Abstract
Purpose of review
Over 70 million people worldwide, including those with neurodegenerative disease (NDD), have been diagnosed with coronavirus disease 2019 (COVID-19) to date. We review outcomes in patients with NDD and COVID-19 and discuss the hypothesis that due to putative commonalities of neuropathogenesis, COVID-19 may unmask or trigger NDD in vulnerable individuals.
Recent findings
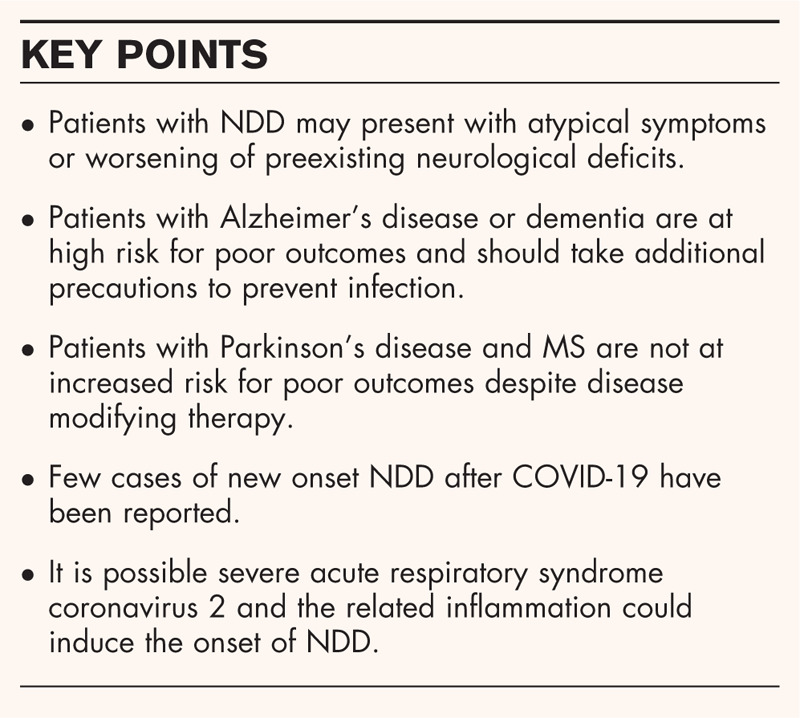
Based on a systematic review of published literature, patients with NDD, including dementia, Parkinson's disease, and multiple sclerosis (MS) make up a significant portion of hospitalized COVID-19 patients. Such patients are likely to present with altered mental status or worsening of their preexisting neurological symptoms. Patients with NDD and poor outcomes often have high-risk comorbid conditions, including advanced age, hypertension, diabetes, obesity, and heart/lung disease. Patients with dementia including Alzheimer's disease are at higher risk for hospitalization and death, whereas those with preexisting Parkinson's disease are not. MS patients have good outcomes and disease modifying therapies do not increase the risk for severe disease. Viral infections and attendant neuroinflammation have been associated with the pathogenesis of Alzheimer's disease, Parkinson's disease, and MS, suggesting that COVID-19 may have the potential to incite or accelerate neurodegeneration.
Summary
Since patients with Alzheimer's disease are at higher risk for hospitalization and death in the setting of COVID-19, additional precautions and protective measures should be put in place to prevent infections and optimize management of comorbidities in this vulnerable population. Further studies are needed to determine whether COVID-19 may lead to an increased risk of developing NDD in susceptible individuals.
Keywords: Alzheimer's disease, coronavirus disease 2019, neurodegenerative disease, Parkinson's disease, severe acute respiratory syndrome coronavirus 2
INTRODUCTION
The novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has swept across the world and caused a devastating pandemic. SARS-CoV-2 causes the syndrome known as coronavirus disease 2019 (COVID-19). Neurodegenerative diseases (NDDs) encompass both dementia syndromes and other autoimmune, genetic, and sporadic conditions. Broadly, NDDs are characterized by the progressive degeneration of the central nervous system and/or peripheral nervous system. Many patients with NDD are older with comorbidities and others are on immunosuppressive therapies. Older patients and those with comorbidities such as hypertension, diabetes, obesity, immunosuppression, and heart/lung disease are at increased risk for poor outcomes related to COVID-19 [1▪▪,2]. High levels of inflammatory cytokines and chemokines develop in COVID-19 and are predictive of disease severity and survival [3,4]. Several cytokines and chemokines have been identified, including IL-2, IL-6, IL-7, IL-10, interferon-inducible protein-10 (IP-10), IFN-I, monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, and TNF-α [5,6▪▪]. Here we provide a review of outcomes in patients with COVID-19 and NDD. In addition, we explore the hypothesis that COVID-19 may lead to a dysregulated immune and inflammatory response that puts patients at higher risk of developing or accelerating NDD.
Box 1.
no caption available
METHODS
In September of 2020, we performed a systematic review of literature published since January 2020 using PubMed and Google Scholar. We sought to identify clinical studies reporting outcomes on patients who contracted COVID-19 and were known to have an NDD or who developed NDD during or after their COVID-19 course. In addition, we sought to identify articles discussing viral infections as causative agents of NDD to highlight potential mechanisms SARS-CoV-2 may use to trigger NDD.
Our search terms included: ‘neurodegenerative disease’; ‘neurodegeneration’; ‘COVID-19’; ‘SARS-CoV-2’; ‘dementia’; ‘Parkinson's disease’; ‘Alzheimer's disease’; ‘multiple sclerosis’; ‘Lewy body dementia’; ‘vascular dementia’; ‘corticobasal degeneration’; ‘frontal temporal dementia’; ‘multiple system atrophy’; ‘progressive supranuclear palsy’; ‘amyotrophic lateral sclerosis’; ‘Charcot-Marie-Tooth’; ‘Huntington's disease’; ‘prion disease’; ‘spinal muscular atrophy’; ‘spinocerebellar ataxia’; ‘mitochondrial disease’.
The following inclusion and exclusion criteria were used to identify appropriate articles. A breakdown of the articles reviewed is detailed in Fig. 1. Out of 242 articles reviewed for inclusion, 66 were included in the final review.
FIGURE 1.
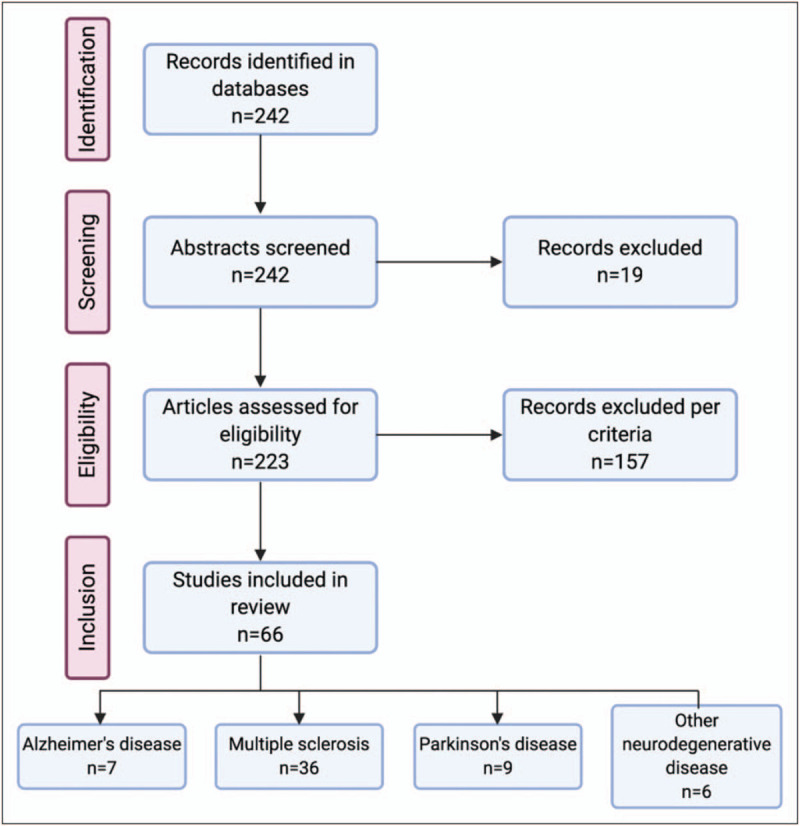
PRISMA flow diagram of articles reviewed and included.
Inclusion:
-
(1)
Patients with confirmed SARS-CoV-2 or high suspicion of clinical COVID-19
-
(2)
Patients with an NDD
-
(3)
Patients who developed symptoms or signs of an NDD during or after a COVID-19 course
Exclusion:
-
(1)
Articles which proposed hypotheses regarding NDD and COVID-19
-
(2)
Studies focusing on adapting care management of patients with NDD but without COVID-19
-
(3)
Preprint publications
-
(4)
Quantitative outcomes not provided
CLINICAL REPORTS OF NEURODEGENERATIVE DISEASE AND CORONAVIRUS DISEASE 2019 IN THE LITERATURE TO DATE
According to recent studies, a large proportion of patients with COVID-19 have underlying NDDs, 9–16% [7,8]. Based on our selection criteria, 66 articles were included in the review. Nineteen articles report on patients with Alzheimer's disease or dementia, 36 on multiple sclerosis (MS), 10 on Parkinson's disease, and six on rare diseases or NDD in general.
Alzheimer's disease
Alzheimer's disease affects an estimated 5 million Americans and is the most common form of dementia [9]. Patients with Alzheimer's disease may be more likely to present with atypical COVID-19 symptoms, such as diarrhea, altered mental status, poor functional status, or worsening preexisting neurologic deficits [10,11]. Up to 20% of older adults can present with altered mental status as the primary COVID-19 symptom [12]. Alzheimer's disease and patient with other types of dementia are also at higher risk for hospitalization and death according to numerous studies [11,13–21]. Most Alzheimer's disease patients have one or more high-risk comorbid medical conditions, which significantly contributes to increased risk of poor outcomes [1▪▪,14]. Even Alzheimer's disease patients who do not contract COVID-19 are at risk for worsening of neuropsychiatric symptoms during the pandemic due to isolation, psychosocial stressors, and lack of optimal care [22].
Significantly, an 18F-FDG PET study in two patients with prolonged anosmia demonstrated hypometabolism in the amygdala, hippocampus, parahippocampus, cingulate cortex, and pre/postcentral gyrus. These findings are concerning since hypometabolism in these same areas is commonly seen in mild cognitive impairment and Alzheimer's disease [23]. In addition, hyposmia can be an early sign of Alzheimer's disease and is a prominent feature of COVID-19. The timing of Alzheimer's disease onset is affected by numerous genetic and environmental factors as well as oxidative stress, vascular disease, and neuroinflammation [24]. COVID-19 is associated with endotheliopathy and neuroinflammation in at least some individuals, though whether COVID-19 can precipitate or facilitate the onset of Alzheimer's disease in susceptible individuals is unknown.
Parkinson's disease
Parkinson's disease is a common neurodegenerative condition that affects approximately 1 million people in the United States [25]. Several patients with comorbid Parkinson's disease and COVID-19 have been reported in the literature [26–28]. Parkinson's disease patients have been found to exhibit atypical symptoms, such as poor mood, fatigue, anosmia, flushing, and joint pain, and in some cases may mimic a Parkinson's disease exacerbation [29]. Overall, the consensus is that Parkinson's disease patients with COVID-19 are at higher risk for significant worsening of motor symptoms and may require up-titration of their dopaminergic regimen [29,30,31▪,32]. However, preexisting Parkinson's disease alone does not impart an increased risk of hospitalization or death [13]. Only one study reported a high mortality rate (40%) in an older patient cohort (mean 78 years old). Parkinson's disease patients with other comorbidities such as obesity, chronic obstructive pulmonary disease, and low vitamin D were also at higher risk [33].
One patient in the literature developed a parkinsonian syndrome associated with COVID-19 [34▪]. The 58-year-old man had hyposmia as an initial symptom along with fever, cough, and dyspnea. Subsequently, he developed an asymmetric hypokinetic-rigid syndrome with generalized myoclonus, fluctuating mental status, and opsoclonus. MRI of the brain was normal, but a dopamine transport single photon emission computed tomography (DaT-SPECT) scan showed decreased presynaptic dopamine uptake asymmetrically in bilateral putamina. In Parkinson's disease patients, the DaT-SPECT typically shows decreased dopamine uptake and nigrostriatal cell loss along the nigrostriatal pathway in the substantia nigra pars compacta (SNc) and striatum [35]. This patient developed dysfunction of the nigrostriatal pathway at its termination in the putamen. The myoclonus resolved without treatment and his parkinsonism improved, but did not completely resolve at the time of publication [34▪].
Multiple sclerosis
MS is a neurodegenerative, autoimmune disease that affects nearly 1 million people in the United States [36]. It is common for MS patients to experience worsening of their preexisting neurological deficits in the setting of infection, and this has been reported in COVID-19 [37,38]. However, MS patients with severe disease requiring hospitalization during COVID-19 were more likely to have comorbid medical conditions, advanced age, obesity, significant disability, or progressive disease [38,39▪▪]. Early in the pandemic, there was a concern that MS patients immunosuppressed by disease modifying therapies (DMTs), would be at higher risk of poor outcomes related to COVID-19. Fortunately, this has not been borne out. Numerous case reports have demonstrated patients on rituximab or ocrelizumab, an anti-CD20 B-cell depleting mAb therapies, have better outcomes, experience mild-to-moderate symptoms, and rarely require hospitalization [40–49]. Significantly, several case reports have also demonstrated MS patients on ocrelizumab clear SARS-CoV-2, but do not always produce a measurable antibody response [41,47,50,51]. A few patients have been reported to mount IgG or IgA antibodies on anti-CD20 therapies [52,53]. Two case reports have described patients taking natalizumab who also experienced mild COVID-19 courses [54,55].
Generally, as reported to date in the literature, MS patients have experienced mild-to-moderate COVID-19 disease course and without evidence that DMT use increases the risk of hospitalization or death [38,56]. In fact, evidence showed MS patients on DMTs had milder symptoms compared with MS patients not on DMTs [39▪▪]. However, this finding may be skewed by the fact that patients who have discontinued DMT are more likely to have primary and secondarily progressive MS and greater baseline disability. Several case reports described severe disease in patients on fingolimod requiring hospitalization and respiratory support [37,57,58]. Significantly, one patient recovered well after tocilizumab administration and another patient's COVID-19 symptoms worsened significantly when fingolimod was stopped [58]. Only one report of two young patients, 22 and 26 years old, described asymptomatic COVID-19 infections while taking fingolimod [59]. One study reported severe COVID-19 symptomatology in the setting of alemtuzumab and cladribine when compared with interferon or glatiramer acetate [60]. However, two other case reports showed patients on alemtuzumab and cladribine who recovered without complication [61,62].
There have been several cases of demyelinating disease in the setting of COVID-19 reported in the literature, but only one case of MS diagnosis after a COVID-19 infection has been described [63▪,64–66]. A 29-year-old female with COVID-19 presented with right optic neuritis and was found to have numerous demyelinating lesions of various ages. It was hypothesized that the patient's SARS-CoV-2 infection unmasked a case of undiagnosed MS, in turn leading to an MS flare.
LINKS TO NEURODEGENERATIVE DISEASE PATHOGENESIS
The literature on NDD and COVID-19 will likely become more robust with time. It is important for all NDD patients to optimize their comorbidities to reduce the risk of severe disease. Patients with dementia are at particularly high risk for morbidity and mortality. It is vital to put new policies and produces in place to protect this vulnerable population. Significantly, Parkinson's disease and MS patients are generally not at high risk for severe disease, but COVID-19 may provoke new onset parkinsonism and demyelinating disease in rare cases. Given less than a year has passed since the pandemic started, it is too early to detect the onset of NDD due to COVID-19 patients; however, previously viral infections and inflammation have been strongly linked to the pathogenesis of Alzheimer's disease, Parkinson's disease, and MS.
Alzheimer's disease
It is well known that both severe systemic and central nervous sysetm infections can lead to permanent neurologic deficits. Memory, cognitive, and learning deficits, as well as behavioral changes have been reported following arboviral infections [67▪▪]. Severe sepsis in older adults is associated with a significant decline in cognitive function and functional status [68]. The extent of systemic inflammation correlates strongly with cognitive deficits. In the 6 months after an infection, TNF-α, a proinflammatory cytokine that is significantly elevated in severe COVID-19, has been associated with a two-fold increase in cognitive decline [69]. Another key inflammatory marker in COVID-19, IL-6, is significantly associated with hippocampal atrophy [70]. Analysis of the cerebrospinal fluid in patients with COVID-19 has revealed markedly elevated inflammatory levels, including IL-6, IL-8, IL-10, IP-10, and TNF-α [71,72]. The severe inflammation in COVID-19 is likely to impart long-lasting detrimental effects, similar to other causes of severe sepsis.
Many viruses have been implicated in β-Amyloid-42 (Aβ42) accumulation and other neuropathological features of Alzheimer's disease, including herpes simplex virus-1 (HSV-1), HSV-2, cytomegalovirus, Epstein–Barr virus (EBV), varicella zoster virus, hepatitis C virus, human herpes viruses-6 (HHV-6), and HHV-7 [73]. Aβ42 is a peptide with antimicrobial and antiviral properties. Majde et al. proposed a ‘hit and run’ mechanism where an infection activates astrocytes and induce microglia to release proinflammatory cytokines and chemokines, which then stimulates Aβ42 release [74,75]. At some point, the inflammatory response becomes dysregulated and Aβ42 misfolds and accumulates. Misfolded Aβ42 and antigen from a new viral infection can bind to and activate microglia leading to additional proinflammatory cytokine and chemokine release. This creates a positive feedback cycle that stimulates chronic neuroinflammation and further neuronal damage and loss [75,76]. On pathology, HHV-6 and HHV-7 has been detected at higher levels in the brains of Alzheimer's disease patients compared with controls suggesting chronic infection is present in many individuals [77].
Heneka et al.[78] hypothesize that the dramatic inflammation of COVID-19 strongly induces the neurodegenerative process through the upregulation of the NLRP3 inflammasome-mediated pathway, a component of the innate immune system. The NLRP3 inflammasome leads to the accumulation of Aβ42 and formation of neurofibrillary tangles and has been proposed as a disease modifying target for both Alzheimer's disease and Parkinson's disease prevention [78,79]. The NLRP3 inflammasome is also a suspected culprit in propagating the cytokine storm seen in COVID-19 and could also contribute Alzheimer's disease pathogenesis in patients with severe disease [80,81]. In addition, patients with apoE4 gene have an increased risk for developing Alzheimer's disease and a vulnerability to HSV-1 infections and patients with both are at significantly increased risk for developing Alzheimer's disease [76,82]. This suggests that the interplay between genes, infections, and neuroinflammation is critical to the pathogenesis of Alzheimer's disease. It is unknown based on current studies whether COVID-19 may trigger the onset of NDD in susceptible individuals, but neuroinflammation may be a trigger for some of the pathogenetic pathways that eventually manifest as Alzheimer's disease.
Parkinson's disease
Several viruses have been implicated in the pathogenesis of Parkinson's disease, including influenza, coronavirus, herpes viruses, coxsackievirus, Japanese encephalitis virus, St. Louis encephalitis virus, West Nile virus, and HIV [83]. Hawkes et al.[84] hypothesize that Parkinson's disease patients undergo a ‘dual-hit’, where a neuroinvasive virus enters the nervous system and triggers immune dysregulation and propagation of abnormal protein accumulation. α-Synuclein (αSyn) is an antimicrobial peptide that is secreted in response to infection and along with its infectious target can be transported retrograde from an infected organ into the central nervous system along cranial nerves, including the olfactory and vagus nerves. Cell autophagy and lysosomal degradation are critical components of the innate immune system and viruses often target this pathway to impair the cell's ability to degrade or recycle pathogenic or cellular materials [85]. By hijacking this pathway, viruses can cause the inadvertent aggregation of intracellular proteins, such as αSyn, which over time may lead to NDD. Genetic and environmental factors predispose certain patients to develop Parkinson's disease [86].
It is plausible that systemic and neuroinflammation and altered immune response associated with COVID-19 could induce Parkinson's disease in susceptible patients. Parkinson's disease has been found in ‘clusters’ of persons living or working in close quarters, suggestive of a shared infectious insult [83]. In fact, antibodies to common coronaviruses have been detected at significantly higher levels in the cerebrospinal fluid of Parkinson's disease patients compared with controls [87]. In murine models, H5N1 influenza virus induced microglial activation followed by αSyn aggregation and significant loss of SNc dopaminergic neurons [88]. The 1918 influenza A pandemic was followed by an outbreak of encephalitic lethargica, parkinsonian type, which is characterized by resting tremor, bradykinesia, and catatonia. Amantadine and memantine, NMDA receptor antagonists and antiviral agents, have long been used to treat the tremor of Parkinson's disease. In an observational study, five Parkinson's disease patients taking adamantanes who tested positive for SARS-CoV-2 had asymptomatic courses and no change in neurologic symptoms, suggesting a possible protective effect of adamantanes against COVID-19 [28]. The regular use of NSAIDs was also found to be protective against developing Parkinson's disease [89]. Inflammatory mediators associated with onset and progression of Parkinson's disease include IL-1, IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, transforming growth factor-α, IL-6, MMP-3, IL-17, and IL-18, many of which are elevate in COVID-19 [90]. High concentrations of IL-1β and TNF-α have been shown to be toxic to the SNc and therapies targeting TNF-α have been proposed to treat Parkinson's disease [91].
Many have highlighted the hyposomia link between Parkinson's disease and COVID-19 [92▪▪]. Anosmia or hyposmia is characteristic of early Parkinson's disease and a prevalent symptom in COVID-19 [86,93]. It is hypothesized that SARS-CoV-2 can enter the central nervous sysetm via the olfactory nerve [94▪▪]. An MRI study of a patient with anosmia in the setting of COVID-19 showed a subtle hyperintensity of the olfactory bulb when symptomatic [95]. One month later the hyperintensity resolved and the olfactory bulbs were notably smaller, providing evidence for resolved edema and possible viral invasion. Another study noted hyperintensity of the olfactory bulb on MRI in five patients with COVID-19. Only three of the patients had anosmia, which suggests neuroinflammation or neuroinvasion may be present without hyposmia. It is currently unknown whether inflammation and/or damage to the basal ganglia associated with COVID-19 predisposes patients to develop Parkinson's disease.
Multiple sclerosis
There is a strong correlation between viral infections and new onset MS as well as MS flares [96,97]. HHVs, including EBV and HHV-6, have been shown to increase the risk of developing MS in vulnerable individuals [98]. Human coronavirus, HCoV-OC43 specifically, has been found in the cerebrospinal fluid and brains of MS patients at significantly higher rates than controls [99,100]. The pathogenesis of MS is still unknown, but the heterogeneity seen both clinically and pathologically alludes to multiple possible mechanisms of immune dysfunction. The ‘bystander’ theory hypothesizes the secretion of proinflammatory cytokines and upregulation of inflammation during a viral infection may be neurotoxic and inadvertently lead to demyelination [99]. Coronaviruses have been shown to stimulate astrocytes and microglia to produce inflammatory mediators, including IL-6 and TNF-α [101]. The molecular mimicry theory hypothesizes viruses share epitopes which cross react with myelin stimulating autoreactive T-cell and/or B-cell production and ultimately leading to demyelination [102]. Autoreactive T cells in MS patient have been shown to cross-react with both HCoV-OC43 antigen and myelin basic protein validating this theory [103]. In addition, antibodies in MS patients have been shown to crosses react with EBV antigen and myelin basic protein and to reliably trigger experimental autoimmune encephalitis in murine models [104]. The presence of persistent virus or viral proteins may continue to stimulate the immune system and perpetuate chronic demyelination.
Several cases of demyelinating disease have been reported in connection with COVID-19. To date, it is unclear whether COVID-19 can predispose patients to relapsing-remitting and progressive MS or whether it only triggers monophasic demyelinating events such as postviral transverse myelitis and acute demyelinating encephalomyelitis. Further studies elucidating the pathophysiology of COVID-19 and central nervous system demyelination will be critical in preventing neurologic damage in susceptible individuals.
STRENGTHS AND LIMITATIONS
The current review is based on a comprehensive and systematic review of the literature available to date. The review is limited by the number of cases reported, the lack of systematic studies to date, and the short-term follow-up of individuals with acute COVID-19 given the recent onset of the pandemic. Longitudinal systematic studies and disease registries to exam the long-term neurologic effects of acute COVID-19 are warranted to fully understand the implications of COVID-19 for the development and progression of neurodegenerative diseases.
CONCLUSION
The findings of this systematic review suggest that patients with underlying dementia who contract COVID-19 are at high risk for hospitalization and death. Additional precautions and protective measures should be put in place to prevent infections and optimize aggressive COVID-19 care for this vulnerable population. In addition, it will be important to address modifiable comorbidities in these patients to reduce the risk of severe disease. Lastly, viral infections and the related inflammation have been associated with the pathogenesis of Alzheimer's disease, Parkinson's disease, and MS, suggesting potential links between COVID-19 and the risk of developing NDD in susceptible individuals. Further research and longitudinal studies are needed to determine if COVID-19 can trigger Alzheimer's disease, Parkinson's disease, or MS in the postinfectious period.
Acknowledgements
None.
Financial support and sponsorship
The researchers did not receive any specific grant funding from any funding agencies for this article.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪▪.Chen T, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci 2020; 75:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]; A retrospective study of 203 patients that demonstrates patients over 65 have more comorbidities and are at higher risk for severe disease and death.
- 2.Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism 2020; 108:154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Valle DM, Kim-Schulze S, Huang H-H, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 2020; 26:1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol 2020; 11:1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6▪▪.Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020; 20:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent review that details the pathophysiology of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immune response and how a dysfunctional immune response contributes to disease progression and tissue damage.
- 7.COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, Korea Centers for Disease Control and Prevention. Coronavirus disease-19: the first 7,755 cases in the Re∗∗∗public of Korea. Osong Public Health Res Perspect 2020; 11:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet 2020; 395:1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement 2019; 15:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isaia G, Marinello R, Tibaldi V, et al. Atypical presentation of Covid-19 in an older adult with severe Alzheimer disease. Am J Geriatr Psychiatry 2020; 28:790–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianchetti A, Rozzini R, Guerini F, et al. Clinical presentation of COVID19 in dementia patients. J Nutr Health Aging 2020; 24:560–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAlpine LS, Zubair AS, Moeller J, et al. Lessons from a neurology consult service for patients with COVID-19. Lancet 2020; 19:806–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang J-M, Kim J-H, Park J-S, et al. Neurological diseases as mortality predictive factors for patients with COVID-19: a retrospective cohort study. Neurol Sci 2020; 41:2317–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyashita S, Yamada T, Mikami T, et al. Impact of dementia on clinical outcomes in elderly patients with coronavirus 2019 (COVID-19): an experience in New York. Geriatr Gerontol Int 2020; 20:732–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Covino M, De Matteis G, Santoro M, et al. Clinical characteristics and prognostic factors in COVID-19 patients aged ≥80 years. Geriatr Gerontol Int 2020; 20:704–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang TS, Ding Y, Freund MK, et al. Prior diagnoses and medications as risk factors for COVID-19 in a Los Angeles Health System. medRxiv 2020; doi: 10.1101/2020.07.03.20145581. PMCID: PMCID: PMC7340203. [Google Scholar]
- 17.Giorgi Rossi P, Marino M, Formisano D, et al. Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLoS One 2020; 15:e0238281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan Y, Wu J, Ni L, et al. Prognosis analysis of patients with mental disorders with COVID-19: a single-center retrospective study. Aging 2020; 12:11238–11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyes-Bueno JA, Mena-Vázquez N, Ojea-Ortega T, et al. Case fatality of COVID-19 in patients with neurodegenerative dementia. Neurologia 2020; 35:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esme M, Koca M, Dikmeer A, et al. Older adults with coronavirus disease 2019; a nationwide study in Turkey. J Gerontol A Biol Sci Med Sci 2020; glaa219. doi: 10.1093/gerona/glaa219. PMCID: PMCID: PMC7499528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison SL, Fazio-Eynullayeva E, Lane DA, et al. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med 2020; 17:e1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boutoleau-Bretonnière C, Pouclet-Courtemanche H, Gillet A, et al. The effects of confinement on neuropsychiatric symptoms in Alzheimer's disease during the COVID-19 crisis. J Alzheimers Dis 2020; 76:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 2011; 32:1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lane CA, Hardy J, Schott JM. Alzheimer's disease. Eur J Neurol 2018; 25:59–70. [DOI] [PubMed] [Google Scholar]
- 25.MedlinePlus [Internet]. Bethesda (MD): National Library of Medicine (US); Parkinson's disease [updated 2020 August 17]. Available from https://medlineplus.gov/genetics/condition/parkinson-disease/#causes [Accessed 17 September 2020]
- 26.Wichmann D, Sperhake J-P, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 2020; 173:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deigendesch N, Sironi L, Kutza M, et al. Correlates of critical illness-related encephalopathy predominate postmortem COVID-19 neuropathology. Acta Neuropathol 2020; 140:583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rejdak K, Grieb P. Adamantanes might be protective from COVID-19 in patients with neurological diseases: multiple sclerosis, parkinsonism and cognitive impairment. Mult Scler Relat Disord 2020; 42:102163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hainque E, Grabli D. Rapid worsening in Parkinson's disease may hide COVID-19 infection. Parkinsonism Relat Disord 2020; 75:126–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Long X, Zhu C, et al. Management of a Parkinson's disease patient with severe COVID-19 pneumonia. Ther Adv Chronic Dis 2020; 11:2040622320949423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31▪.Antonini A, Leta V, Teo J, Chaudhuri KR. Outcome of Parkinson's disease patients affected by COVID-19. Mov Disord 2020; 35:905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]; A case series reporting 10 Parkinson's patients with COVID-19 and highlight the need for increased dopaminergic therapy, worsening nonmotor symptoms, and high mortality rate.
- 32.Cilia R, Bonvegna S, Straccia G, et al. Effects of COVID-19 on Parkinson's disease clinical features: a community-based case–control study. Mov Disord 2020; 35:1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fasano A, Cereda E, Barichella M, et al. COVID-19 in Parkinson's disease patients living in Lombardy, Italy. Mov Disord 2020; 35:1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34▪.Méndez-Guerrero A, Laespada-García MI, Gómez-Grande A, et al. Acute hypokinetic-rigid syndrome following SARS-CoV-2 infection. Neurology 2020; 95:e2109–e2118. [DOI] [PubMed] [Google Scholar]; The first case report of a patient with an asymmetric hypokinetic-rigid syndrome and decreased dopamine uptake in bilateral putamina on dopamine transport single photon emission computed tomography after COVID-19.
- 35.Suwijn SR, van Boheemen CJ, de Haan RJ, et al. The diagnostic accuracy of dopamine transporter SPECT imaging to detect nigrostriatal cell loss in patients with Parkinson's disease or clinically uncertain parkinsonism: a systematic review. EJNMMI Res 2015; 5:12–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology 2019; 92:e1029–e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barzegar M, Mirmosayyeb O, Nehzat N, et al. COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Neurol Neuroimmunol Neuroinflamm 2020; 7:e753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parrotta E, Kister I, Charvet L, et al. COVID-19 outcomes in MS: observational study of early experience from NYU Multiple Sclerosis Comprehensive Care Center. Neurol Neuroimmunol Neuroinflamm 2020; 7:e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39▪▪.Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 2020; 77:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]; A cohort study of 347 multiple sclerosis patients found disease modifying therapies were not associated with severe COVID-19 disease, but patients with advanced age, obesity, and neurological disability were at higher risk for developing severe disease.
- 40.Novi G, Mikulska M, Briano F, et al. COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult Scler Relat Disord 2020; 42:102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thornton JR, Harel A. Negative SARS-CoV-2 antibody testing following COVID-19 infection in two MS patients treated with ocrelizumab. Mult Scler Relat Disord 2020; 44:102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montero-Escribano P, Matías-Guiu J, Gómez-Iglesias P, et al. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: a case series of 60 patients from Madrid, Spain. Mult Scler Relat Disord 2020; 42:102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Safavi F, Nourbakhsh B, Azimi AR. B-cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Mult Scler Relat Disord 2020; 43:102195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iannetta M, Cesta N, Stingone C, et al. Mild clinical manifestations of SARS-CoV-2 related pneumonia in two patients with multiple sclerosis under treatment with ocrelizumab. Mult Scler Relat Disord 2020; 45:102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suwanwongse K, Shabarek N. Benign course of COVID-19 in a multiple sclerosis patient treated with Ocrelizumab. Mult Scler Relat Disord 2020; 42:102201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devogelaere J, D’Hooghe MB, Vanderhauwaert F, D’Haeseleer M. Coronavirus disease 2019: favorable outcome in an immunosuppressed patient with multiple sclerosis. Neurol Sci 2020; 41:1981–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wurm H, Attfield K, Iversen AK, et al. Recovery from COVID-19 in a B-cell-depleted multiple sclerosis patient. Mult Scler 2020; 26:1261–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghajarzadeh M, Mirmosayyeb O, Barzegar M, et al. Favorable outcome after COVID-19 infection in a multiple sclerosis patient initiated on ocrelizumab during the pandemic. Mult Scler Relat Disord 2020; 43:102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Creed MA, Ballesteros E, John Greenfield L, Jr, Imitola J. Mild COVID-19 infection despite chronic B cell depletion in a patient with aquaporin-4-positive neuromyelitis optica spectrum disorder. Mult Scler Relat Disord 2020; 44:102199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meca-Lallana V, Aguirre C, Beatrizdel R, et al. COVID-19 in 7 multiple sclerosis patients in treatment with ANTI-CD20 therapies. Mult Scler Relat Disord 2020; 44:102306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conte WL. Attenuation of antibody response to SARS-CoV-2 in a patient on ocrelizumab with hypogammaglobulinemia. Mult Scler Relat Disord 2020; 44:102315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiorella C, Lorna G. COVID-19 in a multiple sclerosis (MS) patient treated with alemtuzumab: insight to the immune response after COVID. Mult Scler Relat Disord 2020; 46:102447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lucchini M, Bianco A, Del Giacomo P, et al. Is serological response to SARS-CoV-2 preserved in MS patients on ocrelizumab treatment? A case report. Mult Scler Relat Disord 2020; 44:102323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aguirre C, Meca-Lallana V, Barrios-Blandino A, et al. Covid-19 in a patient with multiple sclerosis treated with natalizumab: may the blockade of integrins have a protective role? Mult Scler Relat Disord 2020; 44:102250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borriello G, Ianniello A. COVID-19 occurring during Natalizumab treatment: a case report in a patient with extended interval dosing approach. Mult Scler Relat Disord 2020; 41:102165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crescenzo F, Marastoni D, Bovo C, Calabrese M. Frequency and severity of COVID-19 in multiple sclerosis: a short single-site report from northern Italy. Mult Scler Relat Disord 2020; 44:102372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiarini M, Paghera S, Moratto D, et al. Immunologic characterization of a immunosuppressed multiple sclerosis patient that recovered from SARS-CoV-2 infection. J Neuroimmunol 2020; 345:577282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valencia-Sanchez C, Wingerchuk DM. A fine balance: immunosuppression and immunotherapy in a patient with multiple sclerosis and COVID-19. Mult Scler Relat Disord 2020; 42:102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mallucci G, Zito A, Fabbro BD, Bergamaschi R. Asymptomatic SARS-CoV-2 infection in two patients with multiple sclerosis treated with fingolimod. Mult Scler Relat Disord 2020; 45:102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dalla Costa G, Leocani L, Montalban X, et al. Real-time assessment of COVID-19 prevalence among multiple sclerosis patients: a multicenter European study. Neurol Sci 2020; 41:1647–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernández-Díaz E, Gracia-Gil J, García-García JG, et al. COVID-19 and multiple sclerosis: a description of two cases on alemtuzumab. Mult Scler Relat Disord 2020; 45:102402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Angelis M, Petracca M, Lanzillo R, et al. Mild or no COVID-19 symptoms in cladribine-treated multiple sclerosis: two cases and implications for clinical practice. Mult Scler Relat Disord 2020; 45:102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63▪.Palao M, Fernández-Díaz E, Gracia-Gil J, et al. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord 2020; 45:102377. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first case report of a patient who developed optic neuritis post-COVID-19 demonstrating the potential for SARS-CoV-2 to trigger demyelination.
- 64.Parsons T, Banks S, Bae C, et al. COVID-19-associated acute disseminated encephalomyelitis (ADEM). J Neurol 2020; 267:2799–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zanin L, Saraceno G, Panciani PP, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien) 2020; 162:1491–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zoghi A, Ramezani M, Roozbeh M, et al. A case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult Scler Relat Disord 2020; 44:102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67▪▪.Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 2019; 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]; A timely review of the neuroinvasive potential of coronaviruses as well as the neurological diseases that may be triggered by immune-mediated tissue damage and autoimmunity.
- 68.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010; 304:1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holmes C, Cunningham C, Zotova E, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology 2009; 73:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lindlau A, Widmann CN, Putensen C, et al. Predictors of hippocampal atrophy in critically ill patients. Eur J Neurol 2015; 22:410–415. [DOI] [PubMed] [Google Scholar]
- 71.Benameur K, Agarwal A, Auld SC, et al. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg Infect Dis 2020; 26:2016–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farhadian S, Glick LR, Vogels CBF, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol 2020; 20:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bourgade K, Le Page A, Bocti C, et al. Protective effect of amyloid-β peptides against herpes simplex virus-1 infection in a neuronal cell culture model. J Alzheimers Dis 2016; 50:1227–1241. [DOI] [PubMed] [Google Scholar]
- 74.Majde JA. Neuroinflammation resulting from covert brain invasion by common viruses – a potential role in local and global neurodegeneration. Med Hypotheses 2010; 75:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol 2014; 14:463–477. [DOI] [PubMed] [Google Scholar]
- 76.Abate G, Memo M, Uberti D. Impact of COVID-19 on Alzheimer's disease risk: viewpoint for research action. Healthcare (Basel) 2020; 8:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Readhead B, Haure-Mirande J-V, Funk CC, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron 2018; 99:64–82.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heneka MT, Golenbock D, Latz E, et al. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res Ther 2020; 12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duan Y, Kelley N, He Y. Role of the NLRP3 inflammasome in neurodegenerative diseases and therapeutic implications. Neural Regen Res 2020; 15:1249–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lara PC, Macías-Verde D, Burgos-Burgos J. Age-induced NLRP3 inflammasome over-activation increases lethality of SARS-CoV-2 pneumonia in elderly patients. Aging Dis 2020; 11:756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moolamalla STR, Chauhan R, Deva Priyakumar U, Vinod PK. Host metabolic reprogramming in response to SARS-Cov-2 infection. bioRxiv 2020; 10.1101/2020.08.02.232645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol 2011; 10:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jang H, Boltz DA, Webster RG, Smeyne RJ. Viral parkinsonism. Biochim Biophys Acta 2009; 1792:714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 2007; 33:599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lippi A, Domingues R, Setz C, et al. SARS-CoV-2: at the crossroad between aging and neurodegeneration. Mov Disord 2020; 35:716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tulisiak CT, Mercado G, Peelaerts W, et al. Can infections trigger alpha-synucleinopathies? Prog Mol Biol Transl Sci 2019; 168:299–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fazzini E, Fleming J, Fahn S. Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson's disease. Mov Disord 1992; 7:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jang H, Boltz D, Sturm-Ramirez K, et al. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci U S A 2009; 106:14063–14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gagne JJ, Power MC. Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology 2010; 74:995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chao Y, Wong SC, Tan EK. Evidence of inflammatory system involvement in Parkinson's disease. Biomed Res Int 2014; 2014:308654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leal MC, Casabona JC, Puntel M, Pitossi FJ. Interleukin-1β and tumor necrosis factor-α: reliable targets for protective therapies in Parkinson's disease? Front Cell Neurosci 2013; 7:53–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92▪▪.Sulzer D, Antonini A, Leta V, et al. COVID-19 and possible links with Parkinson's disease and parkinsonism: from bench to bedside. NPJ Parkinsons Dis 2020; 6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent review that discusses the potential for SARS-CoV-2 to contribute to the pathogenesis of Parkinson's disease in susceptible patients, from a basic science and clinical perspective.
- 93.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020; 277:2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94▪▪.Zubair AS, McAlpine LS, Gardin T, et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease. A Review. JAMA Neurol 2019; 77:1018–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review that outlines the historical impact of coronaviruses on the nervous system, reviews the potential routes of entry of SARS-CoV-2 into the central nervous system, and summarizes the clinical neurological complications that have been reported.
- 95.Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol 2020; 77:1028–1029. [DOI] [PubMed] [Google Scholar]
- 96.Panitch HS. Influence of infection on exacerbations of multiple sclerosis. Ann Neurol 1994; 36: Suppl: S25–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andersen O, Lygner PE, Bergström T, et al. Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological study. J Neurol 1993; 240:417–422. [DOI] [PubMed] [Google Scholar]
- 98.Handel AE, Williamson AJ, Disanto G, et al. An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS One 2010; 5:e12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol 2000; 74:8913–8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cristallo A, Gambaro F, Biamonti G, et al. Human coronavirus polyadenylated RNA sequences in cerebrospinal fluid from multiple sclerosis patients. New Microbiol 1997; 20:105–114. [PubMed] [Google Scholar]
- 101.Li Y, Fu L, Gonzales DM, Lavi E. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J Virol 2004; 78:3398–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang J, Markovic-Plese S, Lacet B, et al. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med 1994; 179:973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boucher A, Mercier G, Duquette P, Talbot PJ. Clonal T-cell cross-reactivity between myelin antigens MBP and PLP and human respiratory coronaviruses in multiple sclerosis. J Neuroimmunol 1998; 90:33–133. [Google Scholar]
- 104.Jog NR, McClain MT, Heinlen LD, et al. Epstein Barr virus nuclear antigen 1 (EBNA-1) peptides recognized by adult multiple sclerosis patient sera induce neurologic symptoms in a murine model. J Autoimmun 2020; 106:102332. [DOI] [PMC free article] [PubMed] [Google Scholar]


