lower income patients. Development of comorbidities after diagnosis of sarcoidosis occurred in 63% of patients and were strong independent predictors of poor outcomes. In random forest modeling, income was consistently a leading predictor of outcome.
Conclusions: These results suggest the burden from sarcoidosis preferentially impacts the economically disadvantaged.
Keywords: sarcoidosis, socioeconomic factors, healthcare disparities, comorbidity
At a Glance Commentary
Scientific Knowledge on the Subject
Low socioeconomic status is known to be related to more severe disease on presentation in patients with sarcoidosis. It has not been elucidated if this is due to more severe disease effect or due to late presentation to care.
What This Study Adds to the Field
In this study, we show a consistent, strong association of low income with worse health-related, quality of life, and financial outcomes in a large sample of patients with sarcoidosis in the United States years after the diagnosis using a prospectively collected online survey (the Sarcoidosis Advanced Registry for Cures database). We also demonstrate significant morbidity related to sarcoidosis, its treatment, and comorbidities associated with the disease.
Sarcoidosis has a wide spectrum of disease manifestations, with some patients experiencing spontaneous remission (1) and others developing multiorgan involvement with significant morbidity and impairment of quality of life (QoL) (2). In addition to the burden of the disease itself, the course of sarcoidosis is frequently complicated by comorbid conditions (3), especially in those with multiorgan involvement (4). Past studies have demonstrated that patients with sarcoidosis who develop comorbidities are less physically active and more fatigued (5, 6), have higher health care costs (7), and have higher hospitalization and mortality rates (8). Although the median yearly treatment costs for sarcoidosis are low, for those with severe disease, the costs of diagnostic testing and appropriate treatment can be quite high (9). These data suggest that severe forms of sarcoidosis result in significant morbidity and are associated with significant medical costs.
Severe sarcoidosis is more common in populations with fewer resources and significant barriers to care. Past studies noted increased disease severity at presentation in low-income patients, black patients, the less educated, and those without Medicare or private insurance (10, 11). Potentially compounding the situation, sarcoidosis can also have a significant impact on patients’ finances. A recent Swedish population-based study demonstrated a significant increase in the number of days of work missed in the year of diagnosis (12). In that analysis, those with the most days missed tended to be older and less educated, a group that is less likely to be able to tolerate financial setbacks (12). These analyses demonstrated that the burden of this disease may be falling disproportionately on the economically disadvantaged.
The aim of this study was to evaluate the overall burden of sarcoidosis on a range of health-related, social, and QoL indicators in patients with sarcoidosis. We hypothesized that low income would be associated with worse outcomes after correction for other socioeconomic factors and for disease severity. We also sought to investigate the impact of comorbidity burden on outcomes. Some of these results of these studies have been previously reported in the form of an abstract (13).
Methods
Study Population
The study population was composed of 2,318 adult respondents to the Foundation for Sarcoidosis Research–Sarcoidosis Advanced Registry for Cures questionnaire (FSR-SARC) (14). This questionnaire is a Web-based, patient opt-in, observational cohort platform. The item bank consists of 72 questions regarding patient demographics, disease involvement, diagnostic testing, treatment modalities, and health and social outcomes. The database collects longitudinal and cross-sectional data, allowing patients to update responses over time. Participants are recruited by the Foundation for Sarcoidosis Research via in-person forums and online websites. Patients are also recruited through their medical providers and national and international professional organizations. For this analysis, we used only the baseline survey responses.
We included all surveys completed between June 1, 2014, and December 31, 2016, with a total of 2,630 respondents. From those 2,630 respondents, we excluded 312 persons who were not U.S. citizens (11% of the total respondents) to avoid biases arising from differing social support and insurance systems in other countries, leaving us with our final sample of 2,318. All predictor, confounding, and outcome measures were collected via electronic survey. “Unsure” was coded as “missing” for data analysis. For each outcome, only complete cases were analyzed. Differences in rates of predictor variables and outcomes between patients with and without income data were analyzed to assess for systematic differences (reported in the online supplement).
Our primary exposure was patient-reported household income (defined in the online supplement). We included prespecified covariates related to patient disease burden and socioeconomic status in our models. These variables were collected by patient self-report (see online supplement). We included age at time of survey, household size, sex, race, insurance status, education, organ involvement, medication history, and development of new comorbidities after the diagnosis of sarcoidosis. We divided newly arising comorbidities into those more likely to be related to sarcoidosis (heart failure, fibromyalgia, chronic pain, sleep disorder, chronic fatigue, depression, and lymphoma) and comorbidities more likely to be related to steroid use (glaucoma, diabetes, osteoporosis, cataracts, hypertension, obesity, and sleep apnea). We investigated medical outcomes (ever hospitalized for sarcoidosis, supplemental oxygen use, and mobility device use), health-related QoL as defined by the Sarcoidosis Health Questionnaire (15), and social outcomes (effect on family finances and loss of employment after diagnosis of sarcoidosis). See the online supplement for detailed description of survey questions and coding assessing predictors and outcomes.
The study variables were described using mean with SD for continuous variables and counts with percentage for categorical variables. Odds ratios (ORs) are shown with 95% confidence intervals. Continuous variables were compared using the two-sample independent t test, whereas categorical variables were compared using the Pearson’s chi-square test. To avoid overfitting and miscalibration issues in model building, we applied a shrinkage procedure for variable selection (16, 17). Specifically, the group least absolute shrinkage and selection operator (LASSO) method with Bayesian information criterion was used here. The method is a variant of the standard LASSO method that is specifically designed for the models that have multiple categorical predictors. Correlation analysis of the predictors was conducted to avoid the multicollinearity in a regression model. Only complete cases were included in each model. The regression equation was constructed with predictors selected by the group LASSO method. To determine the relative importance of each predictor, we also performed random forest modeling. The variable importance plots based on the Gini index were generated for each outcome (18). Results are reported in accordance with the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) guidelines (19). All analyses were two-tailed and were performed at a significance level of 0.05. SAS 9.3 software (SAS Institute) was used for all analyses.
Results
Study Population
A total of 2,318 patients were identified for analysis. Social demographic data are provided in Table 1 and disease demographic data are provided in Table 2. The median income range of the cohort was $60,000 to $74,999, with 48% of the participants having completed college. The population was predominantly white (73%) and female (74%) (Table 1). The disease burden was high, with 56% currently requiring medical therapy for sarcoidosis (Table 2). The high disease burden translated into a high prevalence of our prespecified outcomes, with 47% developing a new sarcoidosis-associated comorbidity following the diagnosis of sarcoidosis (mean number of new sarcoidosis comorbidities of 1.2 ± 1.4), 48% developing a new steroid-associated comorbidity following the diagnosis of sarcoidosis (mean number of new steroid-related comorbidities of 1.1 ± 1.3), 36% reporting at least one hospitalization for sarcoidosis (not for diagnostic testing), 44% reporting a great or severe effect on family finances, 21% using supplemental oxygen, 17% using a walker or wheelchair, and 29% having lost their job due to their sarcoidosis (Table 3).
Table 1.
Social Demographics
| Age, yr, mean (SD) |
52.2 (10.7) |
| Sex, n (%) | |
| F | 1,718 (74) |
| M | 600 (26) |
| Missing | 0 (0) |
| Race, n (%) | |
| African American | 418 (18) |
| White | 1,689 (73) |
| Other | 191 (8) |
| Missing | 20 (1) |
| Household income, n (%) | |
| ≥$85,000 | 685 (30) |
| $35,000–84,999 | 795 (34) |
| $0–34,999 | 577 (25) |
| Missing | 261 (11) |
| Insurance type, n (%) | |
| Private | 1,521 (66) |
| Government | 673 (29) |
| None | 78 (3) |
| Missing | 46 (2) |
| Education, n (%) | |
| >College | 457 (20) |
| College | 675 (29) |
| >HS without college degree | 654 (28) |
| HS or less | 369 (16) |
| Missing | 163 (7) |
| People in home, mean (SD) | 2.7 (1.5) |
Definition of abbreviation: HS = high school.
Data are expressed as absolute n (%) or mean (SD) if appropriate.
Table 2.
Disease Demographics
| Duration of disease, yr, mean (SD) | 11.7 (11.9) |
| Medications for sarcoidosis, n (%) | |
| Current | 1,312 (56) |
| Past | 634 (27) |
| None | 313 (14) |
| Missing | 59 (3) |
| Develop sarcoidosis comorbidity, n (%) | |
| Yes | 1,084 (47) |
| No | 976 (42) |
| Missing | 258 (11) |
| Develop steroid comorbidity, n (%) | |
| Yes | 1,123 (48) |
| No | 937 (40) |
| Missing | 258 (11) |
| Pulmonary involvement, n (%) | |
| Yes | 1,794 (77) |
| No | 384 (17) |
| Missing | 140 (6) |
| Skin involvement, n (%) | |
| Yes | 1,357 (59) |
| No | 753 (32) |
| Missing | 208 (9) |
| Ocular involvement, n (%) | |
| Yes | 756 (33) |
| No | 1,243 (54) |
| Missing | 319 (14) |
| Cardiac involvement, n (%) | |
| Yes | 487 (21) |
| No | 1,360 (59) |
| Missing | 471 (20) |
| Neurologic involvement, n (%) | |
| Yes | 381 (16) |
| No | 1,514 (65) |
| Missing | 423 (18) |
Data are expressed as absolute n (%) or mean (SD) if appropriate.
Table 3.
Outcomes by Income Level (Univariate)
| Total | $0–34,999 | $35,000–84,999 | ≥$85,000 | P Value | |||
|---|---|---|---|---|---|---|---|
| n (%) | 2,318 | 577 (25) | 795 (34) | 685 (30) | |||
| New sarcoidosis comorbidity, n (%) |
<0.001 | ||||||
| Yes | 1,084 (47) | 349 (61) | 380 (49) | 250 (37) | |||
| No | 976 (40) | 159 (28) | 333 (42) | 361 (53) | |||
| Missing | 258 (11) | 69 (12) | 82 (10) | 74 (11) | |||
| New sarcoidosis comorbidity, mean (SD) | 1.2 (1.4) | 1.7 (1.6) | 1.2 (1.4) | 0.8 (1.1) | <0.001 | ||
| New steroid comorbidity, n (%) |
<0.001 | ||||||
| Yes | 1,123 (48) | 323 (56) | 409 (51) | 270 (39) | |||
| No | 937 (40) | 185 (32) | 304 (38) | 341 (50) | |||
| Missing | 258 (11) | 69 (12) | 82 (10) | 74 (11) | |||
| New steroid comorbidity, mean (SD) | 1.1 (1.3) | 1.4 (1.5) | 1.1 (1.3) | 0.8 (1.1) | <0.001 | ||
| SHQ, mean (SD) | 3.7 (1.1) | 3.1 (1.0) | 3.6 (1.0) | 4.1 (1.1) | <0.001 | ||
| Hospitalized ever, n (%) |
<0.001 | ||||||
| Yes | 831 (36) | 264 (46) | 258 (33) | 225 (33) | |||
| No | 1,362 (59) | 269 (47) | 495 (62) | 442 (65) | |||
| Missing | 125 (5) | 44 (8) | 42 (5) | 18 (3) | |||
| Finances affected, n (%) |
<0.001 | ||||||
| Greatly or severely affected | 1,025 (44) | 415 (72) | 364 (46) | 161 (24) | |||
| No or slight effect | 1,203 (52) | 136 (24) | 418 (53) | 505 (74) | |||
| Missing | 90 (4) | 26 (5) | 13 (2) | 19 (3) | |||
| Supplemental oxygen, n (%) |
<0.001 | ||||||
| Oxygen device | 476 (21) | 159 (28) | 169 (21) | 91 (13) | |||
| None | 1,791 (77) | 410 (71) | 609 (77) | 577 (84) | |||
| Missing | 51 (2) | 8 (1) | 17 (2) | 17 (3) | |||
| Mobility device, n (%) |
<0.001 | ||||||
| Yes | 383 (17) | 171 (30) | 127 (16) | 50 (7) | |||
| No | 1,862 (80) | 386 (67) | 645 (81) | 620 (91) | |||
| Missing | 73 (3) | 20 (4) | 23 (3) | 15 (2) | |||
| Lost job, n (%) |
<0.001 | ||||||
| Yes | 678 (29) | 290 (50) | 224 (28) | 114 (17) | |||
| No | 1,227 (53) | 152 (26) | 447 (56) | 493 (72) | |||
| Not applicable, did not work | 265 (11) | 94 (16) | 77 (10) | 49 (7) | |||
| Missing | 148 (6) | 41 (7) | 47 (6) | 29 (4) | |||
Definition of abbreviation: SHQ = Sarcoidosis Health Questionnaire.
Health Outcomes: Predictors of Development of New Sarcoidosis-associated Comorbidities
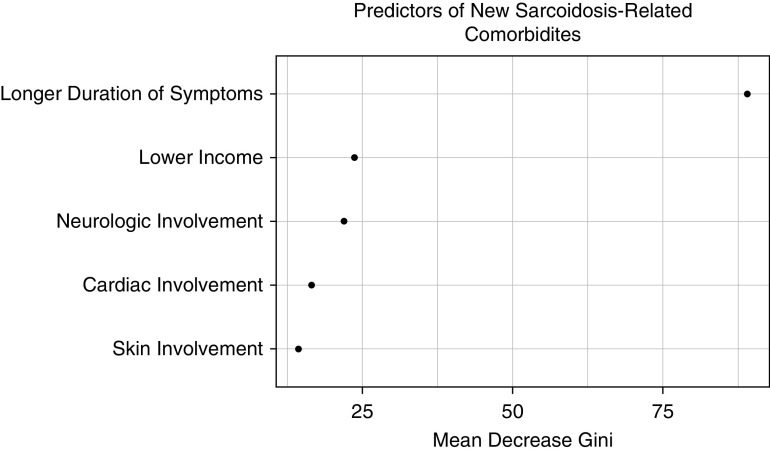
The most common sarcoidosis-related comorbidities were depression (28%), sleep disorders (22%), chronic fatigue (19%), and chronic pain (19%) (see Figure E1 in the online supplement). In multivariate logistic regression, income was a significant predictor of development of new sarcoidosis-related comorbidity (<$35,000, OR, 2.4 [1.7–3.3] and $35,000–84,999, OR, 1.4 [1.1–1.9]) (Table 4). Other independent predictors of development of sarcoidosis-associated comorbidity were neurologic involvement, cardiac involvement, skin involvement, and duration of symptoms. In random forest analysis, income was a leading predictor of development of new sarcoidosis-related comorbidity, following duration of symptoms (Figure 1).
Table 4.
Development of Sarcoidosis Comorbidity (Predictors of Development of Disease-related Comorbidity after Diagnosis with Sarcoidosis)
| Univariate [OR (95% CI)] | Multivariate, LASSO [OR (95% CI)] (n = 1,118) | |
|---|---|---|
|
Income | ||
| ≥$85,000 | Ref | Ref |
| $35,000–84,999 | 1.6 (1.3–2.0) | 1.4 (1.1–1.9) |
| <$35,000 | 3.2 (2.5–4.1) | 2.4 (1.7–3.3) |
| Age, yr | 1.001 (0.99–1.01) | — |
| Sex, M | 0.8 (0.7–1.03) | — |
| Race |
— | |
| White | Ref | |
| African American | 1.8 (1.4–2.2) | |
| Other | 1.5 (1.1–2.1) | |
| Insurance type |
— | |
| Private | Ref | |
| Government | 2.0 (1.7–2.5) | |
| No coverage | 2.0 (1.2–3.2) | |
| Household size | 1.02 (0.97–1.09) | — |
| Patient education |
— | |
| >College | Ref | |
| College | 1.6 (1.2–2.0) | |
| Post-HS education | 2.7 (2.1–3.5) | |
| HS | 2.5 (1.8–3.3) | |
| Pulmonary involvement | 1.7 (1.4–2.2) | — |
| Neurologic involvement | 3.0 (2.4–3.9) | 2.6 (1.9–3.7) |
| Cardiac involvement | 2.0 (1.6–2.5) | 2.2 (1.7–3.0) |
| Ocular involvement | 2.2 (1.8–2.7) | — |
| Skin involvement | 2.5 (2.0–3.0) | 1.6 (1.2–2.1) |
| Duration of symptoms | 1.04 (1.04–1.05) | 1.04 (1.03–1.05) |
| Medication history |
— | |
| Never | Ref | |
| Past | 3.0 (2.2–4.0) | |
| Current | 2.7 (2.1–3.7) | |
Definition of abbreviations: CI = confidence interval; HS = high school; LASSO = least absolute shrinkage and selection operator; OR = odds ratio.
Variables that were significant (P < 0.05) in multivariate analysis are in boldface.
Figure 1.
Random forest model: development of new sarcoidosis-related comorbidity. Random forest plots of analysis of variables are included in a multivariate model selected by least absolute shrinkage and selection operator for prediction of development of new sarcoidosis-related comorbidity. Higher values of “Mean Decrease Gini” reflect the increasing proportional importance of the individual variables for predicting this categorical outcome.
Health Outcomes: Development of Steroid-associated Comorbidity
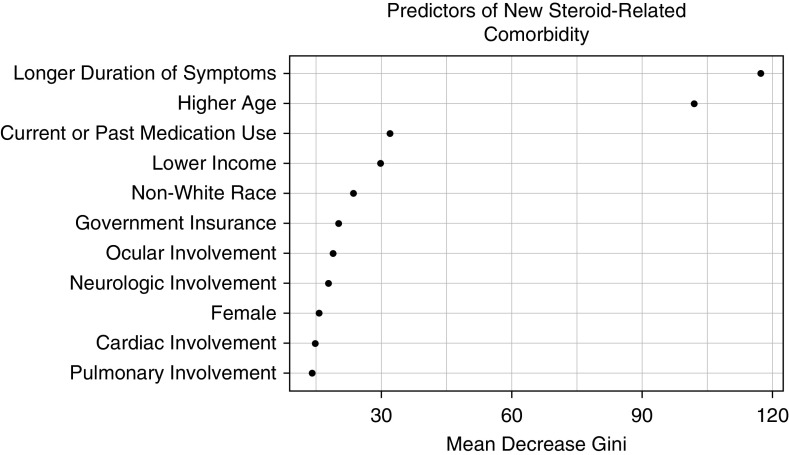
The most common steroid-related comorbidities were obesity (23%), obstructive sleep apnea (22%), and hypertension (17%) (see Figure E1). In multivariate logistic regression, an income of $35,000 to $84,999 was a significant predictor of development of new sarcoidosis-related comorbidity (<$35,000, OR, 1.3 [0.9–2.0]; $35,000–84,999, OR, 1.5 [1.1–2.1]) (Table 5). Other factors independently associated with development of steroid-related comorbidity included age, sex, race, pulmonary involvement, neurologic involvement, ocular involvement, longer duration of symptoms, and past or current use of medication for sarcoidosis. In random forest analysis, income was a leading predictor of development of new steroid-related comorbidity, following duration of symptoms, age, and past or current medication use (Figure 2).
Table 5.
Development of Steroid Comorbidities (Predictors of Development of Steroid-related Comorbidity after Diagnosis with Sarcoidosis)
| Univariate [OR (95% CI)] | Multivariate, LASSO [OR (95% CI)] (n = 988) | |
|---|---|---|
| Income | ||
| ≥$85,000 | Ref | Ref |
| $35,000–84,999 | 1.7 (1.4–2.1) | 1.5 (1.1–2.1) |
| <$35,000 | 2.2 (1.7–2.8) | 1.3 (0.9–2.0) |
| Age | 1.04 (1.03–1.04) | 1.04 (1.02–1.05) |
| Sex, M | 0.8 (0.7–0.97) | 0.7 (0.5–0.95) |
|
Race | ||
| White | Ref | Ref |
| African American | 2.0 (1.6–2.5) | 2.0 (1.4–3.0) |
| Other | 1.7 (1.2–2.3) | 1.9 (1.1–3.3) |
| Insurance type | ||
| Private | Ref | Ref |
| Government | 2.1 (1.7–2.6) | 1.5 (1.1–2.2) |
| No coverage | 1.0 (0.6–1.6) | 0.8 (0.4–1.9) |
| Household size | 0.9 (0.9–0.996) | — |
| Patient education |
— | |
| >College | Ref | |
| College | 1.2 (0.96–1.6) | |
| Post-HS education | 1.5 (1.1–1.9) | |
| HS | 1.6 (1.2–2.1) | |
| Pulmonary involvement | 1.9 (1.5–2.4) | 2.1 (1.4–3.2) |
| Neurologic involvement | 2.4 (1.9–3.1) | 2.4 (1.6–3.5) |
| Cardiac involvement | 1.7 (1.4–2.2) | 1.4 (0.96–1.9) |
| Ocular involvement | 2.4 (1.9–2.9) | 1.6 (1.2–2.2) |
| Skin involvement | 1.8 (1.5–2.2) | — |
| Duration of symptoms | 1.07 (1.06–1.08) | 1.04 (1.02–1.05) |
|
Medication history |
— | |
| Never | Ref | Ref |
| Past | 3.4 (2.5–4.7) | 2.1 (1.3–3.5) |
| Current | 3.0 (2.3–4.0) | 2.6 (1.6–4.1) |
For definition of abbreviations, see Table 4.
Variables that were significant (P < 0.05) in multivariate analysis are in boldface.
Figure 2.
Random forest model: development of new steroid-related comorbidity. Random forest plots of analysis of variables are included in a multivariate model selected by least absolute shrinkage and selection operator for prediction of development of new steroid-related comorbidity. Higher values of “Mean Decrease Gini” reflect the increasing proportional importance of the individual variables for predicting this categorical outcome.
Health Outcomes: Prevalence of Patients Who Have Ever Been Hospitalized for Sarcoidosis
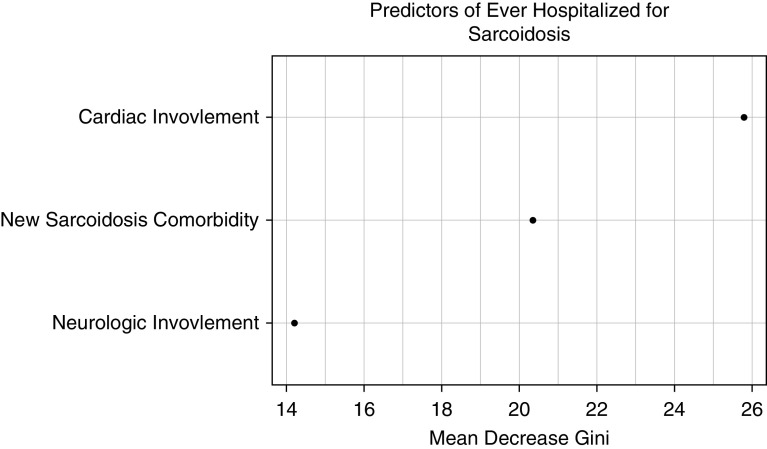
We examined factors associated with patients reporting ever having been hospitalized for sarcoidosis (not diagnostic testing). In multivariate analysis, income was not associated with hospitalization (Table 6). Hospitalization was associated with neurologic involvement, cardiac involvement, and development of new sarcoidosis-related comorbidity (Table 6). In random forest modeling (Figure 3), leading predictors included cardiac involvement and development of new sarcoidosis-related comorbidity.
Table 6.
Ever Hospitalized for Sarcoidosis (Predictors of Hospitalization)
| Univariate [OR (95% CI)] | Multivariate, LASSO [OR (95% CI)] (n = 1,384) | |
|---|---|---|
| Income |
— | |
| ≥$85,000 | Ref | |
| $35,000–84,999 | 1.0 (0.8–1.3) | |
| <$35,000 | 1.9 (1.5–2.4) | |
| Age, yr | 0.99 (0.98–0.997) | — |
| Sex, M | 1.4 (1.1–1.7) | — |
| Race |
— | |
| White | Ref | |
| African American | 1.9 (1.5–2.3) | |
| Other | 1.3 (0.9–1.7) | |
| Insurance type |
— | |
| Private | Ref | |
| Government | 1.7 (1.4–2.1) | |
| No coverage | 2.1 (1.3–3.3) | |
| Household size | 1.06 (0.997–1.1) | — |
| Patient education |
— | |
| >College | Ref | |
| College | 1.4 (1.1–1.9) | |
| Post-HS education | 1.5 (1.2–2.0) | |
| HS | 1.4 (1.0–1.9) | |
| Pulmonary involvement | 1.0 (0.8–1.2) | — |
| Neurologic involvement | 2.6 (2.1–3.3) | 2.4 (1.8–3.2) |
| Cardiac involvement | 3.5 (2.8–4.3) | 3.1 (2.4–4.1) |
| Ocular involvement | 1.6 (1.3–2.0) | — |
| Skin involvement | 1.3 (1.1–1.6) | — |
| Duration of symptoms | 1.02 (1.01–1.03) | — |
| Medication history |
— | |
| Never | Ref | |
| Past | 3.8 (2.6–5.5) | |
| Current | 6.2 (4.3–8.9) | |
| New sarcoidosis comorbidity | 2.8 (2.3–3.3) | 2.2 (1.7–2.7) |
| New steroid comorbidity | 2.2 (1.8–2.6) | — |
For definition of abbreviations, see Table 4.
Variables that were significant (P < 0.05) in multivariate analysis are in boldface.
Figure 3.
Random forest model: hospitalization. Random forest plots of analysis of variables are included in a multivariate model selected by least absolute shrinkage and selection operator for prediction of a patient ever being hospitalized for sarcoidosis. Higher values of “Mean Decrease Gini” reflect the increasing proportional importance of the individual variables for predicting this categorical outcome.
QoL: Sarcoidosis Health Questionnaire
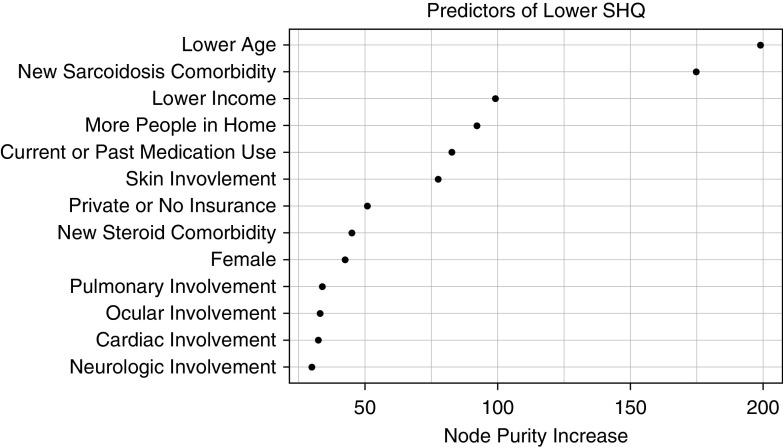
In multivariate linear regression, lower income was predictive of a lower Sarcoidosis Health Questionnaire (SHQ) score (P < 0.001) (Table 7). Other factors associated with lower QoL were lower age; female sex; larger household size; pulmonary, neurologic, or skin involvement; past or current medication use; and development of sarcoidosis-associated or steroid-associated comorbidities. In random forest modeling, low income was the third highest impact variable on SHQ score, behind younger age and development of new sarcoidosis-related comorbidities (Figure 4).
Table 7.
Sarcoidosis Health Questionnaire Total Score (Predictors of Sarcoidosis Health Questionnaire Score)
| Univariate P Values | Multivariate, LASSO P Values (n = 980) | Coefficient Sign (Positive or Negative) | |
|---|---|---|---|
| Income | <0.001 | <0.001 | — |
| ≥$85,000 | Ref | — | |
| $35,000–84,999 | <0.001 | Negative | |
| <$35,000 | <0.001 | Negative | |
| Age, yr | <0.001 | <0.001 | Positive |
| Sex, M | <0.001 | <0.001 | Positive |
| Race | <0.001 | — | — |
| White | |||
| African American | |||
| Other | |||
| Insurance type | <0.001 | 0.07 | — |
| Private | Ref | — | |
| Government | 0.03 | Negative | |
| No coverage | 0.9 | Positive | |
| Household size | <0.001 | 0.005 | Negative |
| Patient education | <0.001 | — | |
| >College | |||
| College | |||
| Post-HS education | |||
| HS | |||
| Pulmonary involvement | <0.001 | <0.001 | Negative |
| Neurologic involvement | <0.001 | 0.007 | Negative |
| Cardiac involvement | 0.005 | 0.07 | Negative |
| Ocular involvement | <0.001 | 0.2 | Negative |
| Skin involvement | <0.001 | <0.001 | Negative |
| Duration of symptoms | <0.001 | — | — |
| Medication history | <0.001 | — | — |
| Never | Ref | — | |
| Past | <0.001 | Negative | |
| Current | <0.001 | Negative | |
| New sarcoidosis comorbidity | <0.001 | <0.001 | Negative |
| New steroid comorbidity | <0.001 | 0.003 | Negative |
Definition of abbreviations: HS = high school; LASSO = least absolute shrinkage and selection operator.
Variables that were significant (P < 0.05) in multivariate analysis are in boldface.
Figure 4.
Random forest model: Sarcoidosis Health Questionnaire (SHQ). Random forest plots of analysis of variables are included in a multivariate model selected by least absolute shrinkage and selection operator for prediction of SHQ score. Higher values of “Node Purity Increase” reflect the increasing proportional importance of the individual variables for predicting this continuous outcome.
Social Outcome: Impact on Family Finances
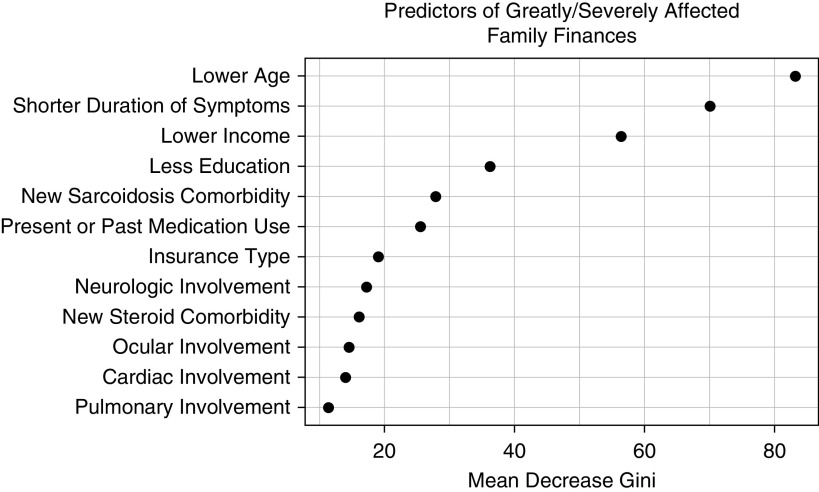
Patients were asked to rate the effect sarcoidosis had on their family finances. In multivariate logistic regression, lower income was strongly associated with great or severe impact on household finances (<$35,000, OR, 7.9 [4.9–12.7] and $35,000–84,999, OR, 2.7 [1.9–3.9]) (Table 8). Other characteristics predictive of severe financial impairment were lower age, pulmonary, cardiac or neurologic involvement, past or current medication use, and new sarcoidosis-associated or steroid-associated comorbidities. In random forest analysis, income was a leading independent predictor of great or severe effect on family finances, behind lower age (Figure 5).
Table 8.
Impact on Family Finances (Predictors of Sarcoidosis Greatly Impacting Family Finances)
| Univariate [OR (95% CI)] | Multivariate, LASSO [OR (95% CI)] (n = 917) | |
|---|---|---|
|
Income | ||
| ≥$85,000 | Ref | Ref |
| $35,000–84,999 | 2.7 (2.2–3.4) | 2.7 (1.9–3.9) |
| <$35,000 | 9.6 (7.4–12.4) | 7.9 (4.9–12.7) |
| Age, yr | 0.97 (0.96–0.98) | 0.96 (0.95–0.98) |
| Sex, M | 0.8 (0.7–0.97) | — |
| Race | ||
| White | Ref | — |
| African American | 1.6 (1.3–2.0) | — |
| Other | 1.8 (1.4–2.5) | — |
| Insurance type | ||
| Private | Ref | Ref |
| Government | 2.1 (1.7–2.5) | 1.0 (0.7–1.4) |
| No coverage | 6.5 (3.6–11.5) | 2.3 (0.8–6.7) |
| Household size | 1.07 (1.01–1.1) | — |
| Patient education | ||
| >College | Ref | Ref |
| College | 1.4 (1.1–1.8) | 0.9 (0.6–1.3) |
| Post-HS education | 2.4 (1.8–3.1) | 1.2 (0.8–1.9) |
| HS | 2.3 (1.7–3.0) | 0.8 (0.5–1.4) |
| Pulmonary involvement | 1.3 (1.1–1.7) | 1.7 (1.1–2.6) |
| Neurologic involvement | 2.9 (2.3–3.7) | 2.2 (1.5–3.3) |
| Cardiac involvement | 1.6 (1.3–2.0) | 1.6 (1.1–2.4) |
| Ocular involvement | 2.0 (1.6–2.4) | 1.2 (0.9–1.7) |
| Skin involvement | 1.9 (1.6–2.3) | — |
| Duration of symptoms | 1.01 (1.01–1.02) | 1.0 (0.976–1.01) |
|
Medication history | ||
| Never | Ref | Ref |
| Past | 2.9 (2.1–4.1) | 2.4 (1.3–4.4) |
| Current | 4.4 (3.3–6.0) | 2.8 (1.6–4.9) |
| New sarcoidosis comorbidity | 4.0 (3.3–4.8) | 2.2 (1.6–3.1) |
| New steroid comorbidity | 2.3 (1.9–2.8) | 1.5 (1.1–2.1) |
For definition of abbreviations, see Table 4.
Variables that were significant (P < 0.05) in multivariate analysis are in boldface.
Figure 5.
Random forest model: greatly impacts family finances. Random forest plots of analysis of variables are included in a multivariate model selected by least absolute shrinkage and selection operator for prediction of family finances being greatly or severely impacted by sarcoidosis. Higher values of “Mean Decrease Gini” reflect the increasing proportional importance of the individual variables for predicting this categorical outcome.
Other Outcomes: Oxygen Use, Mobility Device Use, and Joblessness
In multivariate logistic regression, income was a significant predictor of supplemental oxygen use (<$35,000, OR, 1.5 [1.04–2.3] and $35,000–84,999, OR, 1.7 [1.04–2.6]) (see Table E1); wheelchair or walker use (<$35,000, OR, 3.3 [2.0–5.4] and $35,000–84,999, OR, 1.8 [1.2–2.8]) (see Table E2); and joblessness, either having to quit work due to sarcoidosis (<$35,000, OR, 4.7 [3.1–7.2] nd $35,000–84,999, OR, 1.7 [1.2–2.3]) (see Table E3a) or not having worked prior to sarcoidosis diagnosis (<$35,000, OR, 4.1 [2.3–7.2] and $35,000–84,999, OR, 1.4 [0.9–2.4]) (see Table E3b). In random forest analysis, income was an independent leading predictor of oxygen use, mobility device use, and joblessness (see Figures E2–E4).
Complete statistical models for each outcome are presented in the Tables E4 to E12.
Discussion
In this analysis, we have demonstrated consistent associations of income on a range of health and social outcomes. Patients of lower income were more likely to develop new sarcoidosis-related and steroid-related comorbidities, had worse health-related QoL, and experienced a greater negative effect on their family finances. This effect persisted even after correction for other markers of socioeconomic status and disease severity, including insurance status. In addition, we demonstrated a gradient of effect, with low-income respondents having the worse outcomes noted above, followed by middle-income respondents, and high-income respondents having the best outcomes. Taken together, these results demonstrate a higher burden of disease impacting patients with sarcoidosis who are economically disadvantaged, even years after diagnosis. The effect of income was consistent across almost all outcomes we measured, which argues against a spurious association.
Low socioeconomic status has been associated with severe sarcoidosis phenotypes, even after controlling for race, sex, and education. Early reports demonstrated that patients with sarcoidosis at a municipal hospital, compared with a university hospital, had worse dyspnea, pulmonary function, and radiographic stage at presentation (20). A subsequent study of 100 patients by Rabin and colleagues identified public insurance and low income as risk factors for increased symptoms and emotional and physical disability at diagnosis (11). The follow-up ACCESS (A Case Control Etiologic Study for Sarcoidosis) study confirmed the association of low income and lack of private insurance or Medicare with increased disease severity on presentation, as measured by number of organs involved and pulmonary function tests (10). Since the ACCESS study, little research has been done on the impact of socioeconomic status on health outcomes in sarcoidosis. The most notable gap in the literature is the lack of data regarding the effects of low socioeconomic status on the progression of sarcoidosis symptoms and outcomes.
This study, rather than looking at patients with sarcoidosis at presentation, investigated the association between disease characteristics, socioeconomic status, and longer-term outcomes. In our sample, respondents reported an average disease duration of 11.7 ± 11.9 years. In addition, this study investigated several patient-centered outcomes that are difficult or impossible to capture from electronic medical record review. In agreement with past studies (21), we found an association of worse outcomes with lower income. Patients of lower income had worse health outcomes (development of new sarcoidosis-associated and steroid-associated comorbidities, and need of mobility device and supplemental oxygen), worse health-related QoL (lower SHQ scores), and worse financial outcomes (loss of job or impact on finances). These differences persisted when correcting for race, organ involvement, medication history, and other socioeconomic factors, such as education and insurance status. These results suggest that some aspects of sarcoidosis care, be it medical treatment, social support, or stress management, might be associated with better outcomes in patients of higher socioeconomic status. Hopefully, future studies will be able to determine the best outcome measures of disparity based on socioeconomic factors and elucidate strategies that can improve sarcoidosis care for patients.
A novel finding of our study was the effect of sarcoidosis diagnosis on family finances. Although Arkema and coworkers found that the mean annual income of their Swedish sarcoidosis cohort was not different from the general population by 3 years after diagnosis (12), we found that 46% of patients in our sarcoidosis cohort reported that sarcoidosis had severely impacted their family finances. This is mirrored by a study by Hendriks and colleagues that noted a high rate of disability claims in a population of Dutch patients with sarcoidosis who responded to an online survey regarding the impact of sarcoidosis on employment (22). In our sample, 31% reported quitting their job due to health effects from sarcoidosis. Those of low income were more likely to have to quit their job. Unlike Arkema and colleagues (12), we were able to correct for disease severity, medication use, and comorbidity burden. The retrospective and cross-sectional nature of our study make it impossible to determine if the effect on family finances is causing reduced income or if individuals with limited income are more susceptible to financial harm from a chronic illness, or are in jobs that are more physically demanding or ones that aggravate their disease. Additionally, our study focuses on a self-selected, likely sicker, sample of patients with sarcoidosis. Additionally, the differing levels of social support structures in the United States as compared with Sweden may have played a role in these findings. Although our sample is not representative of the total sarcoidosis population, these results suggest that there is a subset of patients with sarcoidosis who suffer significant financial harm from their disease.
A second novel feature of this study was the detailed inspection of the association of comorbidity with outcomes. We separated comorbidities related to the sarcoidosis disease process and those related to steroid use to examine if poor outcomes were associated with uncontrolled disease or toxic effect from corticosteroids. Sarcoidosis-related comorbidities were associated with all our measured outcomes except for supplemental oxygen use. Steroid-related comorbidities were associated with each outcome besides hospitalizations and mobility device use. In random forest analysis, we generally found sarcoidosis-associated comorbidities more closely associated with each outcome than steroid-associated comorbidities. Interestingly, both sarcoidosis-related and steroid-related comorbidities were significantly related to income, even when corrected for socioeconomic factors and organ involvement. This may reflect patients of low income having higher rates of comorbidity unrelated to sarcoidosis. However, given that the selected comorbidities were common in sarcoidosis (fatigue and chronic pain) and steroid use (obesity and osteoporosis), the data may suggest that low-income patients experience higher rates of steroid toxicity. These findings are in agreement with studies in other chronic diseases, showing an association with income, comorbidity burden, and outcomes (23, 24). Given that this patient cohort had significant disease burden, it is unclear if this finding is generalizable to the whole sarcoidosis population, including those with milder disease. What is certain, is that patients with sarcoidosis of low income have a higher rate of development of new comorbidities, many of which are easily overlooked (fatigue, fibromyalgia, and depression) and are associated with worse outcomes.
Our study has several strengths. We were able to investigate outcomes in a large cohort of patients with sarcoidosis from across the United States. The use of a survey allowed for investigation of various patient-centered outcomes that are difficult to obtain from electronic medical record review. Likewise, the survey allowed us to investigate the role of several social factors, such as income and education, which are often not available in the electronic medical record. In addition, the detailed nature of the Sarcoidosis Advanced Registry for Cures questionnaire allowed for robust statistical correction for disease severity–related factors such as medication use and organ involvement. Finally, we used random forest modeling to accurately rank the importance of our predictors for each outcome. A key advantage of random forest over alternative models is its output of variable importance measures, which can be used to identify relevant features across populations regardless of the frequency of events.
The present study has several limitations. Skewed recruitment and participation may limit the generalizability of our findings and increases the possibility of confounding. Compared with reported sarcoidosis cohorts (10, 25), we had higher rates of extrapulmonary involvement with higher rates of skin involvement (59% vs. 16% and 16%), ocular involvement (33% vs. 12% and 8%), neurologic involvement (16% vs. 5% and 3%), and cardiac involvement (21% vs. 2% and 3%) (10, 25). The survey overrepresented white patients with more severe disease, likely related to access, engagement, and lack of awareness about the registry in some populations. We addressed this issue with robust statistical analysis to correct for known confounders. The self-reported nature of the data also introduces the potential of inaccurate information, including selection bias favoring patients with persistent bothersome disease who may be more likely to participate in a disease registry. However, within the participating individuals, we observed consistent predictors of worse outcomes, arguing against a spurious result. Second, we chose to use the Cox sarcoidosis health-related QoL instrument rather than some of the newer QoL instruments. This was because the newer instruments had not yet been developed for online use at the time of initiating our study. Future studies using QoL instruments with specific organ involvement modules may provide more insight. Finally, the observational nature of our study prevents us from drawing causal associations between respondent’s income; development of comorbidities; and QoL, health, and social outcomes. Specifically, it is unclear if the development of new comorbidities is a driver of worse health outcomes or if the development of new comorbidities is a marker of patients with more severe disease who required more aggressive therapy.
Conclusions
We found a consistent strong negative effect of low income on numerous outcomes in a large population of patients with sarcoidosis. We likewise observed a gradient of effect, with worse outcomes associated with lower incomes. It is plausible that those of higher income have access to resources that those of low income do not, or that lower income predisposes patients to exposure(s) that worsen their disease manifestations. We have also identified an important association between development of comorbidities and disease morbidity in sarcoidosis. Although this study highlights unfortunate disparities in sarcoidosis outcomes, these results also suggest that it is possible to improve sarcoidosis outcomes across several functional domains, especially for economically disadvantaged patients. Future studies should assess the role of comorbidities (fatigue and depression) and steroid effects on disease outcomes in sarcoidosis. In addition, future studies are needed to investigate the cause of the disparity in outcomes between patients of high and low income, which may highlight effective but nonuniformly used therapies for sarcoidosis care.
Supplementary Material
Footnotes
Supported by the Foundation for Sarcoidosis Research and the Ann Theodore Foundation.
Author Contributions: L.J.H., X.-F.W., and D.A.C. were primarily responsible for study design. X.-F.W. performed statistical analysis. A.K.G., M.L.R.N., R.P.B., K.B., M.D., M.A.J., L.A.M., L.S., N.S., and D.A.C. were responsible for registry design and implementation. M.L.R.N., R.P.B., M.D., M.A.J., L.A.M., and L.S. provided substantive study design input. L.J.H., M.L.R.N., and D.A.C. were responsible for article drafting. All authors contributed substantially on article revision and gave final approval for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201906-1250OC on December 11, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Grunewald J, Grutters J, Arkema E, Saketkoo L, Moller D, Muller-Quernheim J. Sarcoidosis. Nat Rev Dis Primer. 2019;5 doi: 10.1038/s41572-019-0096-x. [DOI] [PubMed] [Google Scholar]
- 2.Wells AU. Sarcoidosis: a benign disease or a culture of neglect? Respir Med. 2018;144S:S1–S2. doi: 10.1016/j.rmed.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Cozier YC, Berman JS, Palmer JR, Boggs DA, Serlin DM, Rosenberg L. Sarcoidosis in black women in the United States: data from the Black Women’s Health Study. Chest. 2011;139:144–150. doi: 10.1378/chest.10-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martusewicz-Boros MM, Boros PW, Wiatr E, Roszkowski-Śliż K. What comorbidities accompany sarcoidosis? A large cohort (n=1779) patients analysis. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:115–120. [PubMed] [Google Scholar]
- 5.Kostorz S, Jastrzębski D, Sikora M, Zebrowska A, Margas A, Stepanik D, et al. Predominance of comorbidities in the detriment of daily activity in sarcoidosis patients. Adv Exp Med Biol. 2018;1040:7–12. doi: 10.1007/5584_2017_87. [DOI] [PubMed] [Google Scholar]
- 6.Fleischer M, Hinz A, Brähler E, Wirtz H, Bosse-Henck A. Factors associated with fatigue in sarcoidosis. Respir Care. 2014;59:1086–1094. doi: 10.4187/respcare.02080. [DOI] [PubMed] [Google Scholar]
- 7.Rice JB, White A, Lopez A, Nelson WW. High-cost sarcoidosis patients in the United States: patient characteristics and patterns of health care resource utilization. J Manag Care Spec Pharm. 2017;23:1261–1269. doi: 10.18553/jmcp.2017.17203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pohle S, Baty F, Brutsche M. In-hospital disease burden of sarcoidosis in Switzerland from 2002 to 2012. PLoS One. 2016;11:e0151940. doi: 10.1371/journal.pone.0151940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baughman RP, Field S, Costabel U, Crystal RG, Culver DA, Drent M, et al. Sarcoidosis in America: analysis based on health care use. Ann Am Thorac Soc. 2016;13:1244–1252. doi: 10.1513/AnnalsATS.201511-760OC. [DOI] [PubMed] [Google Scholar]
- 10.Rabin DL, Thompson B, Brown KM, Judson MA, Huang X, Lackland DT, et al. Sarcoidosis: social predictors of severity at presentation. Eur Respir J. 2004;24:601–608. doi: 10.1183/09031936.04.00070503. [DOI] [PubMed] [Google Scholar]
- 11.Rabin DL, Richardson MSA, Stein SR, Sr, Yeager H., Jr Sarcoidosis severity and socioeconomic status. Eur Respir J. 2001;18:499–506. doi: 10.1183/09031936.01.00056201. [DOI] [PubMed] [Google Scholar]
- 12.Arkema EV, Eklund A, Grunewald J, Bruze G. Work ability before and after sarcoidosis diagnosis in Sweden. Respir Med. 2018;144S:S7–S12. doi: 10.1016/j.rmed.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Harper L, Gerke A, Baughman B, Judson M, Serchuck L, Maier L, et al. Social predictors of hospitalization in the Sarcoidosis Advanced Registry for Cures (SARC) [abstract] Am J Respir Crit Care Med. 2018;197:A5360. [Google Scholar]
- 14.Serchuck L, Spitzer G, Rossman M, Baughman R, Culver D, Drent M, et al. The Foundation for Sarcoidosis Research Sarcoidosis Advanced Registry for Cures [abstract] Am J Respir Crit Care Med. 2016;193:A6184. [Google Scholar]
- 15.Cox CE, Donohue JF, Brown CD, Kataria YP, Judson MA. The Sarcoidosis Health Questionnaire: a new measure of health-related quality of life. Am J Respir Crit Care Med. 2003;168:323–329. doi: 10.1164/rccm.200211-1343OC. [DOI] [PubMed] [Google Scholar]
- 16.Moons KGM, Altman DG, Reitsma JB, Ioannidis JPA, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–W73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 17.Yuan M, Lin Y. Model selection and estimation in regression with grouped variables. J R Stat Soc Series B Stat Methodol. 2006;68:49–67. [Google Scholar]
- 18.Breiman L. Random forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- 19.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162:55–63. doi: 10.7326/M14-0697. [Published erratum appears in Ann Intern Med 162:600.] [DOI] [PubMed] [Google Scholar]
- 20.Yeager H, Jr, Rabin DL, Stein SR, Richardson MS, Singh R, Devine MA, et al. Pulmonary sarcoidosis: comparison of patients at a university and a municipal hospital. J Natl Med Assoc. 1999;91:322–327. [PMC free article] [PubMed] [Google Scholar]
- 21.Judson MA, Baughman RP, Thompson BW, Teirstein AS, Terrin ML, Rossman MD, et al. ACCESS Research Group. Two year prognosis of sarcoidosis: the ACCESS experience. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:204–211. [PubMed] [Google Scholar]
- 22.Hendriks CMR, Saketkoo LA, Elfferich MDP, De Vries J, Wijnen PAHM, Drent M. Sarcoidosis and work participation: the need to develop a disease-specific core set for assessment of work ability. Lung. 2019;197:407–413. doi: 10.1007/s00408-019-00234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callhoff J, Luque Ramos A, Zink A, Hoffmann F, Albrecht K. The association of low income with functional status and disease burden in German patients with rheumatoid arthritis: results of a cross-sectional questionnaire survey based on claims data. J Rheumatol. 2017;44:766–772. doi: 10.3899/jrheum.160966. [DOI] [PubMed] [Google Scholar]
- 24.McCurley JL, Gutierrez AP, Bravin JI, Schneiderman N, Reina SA, Khambaty T, et al. Association of social adversity with comorbid diabetes and depression symptoms in the Hispanic community health study/study of Latinos sociocultural ancillary study: a syndemic framework. Ann Behav Med. 2019;53:975–987. doi: 10.1093/abm/kaz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schupp JC, Freitag-Wolf S, Bargagli E, Mihailović-Vučinić V, Rottoli P, Grubanovic A, et al. Phenotypes of organ involvement in sarcoidosis. Eur Respir J. 2018;51:1700991. doi: 10.1183/13993003.00991-2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.