Abstract
Purpose of review
The COVID-19 infection results in various viral-related physical and mental health problems, joined with the long-term psychological impact of the pandemic in general. However, the accompanying neurocognitive changes remain poorly understood.
Recent findings
We synthetize the current knowledge of viral (SARS-CoV-2) induced inflammation, mechanisms to viral entry into the central nervous system and altered neurotransmitter systems to provide an informed neurobiological explanation for the rise of neurocognitive disorders (defined as per the DSM-5 criteria).
Summary
The mild and major neurocognitive disorder symptoms due to the COVID-19 pandemic provide a unique opportunity to address the early changes underlying neurocognitive impairment at both clinical and molecular level. We discuss the utilization of the available evidence for their management and future novel therapeutic opportunities.
Keywords: COVID-19 neurocognitive disorders, inflammation, neurotransmitter, stress, viral infection
INTRODUCTION
The COVID-19 pandemic has affected all segments of the world population and has proven detrimental especially to the most vulnerable groups in society. It has gravely impacted those living in poverty, that is homeless people, people unable to secure adequate shelter, refugees, migrants, displaced persons, as well as older people with disabilities or underlying health conditions. Young and indigenous people also stand to suffer disproportionately both from the pandemic as such and from its indirect effects. Importantly, many people with lower socioeconomic status already have health problems (e.g. higher rates of chronic illness, compromised immune systems and so on), which constitute important risk factors for developing a more serious manifestation of the COVID-19 infection.
The record levels of unemployment due to lockdown measures implemented to curb virus transmission, social isolation and limits on nonessential travel outside the home, closure of shops and entertainment venues, bans on mass gatherings (i.e. sports and art events) and remote working have cumulatively contributed to psychological distress, promoting a myriad of ‘problem behaviours’ such as increased alcohol use, excess smoking, physical inactivity, as well as associated physical problems (i.e. back pain through poor ergonomic posture, scaled-back access to health services and so on).
Box 1.
no caption available
COVID-19 AND MENTAL HEALTH
Apart from the broad global mental health effects of the pandemic, SARS-CoV-2 has caused an additional mental health burden directly linked to the COVID-19 infection. To illustrate, nearly 20% of COVID-19 survivors develop mental health problems, that is anxiety and depression, very early in their convalescence period (14–90 days post diagnosis), with 5.8% developing the first episode of a psychiatric disorder. The incidence of a first diagnosis of dementia in the 14–90 days after COVID-19 diagnosis is 1.6% [95% confidence interval (95% CI) 1.2–2.1] in people older than 65 years [1▪]. A relevant minority of COVID-19 patients have suffered from encephalitis and stroke as a result of the viral infection and delirium has been reported frequently in those patients who required intensive care treatment (reviewed in [2▪]). In addition, the pandemic brought unexpected premature deaths. Over the last year, the SARS-CoV-2 (COVID-19) pandemic has claimed more than 1.25 million lives out of the 54 million infected (2.5%). The death rate is particularly high in the UK, reaching 4% (50 000 deaths in 1.17 million infected, as per the government statistic of 08 November 2020), in contrast to the reported 1.7–2.5% death rate in the Russian Federation and the USA, respectively.
Deaths have been recorded mostly among older people and those with chronic health conditions such as diabetes, respiratory infections, cancer and dementia, indicating that physical and mental frailty alongside old age constitute the main risk factors for SARS-CoV-2 deaths. According to the latest UK figures, COVID-19 accounted for one in 10 deaths in England [23 October 2020 (https://www.ons.gov.uk/]. Living in a care home appears to be the most relevant factor for both an increased risk of COVID-19 infection and consequent death, with Alzheimer's disease patients having a higher risk than those with other neurodegenerative dementias [3▪]. One of the reasons for this state of affairs may be insufficient testing in care homes to detect the virus and stop it from spreading. Also, elderly care home residents may not always show typical symptoms of COVID-19. Joint use of audio and video devices including mobile phones (not sanitised adequately before sharing) has also been mentioned as a source of passing on the viral infection. Additional factors, including social isolation, poor sleeping pattern, anxiety and psychological distress, may have all aggravated the problems in care homes. Although, to date, there is a paucity of research on how this situation has affected the families of the deceased and the wider population, initial reports are starting to emerge reporting that 55% of those who lost loves ones had intense grief reactions (N.M. Melhem et al., unpublished data).
All of the above illustrates the multiple adverse effects of the pandemic on physical and mental health and, in particular, on the development of neurocognitive disorders (Table 1).
Table 1.
Causes and risk factors for neurocognitive disorders
| Cause | Symptoms, mental health and physical conditions as risk factors for neurocognitive disorder |
| Societal experiences of living in and with COVID-19 pandemic | Social isolationNutritionAccess to healthcareStressIntense grief reactionsAnxietyOCDAlcohol and drug abuseAutoimmune diseases (RA, T1DM) |
| Respiratory COVID-19 (respiratory failure or laboratory biomarkers showing inflammation or organ damage) | HypoxiaPTSDAnxietyDepressionPhysical disabilityDelirium |
| CNS COVID-19 | EncephalitisStrokeDelirium |
| Previous Dementia Diagnosis/Down syndrome | Increase in psychological symptoms (communication/mood: apathy, anxiety)Increase in behavioural problems (agitation, compliance with new measures)Altered routine daily activity (i.e. movement, physical inactivity)Faster cognitive declineCaregiver burden (psychological and physical, esp. in rural areasDelirium |
CNS, central nervous system; OCD, obsessive compulsive disorder; PTSD, post-traumatic stress disorder; RA, rheumatoid arthritis; T1DM, type 1 diabetes mellitus.
DSM-5 CRITERIA FOR NEUROCOGNITIVE DISORDER
As the observed mental health symptoms are a result of a prolonged and repetitive stress situation (COVID-19 pandemic), affecting multiple domains of social and personal life, many of them are, thus, trauma inflicted. However, they fall short of the ‘Trauma- and Stressor-Related Disorders’ diagnosis as per the DSM-5 criteria that includes traumatic events as exposure to actual or threatened death/serious injury/sexual violence and experiencing repeated or extreme exposure to aversive details of the traumatic event [4]. Namely, the DSM-5 clearly states that vicarious trauma cannot be the result of repeated exposure via electronic or print media. It is understandable that the unexpected and emotionally traumatic experiences (caused by social and self-isolation, closure of schools, working from home, families kept apart and so on) and the scale we have and still continue to be witnessing could not have been predicted and considered at the time. However, the protracted repetitive psychological insults, either as result of the infection per sé or by proxy have already resulted in long-term mental health constructs within the long-COVID terminology or affecting the cognitive domains.
Neurocognitive disorders (NCDs) are one of these consequences. The latest DSM-5 classification introduced levels of impairment, mild and major. The latter new category encompasses the set of existing cognitive disorders contained in the DSM-IV [5], including dementia and amnestic disorder. The introduction of mild NCDs, as per the DSM-5, aimed to facilitate both clinical diagnostic and therapeutic advances [i.e. early detection and treatment of cognitive decline prior it becoming more pronounced and progress to dementia (major neurocognitive)] and researchers to advance diagnostic and therapeutic opportunities. Most importantly, the latest NCD criteria enable the stratification of NCD categories not only in terms of the known dementia subtypes, that is Alzheimer's disease, vascular dementia, Lewy Body diseases (dementia with Lewy bodies and Parkinson's disease dementia), but also NCD due to (another) medical condition and multiple causes, apart from the substance/medication-induced NCD and unspecified NCD that are also included as diagnoses. The NCD now can also be used for the more subtle cognitive problems including subclinical, and even transient disorders of cognition, or their exacerbation as a result of the psychological response to the pandemic, irrespective of the age at onset (reviewed in [6]). This is of utmost importance to prevent further cognitive deterioration.
Among the number of modifiable dementia risk factors, depression, social isolation, physical inactivity, smoking and diabetes count for 16% of the total of 40% identified modifiable dementia risk factors [7▪▪]. Posttraumatic stress disorder (PTSD) is, similarly, a strong and potentially modifiable risk factor for all-cause dementia. The latest meta-analysis based on nine electronic databases, found PTSD hazard ratio to be 1.61 (n = 905 896; five studies) in veterans, and 2.11 (n = 787 782; three studies) in the general population [8▪]. Obsessive compulsive disorder (OCD) has been described to segregate in families with dementia [9] and late-onset OCD has been reported as a precursor for several neurodegenerative conditions characterized by neuropathological involvement of neocortical and/or basal ganglia areas, including Alzheimer's disease [10], Lewy body dementia [11], fronto-temporal lobe dementia, amyotrophic lateral sclerosis [12] and supranuclear palsy [13]. Furthermore, disruptions in the corticostriatal activity as present in amyotrophic lateral sclerosis, fronto-temporal dementia and supranuclear palsy, also underlie the development of Parkinson's disease [14].
Although the mental health disorders per sé are not linked directly to Parkinsonian syndromes, the latest single case reports indicate that the COVID-19 infection alone may present with reduced nigrostriatal dopamine function, and result in acute transient Parkinson's disease in younger people (reviewed in [15▪]) and may suggest a particular susceptibility of the basal ganglia to both stress and nootropism of the SARS-CoV-2 virus. In support of this is the latest study based on a mathematical model demonstrating that the neuroanatomical distribution of small neurological symptoms due to COVID-19 infection, as seen on neuroradiological (MRI) brain scans, spreads outward from the basal ganglia to other cortical (i.e. temporo-occipital cortices) and spinal areas [16], thus placing the subcortex as one of the main brain areas that are susceptible to SARS-CoV-2 infection.
DELIRIUM IN OLDER PATIENTS WITH COVID-19
Delirium is known to be a common presenting symptom for older adults with severe disease in the emergency department but goes undetected in two-thirds of cases [17]. Delirium is an acute state of confusion characterized by altered level of consciousness, disorientation, inattention and other cognitive disturbances. It commonly affects older persons and is associated with adverse outcomes, including prolonged hospitalization and death [18]. Under-detection of delirium during COVID-19 infection may also contribute to the rise of NCD irrespective of the infectious agents, that is SARS-CoV-2 or other untreated medical conditions due to the access to medical care, or being undetected as it is the case for hypoactive delirium [19].
Most of the published evidence on delirium in COVID-19 infection relates to older people, and then predominantly for those presenting with overt, hyperactive delirium. In contrast, reports for delirium incidence and its characteristics in younger people are missing, similarly to those for hypoactive and subsyndromal delirium in both young and older people [2▪]. Bearing in mind that delirium can result in delayed neurocognitive recovery for up to 1.5 years post serious physical illness [20], it is imperative to be timely diagnosed to prevent long-term poor neurocognitive outcomes.
NEUROPATHOLOGICAL MECHANISM OF CENTRAL NERVOUS SYSTEM DAMAGE IN COVID-19 INFECTION AND THEIR RELEVANCE TO NEUROCOGNITIVE DISORDER
Many viral infections can cause serious damage to the structure and function of the central nervous system (CNS), including severe encephalitis due to coronaviruses (CoVs), toxic encephalopathy caused by severe systemic viral infection (e.g. SARS-CoV and SARS-CoV-2) and severe acute demyelinating lesions developing after viral infection [21]. Some viruses (including the SARS-CoV-2) are neurotropic and can invade nervous tissues and cause infections of immune-functioning macrophages, microglia or astrocytes in the CNS [22]. Acute viral infection is also an important cause of this disease, exemplified by a respiratory infection caused by CoVs [23]. Patients with COVID-19 often suffer from severe hypoxia and viremia, which has the potential to cause toxic encephalopathy. Its clinical symptoms are complex and diverse: patients with a mild course of the disease may develop headache, dysphoria, mental disorder and delirium, whereas those seriously affected may experience loss of consciousness, coma and paralysis [24,25].
Hypoxic brain injury
Severe pneumonia can result in systemic hypoxia leading to brain damage. The contributing factors include peripheral vasodilatation, hypercarbia, hypoxia and anaerobic metabolism with accumulation of toxic compounds. These can result in neuronal swelling and brain oedema, which ultimately result in neurological damage [26▪▪,27].
Blood circulation pathway
Proteins of various viruses can often be detected in nervous system tissue samples (such as cerebrospinal fluid or brain), suggesting that viruses can directly invade the nervous system and cause neuronal damage [28]. A typical virus enters the CNS through the blood circulation, with the virus multiplying in the vasculature and choroid plexus [29▪]. The virus is subsequently released into the blood stream to reproduce in mononuclear macrophages throughout the body. The secondary release into the blood may increase the permeability of the blood–brain barrier through the produced cytokines, thereby promoting the virus to enter the brain and cause viral encephalitis [28]. The low detectable SARS-CoV-2 viral load in the brain tissue postmortem [30▪▪] argues for blood-derived viruses presence in some of them.
Neuronal pathway
The neuronal pathway is an important vehicle for neurotropic viruses to enter the CNS. Viruses can migrate by infecting sensory or motor nerve endings, achieving retrograde or anterograde neuronal transport through the motor proteins, dynein and kinesins [31]. One of the important examples of a neuronal pathway is that of olfactory neuron transport. The unique anatomical organization of olfactory nerves and the olfactory bulb in the nasal cavity and in main olfactory bulb in forebrain effectively makes it a channel between the nasal epithelium and the CNS [28]. As a consequence, SARS-CoV-2 can enter the brain through the olfactory tract in the early stages of infection [32]. Anosmia and chemosensory dysfunction were reported as both one of the first clinical symptoms and being at least 10-fold more common in COVID-19 infection [33▪]. Although the neuroimaging reports are not conclusive, anosmia has been linked to atrophy [34] or hypometabolism of the olfactory bulb (Niesen et al., unpublished data), as well as transient morphological changes in the olfactory bulb [35]. These observed clinical and neuroradiological changes may be in particularly important for the early detection of post-COVID-19 NCDs, as olfactory dysfunction has been now associated with amnestic mild cognitive impairment in HIV adults [36▪].
One of the dopaminergic pathways also originates in the olfactory bulb and it makes it, thus, another candidate for SARS-CoV-2 entry and propagation into the CNS. Dopaminergic receptors modulate the innate immune response to a viral infection (i.e. HIV [37], Ebola virus, [38]) and some viruses, such as the Japanese Encephalitis Virus (JEV), utilize the dopaminergic signal transduction pathway to increase neuronal susceptibility to infection [39]. It is, thus, not surprising that these viruses (i.e. JEV) are found in dopaminergic rich areas, such as thalamus and the midbrain (reviewed in [40]) and therapies targeting dopamine receptors are also being investigated to mitigate viral infections [38]. SARS-CoV-2 may utilize the same pathway to gain entry in the human body [41] and also influence the autoimmune innate response.
IMMUNE-MEDIATED INJURY AND ROLE FOR CYTOKINES
Nervous system damage caused by viral infection may be mediated by the immune system [22]. The cytokine storm is due to the release of high levels of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, tumour necrosis factor (TNF) and chemokines (CCL-2, CCL-3 and CCL-5) by respiratory epithelial and dendritic cells, and macrophages [42▪]. COVID-19 disease severity is characterized by increased IL-2, IL-7, granulocyte-colony stimulating factor, interferon-γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α and hyperferritinimia. The release of IL-6 causes vascular leakage, activation of complement and coagulation cascade, suggesting that mortality in COVID-19 infection might be due to virally driven hyperinflammation [43].
The persistence of COVID-19 infections and its ability to infect macrophages, microglia and astrocytes in the CNS are particularly important. A neurotropic virus can activate glial cells and induce a pro-inflammatory state [44]. IL-6, an important member of the cytokine storm, is positively correlated with the severity of COVID-19 symptoms [43]. In addition, experiments have confirmed that primary glial cells cultured in vitro secrete a large amount of inflammatory factors such as IL-6, IL-12, IL-15 and TNF-α after being infected with CoVs [23]. Furthermore, activation of immune cells in the brain will cause chronic inflammation and brain damage.
THE CHOLINERGIC ANTI-INFLAMMATORY PATHWAY AND THE VAGUS NERVE
The cholinergic system plays an important role in supressing excessive inflammation, as shown in experimental models of disease such as sepsis, ischaemia-reperfusion injury, haemorrhagic shock, abdominal surgery and infections (i.e. pancreatitis, colitis; [45]). The cytokine storm and the worsening of patients’ health status can be dampened or even prevented by specifically targeting the vagal-driven cholinergic anti-inflammatory pathway (CAP) [46]. The CAP is a concept that involves an anti-inflammatory effect of vagal efferents by the release of acetylcholine (ACh) [47]. Nicotinic acetylcholine receptor alpha7 subunit (α7nAChRs) is required for ACh inhibition of macrophage-TNF release and cytokine modulation [48]. Apart from TNF, other pro-inflammatory cytokines such as IL-6, IL-1β were significantly decreased by vagus nerve stimulation (VNS) but not the anti-inflammatory cytokine IL-10 [46].
The vagus nerve, the longest nerve of the organism, innervates the lungs and the gastrointestinal tract, two organs which are targeted by SARS-CoV-2 (COVID-19). ACh released at the distal end of the vagus nerve acts on intrinsic neurons of the enteric nervous system, for example at the level of the gastrointestinal tract to inhibit the release of TNF by macrophages [49]. The intracellular signalling of α7nAChRs inhibits transactivational activity of the transcription factor NF-kB p65 [48] and activates Jak2 and STAT3 signalling [50]. Consequently, α7nAChRs could be a candidate, as they are expressed on immune cells regulating antigen-specific antibody and pro-inflammatory cytokines production and likely regulate the intensity of immuneresponses [51▪▪].
ROLE FOR CYTOKINES AND CHOLINERGIC SYSTEM IN NEUROCOGNITIVE DISORDER
Cognitive changes are present in both infection and neurodegenerative diseases and are associated with increased inflammatory cytokines. The cytokine and chemokine disbalance not necessarily is initiated directly from an infection, but can also arise from stressful conditions [52,53], and results in a variety of health consequences (Fig. 1), due to neurotransmitter and hormonal imbalance, neurobiological changes and even autoimmune illnesses (including rheumatoid arthritis, Type 1 diabetes mellitus).
FIGURE 1.
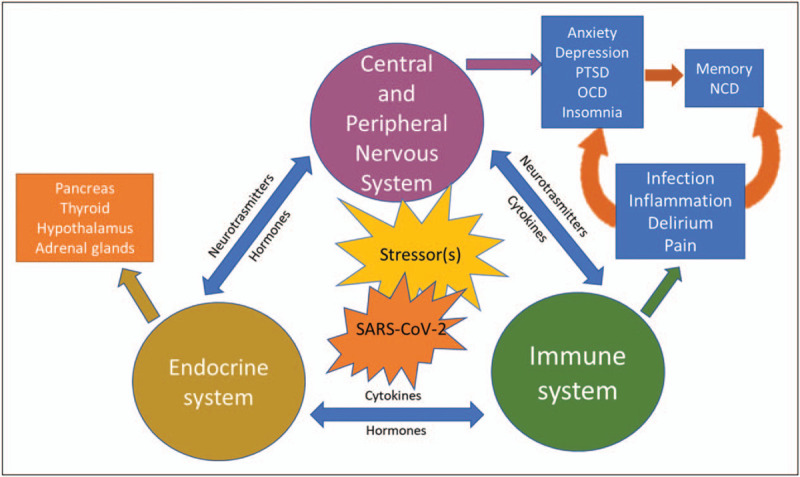
Impact of infective (i.e. SARS-CoV-2) and psychological stressors on the nervous, immune, and endocrine systems. For a more detailed explanation, see the main text. NCD, neurocognitive disorder; OCD, obsessive compulsive disorder; PTSD, post-traumatic stress disorder.
Elevation of proinflammatory cytokines, including IL-1, IL-6 and TNF-alpha, is associated with fatigue, depression and anxiety, as well as hostility and irritability (also referred as sickness behaviours), irrespectively of the cause of the neuropsychiatric symptoms, that is whether they are triggered by an infection or prolong and repetitive stress. In support of this is the wide spectrum of cytokines and chemokines described in PTSD, that is higher levels of peripheral cytokines (IL-2, IL-4, IL-6, IL-8, IL-10 and TNF-α) than those detected in age and sex-matched healthy controls [52], suggesting a generalized inflammatory state in these patients [54▪▪]. Similarly, altered immune response, though with somewhat conflicting results, has been described in OCD (reviewed in [55,56]). Nevertheless, the latest study demonstrated plasma levels of IL-1β, IL-6 and TNF-α were significantly higher in patients with OCD than the healthy controls [57].
The vagal nerve has been now considered as one of the potential viral CNS access routes in COVID-19 [30▪▪]. This is in particularly important in relation to long-term consequences of SARS-CoV-2 infection, including the development of NCD in the COVID-19 survivors. The TNF-α in physiological condition is expressed only in minor, hardly detectable levels in the brain, and is involved in higher cognitive functions, such as memory and learning. Its increase in infection and in stress conditions [58] is ameliorated by acetylcholine, or via the stimulation of the vagal nerve, that attenuates the cytokine production. It is the alpha7 nicotinic acetylcholine receptors (α7nAChRs) that are involved in mediating this anti-inflammatory response to insults due to stress, sepsis, ischemia, haemorrhage and so on.
Majority of people with dementia are now treated with antidementia drugs, predominantly cholinesterase inhibitors (i.e. donepezil, rivastigmine and galantamine). Although these drugs enhance the neurodegeneration-induced working memory decline, they appear not to be as efficient when compared to the α7nAChRs. Namely, in animal studies, the α7nAChR activation has been reported to restore both the amyloid Aβ42 induced long-term potentiation [59] and improve the Aβ25-35-induced cerebral blood flow [60]. However, the observed increase in cortical α7nAChRs may be an indirect effect of increased ACh levels in vivo, thus further providing support for lack of receptors activation in dementia [61]. This suggests that the antidementia drugs alone may not be sufficient to improve the anti-inflammatory and autoimmune response once the innate ACh has declined and may explain the failure of cholinesterase inhibitors clinical trials in delirium [62].
CONCLUSION
Mild NCD symptoms as a result of the COVID-19 pandemic provide a unique opportunity for researchers to address the early changes that underlie neurocognitive impairment at a clinical and molecular level and to longitudinally follow them with a view to modify their outcomes. From the studies published to date, we know that the biological markers for major NCD of Alzheimer's type develop several decades prior to overt clinical symptoms [63] and it is the convergence of multiple diseases that underpins most clinical dementia syndromes [64].
The stress related to the COVID-19 pandemic affects similarly the cytokine system and cholinergic pathways, resulting in depression and poor cognitive performance. It is, thus, important to raise awareness for these consequences among the wider population and put into place ways of increasing people's resilience across the lifespan. Identifying biomarkers that may aid in gauging physical and mental resilience will facilitate timely interventions in preparedness for future similar health events.
The properties of SARS-CoV-2 as both a catalyzer and accelerator to brain protein aggregation [65] is another research opportunity for the dementia field in preventing the aggregate prone brain proteins, such as tau protein, β-amyloid and α-synuclein, to form the insoluble and neuronal detrimental deposits. In doing so, we can expect a new generation of therapeutics to be developed, focused on biological mechanisms to prevent and eventually reverse the neurodegenerative processes occurring with ageing and dementia.
The currently ongoing preclinical research to expand the VNS treatment in inflammatory disorders [66] is an overlooked opportunity for the management of both the SARS-CoV-2 infection and the stress-related psychological consequences living with the pandemic. Targeting the α7nAChRs through VNS could hence be another area of interest in the management of COVID-19 related physical (respiratory and gastrointestinal symptoms) and mental health symptoms, including NCD irrespective of its cause and severity.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry 2020; S2215-0366:30462–30464. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study was based on TriNetX Analytics Network that contains anonymized data from electronic health records in 54 healthcare organizations in the USA, totalling 69.8 million patients. The analysis included 62,354 patients diagnosed with COVID-19 between 20 January and 1 August 2020. The authors present incidence and hazard ratios measures for psychiatric disorders, dementia and insomnia, during the first 14--90 days after a diagnosis of COVID-19.
- 2▪.Mukaetova-Ladinska EB, Kronenberg G. Psychological and neuropsychiatric implications of COVID-19. Eur Arch Psychiatry Clin Neurosci 2020; 1–14. doi: 10.1007/s00406-020-01210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; The review draws on evidence from previous coronavirus outbreaks (i.e. SARS, MERS) and emerging evidence from China, Europe, Asia and the USA to synthesize the current knowledge regarding the psychological and neuropsychiatric implications of the COVID-19 pandemic.
- 3▪.Matias-Guiu JA, Jorge Matías-Guiu J. Death rate due to COVID-19 in Alzheimer's disease and frontotemporal dementia. J Alzheimers Dis 2020; 78:537–541. [DOI] [PubMed] [Google Scholar]; In this observational case series, 204 patients with Alzheimer's disease and frontotemporal dementia were enrolled and the frequency and mortality of COVID-19 determined. COVID-19 occurred in 7.3% of patients living at home and 72.0% of those living at care homes. Living in care facilities and diagnosis of Alzheimer's disease were independently associated with a higher probability of death.
- 4.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 6.Ritchie K, Chan D, Watermeyer T. The cognitive consequences of the COVID-19 epidemic: collateral damage? Brain Commun 2020; 2:fcaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪▪.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020; 396:413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]; Three new modifiable risk factors for dementia were added, excessive alcohol consumption, head injury and air pollution to the 2017 Lancet Commission on dementia prevention, intervention, and care life-course model of nine factors (less education, hypertension, hearing impairment, smoking, obesity, depression, physical inactivity, diabetes, and infrequent social contact). These modifying 12 risk factors might prevent or delay up to 40% of dementias.
- 8▪.Günak MM, Billings J, Carratu E, et al. Posttraumatic stress disorder as a risk factor for dementia: systematic review and meta-analysis. Br J Psychiatry 2020; 217:600–608. [DOI] [PubMed] [Google Scholar]; This meta-analysis addresses the association of PTSD and risk of dementia and reports that PTSD is a strong and potentially modifiable risk factor for all-cause dementia.
- 9.Mrabet Khiari H, Achouri A, Ben Ali N, et al. Obsessive-compulsive disorder: a new risk factor for Alzheimer disease? Neurol Sci 2011; 32:959–962. [DOI] [PubMed] [Google Scholar]
- 10.Frydman I, Ferreira-Garcia R, Borges MC, et al. Dementia developing in late-onset and treatment-refractory obsessive-compulsive disorder. Cogn Behav Neurol 2010; 23:205–208. [DOI] [PubMed] [Google Scholar]
- 11.Frileux S, Millet B, Fossati P, et al. Late-onset OCD as a potential harbinger of dementia with Lewy bodies: a report of two cases. Front Psychiatry 2020; 11:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bersano E, Sarnelli MF, Solara V, et al. A case of late-onset OCD developing PLS and FTD. Amyotroph Lateral Scler Frontotemporal Degener 2018; 19:463–465. [DOI] [PubMed] [Google Scholar]
- 13.Karnik NS, D’Apuzzo M, Greicius M. Nonfluent progressive aphasia, depression, and OCD in a woman with progressive supranuclear palsy: neuroanatomical and neuropathological correlations. Neurocase 2006; 12:332–338. [DOI] [PubMed] [Google Scholar]
- 14.Gao L-l, Wu T. The study of brain functional connectivity in Parkinson's disease. Transl Neurodegener 2016; 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪.Brundin P, Nath AJ, David Beckham JD. Is COVID-19 a perfect storm for Parkinson's disease? Trends Neurosci 2020; 43:931–933. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors reflect on underlying cellular and molecular mechanisms, of Parkinson's disease, and discuss whether COVID-19 might be associated with higher long-term risk of Parkinson's disease.
- 16.Parsons N, Outsikas A, Parish A, et al. Modelling the anatomical distribution of neurological events in COVID-19 patients: a systematic review. medRxiv 2020; doi: 10.1101/2020.10.21.20215640. [Google Scholar]
- 17.MacLullich AM, Hall RJ. Who understands delirium? Age Ageing 2011; 40:412–414. [DOI] [PubMed] [Google Scholar]
- 18.Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA 2017; 318:1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalaria RN, Mukaetova-Ladinska EB. Delirium, dementia and senility. Brain 2012; 135:2582–2584. [DOI] [PubMed] [Google Scholar]
- 20.Salluh JIF, Wang H, Schneider EB, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ 2015; 350:h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright EJ, Brew BJ, Wesselingh SL. Pathogenesis and diagnosis of viral infections of the nervous system. Neurol Clin 2008; 26:617–633. [DOI] [PubMed] [Google Scholar]
- 22.Klein RS, Garber C, Howard N. Infectious immunity in the central nervous system and brain function. Nat Immunol 2017; 18:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohmwald K, Galvez NMS, Rios M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci 2018; 12:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeshi M, Norikazu H, Goto G, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infectious Dis 2020; 94:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dobbs MR. Toxic encephalopathy. Semin Neurol 2011; 31:184–193. [DOI] [PubMed] [Google Scholar]
- 26▪▪.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med 2020; 382:2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors describe the neurologic features in an observational series of 64 consecutive patients admitted to hospital because of acute respiratory distress syndrome due to COVID-19. In acute respiratory distress syndrome due to COVID-19, infection was associated with encephalopathy, prominent agitation and confusion, and corticospinal tract signs. Two of 13 patients who underwent brain MRI had single acute ischemic strokes.
- 27.Yeshun W, Xiaolin X, Zijun C, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 2020; 87:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe 2013; 13:379–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪.Raha AA, Chakraborty S, Henderson J, et al. Investigation of CD26, a potential SARS-CoV-2 receptor, as a biomarker of age and pathology. Biosci Rep 2020; 40:BSR20203092. [DOI] [PMC free article] [PubMed] [Google Scholar]; CD26 expression in ageing and people with diabetes and dementia was explored in serum. The authors found low serum sCD26 levels in at-risk people for COVID-19 infection. They conclude that high serum CD26 levels could protect from viral infection by competitively inhibiting the virus binding to cellular CD26, whereas low sCD26 levels could increase the risk of infection.
- 30▪▪.Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a postmortem case series. Lancet Neurol 2020; 19:919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]; Autopsy findings of 43 adults with COVID-19 reported. The neuropathological changes in patients with COVID-19 seem to be mild, with brainstem neuroinflammatory being the most common finding. There was no evidence for CNS damage caused directly by SARS-CoV-2.
- 31.Swanson PA, II, McGavern DB. Viral diseases of the central nervous system. Curr Opin Virol 2015; 11:44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori I. Transolfactory neuroinvasion by viruses threatens the human brain. Acta Virol 2015; 59:338–349. [DOI] [PubMed] [Google Scholar]
- 33▪.Yan CH, Faraji F, Prajapati DP, et al. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol 2020; 10:806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]; A total of 1480 patients with influenza-like symptoms underwent COVID-19 testing: 59 out of 102 (58%) COVID-19-positive patients and 203 out of 1378 (15%) COVID-19-negative patients. Smell and taste loss were reported in 68% (40/59) and 71% (42/59) of COVID-19-positive individuals, respectively, compared with 16% (33/203) and 17% (35/203) of COVID-19-negative patients (P < 0.001). Smell and taste impairment were independently and strongly associated with COVID-19-positivity and sore throat with COVID-19-negativity patients.
- 34.Chiu A, Fischbein N, Wintermark M, et al. COVID-19-induced anosmia associated with olfactory bulb atrophy. Neuroradiology 2020; 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Politi LS, Salsano E, Grimaldi M. Magnetic Resonance Imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol 2020; 77:1028–1029. [DOI] [PubMed] [Google Scholar]
- 36▪.Sundermann EE, Fields A, Saloner R, et al. The utility of olfactory function in distinguishing early stage Alzheimer's disease from HIV-associated neurocognitive disorder. AIDS 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; This observational study was based on 81 older (≥50 years) people with HIV [PWH (83% male, 65% white]. They completed the University of Pennsylvania Smell Identification Test (UPSIT), a seven-domain neuropsychological test battery and neuromedical evaluation. Fifty-seven participants (70%) were classified with HIV-associated neurocognitive disorders (HANDs) and 35 (43%) were classified as amnestic mild cognitive impairment (aMCI). UPSIT scores were lower (worse) in the high versus low aMCI group (P = 0.002) but did not differ by HAND status. The authors conclude that olfactory assessments may help in detecting early aMCI and dementia among PWH.
- 37.Basova L, Najera JA, Bortell N, et al. Dopamine and its receptors play a role in the modulation of CCR5 expression in innate immune cells following exposure to methamphetamine: implications to HIV infection. PLoS One 2018; 13:e0199861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penny CJ, Vassileva K, Jha A, et al. Mining of Ebola virus entry inhibitors identifies approved drugs as two-pore channel pore blockers. Biochim Biophys Acta Mol Cell Res 2019; 1866:1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simanjuntak Y, Liang JJ, Lee YL, Lin YL. Japanese encephalitis virus exploits dopamine D2 receptor-phospholipase C to target dopaminergic human neuronal cells. Front Microbiol 2017; 8:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laureti M, Narayanan D, Rodriguez-Andres J, et al. Flavivirus receptors: diversity, identity, and cell entry. Front Immunol 2018; 9:2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khalefah M, Khalifah A. Determining the relationship between SARS-CoV-2 infection, dopamine, and COVID-19 complications. J Taibah Univ Medical Sci 2020; 15:550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42▪.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect 2020; 80:607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article reviews the mechanism and treatment of COVID-19 virus-induced inflammatory storm and provides medication guidance for its clinical treatment.
- 43.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Fu L, Gonzales DM, Lavi E. Coronavirus neurovirulence correlates with the ability of the virus to induce pro-inflammatory cytokine signals from astrocytes and microglia. J Virol 2004; 78:3398–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavlov VA. Cholinergic modulation of inflammation. Int J Clin Exp Med 2008; 1:203–212. [PMC free article] [PubMed] [Google Scholar]
- 46.Bonaz B, Sinniger V, Pellissier S. Targeting the cholinergic anti-inflammatory pathway with vagus nerve stimulation in patients with Covid-19? Bioelectron Med 2020; 6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borovikova LV, Ivanova S, Nardi D, et al. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci 2000; 85:141–147. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003; 421:384–388. [DOI] [PubMed] [Google Scholar]
- 49.Matteoli G, Gomez-Pinilla PJ, Nemethova A, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 2014; 63:938–948. [DOI] [PubMed] [Google Scholar]
- 50.de Jonge WJ, van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 2005; 6:844–851. [DOI] [PubMed] [Google Scholar]
- 51▪▪.Mashimo M, Fujii T, Ono S, et al. Minireview: divergent roles of alpha7 nicotinic acetylcholine receptors expressed on antigen-presenting cells and CD4(+) T cells in the regulation of T cell differentiation. Int Immunopharmacol 2020; 82:106306. [DOI] [PubMed] [Google Scholar]; Using spleen cells from ovalbumin (OVA)-specific T cell receptor transgenic DO11.10 mice and the α7nAChR agonist GTS-21, the authors demonstrated that α7nAChRs on antigen presenting cells (APCs) downregulate T cell differentiation by inhibiting antigen processing and thereby interfering with antigen presentation, and α7nAChRs on T cells upregulate differentiation into Tregs and effector T cells. They conclude that the divergent roles of α7nAChRs on APCs and T cells are likely involved in regulating the intensity of immune responses.
- 52.Maloley PM, England BR, Sayles H, et al. Posttraumatic stress disorder and serum cytokine and chemokine concentrations in patients with rheumatoid arthritis. Semin Arthritis Rheum 2019; 49:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Köhler CA, Freitas TH, Maes M, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 2017; 135:373–387. [DOI] [PubMed] [Google Scholar]
- 54▪▪.Zhang L, Hu XZ, Li X, et al. Potential chemokine biomarkers associated with PTSD onset, risk and resilience as well as stress responses in US military service members. Transl Psychiatry 2020; 10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors measured 40 chemokines using the Bio-Plex Pro Human Chemokine Panel Assays in blood samples from soldiers pre and post deployment (pre, post). A number of potential markers were identified: CCL2, CCL15, CCL22, CCL25, CXCL2 and CXCL12 were associated with PTSD onset, and CCL3, CXCL11 and CXCL16 were related to stress response; CCL13, CCL20 and CXCL6 were possible PTSD risk markers, whereas CX3CL1 might be a resilience marker. The authors conclude that the dysregulated chemokines may serve as biomarkers for PTSD onset, risk, and resilience as well as stress responses.
- 55.Marazziti D, Mucci F, Lombardi A, et al. The cytokine profile of OCD: pathophysiological insights. Int J Interferon Cytokine Mediat Res 2015; 7:35–42. [Google Scholar]
- 56.Cosco TD, Pillinger T, Emam H, et al. Immune aberrations in obsessive-compulsive disorder: a systematic review and meta-analysis. Mol Neurobiol 2019; 56:4751–4759. [DOI] [PubMed] [Google Scholar]
- 57.Karagüzel EÖ, Arslan FC, Uysal EK, et al. Blood levels of interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha and cognitive functions in patients with obsessive compulsive disorder. Compr Psychiatry 2019; 89:61–66. [DOI] [PubMed] [Google Scholar]
- 58.Madrigal JLM, Hurtado O, Moro MA, et al. The increase in TNF-alpha levels is implicated in NF-kappaB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology 2002; 26:155–163. [DOI] [PubMed] [Google Scholar]
- 59.Kroker KS, Moreth J, Kussmaul L, et al. Restoring long-term potentiation impaired by amyloid-beta oligomers: comparison of an acetylcholinesterase inhibitor and selective neuronal nicotinic receptor agonists. Brain Res Bull 2013; 96:28–38. [DOI] [PubMed] [Google Scholar]
- 60.Sadigh-Eteghad S, Mahmoudi J, Babri S, Talebi M. Effect of alpha-7 nicotinic acetylcholine receptor activation on beta-amyloid induced recognition memory impairment. Possible role of neurovascular function. Acta Cir Bras 2015; 30:736–742. [DOI] [PubMed] [Google Scholar]
- 61.Reid RT, Sabbagh MN. Effects of donepezil treatment on rat nicotinic acetylcholine receptor levels in vivo and in vitro. J Alzheimers Dis 2003; 5:429–436. [DOI] [PubMed] [Google Scholar]
- 62.Van Eijk MM, Roes KC, Honing ML, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet 2010; 376:1829–1837. [DOI] [PubMed] [Google Scholar]
- 63.Younes L, Albert M, Moghekar A, et al. Identifying changepoints in biomarkers during the preclinical phase of Alzheimer's Disease. Front Aging Neurosci 2019; 11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woodward M, Mackenzie IRA, Hsiung G-YR, et al. Multiple brain pathologies in dementia are common. Eur Geriatr Med 2010; 1:259–265. [Google Scholar]
- 65.Tavassoly O, Safavi F, Tavassoly I. Seeding brain protein aggregation by SARS-CoV-2 as a possible long-term complication of COVID-19 infection. ACS Chem Neurosci 2020; 11:3704–3706. [DOI] [PubMed] [Google Scholar]
- 66.Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res 2018; 11:203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]


