Summary
Background
Data on COVID-19-related mortality and associated factors from low-resource settings are scarce. This study examined clinical characteristics and factors associated with in-hospital mortality of COVID-19 patients in Jakarta, Indonesia, from March 2 to July 31, 2020.
Methods
This retrospective cohort included all hospitalised patients with PCR-confirmed COVID-19 in 55 hospitals. We extracted demographic and clinical data, including hospital outcomes (discharge or death). We used logistic regression to examine factors associated with mortality.
Findings
Of 4265 patients with a definitive outcome by July 31, 3768 (88%) were discharged and 497 (12%) died. The median age was 46 years (IQR 32–57), 5% were children, and 31% had >1 comorbidity. Age-specific mortalities were 11% (7/61) for <5 years; 4% (1/23) for 5–9; 2% (3/133) for 10–19; 2% (8/638) for 20–29; 3% (26/755) for 30–39; 7% (61/819) for 40–49; 17% (155/941) for 50–59; 22% (132/611) for 60–69; and 34% (96/284) for ≥70. Risk of death was associated with higher age, male sex; pre-existing hypertension, diabetes, or chronic kidney disease; clinical diagnosis of pneumonia; multiple (>3) symptoms; immediate ICU admission, or intubation. Across all ages, risk of death was higher for patients with >1 comorbidity compared to those without; notably the risk was six-fold increased among patients <50 years (adjusted odds ratio 5.87, 95%CI 3.28–10.52; 27% vs 3% mortality).
Interpretation
Overall in-hospital mortality was lower than reported in high-income countries, probably due to younger age distribution and fewer comorbidities. Deaths occurred across all ages, with >10% mortality among children <5 years and adults >50 years.
Funding
Wellcome Trust UK.
Keywords: COVID-19, coronavirus, SARS-CoV-2, Mortality, children, Indonesia
Research in context.
Evidence before this study
We searched PubMed on January 27, 2021, for articles that assessed the clinical and demographic factors of in-hospital mortality in patients with coronavirus disease 2019 (COVID-19), using the search terms (“novel coronavirus” OR “SARS-CoV-2″ OR “COVID-19″) AND (“death” OR “mortality” OR “deceased”). Studies from China, North America and Europe have shown COVID-19-related mortality to be associated with older age and common underlying chronic co-morbidities including hypertension, diabetes, obesity, cardiac disease, chronic kidney disease and liver disease. However, most COVID-19 cases have occurred in low- and middle-income countries (LMIC), where reliable data are scarce. In Southeast Asia, by January 26, 2021, COVID-19 case fatality rate had been reported at 2.3% (35/1551) in Vietnam, 2.0% (10,386/516,166) in Philippines, 0.5% (75/14,646) in Thailand, 0.4% (700/190,434) in Malaysia, 0% (0/460) in Cambodia, and <0.1% in Singapore (29/59,366). Indonesia has the highest number of COVID-19 cases and deaths in the region, reporting 2.8% case fatality rate (28,468/1,012,350), with the highest number of cases in the capital city of Jakarta. A preliminary analysis of the first two months of surveillance in Jakarta found that 381 of 4052 (9.4%) patients had died, associated with older age, dyspnea, pneumonia, and hypertension.
Added value of this study
This retrospective hospital-based study of the complete epidemiological surveillance data of Jakarta during the first five months of the epidemic is one of the largest studies in LMIC and the largest in Southeast Asia to date, that analysed the characteristics and outcomes of patients hospitalised with PCR-confirmed COVID-19. Overall in-hospital mortality was lower than reported in high-income countries, which is likely explained by the younger hospital population, fewer comorbidities and less severe disease. Nonetheless, age-specific mortalities were comparable to high-income countries. Although the large majority (78%) of people who died were 50 years or older, deaths occurred across all age groups. A concerning finding was the death of 11% (7/61) of children <5 years hospitalised with COVID-19, which contrasts with previous evidence that severe disease and death among children is rare.
Implications of all available evidence
Differences in patient populations and access to quality health services, among other factors, greatly influence COVID-19 mortality trends in low-resource settings. This study affirmed the vulnerability of elderly and comorbid COVID-19 patients. Increasing burdens of non-communicable diseases in the urban centres of developing nations will impact morbidity and mortality associated with COVID-19. Further studies are needed to understand the extent and underlying causes of death related to COVID-19 in children <5 years in LMIC.
Alt-text: Unlabelled box
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), has spread rapidly around the world since it was first reported in Wuhan, China in December 2019 [1]. Current understanding of COVID-19 mortality mostly comes from clinical epidemiological studies conducted in the early phase of the pandemic in China [2], [3], [4], and in high-income countries of North America [5], [6], [7], [8] and Europe [9,10]. Severe outcomes of COVID-19 in those settings have been consistently associated with older age and pre-existing chronic conditions, such as hypertension, diabetes, obesity, cardiac disease, chronic kidney disease, and liver disease [2,[4], [5], [6], [7], [8], [9], [10].
However, most cases to date have occurred in low- and middle-income countries (LMIC) [11], where differences in age distribution, comorbidities, access to quality health services, and other factors, may greatly influence trends regarding severe outcomes, but data are limited [12], [13], [14], [15], [16], [17]. Indonesia is the fourth most populous country (population 274 million) and the LMIC that has suffered the highest number of COVID-19 confirmed cases and deaths in Southeast Asia, second only to India in Asia [11]. Since the first two laboratory-confirmed SARS-CoV-2 infections were reported on March 2, 2020, Indonesia has reported a total of 1,012,350 cases and 24,468 deaths (2.8% confirmed case fatality rate) up to January 26, 2021 [18], of which 25% (254,580) of cases and 17% (4077) of deaths in the capital city of Jakarta. COVID-19 cases and deaths in Jakarta rapidly escalated during the first two months of the outbreak (March-April 2020), and have steadily trended upward through January 2021 [18].
Indonesia has high burdens of major infectious diseases like malaria, tuberculosis, HIV and other tropical infections [19], as well as non-communicable diseases, with an estimated 73% of deaths caused predominantly by cardiovascular diseases, cancers, chronic pulmonary diseases, diabetes, and others [20]. As in many LMIC, substantial proportions of the population face barriers in accessing quality health care services due to under-resourced and fragile health systems [12], often leaving underlying chronic comorbidities unrecognised and/or poorly managed [21]. These factors may aggravate chronic non-communicable diseases and worsen COVID-19 patient outcomes.
Studies of COVID-19-related mortality in Asia [16,22,23], and in particular from low-resource settings have been limited. Given that several major urban centres of Southeast Asia like Bangkok, Phnom Penh, Ho Chi Minh City, and Kuala Lumpur, have thus far been spared major COVID-19 epidemics, the explosive epidemic in Jakarta can provide insights directly relevant to similar settings in other LMIC. To this end, we analysed the complete clinical epidemiological surveillance data from the Jakarta Health Office, reporting on admissions to 55 COVID-19-designated hospitals within the city, during the first five months of the epidemic (March through July 2020).
Methods
Study design and participants
This was a retrospective cohort study to assess demographic and clinical factors associated with mortality of adults and children hospitalised with COVID-19 in Jakarta, Indonesia. The study population included all PCR-confirmed COVID-19 patients recorded by the Jakarta Health Office who either died or were discharged alive between March 2 and July 31, 2020. In accordance with Indonesia's national COVID-19 guidelines [24], confirmatory SARS-CoV-2 PCR testing was conducted on naso- and/or oropharyngeal swab specimens in COVID-19 reference laboratories, and patients were discharged from hospital after two consecutive PCR-negative tests. All included hospitals had an intensive care unit (ICU) with capability for invasive and non-invasive ventilation [25].
Data collection
Each hospital had a designated surveillance officer responsible for extracting data from the patient medical record to complete the official COVID-19 epidemiological investigation form for each patient. Completed forms were submitted to the Jakarta Health Office for verification and aggregation into a surveillance database. Data regarding dates of onset of illness, SARS-CoV-2 PCR testing, hospital admission, and outcomes (discharge or death) were recorded, along with age, sex, symptoms, comorbidities, and some critical indicators (e.g. immediate admission to intensive care unit). Comorbidities were recorded at admission by attending clinical staff, either based on clinical assessments or patient reporting. Fever was defined as axillary temperature of at least 38 °C [24]. Clinical diagnosis of pneumonia (indicative of moderate or severe disease [26]) was established by the treating physician, based on clinical and radiological evaluation [24].
Statistical analysis
Descriptive statistics included proportions for categorical variables and medians and interquartile ranges (IQRs) for continuous variables. We calculated time in days from symptom onset to hospital admission, and length of hospital stay until death or discharge. We used the Mann-Whitney U test, χ² test, or Fisher's exact test to compare characteristics between deceased and discharged patients. The retrospective nature of the study meant that some of the data were missing, especially on obesity (53%), admission date (39%), time from onset to admission (42%), and time from admission to outcome (44%). Missing-indicator analysis by risk factor stratification and by regression analysis did not identify bias of missing data with respect to mortality, and we excluded those variables from the main regression model. As data were deemed missing at random for mortality as an outcome, we additionally conducted multiple imputation analysis to assess sensitivity of risk factor identification due to missing data. Due to the high proportion of missing data needed to define the time to event variable, time to death was not analysed.
We used bivariable and multivariable logistic regression models to determine the risk of death, expressed as odds ratio with 95% confidential intervals. All independent variables with p-value <0.10 in bivariable analysis were included in the multivariable models. Final model selection was informed by likelihood ratio tests. We reported two final multivariable logistic regression models. In the first model, we assessed the effect of each type of comorbidity (hypertension, diabetes, cardiac disease, chronic obstructive pulmonary disease (COPD), chronic kidney disease, immunocompromised status, and liver disease), and in the second model, the number of comorbidities (0, 1 or >1), along with other demographic and clinical factors. We used interaction terms to examine potential effect modification by age and sex. We set statistical significance at 0.05, and all tests were two sided. All analyses were done in Stata/IC 15.1 (StataCorp, College Station, TX, USA). This study is reported as per Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [27].
Ethics
This study was approved by the Health Research Ethics Committee of the National Institute of Health Research and Development, Ministry of Health Indonesia (LB.02.02/2/KE.554/2020). The requirement for patient consent was waived as this was a secondary analysis of anonymised routine surveillance data [28].
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Between March 2 and July 31, 2020, a total of 21 397 PCR-confirmed COVID-19 cases were recorded by the Jakarta Health Office (Fig. 1). Of those, 4522 (21%) were admitted to hospital because of symptomatic disease and 4265 (94%) had reached a definitive outcome no later than July 31, 2020, i.e. deceased or discharged, and were therefore included in this analysis. There were 257 (6%) who were still hospitalised. The study flow chart and completeness of key data are presented in Fig. 1.
Fig. 1.
Study flowchart and completeness of key variables.
Table 1 presents the characteristics of the 4265 patients included in the analysis. The median age was 46 years (IQR 32–57, range 0.1–99), 217 (5%) were children, and 2217 (52%) were men. The most common presenting symptom was cough (66%, 2788), followed by fever (53%, 2192), malaise (35%, 1460), shortness of breath (32%, 1335), and others (Fig. 2A). The median number of symptoms was 3 (IQR 2–5, range 1–12), 1458 (39%) patients had more than three symptoms. We found a moderate degree of overlap between the three most common symptoms (Fig. 2C). 1488 (37%) patients had a clinical diagnosis of pneumonia, whereas 2777 (63%) could be classified as mild disease. Among 3214 patients with complete records, 102 (3%) patients were directly admitted to ICU, and among 3211 patients with complete records, 55 (2%) were intubated for mechanical ventilation upon admission. Overall, 31% (1299) of patients had a record of one or more pre-existing comorbidities, including hypertension (19%, 795), diabetes mellitus (12%, 501), cardiac disease (10%, 392), COPD (4%, 178), chronic kidney disease (3%, 108), and others (Fig. 2B). There was little overlap between the three most common comorbidities (Fig. 2D).
Table 1.
Demographics, clinical characteristics, and outcomes of COVID-19 hospitalised patients in Jakarta, Indonesia.
| Total N = 4265 |
Deceased N = 497 |
Discharged N = 3768 |
p value | |
|---|---|---|---|---|
| Median age (IQR), years | 46 (32–57) | 58 (51–67) | 44 (31–55) | <0.0001 |
| Age group, years | ||||
| 0–4 | 61 (1%) | 7 (1%) | 54 (1%) | <0.0001 |
| 5–9 | 23 (0.5%) | 1 (0.2%) | 22 (0.6%) | |
| 10–19 | 133 (3%) | 3 (1%) | 130 (3%) | |
| 20–29 | 638 (15%) | 16 (3%) | 622 (17%) | |
| 30–39 | 755 (18%) | 26 (5%) | 729 (19%) | |
| 40–49 | 819 (19%) | 61 (12%) | 758 (20%) | |
| 50–59 | 941 (22%) | 155 (31%) | 786 (21%) | |
| 60–69 | 611 (14%) | 132 (27%) | 479 (13%) | |
| ≥70 | 284 (7%) | 96 (19%) | 188 (5%) | |
| Sex | ||||
| Male | 2217 (52%) | 302 (61%) | 1915 (51%) | <0.0001 |
| Female | 2048 (48%) | 195 (39%) | 1853 (49%) | |
| Presenting symptoms | ||||
| Cough | 2788 (66%) | 372 (76%) | 2416 (64%) | <0.0001 |
| Fever | 2192 (53%) | 303 (63%) | 1889 (51%) | <0.0001 |
| Malaise | 1460 (35%) | 263 (57%) | 1197 (33%) | <0.0001 |
| Shortness of breath | 1335 (32%) | 348 (71%) | 987 (26%) | <0.0001 |
| Headache | 1085 (26%) | 141 (30%) | 944 (26%) | 0.032 |
| Sore throat | 1017 (24%) | 123 (25%) | 894 (24%) | 0.507 |
| Runny nose | 1034 (24%) | 88 (18%) | 946 (25%) | <0.0001 |
| Nausea/vomiting | 1006 (25%) | 154 (34%) | 852 (24%) | <0.0001 |
| Myalgia | 765 (19%) | 100 (22%) | 665 (18%) | 0.052 |
| chills | 442 (11%) | 67 (15%) | 375 (10%) | 0.004 |
| Diarrhoea | 357 (9%) | 53 (12%) | 304 (8%) | 0.022 |
| Abdominal pain | 449 (11%) | 62 (14%) | 387 (11%) | 0.054 |
| Median (IQR) number of symptoms | 3 (2–5) | 4 (3–6) | 3 (1–4) | <0.0001 |
| Number of symptoms | ||||
| ≤3 | 2315 (61%) | 167 (39%) | 2148 (64%) | <0.0001 |
| >3 | 1458 (39%) | 262 (61%) | 1196 (36%) | |
| Number of comorbidities | ||||
| 0 | 2849 (69%) | 184 (38%) | 2665 (73%) | <0.0001 |
| 1 | 836 (20%) | 144 (30%) | 692 (19%) | |
| >1 | 463 (11%) | 155 (32%) | 308 (8%) | |
| Type of comorbidity | ||||
| Hypertension | 795 (19%) | 201 (42%) | 594 (16%) | <0.0001 |
| Diabetes | 501 (12%) | 142 (29%) | 359 (10%) | <0.0001 |
| Cardiac disease | 392 (10%) | 105 (22%) | 287 (8%) | <0.0001 |
| COPD | 178 (4%) | 28 (6%) | 150 (4%) | 0.083 |
| Chronic kidney disease | 108 (3%) | 45 (9%) | 63 (2%) | <0.0001 |
| Immunocompromised | 30 (0.7%) | 7 (2%) | 23 (0.6%) | 0.078 |
| Liver disease | 27 (0.7%) | 7 (2%) | 20 (0.6%) | 0.032 |
| Malignancy | 20 (0.5%) | 2 (0.4%) | 18 (0.5%) | 1.000 |
| Obesity* | 17 (0.8%) | 3 (1%) | 14 (0.8%) | 0.412 |
| Clinical diagnosis of pneumonia | 1488 (37%) | 328 (68%) | 1160 (33%) | <0.0001 |
| Immediate ICU admission | 102 (3%) | 56 (16%) | 46 (2%) | <0.0001 |
| Immediate intubation | 55 (2%) | 39 (11%) | 16 (0.6%) | <0.0001 |
| Median (IQR) time from symptom onset to admission, days* | 6 (3–12) | 5 (3–8) | 6 (3–12) | <0.0001 |
| Median (IQR) hospital length of stay, days* | 24 (13–36) | 6 (2–11) | 26 (18–38) | <0.0001 |
| Period of admission* | ||||
| March | 809 (31%) | 137 (44%) | 672 (30%) | <0.0001 |
| April | 829 (32%) | 91 (29%) | 738 (32%) | |
| May | 463 (18%) | 40 (13%) | 423 (19%) | |
| June | 386 (15%) | 22 (7%) | 364 (16%) | |
| July | 96 (4%) | 20 (7%) | 76 (3%) |
Missing substantial proportion of data, therefore these variables were excluded from further analysis.
Fig. 2.
Presenting symptoms and comorbidities in patients hospitalised with COVID-19 in Jakarta. Symptoms (A) and comorbidities (B) by frequency (see Table 1 for values); Scaled Euler diagrams of overlap of commonest symptoms (C) and comorbidities (D).
Of 4265 patients with a known outcome, 497 (12%) had died, and 3768 (88%) were discharged alive. 36 (7%) deceased patients had been declared dead at hospital arrival. The highest numbers of deaths were observed between March 28 and April 4, 2020 (Fig. 3A). The median time from symptom onset to admission was 6 days (IQR 3–12) overall, 5 days (IQR 3–8) for deceased patients, and 6 days (IQR 3–12) for those discharged. The median length of hospital stay was 24 days (IQR 13–36) overall, 6 days (IQR 2–11) among deceased patients, and 26 days (IQR 18–38) among survivors.
Fig. 3.
Outcomes of hospitalization over time, by age group and pre-existing comorbidity. Number of in-hospital deaths and discharges over time (A), by age groups (B), by number of pre-existing comorbidities (C), and by type of comorbidity (D).
Compared to discharged patients, deceased patients were older (median 58 vs 44 years); more likely to be men (14% vs 10%); to have presenting symptoms of cough, fever, malaise, shortness of breath, headache, nausea/vomiting, chills, diarrhoea, abdominal pain, and >3 symptoms (Table 1); to have pneumonia (68% vs 33%); to be directly admitted to intensive care (16% vs 2%); to receive mechanical ventilation at admission (11% vs 0.6%); and to have a history of any comorbidity (62% vs 27%), 1 comorbidity (30% vs 19%) or >1 comorbidities (32% vs 8%), specifically hypertension (42% vs 16%), diabetes (29% vs 10%), cardiac disease (22% vs 8%), chronic kidney disease (9% vs 2%), and liver disease (2% vs 0.6%) (Table 1; Fig. 3C and 3D).
Although a large majority of deaths (78%, 383) was 50 years or older, death occurred across all age groups. Age-specific mortalities were 11% (7/61) for 0–4 years; 4% (1/23) for 5–9 years; 2% (3/133) for 10–19 years; 3% (8/638) for 20–29 years; 3% (26/755) for 30–39 years; 7% (61/819) for 40–49 years; 16% (155/941) for 50–59 years; 22% (132/611) for 60–69 years; 34% (96/284) for ≥70 years (Supplementary Table 1 and Fig. 3B).
Among the seven children below 5 years who died with COVID-19, four (57%) were boys, with an age range of 0 to 3 years; four (57%) had a clinical diagnosis of pneumonia, four (67%) had >3 symptoms at presentation (1 unknown), two (29%) had a known underlying condition (i.e. cardiac disease and immunocompromised status), and two (29%) were directly admitted to ICU for mechanical ventilation, and five (71%) died within a week after admission. Further details on the clinical course, disease management, underlying conditions and the exact cause of death were not available for this analysis (Supplementary Table 2).
The mortality increased by age and number of pre-existing comorbidities (Supplementary Figure 1). The mortality for the age group <50 years was 5% (110/2372) overall, 3% (59/1945) for those without any comorbidity, 8% (25/331) for those with 1 comorbidity, and 27% (26/96) for those with >1 comorbidities; for the age group 50–59 years 16% (146/2372), 11% (56/515), 18% (42/231), and 32% (48/151), respectively; for the age group 60–69 years 22% (132/601), 16% (47/286), 25% (46/181), and 29% (39/134), respectively; and for the age group ≥70 years 34% (95/278), 21% (22/103), 33% (31/93), and 51% (42/82), respectively.
In bivariable logistic regression analysis (Table 2), the risk of death was associated with older age, sex, number of pre-existing comorbidities, hypertension, diabetes, cardiac disease, chronic kidney disease, liver disease, number of presenting symptoms, pneumonia, immediate ICU admission and mechanical ventilation.
Table 2.
Bivariable analysis of demographic and clinical factors of mortality among 4265 patients hospitalised with COVID-19 in Jakarta, Indonesia.
| Crude Odds Ratio (95% CI) | p value | |
|---|---|---|
| Age group, years | ||
| <50 | 1 (ref) | .. |
| 50–59 | 4.00 (3.10–5.17) | <0.0001 |
| 60–69 | 5.60 (4.28–7.32) | <0.0001 |
| ≥70 | 10.37 (7.61–14.13) | <0.0001 |
| Sex | ||
| Female | 1 (ref) | .. |
| Male | 1.50 (1.24–1.81) | <0.0001 |
| Number of symptoms | ||
| ≤3 | 1 (ref) | .. |
| >3 | 2.82 (2.29–3.46) | <0.0001 |
| Number of comorbidities | ||
| 0 | 1 (ref) | .. |
| 1 | 3.01 (2.39–3.81) | <0.0001 |
| >1 | 7.29 (5.71–9.30) | <0.0001 |
| Type of comorbidity | ||
| None | 1(ref) | |
| Hypertension | 3.63 (2.97–4.44) | <0.0001 |
| Diabetes | 3.80 (3.04–4.76) | <0.0001 |
| Cardiac disease | 3.25 (2.54–4.17) | <0.0001 |
| COPD | 1.44 (0.95–2.18) | 0.084 |
| Chronic kidney disease | 5.82 (3.92–8.64) | <0.0001 |
| Immunocompromised | 2.30 (0.98–5.38) | 0.056 |
| Liver disease | 2.66 (1.12–6.31) | 0.027 |
| Malignancy | 0.83 (0.19–3.60) | 0.807 |
| Clinical diagnosis of pneumonia | ||
| No | 1 (ref) | .. |
| Yes | 4.39 (3.58–5.39) | <0.0001 |
| Immediate ICU admission | ||
| No | 1 (ref) | .. |
| Yes | 11.80 (7.85–17.75) | <0.0001 |
| Immediate intubation | ||
| No | 1 (ref) | .. |
| Yes | 22.54 (12.45–40.81) | <0.0001 |
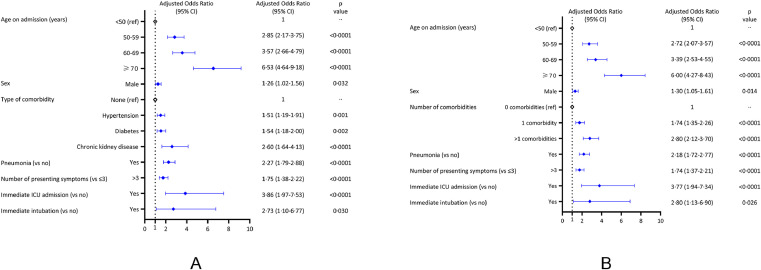
In the first multivariable logistic regression model (Fig. 4A), the risk of death was increased for age groups 50–59 years (aOR 2.85, 95%CI 2.17–3.75), 60–69 years (aOR 3.57, 95%CI 2.66–4.79), and ≥70 years (aOR 6.53, 95%CI 4.64–9.18), compared to <50 years; for males (aOR 1.26, 95%CI 1.02–1.56); for patients with pre-existing hypertension (aOR 1.51, 95%CI 1.19–1.91), diabetes (aOR 1.54, 95%CI 1.18–2.00), or chronic kidney disease (aOR 2.60, 95%CI 1.64–4.13); for patients who had pneumonia (aOR 2.27, 95%CI 1.79–2.88), >3 symptoms at presentation (aOR 1.75, 95%CI 1.38–2.22; reference ≤3 symptoms), immediate ICU admission (aOR 3.86, 95%CI 1.97–7.53), or mechanical ventilation (aOR 2.73, 95%CI 1.10–6.77). We found no associations for cardiac disease, COPD, liver disease, and immunocompromised status. The sensitivity analysis revealed similar findings, suggesting there was no significant bias introduced by missing data in our dataset; it also suggested that the risk of death was increased for patients who had symptom onset <7 days from admission (aOR 1.60, 95%CI 1.21–2.10) compared to those who had ≥7 days symptom onset (Supplementary Table 3).
Fig. 4.
Multivariable logistic regression models. The models present the demographic and clinical risk factors independently associated with in-hospital death (A), and adjusted effect of number of comorbidities on in-hospital death (B). COPD, immunocompromised status, and liver disease were assessed in the multivariable models but were not associated with in-hospital death.
In the second multivariable model (Fig. 4B), we found that the risk of death was increased for patients who had one comorbidity (aOR 1.74, 95%CI 1.35–2.26), and two or more comorbidities (aOR 2.80, 95%CI 2.12–3.70), compared to those without comorbidities, after controlling for age, sex, pneumonia, number of symptoms at presentation, immediate ICU admission, and intubation. Sex was not found to be an effect modifier in either model. Although number of comorbidities was associated with increased risk of death for all age groups, we found that the effect of number of comorbidities on death risk was modified by age (p = 0.009). The effect of number of comorbidities was greater for age <50 years, compared to ≥50 years. For age <50 years, compared to patients without comorbidities, the aOR was 1.73 (95%CI 1.02–2.94) for those with one comorbidity and 5.87 (95%CI 3.28–10.52) for those with two or more comorbidities. For age ≥50 years, compared to those without comorbidities, the aOR was 1.75 (95%CI 1.30–2.34) for those with one comorbidity, and 2.51 (95%CI 1.85–3.42) for those with two or more comorbidities (Supplementary Table 4).
Discussion
This retrospective hospital-based study described the complete epidemiological surveillance data of the Jakarta Health Office, including 4265 adults and children with confirmed COVID-19 admitted in 55 COVID-19-designated referral hospitals, during the first five months of the SARS-CoV-2 epidemic. This analysis represents the largest patient series hospitalised with COVID-19 in Southeast Asia, and one of the largest from LMIC to date. The observed disease pattern broadly reflects that reported globally, with patients usually presenting with multiple symptoms of fever, cough, malaise and/or shortness of breath. Nearly 40% had a clinical diagnosis of pneumonia at admission, indicative of moderate or severe disease. Overall mortality was 12% (497/4265), and deaths occurred across all ages. Although the majority (57%) of all admissions were younger than 50 years, including 5% children, the large majority (78%) of people who died were 50 years or older. Mortality increased with age, from 7% in patients aged 40–49 years to 34% in patients aged ≥70 years. Mortality among children below 5 years was unexpectedly high at 11% (7/61), which has not been reported elsewhere, although available information on any underlying conditions and the exact cause of death of those children was incomplete. Elevated risk of in-hospital death was independently associated with higher age, male sex, history of hypertension, diabetes, chronic kidney disease, pneumonia, multiple (>3) symptoms at presentation, immediate ICU admission, and mechanical ventilation. The increased risk of in-hospital death associated with advancing age was further augmented by the presence of one or more chronic comorbidities, which were recorded in 31% of patients.
The overall COVID-19-related mortality in Jakarta (12%) was substantially lower than those reported in large cohorts in high-income countries, for example, in the US (21%) [7] and the UK (26%) [9], but those populations were substantially older, with more comorbidities and more frequent presentation with severe disease. By contrast, a nationwide analysis of patients hospitalised with COVID-19 in China reported an overall mortality of just 3.1%, but in that population, severe cases and comorbidities were relatively infrequent, while the median age was similar to Jakarta [4]. Compared with other settings, we found age-specific mortality rates in Jakarta to be similar for patients <50 years (5% in Jakarta, 5% in the US [7], and 4% in the UK [9]), but slightly lower for patients ≥50 years (21% in Jakarta, 27% in the US [7], and 29% in the UK [9]. Given that older age has been consistently associated with severe disease and mortality in patients with COVID-19 [2,[5], [6], [7],9], the lower median age (46 years) in Jakarta compared to studies from North America and Europe probably accounts for most of the differences observed in overall in-hospital mortality. The younger age at admission is likely to be mainly related to a younger age distribution in the general population in Jakarta, compared to Europe, the US, and China. Nonetheless, we do not dismiss distinct behavioural factors related to risk of infection, for example adherence to preventive measures, mobility, and health seeking, as also reported in India [16].
National surveillance data in Indonesia to date reported a COVID-19 case fatality rate in children aged 0–5 years of 1.0% (123/11 916) [18]. This is much higher than a case fatality rate of 0.16% in children below 5 years of age in a recent large epidemiological study in Southern India [16]. Several other studies in China [29], Brazil [13, 30], Uganda [14], and South Africa [15] have reported COVID-19-related deaths among children under 5 years to be rare. A review of data from US, Korea and Europe early in the epidemic estimated the overall case fatality rate among children (0–19 years old) to be low at 0.03 per 100 000 (44/42 846) [31]. Although children appear generally less severely affected than older individuals [32], COVID-19 in children can also be characterized by rapid progressive deterioration leading to death, especially in children with comorbidities. Notably, reports from both high-income and LMIC have described a multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19, which can lead to serious illness [33]; to date, to our knowledge, no such cases have been reported in Indonesia. Contemporary background data on childhood case fatality rate due to acute respiratory illness in Jakarta or Indonesia are limited to reliably estimate excess mortality due to COVID-19 [34], [35], [36]. Factors that explain the high COVID-19-related mortality observed among children in Jakarta hospitals may be various, including treatment delays, limited paediatric intensive care capacity, presence of underlying conditions, such as malignancy or malnutrition, among other factors. Further investigations are planned to confirm the extent and cause of death among young children with COVID-19 in Jakarta.
Evidence from previous studies suggests that underlying comorbidities, including pre-existing cardiovascular or cerebrovascular diseases, hypertension, and diabetes mellitus, were associated with poorer COVID-19 outcomes [4,[7], [8], [9]. Chronic comorbidities among COVID-19 patients were less frequent in Jakarta (31% of patients) than reported in large patient series in North America [7,8], South America [13], and Europe [9]. This could reflect the relatively young population admitted to Jakarta hospitals, but also under-reporting of comorbidities by patients, or under-diagnosis due to variable access to quality health services. Presence of one or more comorbidities, especially pre-existing hypertension, diabetes, or chronic kidney disease strongly increased the risk of death in Jakarta, by 51%, 54%, and 160%, respectively, compared to those without such comorbidities. These findings were consistent with similar studies elsewhere [4,8,9]. In contrast, we found no association between cardiac and liver disease and mortality in Jakarta, whereas studies in US [8], and the UK [9] did so. Obesity has also been recognised as an important risk factor of mortality [9,17], but we could not assess that for lack of complete data.
In Jakarta, the median time from symptom onset to death (median 11 days) was shorter than reported in hospital-based studies from Japan (17 days) [22], China (18.5 days) [2], and the US (12.7 days) [6]; the relatively short in-hospital trajectory towards clinical deterioration and death (median 6 days) could suggest possible delays in hospital admission and treatment. Among survivors, the length of hospital stay was much longer (median 26 days) than reported in other studies [2,6,7,9,22], which is most likely due to the requirement of two consecutive PCR-negative test results prior to discharge, with results delayed due to limited PCR testing capacity in Jakarta during the early phase of the epidemic.
This study had some limitations. The retrospective design and reliance on routine hospital surveillance data meant that, for some key baseline variables, data were incomplete or uniformly unavailable (e.g. dates of symptom onset, hospital admission and outcome, vital signs, TB and HIV co-infection, radiology examination, routine laboratory results, and disease severity classification at admission). Comorbidities were often self-reported or could be under-diagnosed, potentially resulting in underreporting and hence underestimation of effect sizes. Details on supportive care and treatment received, particularly respiratory support and management of secondary infections, were also not available for this analysis. Findings from hospitals reported here may not reflect the mortality rate and risk factors associated with COVID-19-related mortality in the general population.
In conclusion, risk factors associated with in-hospital mortality in Jakarta, Indonesia are broadly similar to those in more developed settings in North America, Europe, and Asia, dominated by advanced age and comorbidities. Lower overall mortality in Jakarta was likely driven by the lower median age of the hospital population. While children represented just 5% of admissions, the 11% mortality that occurred among children below 5 years should prompt further investigation of them as potentially highly vulnerable in LMIC settings. The findings highlight the need for enhanced context-specific public health action to reduce infection risk among vulnerable populations and improved care and treatment for the diseased.
Contributors
HS designed the study, cleaned the data, did the analysis, and had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. VA, W, DO, and NS collected and verified the data. HS, IRFE, AHS, JKB, and RLH contributed to the analysis and drafted the paper. All authors critically revised the manuscript for important intellectual content and all authors gave final approval for the version to be published.
Data sharing statement
After publication, the datasets used for this study will be made available to others on reasonable requests to the corresponding author, including a detailed research proposal, study objectives and statistical analysis plan. Deidentified participant data will be provided after written approval from the corresponding author and the Jakarta Health Office.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgments
This work was funded by the Wellcome Trust, UK (106680/Z/14/Z). We acknowledge all health care workers involved in the care for the COVID-19 patients, as well as those involved in the field data collection.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100108.
Contributor Information
Henry Surendra, Email: hsurendra@eocru.org.
Iqbal RF Elyazar, Email: ielyazar@eocru.org.
Bimandra A Djaafara, Email: bimandra.djaafara15@imperial.ac.uk.
Lenny L Ekawati, Email: lekawati@eocru.org.
Kartika Saraswati, Email: ksaraswati@eocru.org.
Rosa N Lina, Email: rlina@eocru.org.
Adhi Andrianto, Email: aandrianto@eocru.org.
Karina D Lestari, Email: kdlestari@eocru.org.
Anuraj H Shankar, Email: anuraj.shankar@ndm.ox.ac.uk.
Guy Thwaites, Email: thwaites@oucru.org.
J. Kevin Baird, Email: kbaird@eocru.org.
Raph L. Hamers, Email: raph.hamers@ndm.ox.ac.uk.
Appendix. Supplementary materials
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A Novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W., Ni Z., Hu Y., Liang W. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W-j, Liang W-h, Zhao Y. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with Coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewnard J.A., Liu V.X., Jackson M.L. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ. 2020;369:m1923. doi: 10.1136/bmj.m1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA - J Am Med Assoc. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison S.L., Fazio-Eynullayeva E., Lane D.A., Underhill P., Lip G.Y.H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17(9) doi: 10.1371/journal.pmed.1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Docherty A.B., Harrison E.M., Green C.A. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giacomelli A., Ridolfo A.L., Milazzo L. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark A., Jit M., Warren-Gash C., Guthrie B. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Heal. 2020;8(8):e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baqui P., Bica I., Marra V., Ercole A., van der Schaar M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Glob Heal. 2020;8(8):e1018–e1026. doi: 10.1016/S2214-109X(20)30285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirenga B., Muttamba W., Kayongo A. Characteristics and outcomes of admitted patients infected with SARS-CoV-2 in Uganda. BMJ Open Resp Res. 2020;7(1) doi: 10.1136/bmjresp-2020-000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boulle A.A., Davies M., Hussey H., Morden E., Vundle Z., Zweigenthal V. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020:ciaa1198. doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laxminarayan R., Wahl B., Dudala S.R. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science. 2020;370(6517):691–697. doi: 10.1126/science.abd7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nachega J.B., Ishoso D.K., Otokoye J.O., Hermans M.P., Machekano R.N., Sam-Agudu N.A. Clinical characteristics and outcomes of patients hospitalized for COVID-19 in Africa: early insights from the democratic republic of the Congo. Am J Trop Med Hyg. 2020:3–5. doi: 10.4269/ajtmh.20-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.https://covid19.go.id/peta-sebaran. 2021 [cited 2021 Jan 27].
- 19.Mboi N., Murty Surbakti I., Trihandini I. On the road to universal health care in Indonesia, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2018;392(10147):581–591. doi: 10.1016/S0140-6736(18)30595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO . WHO; Geneva: 2018. Noncommunicable disease (NCD) country profile, 2018. [Google Scholar]
- 21.Agustina R., Dartanto T., Sitompul R., Susiloretni K.A. Universal health coverage in Indonesia: concept, progress, and challenges. Lancet. 2019;393(10166):75–102. doi: 10.1016/S0140-6736(18)31647-7. [DOI] [PubMed] [Google Scholar]
- 22.Matsunaga N., Hayakawa K., Terada M. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: report of the COVID- 19 REGISTRY JAPAN. Clin Infect Dis. 2020:ciaa1470. doi: 10.1093/cid/ciaa1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung H.K., Kim J.Y., Heo J., Seo H. Clinical course and outcomes of 3, 060 patients with Coronavirus disease 2019 in Korea , January –May 2020. J Korean Med Sci. 2020;35(30):1–11. doi: 10.3346/jkms.2020.35.e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ministry of Health Indonesia . Ministry of Health Indonesia; Jakarta: 2020. Pedoman pencegahan dan pengendalian coronavirus disease (COVID-19). vol. 4; pp. 1–135. https://covid19.go.id/p/protokol?page=1. [Google Scholar]
- 25.http://eis.dinkes.jakarta.go.id/eis/. 2021 [cited 2021 Jan 03].
- 26.WHO . WHO; Geneva: May 2020. Clinical management of COVID-19: interim guidance 27; p. 2020. [Google Scholar]
- 27.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 28.https://corona.jakarta.go.id/id/data-pemantauan. 2020 [cited 2021 Jan 03].
- 29.Lu X., Zhang L., Du H., Zhang J. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos M.M., Lucena E.E.S., Lima K.C., Brito A.A.C., Bay M.B., Bonfada D. Survival and predictors of deaths of patients hospitalised due to COVID-19 from a retrospective and multicentre cohort study in Brazil. Epidemiol Infect. 2020;148:e198. doi: 10.1017/S0950268820002034. 1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhopal S., Bagaria J., Bhopal R. Children's mortality from COVID-19 compared with all-deaths and other relevant causes of death: epidemiological information for decision-making by parents, teachers, clinicians and policymakers Governments. Public Health. 2020;185:19–20. doi: 10.1016/j.puhe.2020.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinman J.B., Lum F.M., Ho P.P.K., Kaminski N., Steinman L. Reduced development of COVID-19 in children reveals molecular checkpoints gating pathogenesis illuminating potential therapeutics. Proc Natl Acad Sci U S A. 2020;117(40):24620–24626. doi: 10.1073/pnas.2012358117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang L., Tang K., Levin M. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20(11):e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutanto A., Gessner B.D., Djlantik I., Steinhoff M., Murphy H., Nelson C. Acute respiratory illness incidence and death among children under two years of age on Lombok Island, Indonesia. Am J Trop Med Hyg. 2002;66(2):175–179. doi: 10.4269/ajtmh.2002.66.175. [DOI] [PubMed] [Google Scholar]
- 35.Azmi S., Aljunid S.M., Maimaiti N., Ali A.A., Muhammad Nur A., De Rosas-Valera M. Assessing the burden of pneumonia using administrative data from Malaysia, Indonesia, and the Philippines. Int J Infect Dis. 2016;49:87–93. doi: 10.1016/j.ijid.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 36.Tan K.K., Dang D.A., Kim K.H., Kartasasmita C., Kim H.M., Zhang X.H. Burden of hospitalized childhood community-acquired pneumonia: a retrospective cross-sectional study in Vietnam, Malaysia, Indonesia and the Republic of Korea. Hum Vaccines Immunother. 2018;14(1) doi: 10.1080/21645515.2017.1375073. 95–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.