Abstract
Background
The prognostic implications of combined pre- and post-capillary pulmonary hypertension (Cpc-PH) in patients with pulmonary hypertension due to left heart disease (PH-LHD) remain controversial. The aim of this retrospective study was to evaluate the new PH-LHD criteria, recommended by the 6th World Symposium on Pulmonary Hypertension and to determine the prognostic value of Cpc-PH.
Methods
A total of 701 patients with symptomatic heart failure who had undergone right-heart catheterization were divided into the following four groups: (i) Isolated post-capillary PH (Ipc-PH) group; mean pulmonary artery pressure (mPAP) >20 mmHg, pulmonary artery wedge pressure (PAWP) >15 mmHg, and pulmonary vascular resistance (PVR) <3 Wood units (WU) (ii) Cpc-PH group; mPAP >20 mmHg, PAWP >15 mmHg, and PVR ≥3 WU (iii) borderline-PH group; mPAP >20 mmHg and PAWP ≤15 mmHg (iv) non-PH group; mPAP ≤20 mmHg. Multivariate Cox hazard analysis was used to investigate whether Cpc-PH was associated with cardiac outcomes.
Results
The study subjects were allocated into the Ipc-PH (n = 268), Cpc-PH (n = 54), borderline-PH (n = 112), or non-PH (n = 267) groups. The Cpc-PH group was associated significantly with adverse cardiac events even after adjustment for clinically relevant confounding factors for heart failure prognosis (vs. non-PH group: HR 2.98 [95% CI 1.81–4.90], P <0.001; vs. Ipc-PH group: HR: 1.92 [95% CI 1.19–3.08], P = 0.007).
Conclusions
The new definitions of PH-LHD stratified patients into 4 categories. Long-term clinical outcomes were significantly different between the four categories, with Cpc-PH having the worst cardiac outcomes.
Introduction
Pulmonary hypertension (PH) due to left heart disease (PH-LHD) caused by elevated left-sided filling pressures is the most common etiology of PH [1]. There is evidence that patients with PH-LHD have a worse clinical prognosis than patients without PH-LHD [1–3]. PH-LHD is further classified into two different subsets according to the presence of a pre-capillary component [combined pre- and post-capillary PH (Cpc-PH) or isolated post-capillary PH (Ipc-PH), respectively] [4]. Cpc-PH is considered a more serious subset than Ipc-PH [5–7]. Current ESC/ERS PH guidelines define PH as a mean pulmonary artery pressure (mPAP) ≥25 mmHg, and define the two subsets of PH-LHD according to the diastolic pressure gradient (DPG) [difference between diastolic PAP and pulmonary artery wedge pressure (PAWP)] and/or pulmonary vascular resistance (PVR) [4]. Previous studies have investigated whether or not Cpc-PH defined by current guidelines predicts clinical outcomes, although the results have varied widely in the patient groups studied [8–11]. The definition of PH using a cut-off value of mPAP ≥25 mmHg is considered empirical [12]. The categorization of two subsets of PH-LHD using DPG has also been considered too restrictive [13]. Reconsideration of these definitions is therefore warranted on the basis of recent analyses and understanding of the pathophysiology of the condition.
The 6th World Symposium on Pulmonary Hypertension suggested a major revision was needed of the new definition for PH of a mPAP >20 mmHg [12], which was based on the fact that mPAP in normal subjects was 14.0 ± 3.3 mmHg [14]. Moreover, in recent analyses [15, 16] only PVR was used as the marker to distinguish between the two subsets of PH-LHD. However, the validity of the new PH definition and categorization of two different subsets of PH-LHD by PVR has not been fully investigated. The aim of this study was to evaluate the new PH-LHD definition for risk stratification in symptomatic heart failure and to investigate the clinical outcomes of Cpc-PH defined by the new criteria.
Methods
Study design
We carried out a retrospective review of patients admitted to our institute. The inclusion criteria were: (1) patients with symptomatic heart failure [New York Heart Association (NYHA) functional classification ≥II and American College of Cardiology Foundation/American Heart Association (ACCF/AHA) classification Stage C or D]; (2) patients who had undergone right heart catheterization (RHC) between January 2007 and December 2016. The exclusion criteria were: (1) acute myocardial infarction, pulmonary arterial hypertension (PAH) (group 1), PH due to lung diseases and/or hypoxia (group 3), chronic thromboembolic PH (group 4), and PH with unclear and/or multifactorial mechanisms (group 5), these patients were excluded at the time of screening; (2) patients with constrictive pericarditis, congenital shunt disease, or receiving hemodialysis. The study patients were divided into four groups according to the criteria of the 6th World Symposium on Pulmonary Hypertension. The patients with symptomatic heart failure but hemodynamically categorized into pre-capillary PH were the undetermined phenotype in the new criteria. We defined this undetermined phenotype as borderline PH: (i) Ipc-PH group, mPAP >20 mmHg, PAWP >15 mmHg, and PVR <3 WU; (ii) Cpc-PH group, mPAP >20 mmHg, PAWP >15 mmHg, and PVR ≥3 WU; (iii) borderline-PH group, mPAP >20 mmHg and PAWP ≤15 mmHg; (iv) non-PH group, mPAP ≤20 mmHg. The study was approved by the institutional review board at Saitama Medical Center, Jichi Medical University (S20-145) and written informed consent was waived because of the retrospective design of the study.
Follow-up
Clinical follow-up was performed at an office visit and by review of medical records. The follow-up period was until December 2018. The day RHC was performed was defined as the index day. The primary endpoint was the composite of cardiac death, re-admission due to heart failure, and implantation of a left ventricular assist device (LVAD). Either cardiac death, first re-admission due to heart failure, or LVAD implantation were considered as an event.
Right heart catheterization
RHC was performed in the study subjects at the compensated stage of heart failure [8]. An external pressure transducer was zeroed at the mid-thoracic line with the patient in the supine position [17]. The average of several consecutive pressure waves over 9 seconds was recorded as the pressure measurement value during RHC. Cardiac output (CO) was measured using thermodilution with cold saline infusion.
Definition of clinical characteristics
Left ventricular (LV) systolic function was expressed as LV ejection fraction (LVEF) measured by echocardiography and was categorized as either reduced LVEF (LVEF <50%) or preserved LVEF (LVEF ≥50%) [18]. Hypertension was defined as a past medical history of hypertension or medical treatment for hypertension before admission [19]. Diabetes mellitus was defined as a hemoglobin A1c level ≥6.5% or treatment for diabetes mellitus before admission [19]. Hyperlipidemia was defined as a low-density lipoprotein cholesterol level ≥140 mg/dL or treatment for hyperlipidemia before admission [19]. Hyperuricemia was defined as a uric acid level >7.0 mg/dL or treatment for hyperuricemia before admission [20]. Anemia was defined as a hemoglobin level <13 g/dL for men and <12 g/dL for women [21]. Renal function was evaluated by the estimated glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease formula modified for the Japanese population [22]. Impaired renal function was defined as eGFR<60 ml/min/1.73 m2 [19]. Estimated right ventricular systolic pressure (eRVSP) measured by echocardiography was calculated as the sum of the peak RV-right atrium (RA) gradient, while RA pressure was estimated by the diameter and respiratory change of the inferior vena cava, as reported previously [23].
Statistical analysis
Data were expressed as mean ± standard deviation (SD) for continuous variables and frequencies and percentages for categorical variables. Analysis of normal or non-normal distributed continuous variables was performed using the Shapiro-Wilk test. Non-parametric continuous variables were analyzed using the Kruskal-Wallis test. Comparison of categorical variables in the four groups was performed using the chi-square test. Kaplan-Meier curves of the Ipc-PH, Cpc-PH, borderline-PH, and non-PH groups were constructed and the curves then compared using the log-rank test. Multivariate Cox hazard analysis was applied to investigate whether each PH group predicted cardiac death, heart failure readmission, or LVAD implantation after adjustment for confounding factors for heart failure (age [24], male sex [25], overweight [26], systolic blood pressure at admission [27], ischemic heart disease [28], anemia [29], hyperuricemia [30], impaired renal function [31], atrial fibrillation or flutter [32], reduced LVEF [33], and use of loop diuretics [34]). The statistical analyses were performed using SPSS 19/Windows statistical software (SPSS Inc, Chicago, IL, USA).
Results
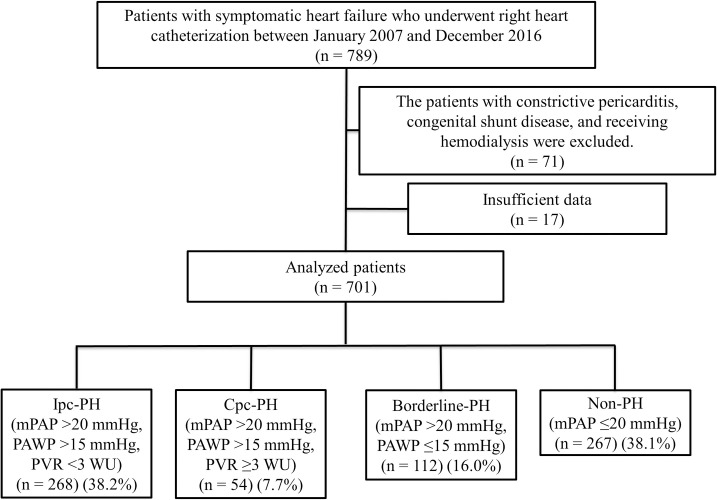
A total of 789 patients were admitted to our hospital for symptomatic heart failure and underwent RHC between January 2007 and December 2016. Seventy-one patients were excluded because of underlying diseases such as constrictive pericarditis, congenital shunt disease, or requirement for hemodialysis. Seventeen patients had insufficient data for RHC and were also excluded from the study. The remaining 701 patients with symptomatic heart failure were included in the analysis. Based on the values of mPAP, PAWP, and PVR the study patients were divided into the Ipc-PH (n = 268), Cpc-PH (n = 54), borderline-PH (n = 112), and non-PH (n = 267) groups. The study flow chart is shown in Fig 1.
Fig 1. Patient enrollment.
Ipc-PH, isolated post-capillary pulmonary hypertension; Cpc-PH, combined pre- and post-capillary pulmonary hypertension; PH, pulmonary hypertension; mPAP, mean pulmonary artery pressure, PAWP, pulmonary artery wedge pressure, PVR, pulmonary vascular resistance; WU, Wood units.
A comparison of the clinical characteristics of the four groups is shown in Table 1. LV systolic function (reduced or preserved LVEF) was not significantly different in the four groups (P = 0.36). There were significant differences in the etiology of heart failure between the 4 groups, with ischemic heart disease being more common in the Cpc-PH group compared to that observed in the other groups. The eGFR level was significantly different in the 4 groups (P = 0.001). Over 70% of patients had received beta blockers and angiotensin converting enzyme inhibitors or angiotensin receptor blockers. The prevalence of taking loop diuretics was significantly different in the 4 groups (P = 0.002). The parameters of RHC are summarized in Table 2 that shows all the parameters (right atrial pressure, systolic pulmonary artery pressure, mPAP, diastolic pulmonary artery pressure, PAWP, CO, cardiac index, heart rate, PVR, DPG, and transpulmonary pressure gradient) were significantly different in the four groups (P <0.001).
Table 1. Clinical characteristics.
| Ipc-PH (n = 268) | Cpc-PH(n = 54) | Borderline-PH (n = 112) | Non-PH(n = 267) | P value | |
|---|---|---|---|---|---|
| Age (years) | 62.6 ± 14.4 | 63.1 ± 13.0 | 66.5 ± 13.6 | 64.9 ± 12.9 | 0.03 |
| Male, n (%) | 193 (72.0%) | 36 (66.7%) | 66 (58.9%) | 174 (65.2%) | 0.08 |
| BMI (kg/m2) | 25.3 ± 5.2 | 25.0 ± 4.8 | 24.5 ± 5.1 | 23.3 ± 4.5 | <0.001 |
| Heart rate on admission (beat/min) | 94.3 ± 28.6 | 87.9 ± 22.8 | 90.9 ± 26.8 | 91.8 ± 29.8 (n = 265) | 0.32 |
| Systolic blood pressure at admission (mmHg) | 125.5 ± 24.2 | 122.9 ± 23.2 | 132.5 ± 25.5 | 135.7 ± 34.2 | 0.002 |
| Left ventricular systolic function | |||||
| Reduced LVEF, n (%) | 173 (64.6%) | 40 (74.1%) | 67 (59.8%) | 171 (64.0%) | 0.36 |
| Preserved LVEF, n (%) | 95 (35.4%) | 14 (25.9%) | 45 (40.2%) | 96 (36.0%) | |
| Principal etiology of heart failure | |||||
| Ischemic heart disease, n (%) | 28 (10.4%) | 13 (24.1%) | 15 (13.4%) | 30 (11.2%) | 0.04 |
| Valvular heart disease, n (%) | 75 (28.0%) | 12 (22.2%) | 31 (27.7%) | 49 (18.4%) | |
| Cardiomyopathy, n (%) | 18 (6.7%) | 4 (7.4%) | 5 (4.5%) | 17 (6.4%) | |
| Others or unknown, n (%) | 147 (54.9%) | 25 (46.3%) | 61 (54.5%) | 171 (64.0%) | |
| Comorbidities | |||||
| Hypertension, n (%) | 138 (51.5%) | 31 (57.4%) | 60 (53.6%) | 124 (46.4%) | 0.35 |
| Diabetes mellitus, n (%) | 91 (34.0%) | 23 (42.6%) | 45 (40.2%) | 79 (29.6%) | 0.11 |
| Hyperlipidemia, n (%) | 118 (44.0%) | 29 (53.7%) | 53 (47.3%) | 108 (40.4%) | 0.27 |
| Hyperuricemia, n (%) | 164 (61.2%) | 35 (64.8%) | 61 (54.5%) | 119 (44.6%) | 0.001 |
| COPD, n (%) | 7 (2.6%) | 1 (1.9%) | 4 (3.6%) | 6 (2.2%) | 0.88 |
| Anemia, n (%) | 90 (33.6%) | 13 (24.1%) | 36 (32.1%) | 79 (29.6%) | 0.50 |
| Impaired renal function (eGFR <60 ml/min/1.73 m2), n (%) | 135 (50.4%) | 28 (51.9%) | 63 (56.3%) | 114 (42.7%) | 0.08 |
| Atrial fibrillation or flutter, n (%) | 122 (45.5%) | 16 (29.6%) | 52 (46.4%) | 105 (39.3%) | 0.09 |
| Echocardiographic characteristics | |||||
| LAD (mm) | 52.8 ± 8.9 (n = 263) | 53.1 ± 6.4 | 51.6 ± 8.1 (n = 108) | 49.3 ± 9.3 (n = 264) | <0.001 |
| LVDd (mm) | 60.4 ± 11.9 (n = 263) | 61.7 ± 10.7 | 58.4 ± 10.2 (n = 108) | 58.2 ± 9.9 (n = 264) | 0.03 |
| LVDs (mm) | 48.2 ± 14.2 (n = 263) | 50.4 ± 13.3 | 45.3 ± 13.0 (n = 107) | 45.8 ± 12.0 (n = 264) | 0.02 |
| LVEF (%) | 41.1 ± 18.1 (n = 263) | 36.4 ± 19.4 | 44.5 ± 17.7 (n = 108) | 42.6 ± 17.2 (n = 264) | 0.02 |
| eRVSP (mmHg) | 40.0 ± 14.9 (n = 258) | 53.1 ± 22.3 (n = 53) | 40.6 ± 16.6 (n = 102) | 31.0 ± 14.4 (n = 245) | <0.001 |
| Laboratory data | |||||
| Hemoglobin (g/dl) | 13.3 ± 2.1 | 13.9 ± 2.6 | 13.3 ± 2.2 | 13.4 ± 2.1 | 0.19 |
| Na (mEq/l) | 139.4 ± 3.7 | 138.5 ± 4.0 | 139.5 ± 3.8 | 139.5 ± 3.0 | 0.23 |
| K (mEq/l) | 4.3 ± 0.5 | 4.3 ± 0.4 | 4.3 ± 0.5 | 4.2 ± 0.5 | 0.94 |
| eGFR (ml/min/1.73 m2) | 58.4 ± 20.3 | 56.9 ± 17.0 | 58.2 ± 22.6 | 64.6 ± 22.1 | 0.001 |
| Uric acid (mg/dl) | 7.5 ± 2.4 (n = 267) | 7.9 ± 2.3 (n = 53) | 7.4 ± 2.4 (n = 111) | 6.7 ± 1.9 (n = 266) | <0.001 |
| BNP (pg/ml) | 723.3 ± 682.8 (n = 261) | 1330.1 ± 1354.9 | 927.9 ± 1127.2 (n = 108) | 672.6 ± 675.2 (n = 261) | <0.001 |
| Medications | |||||
| Angiotensin converting enzyme inhibitor, n (%) | 146 (54.5%) | 31 (57.4%) | 60 (53.6%) | 146 (54.7%) | 0.97 |
| Angiotensin receptor blocker, n (%) | 61 (22.8%) | 8 (14.8%) | 29 (25.9%) | 78 (29.2%) | 0.10 |
| Beta blocker, n (%) | 218 (81.3%) | 44 (81.5%) | 84 (75.0%) | 223 (83.5%) | 0.29 |
| Calcium channel blocker, n (%) | 51 (19.0%) | 11 (20.4%) | 27 (24.1%) | 54 (20.2%) | 0.74 |
| Loop diuretics, n (%) | 244 (91.0%) | 47 (87.0%) | 94 (83.9%) | 212 (79.4%) | 0.002 |
| Thiazide diuretics, n (%) | 5 (1.9%) | 5 (9.3%) | 1 (0.9%) | 11 (4.1%) | 0.01 |
| Mineralocorticoid receptor antagonist, n (%) | 150 (56.0%) | 36 (66.7%) | 51 (45.5%) | 137 (51.3%) | 0.05 |
| Digitalis, n (%) | 18 (6.7%) | 1 (1.9%) | 9 (8.0%) | 17 (6.4%) | 0.49 |
| Oral inotropic agent, n (%) | 2 (0.7%) | 1 (1.9%) | 2 (1.8%) | 1 (0.4%) | 0.47 |
| Statin, n (%) | 104 (38.8%) | 25 (46.3%) | 54 (48.2%) | 96 (36.0%) | 0.11 |
| Amiodarone, n (%) | 22 (8.2%) | 7 (13.0%) | 9 (8.0%) | 18 (6.7%) | 0.49 |
Ipc-PH, isolated post-capillary pulmonary hypertension; Cpc-PH, combined pre- and post-capillary pulmonary hypertension; PH, pulmonary hypertension; BMI, body mass index; LVEF, left ventricular ejection fraction; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; LAD, left atrium dimension; LVDd, left ventricular diastolic dimension; LVDs, left ventricular systolic dimension; eRVSP, estimated right ventricular systolic pressure; BNP, brain natriuretic peptide.
Table 2. Parameters of right heart catheterization.
| Ipc-PH (n = 268) | Cpc-PH(n = 54) | Borderline-PH (n = 112) | Non-PH (n = 267) | P value | |
|---|---|---|---|---|---|
| RAP (mmHg) | 10.5 ± 4.8 | 12.5 ± 5.4 | 7.2 ± 2.8 (n = 111) | 5.2 ± 2.6 (n = 266) | <0.001 |
| sPAP (mmHg) | 43.2 ± 9.8 | 58.0 ± 14.5 | 35.0 ± 5.5 | 26.2 ± 4.7 | <0.001 |
| mPAP (mmHg) | 30.3 ± 6.6 | 40.3 ± 8.9 | 23.4 ± 2.6 | 16.4 ± 2.8 | <0.001 |
| dPAP (mmHg) | 21.8 ± 5.9 | 28.2 ± 7.1 | 15.8 ± 2.7 | 10.8 ± 2.6 | <0.001 |
| PAWP (mmHg) | 22.6 ± 5.6 | 24.0 ± 5.9 | 12.8 ± 2.2 | 9.7 ± 3.0 (n = 266) | <0.001 |
| CO (L/min) | 4.9 ± 1.4 | 3.9 ± 1.0 | 5.0 ± 1.4 (n = 108) | 4.8 ± 1.2 (n = 262) | <0.001 |
| CI (L/min/m2) | 2.9 ± 0.8 | 2.4 ± 0.7 | 3.1 ± 0.8 (n = 108) | 2.9 ± 0.7 (n = 262) | <0.001 |
| Heart rate (beats/min) | 77.9 ± 17.5 (n = 266) | 80.3 ± 13.8 (n = 53) | 71.2 ± 14.2 (n = 109) | 70.4 ± 14.3 (n = 256) | <0.001 |
| PVR (Wood units) | 1.6 ± 0.6 | 4.2 ± 1.4 | 2.3 ± 0.9 (n = 108) | 1.5 ± 0.7 (n = 262) | <0.001 |
| DPG (mmHg) | -0.8 ± 3.7 | 4.2 ± 4.7 | 3.0 ± 3.2 | 1.0 ± 2.5 (n = 266) | <0.001 |
| TPG (mmHg | 7.8 ± 3.3 | 16.3 ± 5.4 | 10.6 ± 3.2 | 6.7 ± 2.4 (n = 266) | <0.001 |
Ipc-PH, isolated post-capillary pulmonary hypertension; Cpc-PH, combined pre- and post-capillary pulmonary hypertension; PH, pulmonary hypertension; RAP, right atrial pressure; sPAP, systolic pulmonary artery pressure; mPAP, mean pulmonary artery pressure; dPAP, diastolic pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; CO, cardiac output; CI, cardiac index; PVR, pulmonary vascular resistance; DPG, diastolic pressure gradient; TPG, transpulmonary pressure gradient.
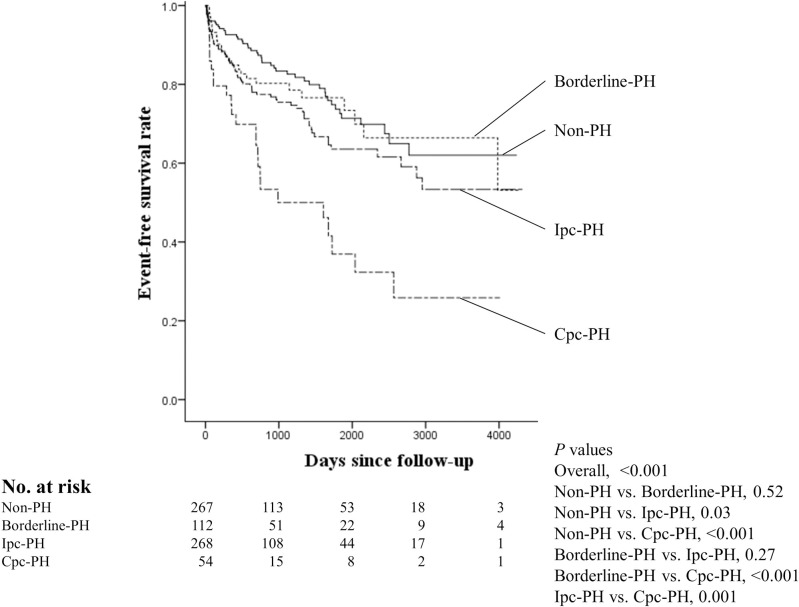
During a median follow-up period of 26 months, there were 166 primary endpoints (S1 Table). The Kaplan-Meier curves for the primary endpoint are shown in Fig 2. Log-rank testing revealed a significant increase in adverse events in the Ipc-PH and Cpc-PH groups compared with that in the non-PH group (P = 0.03 for non-PH group vs. IpC-PH group; P <0.001 for non-PH group vs. Cpc-PH group), while there was no significant difference between the non-PH and borderline-PH groups (P = 0.52). Comparison of the Ipc-PH and Cpc-PH groups showed a significant increase in the risk of adverse events in the Cpc-PH group compared with that observed in the Ipc-PH group (P = 0.001).
Fig 2. Kaplan-Meier curves for primary endpoint in the four groups.
Comparison of the survival curves was performed using the log-rank test. PH, pulmonary hypertension; Ipc-PH, isolated post-capillary pulmonary hypertension; Cpc-PH, combined pre- and post-capillary pulmonary hypertension.
The multivariate Cox regression analysis showed a significant association between adverse cardiac events and the Ipc-PH and Cpc-PH groups compared to that occurring in the non-PH group, even after adjustment for confounding factors (Ipc-PH group, HR 1.56 [95% CI 1.06–2.29], P = 0.02; Cpc-PH group, HR 2.98 [95% CI 1.81–4.90], P <0.001) (Table 3, Model 1). In particular, the Cpc-PH group showed a significant association with cardiac events even when compared to the Ipc-PH group (HR 1.92 [95% CI 1.19–3.08], P = 0.007) (Table 3, Model 2).
Table 3. Multivariate Cox regression analysis predicting primary endpoint.
| Variables | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Classification of PH (vs. Non-PH) | ||||||
| Borderline-PH | 1.08 | 0.66–1.77 | 0.75 | |||
| Ipc-PH | 1.56 | 1.06–2.29 | 0.02 | |||
| Cpc-PH | 2.98 | 1.81–4.90 | <0.001 | |||
| Classification of PH(vs. Ipc-PH) | ||||||
| Non-PH | 0.64 | 0.44–0.94 | 0.02 | |||
| Borderline-PH | 0.70 | 0.43–1.11 | 0.13 | |||
| Cpc-PH | 1.92 | 1.19–3.08 | 0.007 | |||
| Age (10 year increase) | 1.23 | 1.05–1.42 | 0.008 | 1.23 | 1.05–1.42 | 0.008 |
| Male sex (vs. female) | 0.98 | 0.69–1.40 | 0.93 | 0.98 | 0.69–1.40 | 0.93 |
| Overweight (BMI ≥25 kg/m2) | 0.99 | 0.70–1.39 | 0.94 | 0.99 | 0.70–1.39 | 0.94 |
| Systolic blood pressure at admission (10 mmHg increase) | 0.99 | 0.93–1.06 | 0.82 | 0.99 | 0.93–1.06 | 0.82 |
| Ischemic heart disease | 1.77 | 1.16–2.68 | 0.008 | 1.77 | 1.16–2.68 | 0.008 |
| Anemia | 1.34 | 0.95–1.89 | 0.09 | 1.34 | 0.95–1.89 | 0.09 |
| Hyperuricemia | 1.08 | 0.76–1.54 | 0.66 | 1.08 | 0.76–1.54 | 0.66 |
| Impaired renal function (eGFR <60 ml/min/1.73 m2) | 1.07 | 0.76–1.50 | 0.71 | 1.07 | 0.76–1.50 | 0.71 |
| Atrial fibrillation or flutter | 1.06 | 0.76–1.49 | 0.72 | 1.06 | 0.76–1.49 | 0.72 |
| Reduced LVEF (vs. preserved LVEF) | 1.21 | 0.84–1.74 | 0.32 | 1.21 | 0.84–1.74 | 0.32 |
| Loop diuretics use | 1.02 | 0.64–1.62 | 0.94 | 1.02 | 0.64–1.62 | 0.94 |
PH: pulmonary hypertension, Ipc-PH: isolated post-capillary pulmonary hypertension, Cpc-PH: combined pre- and post-capillary pulmonary hypertension, BMI: body mass index, eGFR: estimated glomerular filtration rate, LVEF: left ventricular ejection fraction.
Discussion
The present study included 701 patients with symptomatic heart failure who underwent RHC and investigated whether the new definitions of PH-LHD resulted in better risk stratification of these patients. We found that the new PH-LHD definition clearly stratified patients with symptomatic heart failure and that Cpc-PH was associated significantly with adverse cardiac events even after adjustment for clinically relevant confounding factors.
Validity for the two major changes of the new PH-LHD definition
First, we should discuss about two major issues as follows: whether a change for the mPAP cut-off value to 20 mmHg from 25 mmHg as PH was validated for PH-LHD; which changes were responsible for better prognostication, the cut-off value of mPAP 20 mmHg or using PVR instead of DPG to distinguish IpC-PH and Cpc-PH.
In addition to the fact that mPAP in normal subjects was 14.0 ± 3.3 mmHg, the change of mPAP cut-off value to 20 mmHg was mainly based on the evidence from group 1 population showing worse clinical outcomes in value of mPAP 21–24 mmHg [35, 36]. From the perspective of hemodynamic values in this study, there were few subjects with PAWP >15 mmHg and mPAP ≤20 mmHg. The proportion of PH-LHD (PAWP >15 mmHg and mPAP >20 mmHg) in total LHD (PAWP >15 mmHg) patients during the study period was 98.2%. The mean value of PAWP in the non-PH group was 9.7 ± 3.0 mmHg. Therefore, a value of 20 mmHg as the upper limit of mPAP was appropriate even in PH-LHD. On the other hand, recent study reported that short-term mortality was not statistically different between elevated and normal mPAP group in elevated PVR settings in PH-LHD [37]. We developed further survival analysis using conventional PH criteria (mPAP ≥25 mmHg) and PVR to investigate whether the change of mPAP cut-off value to 20 mmHg from 25 mmHg was meaningful for prognostication. As a result, above conventional criteria also stratified the 4 groups clearly and the Cpc-PH group showed the worst cardiac outcomes (S1 Fig and S2 Table). Thus, the change of mPAP cut-off value to 20 mmHg from 25 mmHg itself did not show meaningful change in terms of risk stratification.
As for the hemodynamic definition of Cpc-PH, there has been discussion on how to distinguish the presence of pre-capillary components in PH-LHD in recent years. The DPG had been used to distinguish between the two subsets of PH-LHD, because an elevated DPG had been shown to be linked to pulmonary vascular remodeling in PH-LHD [38]. However, there are different understanding in real world settings. Conventional PH-LHD definitions according to DPG alone did not show an association between clinical outcomes and Cpc-PH [16, 39]. By contrast, Vanderpool, et al reported that the Cpc-PH defined by elevated DPG also showed statistically significant worse clinical outcomes in a large cohort [40]. In fact, in the current study conventional PH-LHD criteria using only DPG (DPG ≥7 mmHg or not) provided the risk stratification of subjects to some extent, whereas the multivariate Cox regression analysis showed that there was no significant difference in adverse clinical outcomes observed between the Cpc-PH and Ipc-PH groups (S2 Fig and S3 Table). Several studies had revealed PVR as a predictive index for clinical outcomes in patients with heart failure [37, 39]. As for the risk stratification, conventional PH-LHD criteria using PVR alone (PVR ≥3 WU or not) could provide a clear risk stratification even after adjustment for confounding factors of heart failure in this study subjects (S1 Fig and S2 Table). Because the subjects with Cpc-PH categorized by DPG alone were too restrictive compared with that by PVR alone, misclassification of Cpc-PH would show this trend as previous study reported [16]. In fact, the multivariate Cox regression analysis showed that there was no significant difference in cardiac outcomes between the Cpc-PH and Ipc-PH groups, even in the new PH definition (mPAP >20 mmHg) and DPG (DPG ≥7 mmHg or not) (S3 Fig and S4 Table). Hence, we showed that PVR was preferable for definitions of Cpc-PH in terms of clear risk stratification.
Previously, we investigated the clinical features of PH-LHD divided by the values of DPG and transpulmonary pressure gradient (TPPG). We revealed that PH-LHD with DPG ≥7 mmHg (i.e. Cpc-PH) showed worse clinical outcomes as compared with PH-LHD with DPG <7 mmHg and TPPG ≤12 mmHg, but did not show significant difference as compared with PH-LHD with PH-LHD with DPG <7 mmHg and TPPG >12 mmHg [8]. Our previous study indicates that DPG can be a useful marker for the risk stratification of patients with PH-LHD when DPG was combined with TPPG, but DPG alone cannot be a useful marker.
Finally, we compared the outcomes of Cpc-PH between the PH-LHD definition by the “2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension” (S4 Fig and S5 Table) and the new definition by the “6th World Symposium on Pulmonary Hypertension” (Fig 2 and Table 3). The number of patients with Cpc-PH has slightly decreased, while those with Ipc-PH and borderline-PH have increased in the new definition. The both definitions have shown worse long-term outcomes in patients with Cpc-PH compared with Ipc-PH. Notably, the new definition has clarified Cpc-PH as the worst subset of clinical outcomes even compared to other subsets. Thus, the new definition of PH-LHD more clearly stratified the patients with heart failure compared with the definition of 2015 ESC/ERS Guidelines.
Adopt the new PH-LHD definition in real world setting
The feature of our study was that we set not all-cause death but cardiac death as one of the primary composite endpoints in order to strictly determine whether Cpc-PH was associated with heart failure-related clinical outcomes. Under the detailed study design and new definitions of PH-LHD, we found that Cpc-PH was associated significantly with worse clinical outcomes.
In previous studies, the prevalence of Cpc-PH (defined by DPG ≥7 mmHg) was approximately 12% to 13% in patients with heart failure [7, 41]. Other large cohort study showed the prevalence of Cpc-PH (defined by PVR ≥3 WU) was 36.2% within PH-LHD [40]. In our study population, Cpc-PH was 7.7% in patients with heart failure and 16.8% within PH-LHD. This prevalence of Cpc-PH was particularly low compared with above previous studies even in the new PH-LHD definition. The reason was that the severity of heart failure in our study cohort might be mild to moderate, because average mPAP value of our study was 24.7 mmHg, which was lower than average mPAP in previous studies (>30 mmHg) [7, 40]. Another difference between these previous studies and our study was that we strictly selected the study subjects. Because the severity and hemodynamics of heart failure are heterogeneous, we reviewed only patients with symptomatic heart failure. Patients with constrictive pericarditis, congenital shunt disease, and those receiving hemodialysis were excluded from the study, because PH under these conditions may not necessarily be caused by left heart disease. Strict selection of study subjects in our study showed that Cpc-PH was a less common subset of PH-LHD compared to previous studies.
As a result of the new PH-LHD definition in real world settings, patients categorized as borderline-PH represented 16.0% of the subjects in our study. Because pure pre-capillary PH such as groups 1, 3, 4, and 5 PH were not included in the study, these patients were different from pre-capillary PH and had an undetermined phenotype of PH. The borderline-PH group hemodynamically straddled the border of the non-PH and Ipc-PH group. Of course, the patients with borderline-PH might be just well compensated state, which would be the main reason that the event rates in this group showed no statistical difference when compared to the group without PH. However, the mean value of PVR and DPG in this group were 2.3 WU and 3.0 mmHg, respectively, which were higher even compared with Ipc-PH group. Although the values of PVR and DPG in borderline-PH group were close to those in Cpc-PH group, event rate of borderline-PH group straddled across non-PH and Ipc-PH groups. A recent pathological investigation in patients with heart failure with PH showed global pulmonary vascular remodeling with thickening of the media and intima in arteries and thickening of the intima in veins and small pulmonary vessels [42]. That study also showed that the severity of PH correlated most strongly with venous and small vessel remodeling [42]. When this pathological investigation applied to our study, it indicated that the relation between the degree of elevated left heart pressure and PH was not necessarily synonymous even in terms of pulmonary vein and small vessels.
One of the important issues in PH-LHD is whether Cpc-PH may benefit from specific treatment regimens. However, no study has revealed the answer to this question [43–47]. Our study showed that the new definition provided clear risk stratification for heart failure prognosis in the hemodynamically divided groups with symptomatic heart failure. Using this clear risk stratification it is possible that each subset could be different treatments targets. However, the definitive answer to this issue also requires greater understanding of the pathophysiology of PH-LHD.
Study limitations
The study was a retrospective design in a single tertiary center, that may have resulted in higher selection of patients who were hospitalized in our center. It is also possible that incomplete follow-up may have occurred because clinical follow-up was performed at office visits and by review of medical records. Finally, we should mention that we could not clarify whether or not Cpc-PH defined by the new criteria had a pathological pre-capillary component in PH-LHD. Further prospective studies and pathological evaluations are therefore warranted.
Conclusions
The definitions for PH-LHD of the 6th World Symposium on Pulmonary Hypertension recommendations provide clear risk stratification in symptomatic heart failure. Notably, Cpc-PH defined by the new criteria is associated significantly with worse cardiac outcomes.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Comparison of the survival curves was performed using the log-rank test. PH, pulmonary hypertension; PVR, pulmonary vascular resistance; Ipc-PH, isolated post-capillary pulmonary hypertension; Cpc-PH, combined pre- and post-capillary pulmonary hypertension.
(TIF)
Comparison of the survival curves was performed using the log-rank test. PH, pulmonary hypertension; DPG, diastolic pressure gradient; Ipc-PH, isolated post-capillary pulmonary hypertension; Cpc-PH, combined pre- and post-capillary pulmonary hypertension.
(TIF)
Comparison of the survival curves was performed using the log-rank test. PH, pulmonary hypertension; DPG, diastolic pressure gradient; Ipc-PH, isolated post-capillary pulmonary hypertension; Cpc-PH, combined pre- and post-capillary pulmonary hypertension.
(TIF)
Comparison of the survival curves was performed using the log-rank test. PH-LHD, pulmonary hypertension due to left heart disease; PH, pulmonary hypertension; Ipc-PH, isolated post-capillary pulmonary hypertension; Cpc-PH, combined pre- and post-capillary pulmonary hypertension.
(TIF)
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D100–8. 10.1016/j.jacc.2013.10.033 [DOI] [PubMed] [Google Scholar]
- 2.Fang JC, DeMarco T, Givertz MM, Borlaug BA, Lewis GD, Rame JE, et al. World Health Organization Pulmonary Hypertension group 2: pulmonary hypertension due to left heart disease in the adult—a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2012;31(9):913–33. 10.1016/j.healun.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 3.Kjaergaard J, Akkan D, Iversen KK, Kjoller E, Kober L, Torp-Pedersen C, et al. Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol. 2007;99(8):1146–50. 10.1016/j.amjcard.2006.11.052 [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–75. 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 5.Miller WL, Grill DE, Borlaug BA. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart Fail. 2013;1(4):290–9. 10.1016/j.jchf.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 6.Caravita S, Faini A, Carolino D’Araujo S, Dewachter C, Chomette L, Bondue A, et al. Clinical phenotypes and outcomes of pulmonary hypertension due to left heart disease: Role of the pre-capillary component. PLoS One. 2018;13(6):e0199164. 10.1371/journal.pone.0199164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerges M, Gerges C, Pistritto AM, Lang MB, Trip P, Jakowitsch J, et al. Pulmonary Hypertension in Heart Failure. Epidemiology, Right Ventricular Function, and Survival. Am J Respir Crit Care Med. 2015;192(10):1234–46. 10.1164/rccm.201503-0529OC [DOI] [PubMed] [Google Scholar]
- 8.Ibe T, Wada H, Sakakura K, Ikeda N, Yamada Y, Sugawara Y, et al. Pulmonary hypertension due to left heart disease: The prognostic implications of diastolic pulmonary vascular pressure gradient. J Cardiol. 2016;67(6):555–9. 10.1016/j.jjcc.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 9.Tampakakis E, Leary PJ, Selby VN, De Marco T, Cappola TP, Felker GM, et al. The diastolic pulmonary gradient does not predict survival in patients with pulmonary hypertension due to left heart disease. JACC Heart Fail. 2015;3(1):9–16. 10.1016/j.jchf.2014.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palazzini M, Dardi F, Manes A, Bacchi Reggiani ML, Gotti E, Rinaldi A, et al. Pulmonary hypertension due to left heart disease: analysis of survival according to the haemodynamic classification of the 2015 ESC/ERS guidelines and insights for future changes. Eur J Heart Fail. 2018;20(2):248–55. 10.1002/ejhf.860 [DOI] [PubMed] [Google Scholar]
- 11.Rezaee ME, Nichols EL, Sidhu M, Brown JR. Combined Post- and Precapillary Pulmonary Hypertension in Patients With Heart Failure. Clin Cardiol. 2016;39(11):658–64. 10.1002/clc.22579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1). 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vachiery JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53(1). 10.1183/13993003.01897-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34(4):888–94. 10.1183/09031936.00145608 [DOI] [PubMed] [Google Scholar]
- 15.Caravita S, Dewachter C, Soranna D, D’Araujo SC, Khaldi A, Zambon A, et al. Haemodynamics to predict outcome in pulmonary hypertension due to left heart disease: a meta-analysis. Eur Respir J. 2018;51(4). 10.1183/13993003.02427-2017 [DOI] [PubMed] [Google Scholar]
- 16.Dragu R, Hardak E, Ohanyan A, Adir Y, Aronson D. Prognostic value and diagnostic properties of the diastolic pulmonary pressure gradient in patients with pulmonary hypertension and left heart disease. Int J Cardiol. 2019;290:138–43. 10.1016/j.ijcard.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 17.Kovacs G, Avian A, Pienn M, Naeije R, Olschewski H. Reading pulmonary vascular pressure tracings. How to handle the problems of zero leveling and respiratory swings. Am J Respir Crit Care Med. 2014;190(3):252–7. 10.1164/rccm.201402-0269PP [DOI] [PubMed] [Google Scholar]
- 18.Thenappan T, Shah SJ, Gomberg-Maitland M, Collander B, Vallakati A, Shroff P, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4(3):257–65. 10.1161/CIRCHEARTFAILURE.110.958801 [DOI] [PubMed] [Google Scholar]
- 19.Sakakura K, Kubo N, Ako J, Wada H, Fujiwara N, Funayama H, et al. Peak C-reactive protein level predicts long-term outcomes in type B acute aortic dissection. Hypertension. 2010;55(2):422–9. 10.1161/HYPERTENSIONAHA.109.143131 [DOI] [PubMed] [Google Scholar]
- 20.Yamanaka H, Japanese Society of G, Nucleic Acid M. Japanese guideline for the management of hyperuricemia and gout: second edition. Nucleosides Nucleotides Nucleic Acids. 2011;30(12):1018–29. 10.1080/15257770.2011.596496 [DOI] [PubMed] [Google Scholar]
- 21.Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]
- 22.Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Tsukamoto Y, et al. Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am J Kidney Dis. 2007;50(6):927–37. 10.1053/j.ajkd.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 23.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713; quiz 86–8. 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 24.Hamaguchi S, Kinugawa S, Goto D, Tsuchihashi-Makaya M, Yokota T, Yamada S, et al. Predictors of long-term adverse outcomes in elderly patients over 80 years hospitalized with heart failure.—A report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J. 2011;75(10):2403–10. 10.1253/circj.cj-11-0267 [DOI] [PubMed] [Google Scholar]
- 25.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(4 Suppl A):6A–13A. 10.1016/0735-1097(93)90455-a [DOI] [PubMed] [Google Scholar]
- 26.Kenchaiah S, Pocock SJ, Wang D, Finn PV, Zornoff LA, Skali H, et al. Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;116(6):627–36. 10.1161/CIRCULATIONAHA.106.679779 [DOI] [PubMed] [Google Scholar]
- 27.Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O’Connor CM, She L, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296(18):2217–26. 10.1001/jama.296.18.2217 [DOI] [PubMed] [Google Scholar]
- 28.Vedin O, Lam CSP, Koh AS, Benson L, Teng THK, Tay WT, et al. Significance of Ischemic Heart Disease in Patients With Heart Failure and Preserved, Midrange, and Reduced Ejection Fraction: A Nationwide Cohort Study. Circ Heart Fail. 2017;10(6). [DOI] [PubMed] [Google Scholar]
- 29.Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52(10):818–27. 10.1016/j.jacc.2008.04.061 [DOI] [PubMed] [Google Scholar]
- 30.Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, et al. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation. 2003;107(15):1991–7. 10.1161/01.CIR.0000065637.10517.A0 [DOI] [PubMed] [Google Scholar]
- 31.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109(8):1004–9. 10.1161/01.CIR.0000116764.53225.A9 [DOI] [PubMed] [Google Scholar]
- 32.Yamauchi T, Sakata Y, Miura M, Onose T, Tsuji K, Abe R, et al. Prognostic Impact of Atrial Fibrillation and New Risk Score of Its Onset in Patients at High Risk of Heart Failure- A Report From the CHART-2 Study. Circ J. 2017;81(2):185–94. 10.1253/circj.CJ-16-0759 [DOI] [PubMed] [Google Scholar]
- 33.Meta-analysis Global Group in Chronic Heart F. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33(14):1750–7. 10.1093/eurheartj/ehr254 [DOI] [PubMed] [Google Scholar]
- 34.Miura M, Sugimura K, Sakata Y, Miyata S, Tadaki S, Yamauchi T, et al. Prognostic Impact of Loop Diuretics in Patients With Chronic Heart Failure- Effects of Addition of Renin-Angiotensin-Aldosterone System Inhibitors and beta-Blockers. Circ J. 2016;80(6):1396–403. 10.1253/circj.CJ-16-0216 [DOI] [PubMed] [Google Scholar]
- 35.Coghlan JG, Wolf M, Distler O, Denton CP, Doelberg M, Harutyunova S, et al. Incidence of pulmonary hypertension and determining factors in patients with systemic sclerosis. Eur Respir J. 2018;51(4). 10.1183/13993003.01197-2017 [DOI] [PubMed] [Google Scholar]
- 36.Douschan P, Kovacs G, Avian A, Foris V, Gruber F, Olschewski A, et al. Mild Elevation of Pulmonary Arterial Pressure as a Predictor of Mortality. Am J Respir Crit Care Med. 2018;197(4):509–16. 10.1164/rccm.201706-1215OC [DOI] [PubMed] [Google Scholar]
- 37.Crawford TC, Leary PJ, Fraser CD 3rd, Suarez-Pierre A, Magruder JT, Baumgartner WA, et al. Impact of the New Pulmonary Hypertension Definition on Heart Transplant Outcomes: Expanding the Hemodynamic Risk Profile. Chest. 2020;157(1):151–61. 10.1016/j.chest.2019.07.028 [DOI] [PubMed] [Google Scholar]
- 38.Gerges C, Gerges M, Lang MB, Zhang Y, Jakowitsch J, Probst P, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in "out-of-proportion" pulmonary hypertension. Chest. 2013;143(3):758–66. 10.1378/chest.12-1653 [DOI] [PubMed] [Google Scholar]
- 39.Tampakakis E, Shah SJ, Borlaug BA, Leary PJ, Patel HH, Miller WL, et al. Pulmonary Effective Arterial Elastance as a Measure of Right Ventricular Afterload and Its Prognostic Value in Pulmonary Hypertension Due to Left Heart Disease. Circ Heart Fail. 2018;11(4):e004436. 10.1161/CIRCHEARTFAILURE.117.004436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanderpool RR, Saul M, Nouraie M, Gladwin MT, Simon MA. Association Between Hemodynamic Markers of Pulmonary Hypertension and Outcomes in Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2018;3(4):298–306. 10.1001/jamacardio.2018.0128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Assad TR, Hemnes AR, Larkin EK, Glazer AM, Xu M, Wells QS, et al. Clinical and Biological Insights Into Combined Post- and Pre-Capillary Pulmonary Hypertension. J Am Coll Cardiol. 2016;68(23):2525–36. 10.1016/j.jacc.2016.09.942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fayyaz AU, Edwards WD, Maleszewski JJ, Konik EA, DuBrock HM, Borlaug BA, et al. Global Pulmonary Vascular Remodeling in Pulmonary Hypertension Associated With Heart Failure and Preserved or Reduced Ejection Fraction. Circulation. 2018;137(17):1796–810. 10.1161/CIRCULATIONAHA.117.031608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4(1):8–17. 10.1161/CIRCHEARTFAILURE.110.944694 [DOI] [PubMed] [Google Scholar]
- 44.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116(14):1555–62. 10.1161/CIRCULATIONAHA.107.716373 [DOI] [PubMed] [Google Scholar]
- 45.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268–77. 10.1001/jama.2013.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonderman D, Ghio S, Felix SB, Ghofrani HA, Michelakis E, Mitrovic V, et al. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation. 2013;128(5):502–11. 10.1161/CIRCULATIONAHA.113.001458 [DOI] [PubMed] [Google Scholar]
- 47.Vachiery JL, Delcroix M, Al-Hiti H, Efficace M, Hutyra M, Lack G, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J. 2018;51(2). 10.1183/13993003.01886-2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Comparison of the survival curves was performed using the log-rank test. PH, pulmonary hypertension; PVR, pulmonary vascular resistance; Ipc-PH, isolated post-capillary pulmonary hypertension; Cpc-PH, combined pre- and post-capillary pulmonary hypertension.
(TIF)
Comparison of the survival curves was performed using the log-rank test. PH, pulmonary hypertension; DPG, diastolic pressure gradient; Ipc-PH, isolated post-capillary pulmonary hypertension; Cpc-PH, combined pre- and post-capillary pulmonary hypertension.
(TIF)
Comparison of the survival curves was performed using the log-rank test. PH, pulmonary hypertension; DPG, diastolic pressure gradient; Ipc-PH, isolated post-capillary pulmonary hypertension; Cpc-PH, combined pre- and post-capillary pulmonary hypertension.
(TIF)
Comparison of the survival curves was performed using the log-rank test. PH-LHD, pulmonary hypertension due to left heart disease; PH, pulmonary hypertension; Ipc-PH, isolated post-capillary pulmonary hypertension; Cpc-PH, combined pre- and post-capillary pulmonary hypertension.
(TIF)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.