Abstract
Melanogenesis is a key parameter of differentiation in melanocytes and melanoma cells; therefore, search for factors regulating this pathway are strongly desired. Herein, we investigated the effects of melatonin, a ubiquitous physiological mediator that is found throughout animals and plants. In mammals, the pineal gland secretes this indoleamine into the blood circulation to exert an extensive repertoire of biological activities. Our in vitro assessment indicates an oncostatic capacity of melatonin in time-dependent manner (24, 48, 72 hours) in highly pigmented MNT-1 melanoma cells. The similar pattern of regulation regarding cell viability was observed in amelanotic Sk-Mel-28 cells. Subsequently, MNT-1 cells were tested for the first time for evaluation of melanin/melatonin interaction. Thus primary, electron paramagnetic resonance (EPR) spectroscopy demonstrated that melatonin reduced melanin content. Artificially induced disturbances of melanogenesis by selected inhibitors (N-phenylthiourea or kojic acid) were slightly antagonized by melatonin. Additionally, analysis using transmission electron microscopy has shown that melatonin, particularly at higher dose of 10−3 mol/L, triggered the appearance of premelanosomes (stage I-II of melanosome) and MNT-1 cells synthesize de novo endogenous melatonin shown by LC-MS. In conclusion, these studies show a melanogenic-like function of melatonin suggesting it as an advantageous agent for treatment of pigmentary disorders.
Keywords: electron paramagnetic resonance spectroscopy, liquid chromatography-mass spectroscopy, melanogenesis, melanoma cells, melatonin, transmission electron microscopy, tyrosinase activity
1 ∣. INTRODUCTION
Melanogenesis is a multistage process involving melanin synthesis in melanosomes, the transport of melanosomes to the dendrite tips of the melanocytes, and their subsequent release. In melanocytes and in melanoma cells, melanin synthesis is controlled by a cascade of enzymatic reactions beginning with the oxidation of tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA) by tyrosinase (TYR), the rate-limiting enzyme of melanogenesis.1,2 Among the hormonal regulators of melanin synthesis,3 α-melanocyte-stimulating hormone (α-MSH),4-8 a product of POMC processing, plays an important role. It interacts with a specific cell surface melanocortin 1 receptor (MC1R) stimulating melanin synthesis and other differentiated functions of melanocytes.8,9 Furthermore, the cyclic adenosine monophosphate (cAMP) pathway plays a key role in the regulation of melanogenesis through up-regulation of the transcription microphthalmia-associated transcription factor (MITF) and subsequent melanogenic enzymes including TYR.10-12 Thus, considering the enhanced number of individuals with pigment disorders, an agent with potent regulatory capacities of melanogenesis is of broad therapeutic interest.
Melatonin (N-acetyl-5-methoxytryptamine) is a methoxyindole hormone and a bioregulator which is present in almost all biological systems including animals, plants, and microbes.13-17 It is synthesized in the pineal gland18 but also in other cells19-23 including in the human skin.24-28 Melatonin is well-known to regulate circadian rhythm in humans. Additionally, it has many other effects including regulation of immune and endocrine functions, and it exhibits anti-apoptotic,29-32 and anti-inflammatory33 properties against the internal and environmental insults.13,14,16,19,22,28,34-40 These responses are mediated either through binding to membrane-bound melatonin receptors (MT1 or MT2), receptor-independent mechanisms, or through activation of nuclear receptors.13,14,16,22,41,42 Expression of membrane-bound cell surface MT receptors in the skin is variable, depending on the species. For instance, skin from the C57BL/6 mouse predominantly or exclusively expresses MT2.43,44 Differently, human skin expresses both receptors,17,27,45 although with a strong bias toward MT1 (the predominant form found in both whole skin and cultured cells).26,46 We have recently shown that highly pigmented human MNT-1 melanoma cells possess membrane-bound melatonin receptors (MT1 or MT2) indicating high-affinity binding for melatonin.31
To date, extensive studies have been focused on melatonin's role in general regulation of body homeostasis.13,14,16,22 It is known that locally produced melatonin plays a role in the regulation of skin physiology,17,24,27,28,47 and exogenously applied melatonin exerts pro-differentiation effects in human skin.28,48,49 Originally melatonin was alleged as lightening agent based on its action on amphibian skin.21,50 Among mammals, melatonin's role in fur pigmentation has been well established51,52 and subsequently reviewed by Slominski et al.3,53 The anti-melanogenic activity was also documented in rodent melanoma cells54 and murine skin organ culture45 while its oncostatic activity was elucidated in rodent and human melanomas.54-56 Numerous reports showed that melatonin induces cell pigmentation in human amelanotic cell lines, that is, Sk-Mel-1, Sk-Mel-23 or Sk-Mel-28.57,58 Thus, these data may seem to be in conflict to the results of others on melatonin functions in human hair and skin pigmentation.3,27,59 Locally produced melatonin may play a role in the regulation of melanocytic activities via its impact on the peripheral clock. Therefore, testing of topically applied melatonin during defined circadian windows as an external modulator of intracutaneous clock activity as well as describing the mechanism of action of melatonin in human pigmentation requires still better definition.
To better understand the melatonin's capacity of modulation of melanogenesis, we perform our investigations, contrary to the others, on melanotic cell model as well as in presence of inhibitors of melanin synthesis (N-phenylthiourea (PTU), kojic acid). These results together with latest reports undoubtedly contribute a significant enhancement of knowledge to comprehensively describe the biological meaningful of melatonin and its interaction of complexed mechanism of melanogenesis.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Reagents
Minimum essential medium eagle (MEM) with low glucose (1000 mg/L), 1% penicillin-streptomycin solution (10 000 units of penicillin and 10 mg of streptomycin in 1 mL 0.9% NaCl), acetonitrile, bovine serum albumin (BSA), cacodylate buffer (0.1 mol/L), EDTA, ethanol, formic acid, HEPES (1 mol/L), glucose, glutaraldehyde, HCl, isocitrate, isopropanol, KCl, kojic acid, L-DOPA, lead citrate, leucine enkephalin, melatonin, methanol, methylene chloride, MgSO4, MTT, NADPH, NaCl, NaOH, nonessential amino acids (NEAA) (100×), N-phenylthiourea (PTU), 1% OsO4, propylene oxide, serotonin, sodium acetate, Triton® X-100, and uranyl acetate were purchased from . Fetal bovine serum, 0.05% trypsin/0.53 mmol/L EDTA solution, 1 × PBS (pH 7.4), L-glutamine (200 mmol/L), AIM-V™ medium were supplied by Thermo Fisher Scientific.
2.2 ∣. Cell culture
Melanoma cell lines included human melanotic MNT-1 cells acquired as a gift from Dr Cedric Delevoye (Institute Curie), and human amelanotic cells (Sk-Mel-28) (American Type Culture Collection). MNT-1 cells were cultured in MEM medium supplemented with 20% (v/v) heat-inactivated fetal bovine serum, 10% (v/v) AIM-V™ medium, 2 mmol/L L-glutamine, 10 mmol/L HEPES, 1% (v/v) NEAA, 1% (v/v) streptomycin-penicillin solution. Sk-Mel-28 cells were maintained in MEM medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 2 mmol/L L-glutamine, 1% (v/v) streptomycin-penicillin solution. Both cell lines in the logarithmic growth phase were used in all experiments while 80%-90% monolayers of confluent cells were harvested with a mixture of 0.05% trypsin-EDTA solution.
2.3 ∣. Cell treatment
Prior to beginning the treatments, cells were cultured in normal medium for 24 hours to attach to the bottom of the culture dish. Culture medium was replaced with fresh medium containing melatonin versus control sample, that is., 0.2% ethanol in culture medium. Melatonin was dissolved in absolute ethanol and further diluted with 1 × PBS to yield 10−2 mol/L stock solution. Cells were treated with the final concentration of melatonin ranging from 10−11 mol/L to 10−3 mol/L in time-dependent manner for 24, 48, and 72 hours for cell viability (MTT Assay), for melanin synthesis and DOPA oxidase activity of tyrosinase (melatonin range: 10−8, 10−6, 10−3 mol/L for 72 hours) in presence of selected inhibitors, that is, 10−3 mol/L PTU or 200 μg/mL kojic acid. The highest resultant concentration of ethanol 0.2% serving as a solvent was not found to be toxic itself to the cells after 72 hours.
2.4 ∣. Cell viability assay
Cells were seeded on 96-well plates at the density of 0.15 × 105 cells/well in the culture medium and incubated with tested compounds until desired time end-point. Cell viability was evaluated by MTT assay based on the protocol described earlier60 where MTT (5 mg/mL) was dissolved in 1 × PBS, sterilized by filtration through a 0.22 μmol/L Millipore® filter and stored at 4°C. After the treatment, cells were washed twice with 1 × PBS, 100 μL of MTT in culture medium (the final dilution, 1:10) was added to each well, and cells were incubated subsequently for 3 hours at 37°C to allow MTT metabolism. The resultant purple formazan was dissolved in 100 μL isopropanol/0.04N HCl, absorbances were measured at 595 nm using a BioTek EL×808™ microplate reader (BioTek Instruments Inc), and results were normalized to the control cells at 0 hour.
2.5 ∣. Melanin content and electron paramagnetic resonance spectroscopy analysis
Preliminary assessment of melanin content in MNT-1 cells was supported by electron paramagnetic resonance (EPR) spectroscopy.61 After 72 hours incubation with melatonin in dose-dependent manner (10−10, 10−8, 10−6, 10−4, 10−3 mol/L), cells (1 × 106/sample) were pelleted, frozen, and stored at −80°C. EPR analysis was performed in liquid nitrogen using a finger-type quartz Dewar and EMX-AA spectrometer (Bruker BioSpin Corp., Billerica, MA, USA) operating at X-band with 100 kHz magnetic modulation. The amount of melanin in tested samples was determined by comparing double integrals of their EPR signals to the EPR signal of an appropriate melanin standard. For quantification of melanin cells, synthetic DOPA-melanin at the concentration of 0.57 mg/mL was used as described earlier by Sarna et al.62 Resultant data were presented as content of melanin expressed in pg/cell. Comparatively, cysteine-L-DOPA-melanin at a concentration of 1.34 mg/mL was used to show the characteristics of EPR signal of pheomelanin.
Analysis of suppressive actions by used inhibitors and melatonin on melanogenesis was evaluated as follows: Cells were seeded on 6-well plates (Sarstedt) at the density of 0.3 × 106 cells/well and were allowed to attach overnight. Thereafter, cells were incubated for 72 hours in fresh medium containing various concentrations of melatonin or in mixture with 10−3 mol/L PTU or 200 μg/mL kojic acid. For determination of melanin content, the MNT-1 cells were harvested, washed with 1 × PBS, centrifuged at 1000 g for 10 minutes (4°C), and solubilized in 500 μL of 1N NaOH for 2 hours at 80°C. The absorbances were measured at 405 nm using a BioTek EL×808™ microplate reader, and results were presented as the percentage of the control sample.
2.6 ∣. DOPA oxidase activity of tyrosinase
MNT-1 cells were seeded on 6-well plates and incubated with melatonin or in a mixture of melanogenesis modulators for 72 hours. Cells were harvested, washed with 1 × PBS, centrifuged at 1000 g for 10 minutes (4°C), and lysed with 0.5% Triton® X-100 in 1 × PBS on ice. The lysates were subsequently centrifuged at 16 000 g for 15 minutes (4°C), 300 μL resultant supernatant was added to 300 μL of 5 mmol/L L-DOPA in 1 × PBS, and incubated for 1 hour at 37°C. The dopachrome formation was evaluated by measuring absorbance at 475 nm using a BioTek ELx808™ microplate reader, and results were presented as the percentage of the control sample.
2.7 ∣. Liquid Chromatography-Mass Spectrometry (LC-MS) detection of induced production of melatonin
Cells were seeded at the density of 1 × 107, washed with PBS and resuspended with 1 mL HEPES-buffered medium (100 mmol/L HEPES, 120 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L MgSO4, 15 mmol/L sodium acetate, 10 mg/mL BSA and 10 mmol/L glucose; pH 7.4). The reactions were initiated by addition of 100 μmol/L serotonin, 100 μmol/L NADPH, and 1 mmol/L isocitrate. After 24 hours shaking incubation (70 rpm) at 37°C, the extractions were performed twice using 2.5 mL methylene chloride. Resulting samples were dried with liquid nitrogen, redissoved in methanol followed by LC-MS analysis using Xevo G2-XS QTof LC-MS system (Waters). Zorbax Eclipse Plus C18 column (2.1 × 50 mm, 1.8 μm) (Agilent Technology) and Atlantis C18 column (100 × 4.6 mm, 5 μm) (Waters) were used for LC-MS analysis. The flow rates were 0.3 mL/min with isocratic or linear gradient of acetonitrile containing 0.1% formic acid: 15% for 1.5 minute, 15%–30% for 0.1 minutes, 30% for 0.9 minutes, 30%–100% for 0.5 minutes, 100% for 3 minutes for Zorbax Eclipse Plus C18 column, and 0.5 mL/ min with isocratic or linear gradient of acetonitrile containing 0.1% formic acid: 15% for 1.5 minutes, 15%–30% for 3.5 minutes, 30% for 2.5 minutes, 30%–100% for 2.5 minutes, 100% for 5 minutes for Atlantis C18 column. The mass was scanned the range of 100 to 1000 Da in positive mode using the continuum mode with scan time of 1 second. The capillary and cone voltages were 1.7 kV and 40 V, respectively. The desolvation gas flow rate was 800 L/hour with source temperature of 120°C. Leucine enkephalin at the concentration of 200 ng/mL (m/z = 556.2771) was used as the lockspray reference compound at the flow rate of 10 μL/min with lockspray interval of 10 seconds and scan time of 1 second. The mass chromatograms were processed by Waters MassLynx 4.1 software.
2.8 ∣. Transmission Electron Microscopy (TEM)
Briefly, cells were seeded on 6-well plates in the culture medium, and thereafter grown to subconfluence (as judged from light microscopy). Cells were treated with melatonin for 72 hours, harvested, collected by centrifugation (700 rpm. for 5 minutes), washed three times with 1 × PBS, and fixed with 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer for 24 hours at 4°C. Cells were washed three times with 0.1 mol/L cacodylate buffer, postfixed in 1% OsO4 for 2 hours at room temperature (RT), and washed again in distilled water. Cells were embedded in Poly/Bed®812 (Polysciences Inc) after dehydratation in ethanol and propylene oxide. Ultrathin sections (65 nm thick) were counterstained with uranyl acetate and lead citrate before observation with a Jeol JEM 2100 HT transmission electron microscope.
2.9 ∣. Statistical analysis
Experiments were performed at least three times, with results expressed in each case as the mean + standard deviation (SD). Significant differences between results were determined by the univariate analysis of variance (ANOVA) or the Student's ttest and appropriate post hoc analysis using GraphPad Prism 7.05 software. Obtained data for viability assay, melanin content, tyrosinase activity were normalized and are presented as percentage of the control sample, that is, 0.2% EtOH. A P-value of less than .05 was considered statistically significant.
3 ∣. RESULTS AND DISCUSSION
3.1 ∣. The effect of melatonin on melanoma cell proliferation
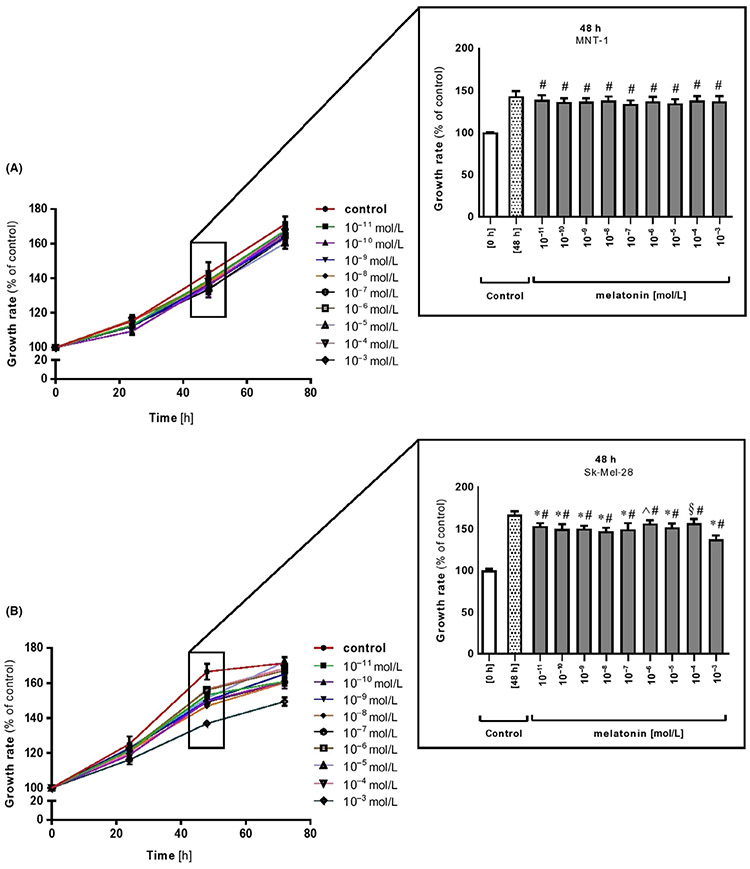
Results of cell viability showed no statistically significant effect of melatonin on cell proliferation in highly pigmented MNT-1 cells, which is in contrast to amelanotic Sk-Mel-28 melanoma cell line Figure 1A,B and inserts). The dose-dependent inhibitory action on Sk-Mel-28 cells was apparent at 48 and 72 hours with the most potent growth inhibition seen at the highest dose of melatonin, that is, 10−3 mol/L Figure 1, inserts for 48 hours). These results, involving especially Sk-Mel-28 cells, are in direct agreement with previous reports performed by Souza et al.58 A similar pattern of regulation upon melatonin in suspension Sk-Mel-1 melanoma cells has been described by Cabrera et al57 or in other melanoma cell lines, indicating an oncostatic effect of melatonin.55,63 Other studies demonstrated in vitro anti-proliferative actions of this molecule in amelanotic rodent melanomas, that is, S-91 mouse and Bomirski AbC1 hamster cell lines,54,64 in human M-6 melanoma cells65 or Sk-Mel-188 melanoma cultured in Ham's F10 medium.63 This inhibitory activity is consistent with a large number of investigations showing the ability of melatonin to reduce cancer cell proliferation in lymphoid, prostate, carcinoma, and neuroblastoma tumor cells.66-73 In contrast to these studies, the melanotic MNT-1 cell line was resistant to significant inhibition of cell proliferation Figure 1A and corresponding insert). We assume that melatonin-mediated arrest of cancer cell proliferation could be connected with the fact that the main target organelles for this indoleamine are mitochondria. Thus, glucose regulation/energy metabolism in cancer cells is critically dependent either on mitochondria or because of fact that most cancer cells use cytosolic glycolysis for ATP synthesis. Moreover, the differences in cancer cell sensitivity to melatonin, which we observed here between MNT-1 and Sk-Mel-28, may also depend on the specific metabolomic fingerprinting of each cancer cell type. Indeed, millimolar concentrations of melatonin decrease S-phase population and trigger apoptosis in colon cancer cells, while the same concentrations only reduce the proportion of cells in G2/M phase in both osteosarcoma and leukemia cells, without any effect on cell death.74,75 In support of these observations, our data also suggest that those effects depend on the overall metabolic and differentiation state of the cancer cells. It should be added that the balance between oxidative and glycolytic metabolism is maintained by hypoxia-inducible factor 1 (HIF-1), a transcription factor which mediates adaptive response to changes in tissue oxygenation. It was shown that increase in HIF-1 dependent mRNAs for genes in the glycolytic pathway is accompanied by increases in total protein levels.76 Thus, it was well-reported that HIF-1-dependent regulation of mitochondrial metabolism may also contribute to the protective effects of ischemic preconditioning.77 Furthermore, HIF-1 protein has been also found as the one that is regulated during process of melanogenesis what is presented and discussed below.
FIGURE 1.
Melatonin displays oncostatic-like effects in melanoma cells. Highly pigmented MNT-1 (A) and amelanotic Sk-Mel-28 (B) melanoma cells were treated with melatonin in dose- (10−11-10−3 mol/L) and time-dependent manner (0, 24, 48, 72 h) and were investigated using the MTT viability assay as described in Materials and Methods. Inserts present inhibitory growth rate at selected time end-point, that is, 48 h for MNT-1 and Sk-Mel-28 melanomas. Data were presented as the mean + SD (n = 6), the values are expressed as percentage of the control sample. Statistically significant differences versus control at 48 h were indicated as §P < .05, ^P < .01, *P < .001 while comparison of melatonin-treated cells versus control sample at 0 h was indicated as #P < .001
3.2 ∣. Effect of melatonin on melanogenesis in melanoma cells
Melanogenesis is regulated at the cellular level via the controlling formation of melanosomes, which can be produced in varying sizes, numbers, and densities depending on melanin content. Finally, melanogenesis is involving at the subcellular level intracellular pathways where the gene expression is encoded by the melanogenesis-related enzymes, including tyrosinase, tyrosinase-related protein 1 and 2 (TRP1 and TRP2). It should be noted that although these three enzymes are engaged in the melanogenesis pathway, only tyrosinase is absolutely necessary for melanogenesis, due to its key role in this process. These pathways are initiated by a variety of hormones, including interleukins, interferons, growth factors, and prostaglandins, which determine not only the quantity but also the quality of the synthesized melanin.78
Herein, highly pigmented MNT-1 melanoma has been identified as an optimal model to assess the efficacy of melanogenic regulators. This selection is also in agreement with previous reports regarding pigmentation research.79-83 Recent studies have shown that melatonin inhibits melanin formation in hair follicles of Siberian hamster51,52 and in rodent melanoma cells.54,84 Thus, we checked the role of melatonin in regulation of melanogenesis in human malignant melanocytes. As evident from EPR results (Figure 2A-C), MNT-1 cells contained predominantly eumelanin as indicated by the characteristic spectral line obtained for the cells in comparison to synthetic DOPA-melanin used as standard for eumelanin. In addition, EPR analysis revealed a significant decrease of melanin content after treatment with melatonin reaching the level of 2.34 ± 0.01 (10−6 mol/L, P < .05), 2.22 ± 0.09 (10−4 mol/L, P < .01) and 2.09 ± 0.07 (10−3 mol/L, P < .001) pg of melanin per cell compared to the values in untreated melanoma cells (2.72 ± 0.13 pg/cell) Figure 2D. Furthermore, melatonin doses applied in these studies which elicit inhibition correspond to the physiological limits. It has reported mammalian plasma melatonin concentrations which fall within the range of 10-200 pg/mL, that is, approximately 5 × 10−8 mol/L to 1 × 10−6 mol/L. The inhibitory action of melatonin on melanogenesis occurs in the absence of effects upon tyrosinase, generally accepted as the rate-limiting enzyme in melanin biosynthesis.85 This confirms earlier in vivo findings of Logan and Weatherhead86 that increased tyrosinase activity does not inevitably result in melanogenesis. This suggests that pigment production can be controlled other than through the regulation of tyrosinase.54 For instance, Logan and Weatherhead52 showed that melatonin (10−6 mol/L) brings inhibition of melanogenesis independently of applied series of blockers of this process. This may imply that melatonin arrests melanogenesis through a mechanism which operates at some post-tyrosinase step in the melanin biosynthethic pathway. Slominski and Pruski54 also presented that melatonin at relatively higher doses, those consistent to this study, is acting as a competitive inhibitor rather than acting through melatonin receptors or via binding sites for a ligand, however, their location remains to be identified.
FIGURE 2.
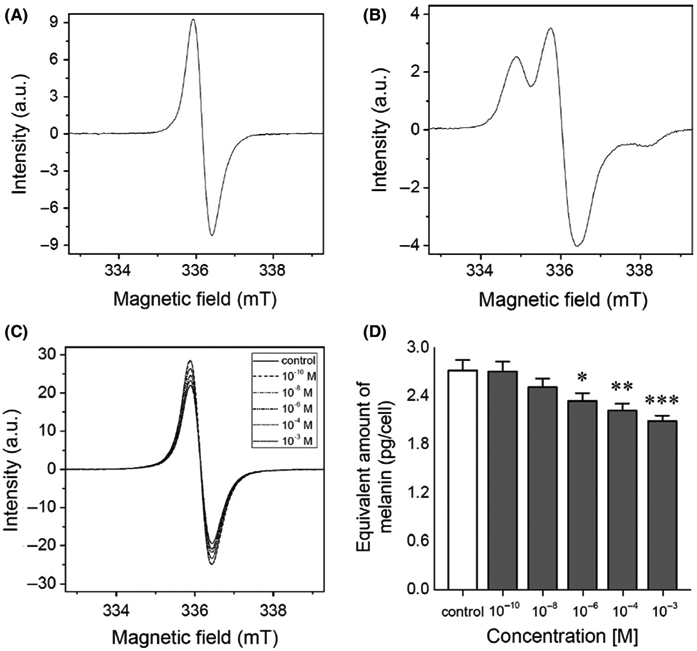
Melatonin decreases melanin content in MNT-1 melanoma cells. Based on assessment carried out by electron paramagnetic resonance (EPR) spectroscopy, here the spectra of (A) synthetic DOPA-melanin used as standard for melanin determination; (B) synthetic cysteine-L-DOPA melanin indicating the characteristic of EPR signal of pheomelanin, and cells after 72 h incubation with melatonin in dose-dependent manner are depicted (C). Bar graph displays mean values + SD (n = 3) of pg of melanin per cell in melatonin-treated cells (D). Statistically significant differences versus control were indicated as *P < .05, **P < .01, ***P < .001
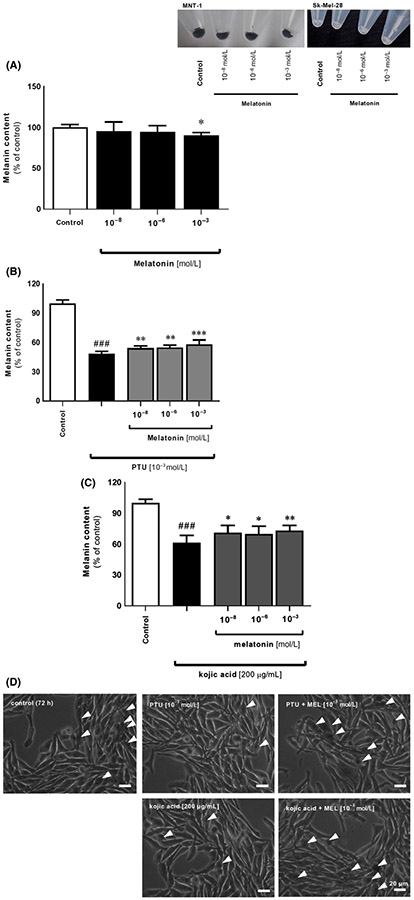
Indeed, inhibitory action of melatonin enclosed here is in agreement with previous reports using mouse S91 and hamster AbC1 melanoma cells cultured in vitro,54,63 and it can be coupled with enzymatic or gene expression-related mechanisms. Melatonin at lower (physiologic) concentrations (0.1-10 nmol/L) affects proliferation with no evident actions toward melanogenesis. High doses (>0.10 μmol/L) inhibited the induction of melanogenesis but was either without an effect or even stimulated (100 μmol/L) proliferation. Thus, differential effects of melatonin on cell proliferation and melanin content could indicate that this indoleamine may regulate or modify both processes through different/independent mechanisms. This was confirmed by subsequent analysis where we noticed a similar pattern of regulation regarding significant drop of pigment content at the dose of 10−3 mol/L (P < .05) Figure 3A. Comparative images of pellets revealed that there was no effect of melatonin on pigmentation in amelanotic Sk-Mel-28 melanoma cell line (Figure 3, inserted images). Furthermore, we assessed the influence of melatonin's responsiveness to alterations in melanogenesis mediated by selected inhibitors, that is, PTU and kojic acid. They decreased melanin content after 72 hours by 52% and 39% (P < .001), respectively Figure 3B,C what was visualized afterward using the light microscope Figure 3D. These data are consistent with PTU-treated Sk-Mel-188 melanomas presented by Brożyna et al87 or Slominski et al.88 Interestingly, co-incubation of melatonin indicates an antagonizing effect of neurohormone, however, the difference was only slightly pronounced. Similar action of melatonin was observed at the level of tyrosinase activity Figure 4A,B as well. Presence of inhibitors decreased it by 45% and 26% (P < .001) for PTU and kojic acid, respectively, while melatonin antagonized these alterations, particularly at the dose of 10−3 mol/L. Above results implicate a logical mechanisms of melanogenesis but clearly suggest to be more complex than originally thought, involving diverse molecular pathways. Slominski et al47 using combined in vitro and in vivo data demonstrated that stimulation of melanogenesis increases the overall expression of HIF-1α and its subsequent nuclear localization. It is also possible that HIF-1α is induced by the production of intermediates of melanogenesis, including ROS and the consumption of intracellular oxygen by the melanogenic process.2,3 Indeed, the authors presented that expression of HIF-1α, but not HIF-2α, protein is prominently up-regulated proportionally to induction of melanogenesis in Bomirski hamster AbC1 and human Sk-Mel-188 melanoma cells.47 As a result, initiation of melanogenesis affects the expression of multiple genes involved in the regulation of melanocyte/melanoma behavior, including the metabolic switch to glycolysis cooridinated by HIF-1. It accompanies the changes in mitochondrial stress-related genes, immunity, angiogenesis, and cell proliferation. This can be linked with our results where we observed significant decrease of melanin synthesis at 1 mmol/L melatonin while Park et al89 detected inhibition of HIF-1α protein at the same dose of this indoleamine. This shows the correlation between melatonin, HIF-1, and melanogenesis.
FIGURE 3.
Melatonin counteracts the inhibitor-mediated alterations in cell pigmentation in MNT-1 cells. Images enclosed as insert present distinct differences in pigmentation ratio between MNT-1 and Sk-Mel-28, representatives for highly pigmented and amelanotic cells, respectively. (A) Evaluation of melanin content has been carried out using melanotic MNT-1 cells cultured in MEM supplemented medium for 72 h with melatonin (10−8, 10−6, 10−3 mol/L) and melanogenic inhibitors, that is, 10−3 mol/L PTU (B) or 200 μmol/L kojic acid (C) as described in Materials and Methods. Data were presented as the mean + SD (n = 5), the values are expressed as percentage of the control sample. Statistically significant differences were indicated as *P < .05, **P < .01, ***P < .001 while comparison of PTU- or kojic acid-treated cells versus control sample was indicated as ###P < .001. (D) Visualization of MNT-1 cells in culture and effect of melatonin versus PTU or kojic acid. Arrowheads point out pigmented cells
FIGURE 4.
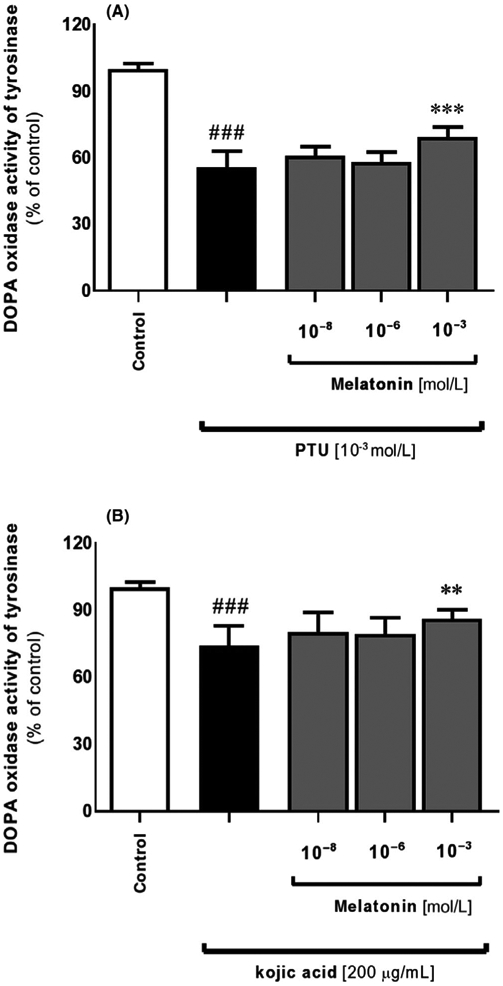
Actions of melatonin on altered tyrosinase activity due to selected inhibitors in MNT-1 melanoma cell line. Applied 10−3 mol/L PTU (A) or 200 μmol/L kojic acid (B) significantly affected tyrosinase activity compared to co-incubated cells with melatonin for 72 hours. Briefly, cells were lysed with 0.5% Triton® X-100, centrifuged at 16 000 g (15 min, 4°C), 5 mmol/L L-DOPA was added to resultant supernatant, and the dopachrome formation was evaluated by measuring absorbance at λ = 475 nm. Data were presented as the mean + SD (n = 5), the values are expressed as percentage of the control sample. Statistically significant differences were indicated as *P < .05, **P < .01, ***P < .001 while comparison of PTU- or kojic acid-treated cells versus control sample was indicated as ###P < .001
Despite all, we suggest that melatonin could be considered as a regulator of pigmentary disorders. Although it is generally agreed that melatonin exerts a hypopigmenting action on most biological systems, its effects in mammalian pigmentation are particularly uncertain and undoubtedly multifaceted. For instance, orally applied melatonin has no noticeable effects on mammalian pigmentation90 especially in man.59 On the other hand, melatonin plays a key role in the control of pigmentation in certain rodent species that undergo seasonal fur color changes.91 Thus, the synthesis of melatonin is inhibited by long days and is maximal under short days.92 However, whether the effect of melatonin is directly exerted on melanocytes or indirectly via an interaction with the pituitary synthesis and release of α-MSH, remains to be clarified.
Earlier in vitro analysis showed that MNT-1 cell line contains abundant melanosomes at all stages (ie, stages I–IV)80 whereas Sk-Mel-28 cells have only stage I and II melanosomes.93,94 In fact, both, stage I and II melanosomes are early melanosomes or premelanosomes because they have not initiated melanin synthesis. In contrast, stage III melanosomes are characterized by the active synthesis of melanin, which results in the deposition of black electron-dense pigment on a fibrillar matrix. Finally, stage IV melanosomes are fully mature and little internal structure is visible because they are completely packed with melanin. According to Chen et al,80 MNT-1 cells have relatively even distribution of melanosomes according to stage (II-III to IV),this is in line with our observation conducted by TEM assessment where we showed distinguish stages of developed melanosomes Figure 5. It was also apparent that 10−3 mol/L melatonin induced a marked development of premelanosomes at stage I-II Figure 5D with simultaneous maintenance of matured melanosomes at stage III-IV for melatonin at lower doses of 10−8 mol/L or 10−6 mol/L Figure 5B,C. Endogenous melatonin in MNT-1 cells is also present as we confirmed using LC-MS analysis (Figure 6A-F). This could alleviate our concerns that treatment with melatonin may not be more apparent (masking effect) or is obvious only at high concentration (10−3 mol/L). Furthermore, this assessment is that currently investigated melanomas have a significant capacity to synthesize melatonin de novo upon exogenous application of 100 μmol/L serotonin. Thus, micromolar concentration of serotonin is present in serum, therefore, not spectacular effect of serotonin on melatonin production could be related to oversaturated endogenous system. We are aware that our approach regarding evaluation of ability of endogenous synthesis of melatonin in MNT-1 cells is undoubtedly a milestone. Thus, the analysis has defined this cell line as a target model related to the widely understood studies about melatonin and its relevance to melanogenesis.
FIGURE 5.
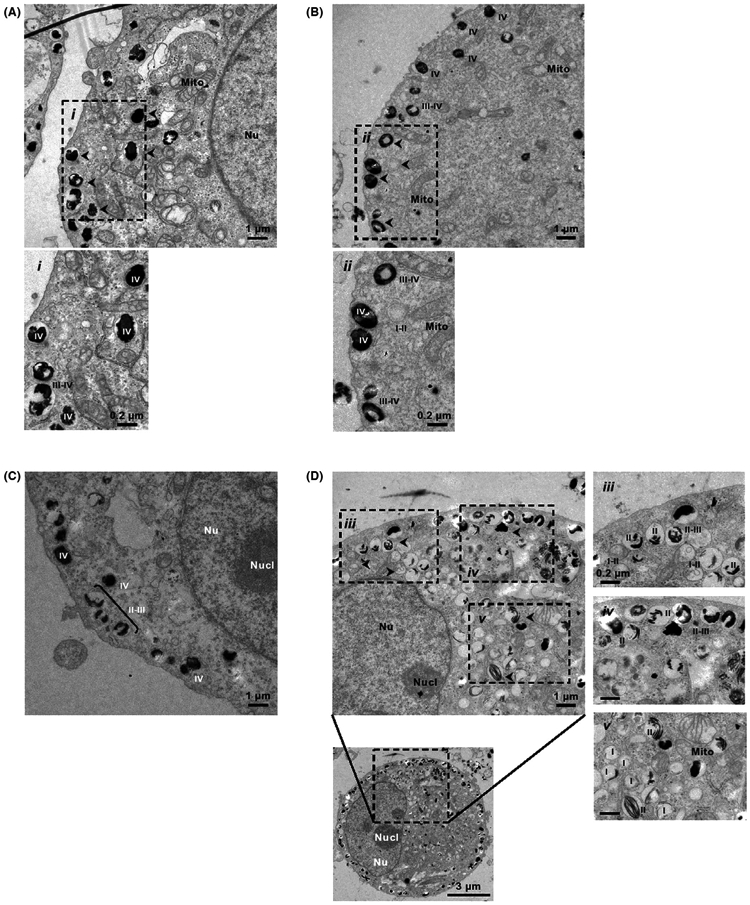
Transmission electron microscopy images were obtained as described in Materials and Methods. This study reveals differences in number of the particular stages of melanosome occurred after 72 hours with melatonin in dose-dependent manner, that is, 10−8 mol/L (B), 10−6 mol/L (C), 10−3 mol/L (D) compared to the control sample (A). Arrowheads indicate some stages of I-IV melanosomes. Nu, nucleus; Nucl, nucleolus; Mito, mitochondria
FIGURE 6.
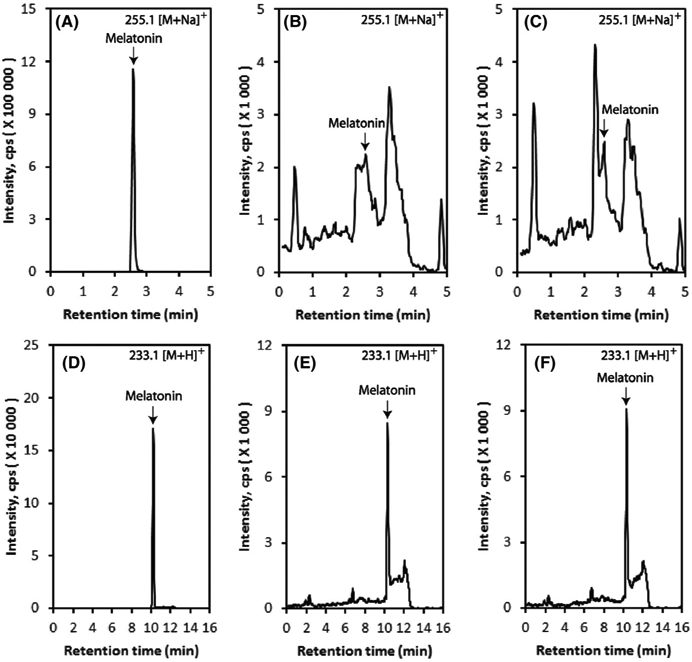
Melatonin production is slightly elevated by endogenous addition of serotonin. Highly pigmented MNT-1 cells were incubated with 100 μmol/L serotonin for 24 hours, and melatonin was extracted with methylene chloride followed by LC-MS using Xevo G2-XS QT of LC-MS system as described in Materials and Methods. Extracted Ion Chromatograms (EIC) with m/z = 255.1 [M + Na]+ using Zorbax Eclipse Plus C18 column are shown: (A) melatonin standard; (B) control (without serotonin); (C) 100 μmol/L serotonin. EICs with m/z = 233.1 [M + H]+ using Atlantis C18 column are shown: (D) melatonin standard; (E) control (without serotonin); (F) 100 μmol/L serotonin
In conclusion, our study provides evidence that melatonin exhibits anti-proliferative actions in MNT-1 or Sk-Mel-28 cells which are consistent with earlier reports with other melanoma cells.54,55,57,58,63-65 Collectively, the findings enrich current knowledge where melatonin is an effective antitumor compound useful in the treatment of melanoma alone or in combination with known chemotherapeutic agents to improve the efficacy of conventional cytotoxic agents. Additionally, melatonin possesses multifaceted effects with regard to regulation of melanogenesis. It lightens highly pigmented MNT-1 cells what was not performed so far. It neutralizes pigmentation disturbances mediated by exogenously applied melanogenic inhibitors. The molecular mode of action of melatonin may be related to the fact that it tends to maintain cellular homeostasis as observed earlier when it attenuates oxidative stress,28,30,31,34,40,95, apoptosis29,30 and inflammation33,35 in normal cells while stimulating it in cancer cells. Herein, melatonin's action on pigmentation opens new regulatory targets for this molecule. However, further examinations of these associations are strongly desired, including studies on primary melanocytes. The ability of melatonin to control this process may serve as a rationale strategy for treatment of pigmentary disorders with this multifunctional molecule.
ACKNOWLEDGEMENTS
We express our gratitude to Olga Woźnicka, PhD (Institute of Zoology and Biomedical Research, Jagiellonian University) for her technical support regarding transmission electron microscopy. The present study was mainly supported by the grant of the German Research Foundation (Deutsche Forschungsgemeinschaft [DFG]); grant number: KL2900/2-1 (KK) with partial funding by grants Sonata-2015/19/D/ST4/01964 from the National Science Center of Poland (MS), 1R01AR056666-01A2, and to some degree 1R01AR073004-01A1 and 1RO1AR071189-01A1 from NIH (ATS).
Funding information
Deutsche Forschungsgemeinschaft (DFG), Grant/Award Number: KL2900/2-1; NIH Clinical Center, Grant/Award Number: 1R01AR056666-01A2, 1R01AR073004-01A1 and 1RO1AR071189-01A1 ; Narodowe Centrum Nauki, Grant/Award Number: Sonata-2015/19/D/ST4/01964
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Hearing VJ, Jiménez M. Analysis of mammalian pigmentation at the molecular level. Pigment Cell Res. 1989;2(2):75–85. [DOI] [PubMed] [Google Scholar]
- 2.Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012;25(1):14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Malek Z, Swope VB, Suzuki I, et al. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc Natl Acad Sci USA. 1995;92(5):1789–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Böhm M, Wolff I, Scholzen TE, et al. α-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J Biol Chem. 2005;280(7):5795–5802. [DOI] [PubMed] [Google Scholar]
- 6.Dunbar JC, Lu H. Proopiomelanocortin (POMC) products in the central regulation of sympathetic and cardiovascular dynamics: studies on melanocortin and opioid interactions. Peptides. 2000;21(2):211–217. [DOI] [PubMed] [Google Scholar]
- 7.Gkogkolou P, Sarna M, Sarna T, Paus R, Luger TA, Böhm M. Protection of glucotoxicity by a tripeptide derivative of α-melanocyte-stimulating hormone in human epidermal keratinocytes. Br J Dermatol. 2019;180(4):836–848. [DOI] [PubMed] [Google Scholar]
- 8.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80(3):979–1020. [DOI] [PubMed] [Google Scholar]
- 9.Park HY, Russakovsky V, Ohno S, Gilchrest BA. The beta isoform of protein kinase C stimulates human melanogenesis by activating tyrosinase in pigment cells. J Biol Chem. 1993;268(16):11742–11749. [PubMed] [Google Scholar]
- 10.Bertolotto C, Abbe P, Hemesath TJ, et al. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J Cell Biol. 1998;142(3):827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halaban R, Pomerantz SH, Marshall S, Lerner AB. Tyrosinase activity and abundance in Cloudman melanoma cells. Arch Biochem Biophys. 1984;230(1):383–387. [DOI] [PubMed] [Google Scholar]
- 12.Wong G, Pawelek J. Melanocyte-stimulating hormone promotes activation of pre-existing tyrosinase molecules in Cloudman S91 melanoma cells. Nature. 1975;255(5510):644–646. [DOI] [PubMed] [Google Scholar]
- 13.Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin – a pleiotropic, orchestrating regulator molecule. Prog Neurogibol. 2011;93(3):350–384. [DOI] [PubMed] [Google Scholar]
- 14.Lanoix D, Lacasse AA, Reiter RJ, Vaillancourt C. Melatonin: the smart killer: the human trophoblast as a model. Mol Cell Endocrinol. 2012;348(1):1–11. [DOI] [PubMed] [Google Scholar]
- 15.Reiter RJ, Tan DX, Zhou Z, Cruz MH, Fuentes-Broto L, Galano A. Phytomelatonin: assisting plants to survive and thrive. Molecules. 2015;20(4):7396–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiter RJ, Rosales-Corral S, Tan DX, Jou MJ, Galano A, Xu B. Melatonin as a mitochondria-targeted antioxidant: one of evolution's best ideas. Cell Mol Life Sci. 2017;74(21):3863–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol Metab. 2008;19(1):17–24. [DOI] [PubMed] [Google Scholar]
- 18.Lerner AB, Case JD, Takahashi Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J Biol Chem. 1960;235:1992–1997. [PubMed] [Google Scholar]
- 19.Bubenik GA. Thirty four years since the discovery of gastrointestinal melatonin. J Physiol Pharmacol. 2008;59:33–51. [PubMed] [Google Scholar]
- 20.Konturek SJ, Konturek PC, Brzozowska I, et al. Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT). J Physiol Pharmacol. 2007;58(3):381–405. [PubMed] [Google Scholar]
- 21.Lerner AB, Case JD, Mori W, Wright MR. Melatonin in peripheral nerve. Nature. 1959;183:1821. [DOI] [PubMed] [Google Scholar]
- 22.Tan DX, Reiter RJ. Mitochondria: the birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019;2(1):44–66. [Google Scholar]
- 23.Zmijewski MA, Sweatman TW, Slominski AT. The melatonin-producing system is fully functional in retinal pigment epithelium (ARPE-19). Mol Cell Endocrinol. 2009;307(1–2):211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi H, Kromminga A, Dunlop TW, et al. A role of melatonin in neuroectodermal-mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J. 2005;19(12):1710–1712. [DOI] [PubMed] [Google Scholar]
- 25.Slominski A, Baker J, Rosano TG, et al. Metabolism of serotonin to N-acetylserotonin, melatonin, and 5-methoxytryptamine in hamster skin culture. J Biol Chem. 1996;271(21):12281–12286. [DOI] [PubMed] [Google Scholar]
- 26.Slominski A, Pisarchik A, Semak I, et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002;16(8):896–898. [DOI] [PubMed] [Google Scholar]
- 27.Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005;19(2):176–194. [DOI] [PubMed] [Google Scholar]
- 28.Slominski AT, Hardeland R, Zmijewski MA, Slominski RM, Reiter RJ, Paus R. Melatonin: A cutaneous perspective on its production, metabolism, and functions. J Invest Dermatol. 2018;138(3):490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrabi SA, Sayeed I, Siemen D, Wolf G, Horn TF. Direct inhibition of the mitochondrial permeability transition pore: A possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J. 2004;18(7):869–871. [DOI] [PubMed] [Google Scholar]
- 30.Janjetovic Z, Nahmias ZP, Hanna S, et al. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J Pineal Res. 2014;57(1):90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleszczyński K, Bilska B, Stegemann A, et al. Melatonin and its metabolites ameliorate UVR-induced mitochondrial oxidative stress in human MNT-1 melanoma cells. Int J Mol Sci. 2018;19(12):e3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slominski AT, Zmijewski MA, Semak I, et al. Melatonin, mitochondria, and the skin. Cell Mol Life Sci. 2017;74(21):3913–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleszczyński K, Zwicker S, Tukaj S, et al. Melatonin compensates silencing of heat shock protein 70 and suppresses ultraviolet radiation-induced inflammation in human skin ex vivo and cultured keratinocytes. J Pineal Res. 2015;58(1):117–126. [DOI] [PubMed] [Google Scholar]
- 34.Fischer TW, Kleszczyński K, Hardkop LH, Kruse N, Zillikens D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2'-deoxyguanosine) in ex vivo human skin. J Pineal Res. 2013;54(3):303–312. [DOI] [PubMed] [Google Scholar]
- 35.Janjetovic Z, Jarrett SG, Lee EF, Duprey C, Reiter RJ, Slominski AT. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci Rep. 2017;7(1):1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleszczyński K, Tukaj S, Kruse N, Zillikens D, Fischer TW. Melatonin prevents ultraviolet radiation-induced alterations in plasma membrane potential and intracellular pH in human keratinocytes. J Pineal Res. 2013;54(1):89–99. [DOI] [PubMed] [Google Scholar]
- 37.Kleszczyński K, Zillikens D, Fischer TW. Melatonin enhances mitochondrial ATP synthesis, reduces reactive oxygen species formation, and mediates translocation of the nuclear erythroid 2-related factor 2 resulting in activation of phase-2 antioxidant enzymes (γ-GCS, HO-1, NQO1) in ultraviolet radiation-treated normal human epidermal keratinocytes (NHEK). J Pineal Res. 2016;61(2):187–197. [DOI] [PubMed] [Google Scholar]
- 38.Luchetti F, Canonico B, Betti M, et al. Melatonin signaling and cell protection function. FASEB J. 2010;24(10):3603–3624. [DOI] [PubMed] [Google Scholar]
- 39.Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12(2):151–180. [DOI] [PubMed] [Google Scholar]
- 40.Skobowiat C, Brożyna AA, Janjetovic Z, et al. Melatonin and its derivatives counteract the ultraviolet B radiation-induced damage in human and porcine skin ex vivo. J Pineal Res. 2018;65(2):e12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27(2):101–110. [DOI] [PubMed] [Google Scholar]
- 42.Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351(2):152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J Cell Physiol. 2003;196(1):144–153. [DOI] [PubMed] [Google Scholar]
- 44.Slominski A, Pisarchik A, Wortsman J. Expression of genes coding melatonin and serotonin receptors in rodent skin. Biochim Biophys Acta. 2004;1680(2):67–70. [DOI] [PubMed] [Google Scholar]
- 45.Slominski A, Chassalevris N, Mazurkiewicz J, Maurer M, Paus R. Murine skin as a target for melatonin bioregulation. Exp Dermatol. 1994;3(1):45–50. [DOI] [PubMed] [Google Scholar]
- 46.Pozo D, García-Mauriño S, Guerrero JM, Calvo JR. mRNA expression of nuclear receptor RZR/RORalpha, melatonin membrane receptor MT, and hydroxindole-O-methyltransferase in different populations of human immune cells. J Pineal Res. 2004;37(1):48–54. [DOI] [PubMed] [Google Scholar]
- 47.Slominski A, Kim TK, Brożyna AA, et al. The role of melanogenesis in regulation of melanoma behavior: melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways. Arch Biochem Biophys. 2014;563:79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim TK, Kleszczynski K, Janjetovic Z, et al. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013;27(7):2742–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slominski AT, Kleszczyński K, Semak I, et al. Local melatoninergic system as the protector of skin integrity. Int J Mol Sci. 2014;15(10):17705–17732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lerner AB. Hormonal control of pigmentation. Annu Rev Med. 1960;11:187–194. [DOI] [PubMed] [Google Scholar]
- 51.Logan A, Weatherhead B. Melatonin-induced inhibition of melanogenesis in hair follicles in vitro [proceedings]. J Endocrinol. 1979;81(2):168P. [PubMed] [Google Scholar]
- 52.Logan A, Weatherhead B. Post-tyrosinase inhibition of melanogenesis by melatonin in hair follicles in vitro. J Invest Dermatol. 1980;74(1):47–50. [DOI] [PubMed] [Google Scholar]
- 53.Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005;124(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slominski A, Pruski D. Melatonin inhibits proliferation and melanogenesis in rodent melanoma cells. Exp Cell Res. 1993;206(2):189–194. [DOI] [PubMed] [Google Scholar]
- 55.Fischer TW, Zmijewski MA, Zbytek B, et al. Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int J Oncol. 2006;29(3):665–672. [DOI] [PubMed] [Google Scholar]
- 56.Slominski A, Fischer TW, Zmijewski MA, et al. On the role of melatonin in skin physiology and pathology. Endocrine. 2005;27(2):137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cabrera J, Negrín G, Estévez F, Loro J, Reiter RJ, Quintana J. Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-MEL-1 cells. J Pineal Res. 2010;49(1):45–54. [DOI] [PubMed] [Google Scholar]
- 58.Souza AV, Visconti MA, Castrucci AM. Melatonin biological activity and binding sites in human melanoma cells. J Pineal Res. 2003;34(4):242–248. [DOI] [PubMed] [Google Scholar]
- 59.McElhinney DB, Hoffman SJ, Robinson WA, Ferguson J. Effect of melatonin on human skin color. J Invest Dermatol. 1994;102(2):258–259. [DOI] [PubMed] [Google Scholar]
- 60.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay; assessment of chemosensitivity testing. Cancer Res. 1987;47(4):936–942. [PubMed] [Google Scholar]
- 61.Sarna T, Swartz HN. The physical properties of melanin. In: Nordlund JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, Ortonne JP, eds. The Pigmentary System, Physiology and Pathophysiology. Oxford, UK: Blackwell Publishing Ltd; 2006:311–341. [Google Scholar]
- 62.Sarna M, Zadlo A, Pilat A, et al. Nanomechanical analysis of pigmented human melanoma cells. Pigment Cell Melanoma Res. 2013;26(5):727–730. [DOI] [PubMed] [Google Scholar]
- 63.Kim TK, Lin Z, Tidwell WJ, Li W, Slominski AT. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol Cell Endocrinol. 2015;404:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Castrucci AM, Almeida AL, Al-Obeidi FA, et al. Comparative biological activities of alpha-MSH antagonists in vertebrate pigment cells. Gen Comp Endocrinol. 1997;105(3):410–416. [DOI] [PubMed] [Google Scholar]
- 65.Ying SW, Niles LP, Crocker C. Human malignant melanoma cells express high-affinity receptors for melatonin: antiproliferative effects of melatonin and 6-chloromelatonin. Eur J Pharmacol. 1993;246(2):89–96. [DOI] [PubMed] [Google Scholar]
- 66.Blask DE, Sauer LA, Dauchy RT, Holowachuk EW, Ruhoff MS, Kopff HS. Melatonin inhibition of cancer growth in vivo involves suppression of tumor fatty acid metabolism via melatonin receptor-mediated signal transduction events. Cancer Res. 1999;59(18):4693–4701. [PubMed] [Google Scholar]
- 67.Cos S, González A, Güezmes A, et al. Melatonin inhibits the growth of DMBA-induced mammary tumors by decreasing the local biosynthesis of estrogens through the modulation of aromatase activity. Int J Cancer. 2006;118(2):274–278. [DOI] [PubMed] [Google Scholar]
- 68.Cucina A, Proietti S, D'Anselmi F, et al. Evidence for a biphasic apoptotic pathway induced by melatonin in MCF-7 breast cancer cells. J Pineal Res. 2009;46(2):172–180. [DOI] [PubMed] [Google Scholar]
- 69.Dauchy RT, Blask DE, Dauchy EM, et al. Antineoplastic effects of melatonin on a rare malignancy of mesenchymal origin: melatonin receptor-mediated inhibition of signal transduction, linoleic acid metabolism and growth in tissue-isolated human leiomyosarcoma xenografts. J Pineal Res. 2009;47(1):32–42. [DOI] [PubMed] [Google Scholar]
- 70.Girgert R, Hanf V, Emons G, Gründker C. Membrane-bound melatonin receptor MT1 down-regulates estrogen responsive genes in breast cancer cells. J Pineal Res. 2009;47(1):23–31. [DOI] [PubMed] [Google Scholar]
- 71.Reiter RJ, Rosales-Corral SA, Tan DX, et al. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int J Mol Sci. 2017;18(4):843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rubio S, Estévez F, Cabrera J, Reiter RJ, Loro J, Quintana J. Inhibition of proliferation and induction of apoptosis by melatonin in human myeloid HL-60 cells. J Pineal Res. 2007;42(2):131–138. [DOI] [PubMed] [Google Scholar]
- 73.Xi SC, Siu SW, Fong SW, Shiu SY. Inhibition of androgen-sensitive LNCaP prostate cancer growth in vivo by melatonin: association of antiproliferative action of the pineal hormone with mt1 receptor protein expression. Prostate. 2001;46(1):52–61. [DOI] [PubMed] [Google Scholar]
- 74.Hong Y, Won J, Lee Y, et al. Melatonin treatment induces interplay of apoptosis, autophagy, and senescence in human colorectal cancer cells. J Pineal Res. 2014;56(3):264–274. [DOI] [PubMed] [Google Scholar]
- 75.Liu L, Xu Y, Reiter RJ. Melatonin inhibits the proliferation of human osteosarcoma cell line MG-63. Bone. 2013;55(2):432–438. [DOI] [PubMed] [Google Scholar]
- 76.Seagroves TN, Ryan HE, Lu H, et al. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol Cell Biol. 2001;21(10):3436–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 2011;1813(7):1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang TS, Chen CT. Inhibitory effect of homochlorcyclizine on melanogenesis in α-melanocyte stimulating hormone-stimulated mouse B16 melanoma cells. Arch Pharm Res. 2012;35(1):119–127. [DOI] [PubMed] [Google Scholar]
- 79.Chen KG, Valencia JC, Lai B, et al. Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas. Proc Natl Acad Sci USA. 2006;103(26):9903–9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen KG, Leapman RD, Zhang G, et al. Influence of melanosome dynamics on melanoma drug sensitivity. J Natl Cancer Inst. 2009;101(18):1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kushimoto T, Basrur V, Valencia J, et al. A model for melanosome biogenesis based on the purification and analysis of early melanosomes. Proc Natl Acad Sci USA. 2001;98(19):10698–10703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Piantelli M, Tatone D, Castrilli G, et al. Quercetin and tamoxifen sensitize human melanoma cells to hyperthermia. Melanoma Res. 2001;11(5):469–476. [DOI] [PubMed] [Google Scholar]
- 83.Valencia JC, Rouzaud F, Julien S, et al. Sialylated core 1 O-glycans influence the sorting of Pmel17/gp100 and determine its capacity to form fibrils. J Biol Chem. 2007;282(15):11266–11280. [DOI] [PubMed] [Google Scholar]
- 84.Valverde P, Benedito E, Solano F, Oaknin S, Lozano JA, García-Borrón JC. Melatonin antagonizes alpha-melanocyte-stimulating hormone enhancement of melanogenesis in mouse melanoma cells by blocking the hormone-induced accumulation of the c locus tyrosinase. Eur J Biochem. 1995;232(1):257–263. [DOI] [PubMed] [Google Scholar]
- 85.Slominski A, Moellmann G, Kuklinska E, Bomirski A, Pawelek J. Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway, L-tyrosine and L-dopa. J Cell Sci. 1988;89(Pt 3):287–296. [DOI] [PubMed] [Google Scholar]
- 86.Logan A, Weatherhead B. Pelage color cycles and hair follicle tyrosinase activity in the Siberian hamster. J Invest Dermatol. 1978;71(5):295–298. [DOI] [PubMed] [Google Scholar]
- 87.Brożyna AA, VanMiddlesworth L, Slominski AT. Inhibition of melanogenesis as a radiation sensitizer for melanoma therapy. Int J Cancer. 2008;123(6):1448–1456. [DOI] [PubMed] [Google Scholar]
- 88.Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer. 2009;124(6):1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Park JW, Hwang MS, Suh SI, Baek WK. Melatonin down-regulates HIF-1 alpha expression through inhibition of protein translation in prostate cancer cells. J Pineal Res. 2009;46(4):415–421. [DOI] [PubMed] [Google Scholar]
- 90.Nordlund JJ, Lerner AB. The effects of oral melatonin on skin color and on the release of pituitary hormones. J Clin Endocrinol Metab. 1977;45(4):768–774. [DOI] [PubMed] [Google Scholar]
- 91.Weatherhead B. Melanotropins and melanin pigmentation in the skin of mammals. In: Hadley ME, ed. The Melanotropic Peptides, vol. 2. Raton, FL: CRC Press, Boca; 1988:1–19. [Google Scholar]
- 92.Reiter RJ. Normal patterns of melatonin levels in the pineal gland and body fluids of humans and experimental animals. J Neural Transm Suppl. 1986;21:35–54. [PubMed] [Google Scholar]
- 93.Korkmaz A, Reiter RJ, Topal T, Manchester LC, Oter S, Tan DX. Melatonin: an established antioxidant worthy of use in clinical trials. Mol Med. 2009;15(1–2):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reiter RJ, Tan DX. What constitutes a physiological concentration of melatonin? J Pineal Res. 2003;34(1):79–80. [DOI] [PubMed] [Google Scholar]
- 95.Reiter RJ, Tan DX, Cabrera J, D'Arpa D. Melatonin and tryptophan derivatives as free radical scavengers and antioxidants. Adv Exp Med Biol. 1999;467:379–387. [DOI] [PubMed] [Google Scholar]