Abstract
Background:
Thrombolytic therapy significantly improves outcomes among patients with acute ischemic stroke. While cancer outcomes have dramatically improved, the utilization, safety, and mortality outcomes of patients with cancer who receive thrombolytic therapy for acute ischemic stroke are unknown.
Methods:
Using a national database, we identified all hospitalizations for acute ischemic stroke requiring thrombolytic therapy between 2003 and 2015. Patients with contraindications to thrombolytic therapy were excluded. Following propensity score matching for comorbidity burden, trends in thrombolytic therapy use and its effect on in-hospital mortality, intracranial or all-cause bleeding, and the combined endpoint of mortality and all-cause bleeding, by presence/absence of cancer were evaluated. We also evaluated 30- and 90-day readmission rates post-thrombolytic therapy administration.
Results:
We identified 237,687 acute ischemic stroke hospitalizations requiring thrombolytic therapy, of which 26,328 (11%) had an underlying cancer. Over the study period, thrombolytic therapy use increased across all acute ischemic stroke admissions, irrespective of cancer presence (12.4/1000 in 2003 to 81.1/1000 in 2015, P<0.0001). However, thrombolytic therapy utilization differed by cancer presence (4.8% cancer vs. 5.1% non-cancer, P=0.001). There was no difference in intracranial bleeding (9.6% vs. 9.7%), all-cause bleeding (13.2% vs. 13.2%), or in-hospital mortality (7.6% vs. 7.2%). While there was no difference in 30-day readmission rates by cancer presence (24% vs. 29%, P=0.40), at 90-days, cancer patients saw higher readmission rates (17.2% vs. 13.3%, P=0.02).
Conclusions:
Contemporary thrombolytic therapy use for acute ischemic stroke has risen, irrespective of presence of cancer. Yet, patients with comorbid cancer appear to see lower rates of thrombolytic therapy use for acute ischemic stroke, despite no difference in the rate of intracranial bleeding or mortality after adjustment for comorbidities.
Keywords: Ischemic stroke, cancer, thrombolytic, in-hospital outcomes, readmission
Introduction
Nearly one in four persons over the age of 25 years will go on to have stroke during their lifetime.1 A large majority of the strokes are acute ischemic stroke (AIS).2 Among patients presenting with AIS, cancer has emerged as an increasingly frequent comorbid disease.3 Recent estimates have placed the incidence of AIS at 3.0% within six months of cancer diagnosis, a value nearly double than that observed in the general population.4 Moreover, with time this rate continues to rise, reaching 6.0% within just two years of an initial diagnosis.4 As cancer outcomes continue to improve and thus cancer patients are increasingly surviving to older age, with a predicted 19 million survivors by 2024, the presentation of AIS among cancer patients is only expected to rise.3,5
Currently, the only Food and Drug Administration approved medical therapy for AIS is intravenous alteplase (tPA). The use of tPA has been associated with dramatically improved outcomes across broad populations, and may be considered equally as effective in the presence of cancer.6 However, the use of tPA is not without its risks.7 Emerging reports have suggested that presence of cancer may be a potential effect modifier after tPA administration, especially in those with metastatic disease.8,9 Yet, the patterns and safety of tPA use among contemporary AIS patients with underlying cancer, particularly in the absence of metastatic disease, are largely unknown.
Accordingly, we sought to assess contemporary patterns of tPA utilization, as well as bleeding and mortality outcomes among patients presenting with AIS, but with an underlying cancer diagnosis. We also assessed trends in readmission etiologies in following the receipt of tPA for AIS in the presence of cancer. We hypothesize that patients with non-metastatic cancer have different utilization, outcomes, and readmissions as compared to non-cancer patients.
Methods
Data sources
From the National Inpatient Sample (NIS), an inpatient database2 developed by the Agency for Healthcare Research and Quality (AHRQ), data elements from January 2003 to September 2015 were collected. Readmission data and statistics were derived using another AHRQ dataset, called the National Readmission Dataset (NRD). It is a nationally representative rehospitalization dataset.10 For this study, we utilized 2013 and 2014 NRD datasets. These datasets and common data refining methods are described in detail in the Supplemental Methods.
Study population and variables
During cohort creation using NIS, we used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes to identify all hospitalized adults, at least 18 years old, who had a primary diagnosis (DX1 of NIS) of AIS (433,434, 436).11 Discharge diagnoses and procedures were recoded using the clinical classification of diseases software (CCS) into broad categories, available as separate variables within the NIS and NRD datasets, respectively. We used the CCS coded discharge diagnoses to further define our initial cohort where we identified AIS with 109 codes (DXCCS1 only). Identification of cancer patients using ICD-9 and CCS codes, cohort refinement using secondary diagnosis, details of Elixhauser’s comorbidities, and certain exclusion criteria are detailed in the Supplemental Methods. A complete list of cancers, by location, can also be found in Table S4.
All patients who did not have either a cancer DXCCS codes or a cancer comorbidity code were considered non-cancer patients. tPA was identified using the ICD-9-CM procedure code of 99.10. Metastatic disease patients were studied independent of those with non-metastatic cancer given the inherently higher risk profile, more likely provider or patient preference against tPA administration, and undetermined locations of disease.
In addition, an important challenge regarding thrombolytic therapy with administrative data has been the inability to calculate reliable NIH stroke scale (NIHSS) scores, which was created to document and standardize the clinical severity of strokes across multicenter clinical trials.12 However, recently a Stroke Administrative Severity Scale (SASI) was devised and applied using administrative data, with strong correlation to measured NIHSS scores (c=0.83).13 SASI score of ≥7 was assigned to high-risk subgroup in accordance with prior validated results.13
During cohort creation using NRD, all hospitalizations with AIS were first identified in the years 2013 and 2014. Among those hospitalizations, we only included admissions where tPA was administered, the patient survived until discharge, and the length of stay was not missing.
Outcomes
The primary outcome was in-hospital mortality, based on the presence or absence of comorbid cancer. Differences in length of stay, hospital charges, and discharge disposition were also used as outcomes of interest. Additional in-hospital outcomes assessed included intracranial hemorrhage (ICH), gastrointestinal (GI) bleed, all bleeding, and the combined outcome of in-hospital mortality and/or all bleeding. These outcomes were identified using ICD-9CM codes (Table S5) and are reflected in a similar NIS-derived tPA analysis focused on venous thromboembolic events among cancer patients.14 It was assumed that ICH was an outcome after tPA administration even though ICD-9 code for primary hemorrhagic stroke is identical. All patients had a primary diagnosis of AIS and secondary diagnosis of ICH to conform to this assumption. Additionally, the authors assume that primary hemorrhagic stroke would have been ruled out prior to tPA administration. Moreover, there is also a possibility that if a massive or symptomatic ICH occurred after AIS, physicians are likely to code the dominant hemorrhagic pathology as the primary diagnosis, despite it occurring after AIS. All charges and costs were inflation adjusted to 2015.15 NRD was utilized to compare and contrast etiologies and rates of 30-day and 90-day readmission between the cancer and non-cancer cohorts.
Statistical analysis
After detailed variance analyses of the NIS and NRD datasets thus created (Supplemental Methods), survey-specific statements with appropriate patient and hospital level weights were used to obtain national estimates.10,16 Rao-Scott Chi-square tests were used for comparing categorical variables, and survey specific t-test were used for continuous variables. For tests of trends, we used the Cochrane Armitage test of trend for categorical variables and survey-specific linear regression for continuous variables. All figures and tables, except Supplemental Figures 3 and 4 (NRD), were generated from NIS analyses. Patient and hospital level difference in characteristics were also assessed using standardized differences given multiple significant associations found using aforementioned methodology (Table S1).17
Three separate triennial cohorts from 2003 to 2005, 2008 to 2010 and 2013 to 2015 were created to present baseline estimates and outcomes over different eras of stroke and tPA use.6 We estimated all cancer and non-cancer admissions (essentially the entire available NIS, from 2003 to 2015) for each year using the methodology described above, to use as denominators for comparative annual trends. Hospitalizations in which tPA was not administered were considered and the data were utilized for comparison purposes in both cancer and non-cancer cohorts. Even though the study was specifically meant to study the tPA cohort, certain trends with non-tPA subgroup were presented for quantitative reference.
Trends and outcomes presented were prepared using a propensity matched cohort, discussed in the Supplemental Methods, which adjusted for age, gender, race, insurance status, hypertension, diabetes, HCUP mortality score, as well as hospital location, teaching status, size and geographic region. A sensitivity analysis excluding cancers with higher rates of bleeding (primary CNS, gastrointestinal, and hematologic cancers) was also performed. Similar assessment among those cancer patients with active anticancer therapy codes (i.e. chemotherapy or radiation) versus those without was performed (Supplemental Methods). Further, subgroup trends based on SASI score, gender, metastatic disease, and age less than 50 were also performed. In addition to aforementioned propensity model, SASI score-adjusted model was presented.
An exploratory multivariable logistic regression analysis (supplemental methods) modelling for outcomes of in-hospital mortality, and the combined outcome of bleeding and in-hospital mortality was presented.
NRD analysis was performed using previously established methodology to define 30-day and 90-day readmission rates after index AIS and tPA use.4,18 Univariate analysis was performed to calculate the odds of readmission, by specific etiology [ex. repeat stroke or transient ischemic attack (TIA), or bleeding]. All analyses were performed using SAS software, version 9.4 (SAS Institute), and the description of study methodology is presented in Figure S5.
Results
Baseline characteristics
Overall, between 2003 and 2015, the study sample included 237,687 AIS hospitalizations where tPA was delivered. Table 1 displays the population characteristics. The mean age was 68.1±0.1 years, 49.5% were female, 82.4% had hypertension, 32.5% had diabetes, and 3.4% an underlying coagulation disorder. Among these, there were 26,328 (11.1%) admissions with a non-metastatic cancer co-diagnosis. Those with concurrent cancer were older, mostly male, more likely on Medicare or Medicaid, non-White, and have a history of coagulation dysfunction (Table 1, S1). Over the entire study period, cancer patients have a proportionately higher amount of Medicare as a payment source when compared to non-cancer patients, 77.3% and 61.3%, respectively (P<0.0001). There was no difference in tPA use among cancer vs. non-cancer patients when stratified by teaching status of hospital (62.6% vs. 63.5% teaching hospitals in cancer vs. non-cancer patients, P=0.24). There was no difference in AIS severity based on presence of cancer (mean SASI score 6.4±0.1 vs. 6.5±0.1, P=0.20).
Table 1.
Patient and hospital level characteristics of strokes with thrombolytic use (crude rates).
| 2003–2005 |
2008–2010 |
2013–2015a |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cancer (n=1316) | Non-cancer (n=15,444) | P-value | Cancer (n=5715) | Non-cancer (n=47,610) | P-value | Cancer (n=1 1,750) | Non-cancer (n=83,375) | P-value | |
| Patient characteristics | |||||||||
| Age, years (mean±SE) | 73.2±0.7 | 66.8±0.3 | <0.0001 | 74.4±0.4 | 67.5±0.2 | <0.0001 | 74.2±0.3 | 67.2±0.1 | <0.0001 |
| Women, % | 44.9 | 46.9 | 0.56 | 51 | 49.7 | 0.40 | 49.8 | 49.4 | 0.77 |
| Race, % | <0.0001b | <0.0001 | <0.0001 | ||||||
| White | 83.9 | 76 | 79.8 | 72.7 | 79.7 | 69.2 | |||
| Black | 7.9 | 12.9 | 11.9 | 14.8 | 10.3 | 15.6 | |||
| Hispanic | 4.5 | 6.2 | 4.1 | 6.5 | 5 | 8.6 | |||
| Asian or Pacific Islander | 1 | 2.4 | 1.3 | 2.8 | 2 | 3.3 | |||
| Native American | 0 | 0.1 | 0.3 | 0.4 | 0.2 | 0.3 | |||
| Other | 2.8 | 2.3 | 2.6 | 2.9 | 2.6 | 3 | |||
| Payment source (%) | <0.0001 | <0.0001 | <0.0001 | ||||||
| Medicare | 78.7 | 58.7 | 76.6 | 58.9 | 77.3 | 61.3 | |||
| Medicaid | 2.5 | 5.6 | 4.3 | 7.2 | 3.8 | 8.7 | |||
| Private | 17.3 | 28.4 | 15.5 | 25.9 | 15.1 | 22.4 | |||
| Self-pay | 0.7 | 4.8 | 1.3 | 4.9 | 2.2 | 4.8 | |||
| No charge | 0 | 0.4 | 0.4 | 0.5 | 0.2 | 0.4 | |||
| Others | 0.8 | 2.1 | 2 | 2.5 | 1.5 | 2.4 | |||
| Comorbidities (%) | |||||||||
| Stroke specific | |||||||||
| Prior stroke | 0c | 0c | 11.6 | 8.9 | 0.0003 | 14.3 | 12.7 | 0.03 | |
| Hypertension | 77.6 | 76.7 | 0.7 | 82 | 81.4 | 0.66 | 84.4 | 84.6 | 0.89 |
| Diabetes | 21.2 | 23.9 | 0.3 | 31.6 | 30.7 | 0.44 | 32 | 36.2 | <0.0001 |
| Obesity | 1.9 | 5.7 | 0.008 | 6.5 | 9.3 | 0.003 | 9.5 | 13.6 | <0.0001 |
| Hyperlipidemia | 22.4 | 24.6 | 0.7 | 42 | 42.3 | 0.86 | 54.8 | 53.8 | 0.36 |
| Chronic kidney disease | 1.8 | 4 | 0.08 | 11.8 | 10.1 | 0.1 | 17.1 | 13 | <0.0001 |
| Coronary artery disease | 30.7 | 28.6 | 0.44 | 32.1 | 29.3 | 0.053 | 33.1 | 28.4 | <0.0001 |
| Atrial arrhythmia | 31.6 | 31.8 | 0.96 | 41.5 | 34.7 | <0.0001 | 38.9 | 32.2 | <0.0001 |
| Peripheral vascular disease | 5.6 | 7.4 | 0.25 | 9.1 | 9.1 | 0.98 | 10.5 | 9.9 | 0.4 |
| Alcoholism | 3.3 | 4.3 | 0.46 | 2.3 | 4.4 | 0.0007 | 3.3 | 5.1 | 0.0002 |
| Smoking | 20.8 | 21.3 | 0.84 | 26.4 | 27.1 | 0.65 | 36.2 | 34.5 | 0.12 |
| Other cardiovascular | |||||||||
| Congestive heart failure | 12.4 | 16 | 0.1 | 15.8 | 16.4 | 0.64 | 17.3 | 16.6 | 0.45 |
| Valvular heart disease | 7.2 | 11.4 | 0.02 | 11.5 | 10.2 | 0.17 | 13.1 | 10.8 | 0.0008 |
| Non-traditional | |||||||||
| Weight loss | 1.5 | 1.7 | 0.79 | 3.3 | 3.6 | 0.63 | 3.9 | 3.4 | 0.22 |
| Anemia | 0.7 | 0.5 | 0.22 | 0.1 | 0.2 | 0.36 | 1.4 | 1.4 | 0.83 |
| Arthritis and collagen vascular disease | 2.5 | 1.9 | 0.52 | 1.8 | 1.1 | 0.80 | 3.3 | 2.6 | 0.06 |
| Chronic liver disease | 0 | 0.5 | d | 0.6 | 0.8 | 0.29 | 1 | 1.3 | 0.31 |
| Chronic lung disease | 13.2 | 14 | 0.7 | 15.9 | 14.1 | 0.45 | 19.5 | 15.2 | <0.0001 |
| Hypothyroidism | 8.7 | 8.7 | 0.99 | 15.3 | 12 | 0.0006 | 18 | 14 | <0.0001 |
| Psychiatric | 7.5 | 6.5 | 0.47 | 9.1 | 10.1 | 0.29 | 13 | 12.9 | 0.9 |
| Fluid/electrolyte disorder | 13.8 | 14.9 | 0.55 | 19.6 | 20.6 | 0.44 | 22.9 | 21.8 | 0.26 |
| Coagulation disorder | 2.2 | 2 | 0.85 | 3 | 2.7 | 0.57 | 5.6 | 3.6 | <0.0001 |
| Substance abuse | 4.1 | 5.4 | 0.36 | 3.1 | 5.8 | <0.0001 | 4.3 | 7.6 | <0.0001 |
| Total Elixhauser’s comorbidities ≥3 | 36.6 | 35.5 | 0.78 | 66.5 | 59.9 | 0.0009 | 70.9 | 65.8 | <0.0001 |
| Elixhauser’s readmission score (mean±SE) | 9.8±0.6 | 8.6±0.2 | 0.06 | 17±0.4 | 14±0.2 | <0.0001 | 18.8±0.3 | 15.2±0.1 | <0.0001 |
| Elixhauser’s mortality score (mean±SE) | 3.8±0.4 | 3.2±0.2 | 0.3 | 7.9±0.3 | 6.5±0.2 | <0.0001 | 8.6±0.2 | 6.4±0.1 | <0.0001 |
| SASI score (mean±SE) | 5.4±0.4 | 5.5±0.2 | 0.67 | 6.1±0.2 | 6.6±0.2 | 0.006 | 6.6±0.1 | 6.6±0.1 | 0.95 |
| Hospital level variables | |||||||||
| Teaching hospital (%) | 59.3 | 52.9 | 0.06 | 56.6 | 58 | 0.45 | 69.1 | 69.1 | 0.98 |
Only data till September 2015 presented.
Excluding Native Americans as value is 0.
V code used for this diagnosis not available for years prior to 2007.
Chi-Square not reported if one of the values is 0.
Sub-group analysis of cancer types demonstrated stroke incidence to be highest in breast cancer (20.5%), prostate cancer (19.8%) and colon cancer (12.0%), respectively (Table 2).
Table 2.
Acute ischemic strokes among cancer patients stratified by type of cancer; thrombolytic therapy among cancer patients stratified by type of cancer and incidence of intracranial hemorrhage among different cancer types who received tPA.
| Stroke |
Stroke hospitalizations receiving thrombolytics |
|||
|---|---|---|---|---|
| Cancer | Percent of all cancersa | Cancer | Percent of all cancersa | Percent with intracerebral hemorrhageb |
| Breast cancer | 20.5 | Breast cancer | 22.1 | 9.7 |
| Prostate cancer | 19.8 | Prostate cancer | 20.2 | 10.9 |
| Colon cancer | 12 | Melanoma and other Skin cancer | 12.5 | 7.8 |
| Melanoma and other Skin cancers | 10.8 | Colon cancer | 10.9 | 10.9 |
| Other cancers | 10.8 | Genitourinary cancers | 10.1 | 9.3 |
| Genitourinary cancers | 9.7 | Hematologic cancers | 8.8 | 9.7 |
| Hematologic cancers | 9.2 | Other cancers | 8.8 | 11.2 |
| Lung cancer | 7.4 | Lung cancer | 6.1 | 11.2 |
| Head and neck cancers (including thyroid) | 3.7 | Head and neck cancers (including thyroid) | 4.1 | 6.6 |
| Renal cancer | 2.8 | Renal cancer | 2.8 | 11.4 |
| Other gastrointestinal cancers (excluding pancreatic and colon) | 2.5 | Other gastrointestinal cancers (excluding pancreatic and colon) | 2.6 | 7.9 |
| Ovarian cancer | 1.3 | Ovarian cancer | 1.5 | 6.3 |
| Pancreatic cancer | 0.5 | Pancreatic cancer | 0.4 | –c |
Percentage adds up to >100% as multiple hospitalization have more than one cancer.
Intracranial hemorrhage incidence from 2003 to 2015. Unadjusted data.
Actual N lower than Agency for Healthcare Research and Quality requirements.
Thrombolytic utilization
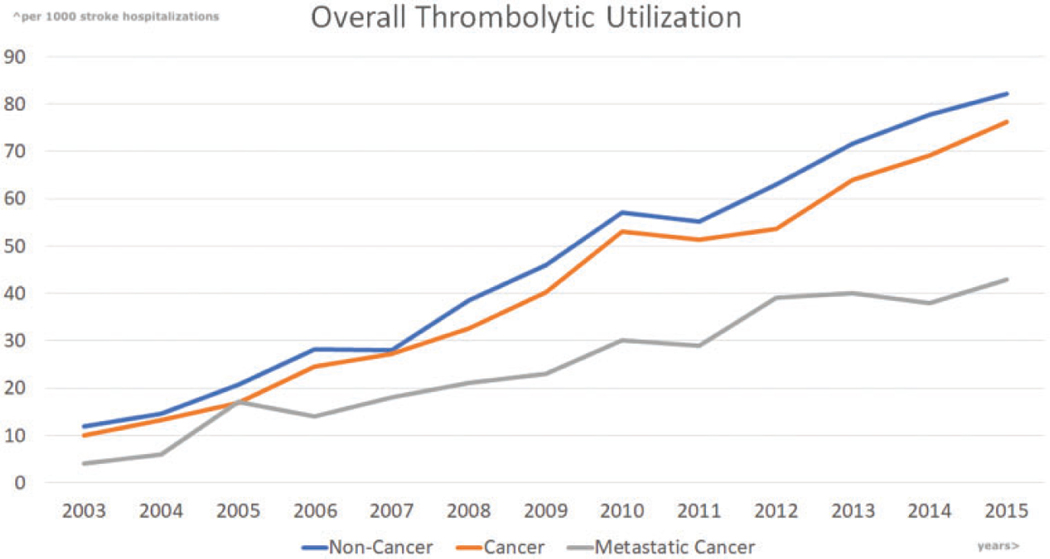
Over the study period, the incidence of tPA use across hospitalizations for AIS increased from 12.4 per 1000 to 81.1 per 1000 (P-trend <0.0001). Among cancer patients, the utilization of tPA increased from 10 to 76 per 1000 AIS hospitalizations (P-trend <0.0001). Among cancer patients with stroke history those who received thrombolytics were 22.1% in breast cancer, 20.1% in prostate cancer, and 10.9% in colon cancer. When compared to non-cancer admissions, tPA utilization was lower in the cancer population after adjustment for comorbid disease (Figure 1, Pcancer vs. non-cancer=0.001). This relationship remained after excluding primary CNS, GI, and hematologic cancers in the years after 2012 (P-trend <0.0001 in either group; Pcancer vs. non-cancer=0.0001; Figure S1(a)). Additionally, among all cancer patients, there was no difference in use, by the presence or absence of anticancer therapy (42.4 vs. 42.8 per 1000 administrations in AIS, Pcancer therapy vs. non-cancer therapy=0.82). SASI score, much like the clinical NIH stroke scale, differentiated hospitalizations into cohorts of higher and lower tPA utilization, and had similar trends across years (P-trend <0.0001, Figure S1(b)). There was no difference in tPA utilization in either of the SASI score risk groups [Pcancer vs. non-cancer (<7)=0.77; Pcancer vs. non-cancer (≥7)=0.61] or even after including SASI score into the adjustment model [Pcancer vs. non-cancer=0.71]. There was increasing utilization of tPA noted in those <50 years of age and gender-based subgroups (Figure S1(c) and (d)), respectively (P-trends <0.0001). There was a difference in tPA utilization among the male subgroup (Pcancer vs. non-cancer<0.0001). However, there was no difference in tPA utilization among females (Pcancer vs. non-cancer=0.23) and those <50-years age (Pcancer vs. non-cancer=0.52).
Figure 1.
Propensity-score match adjusted trends in thrombolytic utilization from 2003 through 2015. Presented per 1000 stroke hospitalizations, with an observed Pcancer vs. non-cancer=0.001.
Adjusted outcomes and predictors of outcomes (Table 3)
Table 3.
Propensity matched outcomes of hospitalizations post-thrombolytics.
| 2003–2005 |
2008–2010 |
2013–2015a |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cancer (n=1316b) | Non-cancer (n=15,444b) | P-value | Cancer (n=5715b) | Non-cancer (n=47,610b) | P-value | Cancer (n=11,750b) | Non-cancer (n=83,375b) | P-value | |
| In-hospital outcomes | |||||||||
| Intracranial hemorrhage, % | 3.1 | 6 | 0.1 | 7.3 | 7.4 | 0.94 | 10.8 | 11.1 | 0.68 |
| GI bleed, % | 0.5 | 1.8 | 0.18 | 2.6 | 1.5 | 0.01 | 1.2 | 1.2 | 0.78 |
| All bleeding, % | 4.6 | 9.5 | 0.02 | 11.7 | 11.1 | 0.57 | 14.1 | 14.5 | 0.53 |
| In-hospital mortality, % | 10.5 | 11.6 | 0.63 | 8.9 | 11.3 | 0.04 | 7.6 | 7.2 | 0.62 |
| Length of stay (mean±SE, days) | 3.9 ±0.1 | 4.2 ±0.04 | 0.0003 | 3.4 ±0.1 | 3.7 ±0.03 | 0.67 | 3.1 ±0.02 | 3.2 ±0.02 | 0.1 |
| Total hospital charges (mean±SE, US$)c | 21,630±436 | 22,131±400 | 0.07 | 28,036±602 | 28,615±571 | 0.19 | 33,382±289 | 33,875±254 | 0.009 |
| Total hospital costs (mean±SE, US$)d | 8406±130 | 8420±124 | 0.06 | 9168±150 | 9276±143 | 0.07 | 9157±68 | 9087±56 | 0.1 |
| Disposition, % | 0.09 | 0.45 | 0.02 | ||||||
| Home | 31.6 | 24.1 | 24 | 23.4 | 30.1 | 29.7 | |||
| Short term hospital | 7.2 | 4.6 | 4.3 | 4.2 | 3.4 | 3.9 | |||
| Skilled care facility | 43 | 52.7 | 51.2 | 50.8 | 45 | 47.8 | |||
| Home health care | 7.1 | 6.8 | 10.7 | 9.6 | 13.1 | 10.7 | |||
| Against medical advice | 0.6 | 0.2 | 0.8 | 0.6 | 0.6 | 0.7 | |||
| Unknown | 0 | 0 | 0.1 | 0.1 | 0.2 | 0.1 | |||
Only data till September 2015 presented.
Absolute number of patients (n) included in the propensity matching model. The percentages and P-values are presented from adjusted model.
Adjusted to inflation.
Using HCUP cost-to-charge, wage index adjustment along with inflation adjustment.
When tPA-associated complications were assessed over the entire study period, there was no difference in the risk-adjusted rates of ICH, GI bleeding, and all-cause bleeding, based on presence of cancer (9.6% vs. 9.7% P=0.87; 1.5% vs. 1.4%, P=0.48; 13.2% in both groups, P=0.94, respectively). Similarly, no difference in risk-adjusted in-hospital mortality was seen (8.4% vs. 9.1%, P=0.15). The combined outcomes of bleeding and/or in-hospital mortality were similar in cancer vs. non-cancer cohort (18.9% vs. 19.6%, P=0.33). However, there was a statistically significant difference in the length of stay, and total hospital costs between the cancer vs. non-cancer cohorts (4.3±0.1 days vs. 4.6±0.04 days, P<0.0001; $18,944±246 vs. $19,289±187, P=0.002). The most common disposition for post tPA cancer cohort is transferred to skilled nursing facility (47.7%).
There has been a significant increase (Figure 2, P-trend <0.0001) in all-cause bleeding and ICH post –tPA administration over the study period, but no significant difference in cancer vs. non-cancer (P=0.68, 0.53 respectively, Table 3). Moreover, the incidence of ICH associated with thrombolytic use was 9.7% in breast cancer, 10.9% in prostate cancer, and 10.9% in colon cancer (Table 2).
Figure 2.
Propensity-score match adjusted intracranial hemorrhage (a), and all-cause bleed (b) rates post thrombolytic. Presented as per 100 thrombolytic administration. Pcancer vs. non-cancer (intracranial hemorrhage)=0.87; Pcancer vs. non-cancer (all cause bleeding)=0.94.
Similar increasing trends were noticed in non-tPA AIS arms of either cohort as well (Figure S2(a) and (b)). There has been a reduction in in-hospital mortality (Figure 3) in both cancer and non-cancer cohorts irrespective of tPA administration. However, among the tPA recipients, there was a lower drop in cancer (2.3%) vs. non-cancer (5.2%, P<0.0001) cohort. No significant differences were observed in either arms of the contemporary years (7.6% vs. 7.2%, P=0.62, Table 3). In contrast to the entire study period, there was no statistical difference in GI bleeding, length of stay, and total hospital costs for comorbid cancer and non-cancer hospitalizations in the contemporary years (P=0.78, 0.1, and 0.1, respectively, Table 3).
Figure 3.
Propensity-score match adjusted in-hospital mortality trends with and without thrombolytic therapy. Presented as per 100 stroke patients. Pcancer vs. non-cancer (thrombolytic)=0.15; Pcancer vs. non-cancer (non-thrombolytic)<0.0001.
In sensitivity analysis after multivariable adjustment, cancer was not found to be associated with an increased risk of in-hospital mortality (P=0.06) or combined outcome of in-hospital mortality and bleeding (P=0.32). Coagulation disorder [adjusted odds ratio (OR)=2.5, CI 1.4–4.4, P=0.002] and SASI score (adjusted OR=1.2, CI 1.1–1.2, P<0.0001) were associated with in-hospital mortality in cancer with AIS hospitalization undergoing tPA therapy. Additional data regarding other risk factors of interest are presented in Table S2(a) and (b).
Metastatic cancer
In addition, among the 63,424 patients with metastatic cancer with AIS over 13-years, there were only 1695 tPA administrations (2.7%). During the study period, increase in tPA utilization from 4 to 43 per 1000 AIS hospitalization was observed (Figure 1). Overall, mortality (15.8% vs. 12.1%), ICH (12.8% vs. 2.8%) and major bleeding (20.2% vs. 7.1%) were higher in those who received tPA compared to those who did not (P<0.0001 for all comparisons).
Readmission after tPA in AIS
There is no significant difference in 30-day readmission with respect to cancer diagnosis (9.5% in cancer vs. 9.1% in non-cancer, P=0.78). Similarly, the most frequent etiology of 30-day readmission across both cohorts was repeat stroke/TIA related (24% vs. 29%, P=0.4, Figure S3). Cancer hospitalizations had higher rates of readmission after tPA administration at 90 days (17.2% vs. 13.3%, P=0.02). Moreover, the most common etiology of 90-day readmission among cancer patients was infectious (21% vs. 15%, P=0.051), while repeat stroke/TIA remained most frequent among those without cancer (27% vs. 19%, P=0.10, Figure S4). Yet, only hematologic (i.e. cancer or non-cancer hematologic) causes of readmission at 90-days were elevated (OR 3.4, CI 1.3–9.2 at 30-days, Table S3), as infection did not reach statistical significance [1.61 (0.99–2.61) P=0.051).
Discussion
This national database study, which included over 200,000 AIS hospitalizations, was designed to determine tPA utilization, bleeding outcomes, and mortality trends in non-metastatic cancer hospitalizations over a 13-year period. This study shows an overall increase, but lower utilization of tPA in cancer vs. non-cancer AIS hospitalizations, as well as a decreasing mortality and increasing risk of bleeding. In sensitivity analyses, we did not find an association between cancer diagnosis and the risk of the above outcomes. Additionally, tPA utilization for AIS was linked with a significantly higher risk of readmission in cancer patients compared to non-cancer patients at 90-days.
When comparing comorbid cancer admissions to non-cancer admissions, we found no difference in mortality between the two groups. Our findings are in line with prior studies that evaluated outcomes in comorbid cancer hospitalizations who received tPA and/or endovascular treatment between 2009 and 2010.19,20 However, one of the major complications that arise from systemic thrombolytics center around internal bleeding and ICH.7 ICH after tPA administration was reported as 6.4% in the landmark National Institute of Neurological Disorders and Stroke trial, and has ranged from 2 to 7% throughout prior literature.21,22 In fact, higher odds of ICH in comorbid cancer along with increased length of stay in the hospital have been reported by a prior study.20 However, the prior study also included metastatic disease patients (almost 30%) and thus findings were not limited to cancer patients without known metastatic disease at presentation. Within the current study, significantly fewer patients with metastatic disease receiving tPA were available (although outcomes were worse). As such, it is unknown if similar results would have been noted with consideration of cancer progression or disease status.
Despite the overall increase in thrombolytic utilization, we found a significant decrease in utilization with respect to cancer diagnosis. To help control for unseen exclusions, primary CNS, GI, and hematologic cancer diagnoses were excluded. The observed differences by presence of cancer remained significant despite these exclusions and general illness adjustments, suggesting unmeasured factors, such as physician–patient perception of overall prognosis and relative hesitation regarding the safety of use in those with cancers.21,22 Yet, a sizable number of patients may have had differences in resuscitation status that could have contributed to this finding, but this could not be captured within this dataset. Moreover, as to our knowledge, this is the first study to evaluate trends of thrombolytic utilization based on presence of cancer, and further studies will be needed to evaluate potential factors underlying these differences.
Readmission for AIS in cancer patients was previously reported to be three times higher than noncancer patients.23 In a 2014 retrospective single-center study, the reported incidence of recurrent stroke at 90-days was estimated at 7% in the absence of metastatic disease.23 However, these observations were not limited to those treated with tPA, and within the present analysis tPA use was not associated with higher rates of recurrent AIS, after accounting for general comorbid risk and level of cancer progression. Contrasting to the 30-day readmission, we found the most common etiology of 90-day readmission among cancer patients to be infectious, while repeat stroke/TIA remained most frequent among those without cancer.
Despite the strengths of this investigation, several limitations should be acknowledged. First, the database relies on clinicians accurately encoding diagnoses into ICD-9 codes which can be subject to criticism. When using NIS data, symptomatic and asymptomatic ICH are reported together, which could also contribute to our high ICH rate and may not necessarily have a clinical correlation. The dataset also does not allow for precise staging of cancer, beyond the presence of advanced or metastatic disease, nor is there provision for ongoing anticancer therapy, timing or type of anticancer therapy (ex. targeted vs. traditional), or code status.23 Furthermore, the precise factors underlying AIS and survival to discharge, patient–physician discussion of goals of care or disease perception, and use of active or recent treatments (i.e. surgery, anticoagulant therapies, etc.) could not be determined and require prospective validation. Moreover, we also acknowledge that SASI score might not be an ideal substitution for NIHSS score.13 Additionally, the time calculation of SASI score unlike the NIHSS score could not be determined in this administrative dataset. Finally, even though statistically necessary, risk matching using propensity score may have masked true and relevant associations that explain physicians’ tPA decisions and stroke outcomes.
Conclusions
Patients with cancer face a high-risk of AIS. Analysis of available contemporary population data suggests a lower rate of tPA use, despite similar bleeding and mortality risk among non-metastatic cancer patients, even after accounting for other comorbid disease and AIS severity. Additional prospective studies evaluating the factors underlying the decision for tPA use and the role of patient–physician perception of cancer prognosis are needed.
Supplementary Material
Acknowledgments
Funding
Dr. Addison and Dr. Mitchell were supported by an NCI K12CA133250 grant. Dr. Awan has received research funding from Innate Pharma, Pharmacyclics, served on the speaker’s bureau of Astra Zeneca, Abbvie, and provided consulting services to Gilead Sciences, Pharmacyclics, Inc., Janssen, Abbvie, Sunesis, and Genentech, and was supported by NIH grant number R35-CA197734. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Collaborators GBDLRoS. Feigin VL, Nguyen G, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med 2018; 379: 2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018; 137: e67–e492. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD and Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 4.Navi BB, Reiner AS, Kamel H, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol 2017; 70: 926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro CL. Cancer survivorship. N Engl J Med 2018; 379: 2438–2450. [DOI] [PubMed] [Google Scholar]
- 6.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 7.Yaghi S, Willey JZ, Cucchiara B, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017; 48: e343–e361. [DOI] [PubMed] [Google Scholar]
- 8.Nam KW, Kim CK, Kim TJ, et al. Intravenous thrombolysis in acute ischemic stroke with active cancer. Biomed Res Int 2017; 2017: 4635829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selvik HA, Naess H and Kvistad CE. Intravenous thrombolysis in ischemic stroke patients with active cancer. Front Neurol 2018; 9: 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khera R, Angraal S, Couch T, et al. Adherence to methodological standards in research using the national inpatient sample. JAMA 2017; 318: 2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thigpen JL, Dillon C, Forster KB, et al. Validity of international classification of disease codes to identify ischemic stroke and intracranial hemorrhage among individuals with associated diagnosis of atrial fibrillation. Circ Cardiovasc Qual Outcomes 2015; 8: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brott T, Adams HP Jr., Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20: 864–870. [DOI] [PubMed] [Google Scholar]
- 13.Simpson AN, Wilmskoetter J, Hong I, et al. Stroke administrative severity index: using administrative data for 30-day poststroke outcomes prediction. J Comp Eff Res 2018; 7: 293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bashir R, Zack CJ, Zhao H, Comerota AJ and Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med 2014; 174: 1494–1501. [DOI] [PubMed] [Google Scholar]
- 15.Miller KD, Goding Sauer A, Ortiz AP, et al. Cancer statistics for Hispanics/Latinos, 2018. CA Cancer J Clin 2018; 68(6): 425–445. [DOI] [PubMed] [Google Scholar]
- 16.Guha A, Dey AK, Armanious M, et al. Health care utilization and mortality associated with heart failure-related admissions among cancer patients. ESC Heart Fail 2019; 6: 733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamdani M, Sykora K, Li P, et al. Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 2005; 330: 960–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwok CS, Martinez SC, Pancholy S, et al. Effect of comorbidity on unplanned readmissions after percutaneous coronary intervention (from the nationwide readmission database). Sci Rep 2018; 8: 11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murthy SB, Karanth S, Shah S, et al. Thrombolysis for acute ischemic stroke in patients with cancer: a population study. Stroke 2013; 44: 3573–3576. [DOI] [PubMed] [Google Scholar]
- 20.Weeda ER and Bohm N. Association between comorbid cancer and outcomes among admissions for acute ischemic stroke receiving systemic thrombolysis. Int J Stroke 2019; 14(1): 48–52. [DOI] [PubMed] [Google Scholar]
- 21.Diedler J, Ahmed N, Sykora M, et al. Safety of intravenous thrombolysis for acute ischemic stroke in patients receiving antiplatelet therapy at stroke onset. Stroke 2010; 41: 288–294. [DOI] [PubMed] [Google Scholar]
- 22.Menon BK, Saver JL, Prabhakaran S, et al. Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue-type plasminogen activator. Stroke 2012; 43: 2293–2299. [DOI] [PubMed] [Google Scholar]
- 23.Navi BB, Singer S, Merkler AE, et al. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology 2014; 83: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.