Abstract
Background:
Randomized clinical trials demonstrated the benefits of percutaneous coronary interventions (PCI) in diverse clinical settings. Patients with cancer were not routinely included in these studies.
Methods/results:
Literature search of PubMed, Cochrane, Medline, SCOPUS, EMBASE, and ClinicalTrials was conducted to identify studies that assessed one-year all-cause, cardiovascular and non-cardiovascular mortality in patients with historical or active cancer. Using the random effects model, we computed risk ratios (RRs) and standardized mean differences and their 95% confidence intervals for the dichotomous and continuous measures and outcomes, respectively. Of 171 articles evaluated in total, 5 eligible studies were included in this meta-analysis. In total, 33,175 patients receiving PCI were analyzed, of whom 3323 patients had cancer and 29,852 no cancer history. Patients in the cancer group had greater all-cause mortality [RR 2.22 (1.51–3.26; p < 0.001)], including cardiovascular mortality [RR 1.34 (1.1–1.65; p = 0.005)] and non-cardiovascular mortality [RR 3.42 (1.74–6.74; p ≤ 0.001], at one-year compared to non-cancer patients. Patients in the cancer group had greater one-month all-cause mortality [RR 2.01 (1.24–3.27; p = 0.005)] and greater non-cardiovascular mortality [RR 6.87 (3.10–15.21; p ≤0.001)], but no difference in one-month cardiovascular mortality compared to non-cancer patients. Meta-regression analyses showed that the difference in one-year all-cause and cardiovascular mortality between both groups was not attributable to differences in baseline characteristics, index PCI characteristics, or medications prescribed at discharge.
Conclusions:
Patients with cancer undergoing PCI have worse mid-term outcomes compared to non-cancer patients. Cancer patients should be managed by a multi-specialist team, in an effort to close the mortality gap.
Keywords: Cancer, Percutaneous coronary intervention, Mortality, Outcomes, Drug eluding stent, Meta-analysis
1. Introduction
Cancer and heart disease are the two most common causes of death in Western populations [1]. Despite an increase in cancer diagnoses in recent years, mortality has decreased in this patient population due to more effective therapeutic methods [2]. Increased life expectancy, clinical risk factors for cancer, cancer itself, and most current anticancer therapies are associated with heart disease, including coronary artery disease (CAD) [3–6]. Therefore, a growing number of patients with both cancer and CAD are commonly encountered in the outpatient and inpatient setting. Management of this patent population is extremely complex and usually requires a multidisciplinary team of specialists, including cardiologists, oncologists, radiation oncologist, interventionalists and surgeons.
Percutaneous coronary intervention (PCI) is the most frequently performed revascularization technique in patients with CAD [7]. Due to a greater prevalence of a prothrombotic state and low platelet counts, PCI, drug-eluding stents and/or dual antiplatelet therapy are not regularly administered in cancer patients [8,9]. Thus, patients with historical or active cancer are typically excluded from cardiovascular randomized clinical trials [10,11]. Most of the clinical outcomes data after PCI in cancer patients comes from observational or single-center studies [12–18]. In this meta-analysis with meta-regression, we aimed to define one-year outcomes after PCI in patients with historical or active cancer.
2. Methods
This meta-analysis was registered with PROSPERO international prospective register of systematic reviews (CRD42018111492). The study protocol was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (PRISMA reference) [19].
2.1. Eligibility criteria
We aimed to evaluate differences in all-cause, non-cardiovascular and cardiovascular mortality at one-year after PCI in patients with active or history of cancer compared to patients with no history of cancer. In order to be included in the meta-analysis, studies had to fulfill the following criteria: (1) studies of human adults (age ≥ 18 years), (2) compared outcomes with historical or active cancer to patients with no cancer history undergoing PCI, and (3) reported one-year all-cause mortality, one year cardiovascular mortality or one-year non-cardiovascular mortality. The studies meeting the above criteria were evaluated and excluded if the following applied: (1) duplicate studies and/or overlap of patients among 2 studies, and/or between abstracts and original research manuscripts, (2) outcomes of interest were not reported. When overlap or duplicate studies were found, the most recent was included.
2.2. Information sources and search
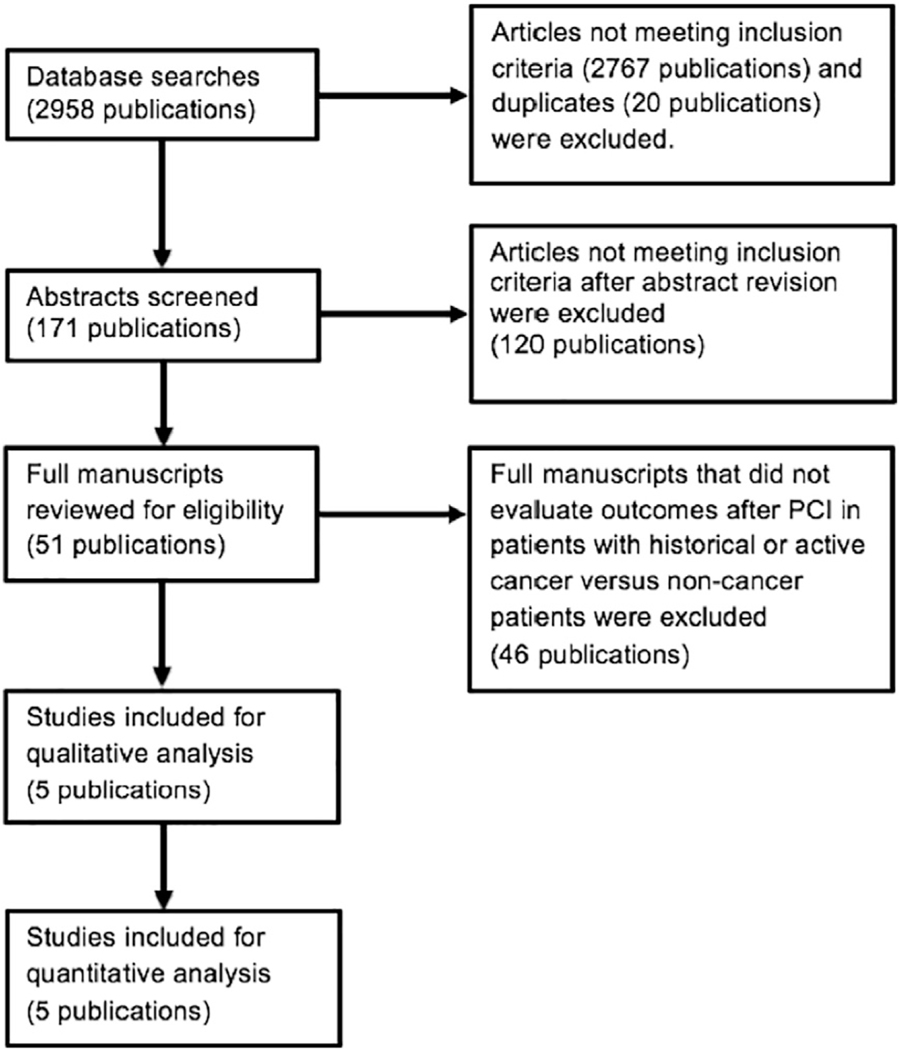
A literature search was performed through the PubMed, Cochrane, Medline, SCOPUS, EMBASE and ClinicalTrials databases from inception until December 2018. Original research manuscripts as well as conference abstracts with detailed information were identified. The following search terms were used: “(“Cancer”[Mesh]) AND (“Percutaneous Coronary Intervention”[Mesh])”. There was no language restriction in our search. The references of included articles and prior meta-analysis were reviewed for additional original manuscripts and abstracts not found with our original search strategy. Additionally, the authors of the manuscripts analyzed were contacted if further information was needed. Fig. 1 illustrates the PRISMA flow diagram of the search strategy.
Fig. 1.
Flowchart of search strategy and literature review process.
2.3. Study selection, data collection and quality assessment
References and abstracts of the search result were screened and reviewed independently by two investigators (R.Q. and H.S.) to determine review of full-text paper. Full-text manuscripts were reviewed in full independently by two investigators (R.Q. and H.S.) using the Critical Appraisal Skills Programme (CASP) tool for each study design (CASP reference) [20]. To be included in the meta-analysis, the study required an affirmative answer in the screening section A of the CASP tool. At any instance in the search, a third independent investigator (D.M.) reviewed abstracts/manuscript to resolve any discrepancies between the first two investigators. Once the studies to be included in the meta-analysis were identified, two investigators (R.Q. and H.S.) independently extracted data from the studies using a standardized data extraction form. Newcastle-Ottawa scale was used to assess risk of bias of each individual study. The certainty of the pooled data for every primary outcome was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) [21].
2.4. Data items
The primary endpoints of this study were one-year all-cause, cardiovascular and non-cardiovascular mortality. Secondary endpoints evaluated in this meta-analysis were one-month all-cause, cardiovascular and non-cardiovascular mortality [22].
2.5. Data analysis
We used risk ratio (RR) with 95% confidence intervals (CIs) as summary measure for dichotomous data. For continuous variables, standardized mean differences (SMD) with 95% CIs were used. For all outcomes, the data was pooled using a random-effects meta-analysis model based on the assumption of differences of treatments provided and/or sampling variability between studies. This assumption was further tested with Cochran’s Q-test (p value <0.1) and I2 statistic for heterogeneity expressed as a percentage. If heterogeneity was found, sensitivity analysis was conducted to explore its potential origin. Sensitivity analysis to evaluate the impact of each study on the observed results entailed performing iterative analyses with sequential exclusion of each individual study and reassessment of the pooled RR. To evaluate for publication bias, a funnel plot was used and quantified with Begg’s test for small-study effects. Meta-regression analysis investigating the effects of study-level characteristics including age, gender, smoking status, hypertension, diabetes mellitus, dyslipidemia, stroke/transient ischemic attack (TIA), peripheral artery disease (PAD), chronic kidney disease (CKD) stage III-IV (eGFR <60 ml/min.1.73m2), heart failure, prior myocardial infarction (MI), prior coronary artery bypass grafting (CABG), acute coronary syndrome on presentation, multivessel CAD on index PCI, coronary artery involved, the use of drug-eluting stents (DES) and medical regimen on discharge was conducted. The baseline patient characteristics from individual studies were used as independent variables in linear meta-regression on the log-transformed risk ratios (RR) of cancer versus non-cancer patients undergoing PCI on one-year all-cause mortality and cardiovascular mortality to calculate the variables’ meta-regression coefficients with 95% CIs, allowing to test for modulation by any of these variables of the effect of cancer on mortality after PCI.
A p-value <0.05 was considered statistically significant unless otherwise specified. All statistical analyses were performed using Stata version 14.2 (StataCorp LP, College Station, Texas). The evidence profile table was created using the GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc.). Available from gradepro.org.
3. Results
3.1. Literature search, study selection and characteristics
Our search identified 2958 potential titles. After exclusion of duplicates and titles not relevant or with exclusion criteria, 5 studies were included in the analysis (Fig. 1). All of the included titles were observational studies. These studies included a total of 33,175 patients, 3323 in the cancer group and 29,852 with no cancer history. Baseline characteristics of the included studies and population are described in Supplemental Table 1. Compared to those without cancer, patients in the cancer group were younger [SMD 0.41 (0.13–0.7; p = 0.005)], were less likely to smoke [RR 0.67 (0.48–0.99, p = 0.041)], have dyslipidemia [RR 0.88 (0.78–0.98; p = 0.026)], receive a DES during index PCI [RR 0.89 (0.85–0.92, p < 0.001)] or be discharged with an angiotensinconverting enzyme inhibitor/angiotensin II receptor blocker (ACEI/ARB) [RR 0.92 (0.87–0.97; p = 0.001)]. Additionally, patients in the cancer group were more likely to have PAD [RR 1.39 (1.12–1.73; p = 0.003)], CKD III-IV [RR1.52 (1.01–2.3; p = 0.044)], prior MI [RR 1.27 (1.06–1.53; p = 0.01)] and plain balloon angioplasties (POBA) during index PCI [RR 1.53 (1.19–1.97; p = 0.001)].
3.2. Primary endpoints
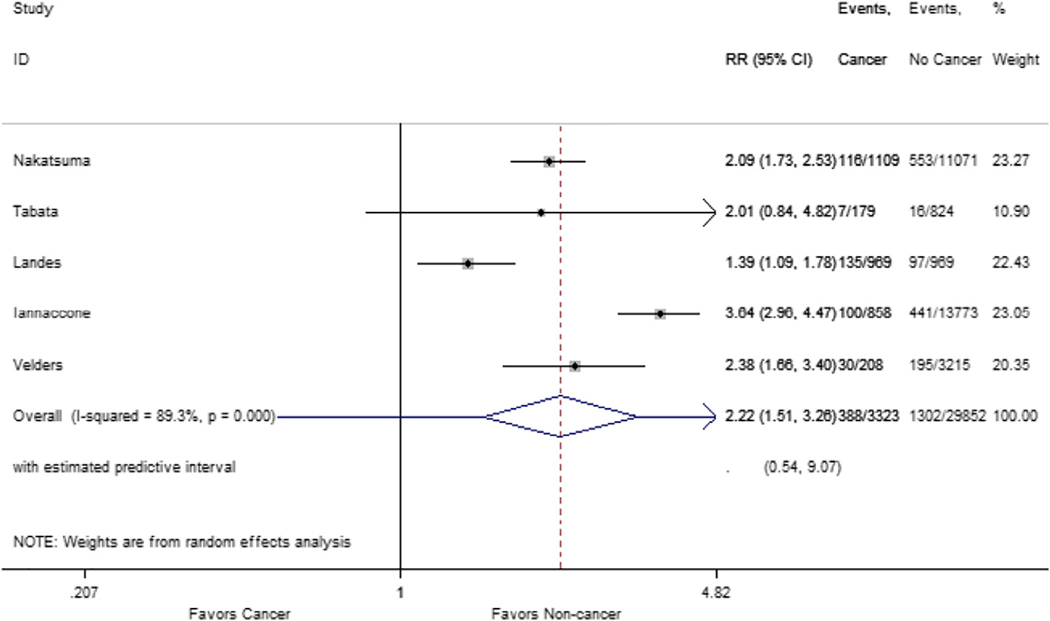
Analysis of one-year all-cause mortality included data from the 5 included studies. This revealed a total of 1690 deaths (5%), of which 388 occurred in the cancer group (12%) and 1302 in the no-cancer group (4%). Patients in the cancer group had greater overall mortality [RR 2.22 (1.51–3.26; p < 0.001)]; there was significant heterogeneity in this analysis (I2 = 89.3%, p < 0.001) (Fig. 2). There was no publication bias in funnel plot analysis and Begg’s test (p = 0.602) was consistent with this finding (Supplemental Fig. 1).
Fig. 2.
Forest plot of one-year all-cause mortality. Plot comparing one-year all-cause mortality in patients with historical or active cancer versus non-cancer patients.
For one-year cardiovascular mortality analysis, data was obtained from 4 studies. These studies included a total of 18,544 patients, 2465 in the cancer group and 16,079 with no cancer history. A total of 733 cardiovascular deaths (2%) were observed, of which 118 occurred in the cancer group (4%) and 615 in the no-cancer group (2%). Patients in the cancer group had greater cardiovascular mortality [RR 1.34 (1.1–1.65; p = 0.005), and there was no significant heterogeneity in this analysis (I2 = 0%, p = 0.418) (Fig. 3). There was no publication bias in funnel plot analysis and Begg’s test was consistent with this finding (p = 0.117) (Supplemental Fig. 2).
Fig. 3.
Forest plot of one-year cardiovascular mortality. Plot comparing one-year cardiovascular mortality in patients with historical or active cancer versus non-cancer patients.
Lastly, for one-year non-cardiovascular mortality analysis, data was obtained from 4 studies. This analysis revealed a total of 18,558 patients, 2472 in the cancer group and 16,086 with no cancer history. There were 414 non-cardiovascular deaths (2.23%) in total, of which 170 occurred in the cancer group (6.88%) and 244 in the no-cancer group (1.52%). Patients in the cancer group had greater non-cardiovascular mortality [RR 3.42 (1.74–6.74; p ≤0.001), and there was significant heterogeneity in this analysis (I2 = 88.3%, p < 0.001) (Fig. 4). Publication bias was found in funnel plot analysis but it was not supported by the Begg’s test (p = 0.317) (Supplemental Fig. 3).
Fig. 4.
Forest plot of one-year non-cardiovascular mortality. Plot comparing one-year non-cardiovascular mortality in patients with historical or active cancer versus non-cancer patients.
3.3. Sensitivity analyses
Sensitivity analysis of all-cause, cardiovascular and non-cardiovascular mortality was performed by iteratively omitting each individual studies from the meta-analysis (Supplemental Table 2). This was conducted to identify outlying studies that could be driving the heterogeneity appreciated in these outcomes. With regard to one-year all-cause mortality, excluding Landes et al. from the meta-analysis led to a mild decrease in heterogeneity and a greater relative risk of this outcome (RR 2.56, 95%CI 1.81–3.63; I2 81.2%; p = 0.001). Excluding the study by Iannaccone et al. had the greatest impact on heterogeneity and on the overall effect estimate of this outcome (RR 1.89; 95%CI 1.44–2.49; I2 66.2%; p = 0.031) (Supplemental Fig. 4). For one-year cardiovascular mortality, removing the study by Nakatsuma et al. from the analysis rendered the RR estimate similar to the analysis including all of the studies, however with 95% CI including a RR 1. Furthermore, excluding this study from the one-year cardiovascular mortality analysis increased the heterogeneity estimate from 0 to 30% (Supplemental Fig. 5).
For one-year non-cardiovascular mortality, excluding Landes et al. from our analysis completely eliminated the heterogeneity and lead to a greater overall relative risk of this outcome (RR 4.37, 95%CI 3.38– 5.65; I2 0%; p = 0.699) (Supplemental Fig. 6).
For one-year all-cause and non-cardiovascular mortality, regardless of which study was removed from the analysis, the RR estimate remained statistically significant.
3.4. Secondary endpoints
Although our literature review was primarily designed to evaluate one-year mortality, we evaluated one-month mortality to gain insight into the events occurring shortly after intervention that could lead to differences in mortality. Three studies provided one-month overall mortality data, including a total of 18,544 patients of which 492 (3%) died within one month of PCI. Of these deaths, 133 occurred in the cancer group (5%) and 359 in the no-cancer group (2%). Patients in the cancer group had greater one-month all-cause mortality [RR 2.01 (1.24–3.27; p = 0.005)], there was significant heterogeneity in this analysis (I2 = 77.1%, p = 0.013) (Supplemental Fig. 7). The same three studies provided one-month cardiovascular mortality data. Of these deaths, 83 occurred in the cancer group (3%) and 346 in the no-cancer group (2%). There was no difference in one-month cardiovascular mortality between the two groups [RR 1.61 (0.89–2.9; p = 0.117)]. These analyses had significant heterogeneity (I2 = 80.8%, p = 0.005) (Supplemental Fig. 8). Three studies provided one-month non-cardiovascular mortality data, includinga total of 18,544 patients of which 63 (0.34%) died within one-month of index PCI. Of these deaths, 50 occurred in the cancer group (2.03%) and 13 in the non-cancer group (0.1%). Patients in the cancer group had greater non-cardiovascular mortality [RR 6.87 (3.10–15.21; p ≤0.001), and there was no significant heterogeneity in this analysis (I2 = 17.7%, p = 0.297) (Supplemental Fig. 9).
3.5. Meta-regression
Meta-regression coefficients revealed that age, female sex, smoking, HTN, DM, dyslipidemia, prior stroke/TIA, PAD, CKD III-V, heart failure, prior MI, prior CABG, acute coronary syndrome at presentation, left anterior descending, left main or multi-vessel disease at index PCI, the use of DES or prescribing aspirin, P2Y12 inhibitors, ACEI/ARBs, beta-blockers, statins and proton pump inhibitors at discharge were not associated with the one-year all-cause mortality outcome (Supplemental Table 3). Meta-regression representative plots are shown in Supplemental Figs. 10 to 32. Meta-regression coefficients revealed that age, female sex, smoking, HTN, DM, left anterior descending artery disease on index PCI, the use of DES were not associated with the one-year cardiovascular mortality outcome (Supplemental Table 4). Meta-regression representative plots are shown in Supplemental Figs. 33 to 39.
3.6. Certainty of the evidence
According to the GRADE certainty analysis, the evidence for the three primary endpoints was low (Supplemental Table 5).
All of the included studies were observational and in most the data was extracted retrospectively carrying serious risk of bias. There was significant inconsistency, by I2 > 85% and Begg’s test, for the one-year overall mortality and non-cardiovascular mortality. There was no significant inconsistency for one-year cardiovascular mortality. The data obtained from all studies included a population in which the question is clinically relevant (all cancer patients), used interventions that would be used in this group of patients and rather than evaluate a surrogate outcome studied a dichotomous and clear outcome. Hence, we did not downgrade for indirectness. The confidence intervals for all outcomes crossed threshold that would make the findings relevant. For the all-cause and non-cardiovascular mortality, optimal information size criteria were achieved. For cardiovascular mortality, although the optimal information size criteria were not met, since the outcome was infrequent and sample size is >2000 for cancer patients and 16,000 for non-cancer patients, we decided not to downgrade. Since the RR was >2 for all-cause and non-cardiovascular mortality, the certainty was upgraded one level for strong association.
4. Discussion
In the present meta-analysis, we have shown that patients with historical or active cancer have greater all-cause mortality, including cardiovascular mortality and non-cardiovascular mortality at one-year compared to non-cancer patients. In addition, patients with historical or active cancer had greater one-month all-cause mortality and non-cardiovascular mortality but similar cardiovascular mortality compared to non-cancer patients. Meta-regression analyses revealed the difference in all-cause and cardiovascular one-year mortality between both groups were not attributable to differences in baseline characteristics, index PCI characteristics or medications prescribed at discharge analyzed in Supplemental Tables 3 and 4.
In the National Heart, Lung, and Blood Institute Dynamic Registry, aimed at comparing outcomes following PCI for ST-elevation and nonST-elevation myocardial infarction, patients with a history of cancer were found to have worse in-hospital and one-year mortality [23]. Hess and colleagues, using data from the Duke Information Systems for Cardiovascular Care and the Duke Tumor Registry, reported patients with a history of cancer undergoing PCI had similar outcomes compared to non-cancer patients [18]. Therefore, due to variable results, mid-term outcomes after PCI in patients with historical or active cancer are not fully understood and required further evaluation. The present meta-analysis of over 33,000 patients, in which approximately 1 in 10 had historical or active cancer, showed that active or historical cancer is associated with a two-fold increased risk of all-cause one-year mortality after PCI compared to non-cancer patients. The cancer cohort was significantly younger, had less exposure to tobacco and dyslipidemia but significantly more PAD, CKD stages III-V, and prior myocardial infarctions. These patients were also less likely to get less ACEI/ARBs at discharge compared to non-cancer patients. Despite this, our meta-regression analysis showed the difference in all-cause one-year mortality and cardiovascular mortality between both groups of patients was not driven by any of the analyzed patient’s baseline characteristics, index PCI characteristics or medications prescribed at discharge. This may suggest impactful ramifications of cancer on outcomes, even beyond traditional comorbid disease burden and short-term treatment strategies. A key finding in the present study is that historical or active cancer has a strong impact in the therapeutic strategy employed during index PCI, causing physicians to abstain from using more effective forms of percutaneous revascularization (11% less DES and 53% more POBA, p ≤ 0.001) in this patient population.
With regard to one-month outcomes, cancer patients were also found to have an increased risk of cardiovascular mortality and close to a six-fold increased risk of non-cardiovascular mortality at one-year after PCI compared to non-cancer patients. Although not reported in the majority of the studies analyzed and thus not included in our analysis, it is likely that mortality due to malignancy itself is the main variable leading to increased 30-day and one-year non-cardiovascular mortality and subsequently increased all-cause mortality at one-year in the cancer group.
In part due to increased risk of bleeding, thrombosis and concomitant affection of other organs, patients with historical or active cancer undergoing PCI are managed less aggressively than non-cancer patients. As shown in our study, this patient population has worse overall and cardiovascular mortality. However, these do not appear to correlate with baseline characteristics of the cancer group.
4.1. Study limitations
The studies included in our analysis are all observational studies, with a consequent risk of bias which is unaccounted for. Due to limited available data of long-term post-PCI outcomes data, this meta-analysis was limited to evaluating one-year outcomes. Thus, long-term outcomes post-PCI in this patient population were not studied. Although we used meta-regression and sensitivity analyzes to evaluate sources of heterogeneity and baseline characteristics between groups that could explain differences in the primary outcomes, the small number of studies included make us interpret these results with caution. Also, in most of these studies patients with either active cancer or history of cancer were grouped together. Similarly, all types of cancer, despite the difference in outcomes were pooled together. Only 2 studies (Landes et al. and Velders et al.) specified that patients with non-melanoma skin cancer were excluded from their studies. The aforementioned limitations could explain the heterogeneity appreciated in the different outcomes analyzed despite various sensitivity analyses.
Lastly, using a systematic approach to the evidence, the certainty for one-year all-cause mortality, cardiovascular mortality and non-cardiovascular mortality is low. This implies, that our confidence in the effect estimate is limited.
4.2. Conclusions
The current meta-analysis supports that patients with historical or active cancer have increased risk of one-year all-cause mortality, cardiovascular and non-cardiac mortality after PCI compared to non-cancer patients. The increased risk of mortality found in the cancer group was not attributable to differences in patient’s baseline characteristics, procedural characteristics and discharge medications. Despite these findings, the presence of historical or active cancer has significant implications in the selection of the therapeutic strategy by physicians, who often defer using more effective percutaneous revascularization options, (i.e. DES deployment) in this patient population. Management of patients with historical or active cancer undergoing PCI should be individualized and discussed in a multidisciplinary fashion with aim to receive optimal and comparable care to non-cancer patients, in an effort to close the mortality gap.
Supplementary Material
Acknowledgements
Funding statement
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Declaration of competing interest
The authors report no relationships that could be construed as a conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2019.09.016.
References
- [1].Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. , Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012, Eur. J. Cancer 49 (2013) 1374–1403. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A, Cancer statistics, CA Cancer J. Clin. 68 (2018) 7–30. [DOI] [PubMed] [Google Scholar]
- [3].Levis BE, Binkley PF, Shapiro CL, Cardiotoxic effects of anthracycline-based therapy: what is the evidence and what are the potential harms? Lancet Oncol. 18 (2017) e445–e456. [DOI] [PubMed] [Google Scholar]
- [4].Hassan SA, Palaskas N, Kim P, Iliescu C, Lopez-Mattei J, Mouhayar E, Mougdil R, Thompson K, Banchs J, Yusuf SW, Chemotherapeutic agents and the risk of ischemia and arterial thrombosis, Curr. Atheroscler. Rep. 20 (2018) 10. [DOI] [PubMed] [Google Scholar]
- [5].Armstrong GT, Kawashima T, Leisenring W, et al. , Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study, J. Clin. Oncol. 32 (2014) 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Abdel-Qadir H, Austin PC, Lee DS, et al. , A population-based study of cardiovascular mortality following early-stage breast cancer, JAMA Cardiol. 2 (2017) 88–93. [DOI] [PubMed] [Google Scholar]
- [7].Mamas MA, Fath-Ordoubadi F, Danzi GB, et al. , Prevalence and impact of co-morbidity burden as defined by the Charlson Co-morbidity Index on 30-day and 1- and 5-year outcomes after coronary stent implantation (from the Nobori-2 Study), Am. J. Cardiol. 116 (2015) 364–371. [DOI] [PubMed] [Google Scholar]
- [8].Franco AT, Corken A, Ware J, Platelets at the interface of thrombosis, inflammation, and cancer, Blood 126 (2015) 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lip GY, Chin BS, Blann AD, Cancer and the prothrombotic state, Lancet Oncol. 3 (2002) 27–34. [DOI] [PubMed] [Google Scholar]
- [10].Wiviott SD, Braunwald E, McCabe CH, et al. , TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes, N. Engl. J. Med. 357 (2007) 2001–2015. [DOI] [PubMed] [Google Scholar]
- [11].Wallentin L, Becker RC, Budaj A, et al. , Ticagrelor versus clopidogrel in patients with acute coronary syndromes, N. Engl. J. Med. 361 (2009) 1045–1057. [DOI] [PubMed] [Google Scholar]
- [12].Nakatsuma K, Shiomi H, Morimoto T, et al. , Influence of a history of cancer on long-term cardiovascular outcomes after coronary stent implantation (an Observation from Coronary Revascularization Demonstrating Outcome Study-Kyoto Registry Cohort-2), Eur. Heart J. Qual. Care Clin. Outcomes 4 (2018) 200–207. [DOI] [PubMed] [Google Scholar]
- [13].Wang F, Gulati R, Lennon RJ, et al. , Cancer history portends worse acute and long-term noncardiac (but not cardiac) mortality after primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction, Mayo Clin. Proc. 91 (2016) 1680–1692. [DOI] [PubMed] [Google Scholar]
- [14].Tabata N, Sueta D, Yamamoto E, et al. , Outcome of current and history of cancer on the risk of cardiovascular events following percutaneous coronary intervention: a Kumamoto University Malignancy and Atherosclerosis (KUMA) study, Eur. Heart J. Qual. Care Clin. Outcomes 4 (2018) 290–300. [DOI] [PubMed] [Google Scholar]
- [15].Iannaccone M, D’Ascenzo F, Vadalà P, et al. , Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: a BleeMACS substudy, Eur. Heart J. Acute Cardiovasc. Care 7 (2018) 631–638. [DOI] [PubMed] [Google Scholar]
- [16].Landes U, Kornowski R, Bental T, et al. , Long-term outcomes after percutaneous coronary interventions in cancer survivors, Coron. Artery Dis. 28 (2017) 5–10. [DOI] [PubMed] [Google Scholar]
- [17].Velders MA, Boden H, Hofma SH, et al. , Outcome after ST elevation myocardial infarction in patients with cancer treated with primary percutaneous coronary intervention, Am. J. Cardiol. 112 (2013) 1867–1872. [DOI] [PubMed] [Google Scholar]
- [18].Hess CN, Roe MT, Clare RM, et al. , Relationship between cancer and cardiovascular outcomes following percutaneous coronary intervention, J. Am. Heart Assoc. (2014) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hutton B, Salanti G, Caldwell DM, et al. , The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations, Ann. Intern. Med. 162 (2015) 777–784. [DOI] [PubMed] [Google Scholar]
- [20].Taylor R, Reeves B, Ewings P, Binns S, Keast J, Mears R, A systematic review of the effectiveness of critical appraisal skills training for clinicians, Med. Educ. 34 (2000) 120–125. [DOI] [PubMed] [Google Scholar]
- [21].Guyatt G, Oxman AD, Akl EA, et al. , GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables, J. Clin. Epidemiol. 64 (2011) 383–394. [DOI] [PubMed] [Google Scholar]
- [22].Hicks KA, Mahaffey KW, Mehran R, et al. , 2017 cardiovascular and stroke endpoint definitions for clinical trials, Circulation. 137 (2018) 961–972. [DOI] [PubMed] [Google Scholar]
- [23].Abbott JD, Ahmed HN, Vlachos HA, Selzer F, Williams DO, Comparison of outcome in patients with ST-elevation versus non-ST-elevation acute myocardial infarction treated with percutaneous coronary intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry), Am. J. Cardiol. 100 (2007) 190–195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.