Abstract
Objective:
The aim of this study was to evaluate Scotland's national HIV preexposure prophylaxis (PrEP) programme in relation to PrEP uptake and associated population-level impact on HIV incidence among MSM.
Design:
A retrospective cohort study within real-world implementation.
Methods:
Comparison of HIV diagnoses from national surveillance data and HIV incidence within a retrospective cohort of HIV-negative MSM attending sexual health clinics from the National Sexual Health information system between the 2-year periods pre(July 2015–June 2017) and post(July 2017–June 2019) introduction of PrEP.
Results:
Of 16 723 MSM attending sexual health services in the PrEP period, 3256 (19.5%) were prescribed PrEP. Between pre-PrEP and PrEP periods, new HIV diagnoses among MSM declined from 229 to 184, respectively [relative risk reduction (RRR): 19.7%, 95% confidence interval (95% CI) 2.5–33.8]; diagnosed recently acquired infections declined from an estimated 73 to 47, respectively (35.6%, 95% CI 7.1–55.4). Among MSM attending sexual health clinics, HIV incidence per 1000 person-years declined from 5.13 (95% CI 3.90–6.64) pre-PrEP to 3.25 (95% CI 2.30–4.47) in the PrEP period (adjusted IRR 0.57, 95% CI 0.37–0.87). Compared with the pre-PrEP period, incidence of HIV was lower in the PrEP period for those prescribed PrEP (aIRR 0.25, 95% CI 0.09–0.70) and for those not prescribed PrEP (aIRR 0.68, 95% CI 0.43–1.05).
Conclusion:
We demonstrate national population-level impact of PrEP for the first time in a real-world setting. HIV incidence reduced in MSM who had been prescribed PrEP and, to a lesser extent, in those who had not. Promotion of the benefits of PrEP needs to extend to MSM who do not access sexual health clinics.
Keywords: epidemiology, HIV preexposure prophylaxis, incidence, MSM, prevention, public health
Introduction
The effectiveness of oral tenofovir disoproxil/emtricitabine (tenofovir-emtricitabine) as HIV preexposure prophylaxis (PrEP) in MSM, a group at high risk of infection, has been established in clinical trials [1–3]. Reduction in HIV incidence of 44–97% [1,4] has been reported with both daily and intermittent dosing regimens. A single large prospective Australian cohort study of rapidly implemented, high coverage daily PrEP [5] reported a 25.1% relative risk reduction (RRR) in population-wide HIV diagnoses in MSM over the time period 12 months before to 12 months after PrEP implementation. The reduction was greater for HIV diagnoses involving recent infections, which decreased by almost one-third.
The majority of HIV infections in Scotland are in MSM, in whom 47% of all new diagnoses and 60% of recently acquired infections are reported [6]. In July 2017, Scotland became one of the first countries to implement a national HIV PrEP programme for those at greatest risk of HIV [7]. The service is provided nationwide, almost exclusively within sexual health clinics. Clinics provide daily and event-based oral tenofovir-emtricitabine, adherence support and associated monitoring [for toxicities and sexually transmitted infection (STI) and blood-borne virus screening] and STI prevention services [8,9]. Medication and associated care is free to Scottish residents aged 16 years and older, who meet the following risk-based eligibility criteria: being a sex partner of someone who is HIV-positive with a detectable viral load; having a documented bacterial rectal STI in the last 12 months; reporting condomless penetrative anal sex with two or more partners in the last 12 months, ‘equivalent high risk of HIV acquisition’ [8]. PrEP has been widely promoted by community-based organizations and within sexual health services. Almost all people who have received PrEP are MSM [10].
Unlike other parts of the UK, there has been an ongoing outbreak of HIV in people who inject drugs (PWID) in Scotland since 2015. Prevalence of HIV among PWID in Glasgow city centre increased 10-fold from 1% pre-outbreak to 11% in 2017–2018 [11]. However, given that oral tenofovir-emtricitabine is only licensed for prevention of sexual HIV transmission in Scotland [12], PrEP provision is limited to PWID with additional high risk of HIV acquisition by sexual routes.
We examined national surveillance data on HIV diagnoses and recent infections among MSM before and after introduction of the PrEP programme. Time trends in new diagnoses may not necessarily reflect population trends in HIV incidence if the uptake of testing among those with a recent infection is suboptimal or indeed varies over time. Therefore, we examined uptake of PrEP and HIV incidence (comparing the 2-year periods pre and post introduction of PrEP) within a national cohort of all MSM attending sexual health clinics in Scotland.
Materials and methods
Study design and data sources
In this retrospective cohort study, we analysed national surveillance data held at Public Health Scotland (PHS) for a 4-year study period (July 2015–June 2019), which encompassed the 2 years pre and 2 years post introduction of the National HIV PrEP programme. We examined the characteristics of MSM attending sexual health clinics and their uptake of PrEP, new HIV diagnoses and associated recent infections among MSM across Scotland (involving those diagnosed within sexual health as well as other settings), and HIV incidence among MSM attending sexual health clinics.
We obtained data on MSM attending sexual health clinics from NaSH, the electronic patient record system used in almost all specialist sexual health settings in Scotland [13]. Clinics, providing access to sexual healthcare for over 98% of the Scottish population, have been submitting data to NaSH since 2011. This includes data on HIV and STI testing from sexual health clinics but does not cover testing in other settings. MSM are defined as assigned male sex at birth and ever having at least one male sexual partner. Using NaSH, we retrospectively identified a cohort of MSM (aged 15 years and over) who had attended a sexual health clinic in Scotland between July 2015 and June 2019. Individuals with a positive HIV test prior to July 2015 were excluded. Data on attendances, HIV and STI test results and PrEP prescriptions for this cohort were extracted from NaSH.
We also used the Scottish HIV diagnosis database to examine data on diagnoses among MSM reported to PHS by NHS laboratories across Scotland [14]. Diagnoses recorded include first ever diagnoses (here referred to as new diagnoses) and diagnoses previously recorded elsewhere but newly reported in Scotland.
Outcomes
Uptake of preexposure prophylaxis
Uptake was defined as at least one prescription for PrEP (daily or event-based) among MSM attending sexual health clinics from July 2017 to June 2019. Individuals who tested positive for HIV within 1 week of their first PrEP prescription were excluded from analysis, as they had acquired infection prior to commencing PrEP.
HIV diagnoses and recent infections
An HIV diagnosis was based on repeat reactive results on more than one laboratory-based fourth generation antibody/antigen assay with further confirmation by western blot.
Among diagnoses, the number recently infected (estimated in the 3–4 months prior to diagnosis) was determined through HIV antibody avidity testing, available in Scotland since April 2014 [15]. To estimate the total number of recent infections, we assumed that the avidity status of diagnoses with missing data (due to insufficient sample for retesting) followed an equivalent pattern to those with an avidity result.
Estimated incidence of HIV
HIV incidence was estimated using the person-years approach for the subcohort of MSM attending sexual health clinics in Scotland during the study period who had at least two HIV tests recorded on NaSH. Participants were included in the follow-up cohort if they had an HIV negative test up to 2 years prior to their first attendance in the study period and had subsequently tested during the study period. An incident HIV infection was defined as the date of a patient's first positive HIV antibody test on NaSH and recorded within the study period. Person-years were calculated from the start of the study period (1 July 2015) if they had tested negative previously (i.e. relating to the two-year period prior to their attendance in the study period) or from the first HIV-negative test within the study period up to either their last negative or their first positive HIV test in the study period. We also undertook a sensitivity analysis which assumed HIV infection occurred midway between an individual's last negative and first positive test. With the midpoint approach, we used additional HIV testing data up to March 2020 to capture diagnoses occurring beyond July 2019 with assumed time of infection within our study period.
Statistical analysis
Characteristics of MSM attending sexual health clinics and their uptake of preexposure prophylaxis
MSM attending sexual health clinics in the pre-PrEP and PrEP periods were compared according to the following characteristics: sex, age, ethnicity, NHS board of residence (or clinic, where missing), residence in the most deprived quintile (according to the Scottish Index of Multiple Deprivation [16]), history of injecting drugs and risk category. We characterized the latter hierarchically in the year prior to and including first attendance at clinic in the study period, based on available data in NaSH, in the following order of risk of infection: prescribed HIV postexposure prophylaxis (PEP) and/or diagnosed with rectal STIs (gonorrhoea and/or chlamydia); diagnosed with a nonrectal STI; tested for HIV and/or STIs; and not tested for HIV or STIs. Odds ratios (ORs) and adjusted odds ratios (aORs) were obtained using logistic regression to identify characteristics associated with uptake of PrEP.
Nationwide trends in surveillance data on HIV diagnosis and recent infections
We compared the number of HIV diagnoses and estimated number of diagnosed recent infections between the pre-PrEP and PrEP periods. We also considered the 2-year period between July 2013 and June 2015 to assess any change occurring during an extended period prior to introduction of PrEP. RRRs of the percentage change in the number of diagnoses between periods were calculated using a denominator of 53 000 MSM (approximately 2% of the Scottish male population [17]) for all periods and 95% confidence intervals (95% CIs) derived assuming a Poisson distribution.
HIV incidence among MSM attending sexual health clinics
We calculated incidence of HIV as the rate per 1000 person-years, with 95% CIs derived assuming a Poisson distribution. We estimated incidence rate ratios (IRRs) and adjusted incidence rate ratios (aIRRs) using the person-years method [18,19], using Poisson regression. A time-dependent variable was used to compare incidence pre and post introduction of PrEP, with person-years and incident cases attributed to the respective periods. The PrEP period was further divided into time accumulated with and without exposure to PrEP, where exposure was defined from the time of first PrEP prescription up to date of censoring.
For this analysis, we adjusted for the characteristics mentioned previously, but risk category was collapsed into those with a high-risk behaviour factor – relating to and above – versus those without. Risk category was included as a time-dependent variable wherein individuals could transition from no high-risk behaviour in the pre-PrEP period to high-risk behaviour in the PrEP period if they were diagnosed with an STI or were prescribed PEP. We also undertook stratified analysis for MSM with and without high-risk behaviour.
Role of the funding source
This study was funded by Health Protection Scotland (HPS). HPS had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Ethical approval
We conducted secondary analysis of anonymized administrative healthcare data for which pan-Scotland Caldicott approval is in place [13]. Formal ethical approval was not required.
Results
Characteristics of MSM attending sexual health clinics and their uptake of preexposure prophylaxis
The number of MSM attending clinics increased by 17% from 14 319 pre-PrEP to 16 723 in the PrEP period (Table 1). Small differences (<5%) in the characteristics of MSM between periods were observed. The median age at first attendance was 28 (IQR 22–37) years and a similar proportion were new to sexual health services (39–44%), of non-White ethnicity (5%), resident in the most deprived quintile (19–20%), attended clinics in Scotland's two largest cities (51–53%) and had a rectal STI or were prescribed PEP in the previous 12 months (12–13%).
Table 1.
Characteristics and uptake of HIV PrEP among 23 062 MSM attending sexual health clinics in Scotland between July 2015 and June 2019.
| Number of MSM attended at least oncea | Uptake of PrEPAmong MSM attending in the PrEP period | |||||
| Pre-PrEP(July 2015 to June 2017)N1 (col %) | PrEP period(July 2017 to June 2019)N2 (col %) | Pc | N3 (% of N2) | OR (95% CI) | aOR (95% CI) | |
| Total MSM | 14 319 | 16 723 | 3256 (19.5%) | |||
| Attendance history | ||||||
| Last attended within the last 2 years | 5404 (37.7%) | 6804 (40.7%) | <0.001 | 1846 (27.1%) | 1.00 | 1.00 |
| Last attended more than 2 years ago | 2670 (18.6%) | 3329 (19.9%) | 0.005 | 541 (16.3%) | 0.52 (0.47–0.58) | 0.53 (0.47–0.59) |
| Never attended in the history of NaSH | 6245 (43.6%) | 6590 (39.4%) | <0.001 | 869 (13.2%) | 0.41 (0.37–0.45) | 0.51 (0.47–0.57) |
| Age at first attendance (years) | ||||||
| Median (IQR) | 28 (22–37) | 28 (22–37) | 31 (25–41) | |||
| 15--24 | 4556 (31.8%) | 4798 (28.7%) | <0.001 | 766 (16.0%) | 1.00 | 1.00 |
| 25--39 | 6074 (42.4%) | 7571 (45.3%) | <0.001 | 1607 (21.2%) | 1.42 (1.29–1.56) | 1.32 (1.20–1.46) |
| 40--49 | 1923 (13.4%) | 2124 (12.7%) | 0.060 | 488 (23.0%) | 1.57 (1.38–1.78) | 1.54 (1.34–1.75) |
| 50+ | 1766 (12.3%) | 2230 (13.3%) | 0.009 | 395 (17.7%) | 1.13 (0.99–1.29) | 1.15 (1.00–1.32) |
| Ethnicity | ||||||
| White | 11 426 (79.8%) | 12 175 (72.8%) | <0.001 | 2577 (21.2%) | 1.00 | 1.00 |
| Non-White | 677 (4.7%) | 745 (4.5%) | 0.263 | 177 (23.8%) | 1.16 (0.98–1.38) | 1.20 (1.00–1.43) |
| Not known | 2216 (15.5%) | 3803 (22.7%) | <0.001 | 502 (13.2%) | 0.57 (0.51–0.63) | 0.67 (0.60–0.75) |
| NHS Board of Residence | ||||||
| Greater Glasgow and Clyde | 4576 (32.0%) | 5242 (31.3%) | 0.253 | 1191 (22.7%) | 1.00 | 1.00 |
| Lothian | 4063 (28.4%) | 4607 (27.5%) | 0.109 | 806 (17.5%) | 0.72 (0.65–0.80) | 0.68 (0.61–0.76) |
| Rest of Scotland | 5680 (39.7%) | 6874 (41.1%) | 0.010 | 1259 (18.3%) | 0.76 (0.70–0.83) | 0.77 (0.70–0.85) |
| Deprivation quintile of residence | ||||||
| 1 (Most deprived) | 2850 (19.9%) | 3140 (18.8%) | 0.013 | 661 (21.1%) | 1.00 | 1.00 |
| 2 | 2708 (18.9%) | 2987 (17.9%) | 0.018 | 615 (20.6%) | 0.97 (0.86–1.10) | 1.04 (0.91–1.18) |
| 3 | 2424 (16.9%) | 2748 (16.4%) | 0.248 | 586 (21.3%) | 1.02 (0.90–1.15) | 1.11 (0.97–1.26) |
| 4 | 2310 (16.1%) | 2601 (15.6%) | 0.168 | 480 (18.5%) | 0.85 (0.74–0.97) | 0.94 (0.82–1.08) |
| 5 | 2603 (18.2%) | 2888 (17.3%) | 0.038 | 500 (17.3%) | 0.79 (0.69–0.89) | 0.89 (0.78–1.02) |
| Not known | 1424 (9.9%) | 2359 (14.1%) | <0.001 | 414 (17.5%) | 0.80 (0.70–0.91) | 0.97 (0.85–1.12) |
| Ever injected drugs | ||||||
| No | 13 047 (91.1%) | 14 968 (89.5%) | <0.001 | 2946 (19.7%) | 1.00 | 1.00 |
| Yes | 277 (1.9%) | 293 (1.8%) | 0.250 | 103 (35.2%) | 2.21 (1.73–2.82) | 1.96 (1.52–2.53) |
| Not Known | 995 (6.9%) | 1462 (8.7%) | <0.001 | 207 (14.2%) | 0.67 (0.58–0.78) | 0.87 (0.74–1.02) |
| Testing and STI history in the last 12 monthsb | ||||||
| Prescribed HIV PEP and/or had a rectal STI | 1693 (11.8%) | 2087 (12.5%) | 0.081 | 746 (35.7%) | 5.23 (4.30–6.37) | 4.82 (3.94–5.89) |
| Had an STI (nonrectal) | 977 (6.8%) | 1081 (6.5%) | 0.213 | 246 (22.8%) | 2.77 (2.21–3.47) | 2.63 (2.09–3.31) |
| Had an STI or HIV test | 10 291 (71.9%) | 12 119 (72.5%) | 0.245 | 2126 (17.5%) | 2.00 (1.67–2.40) | 2.02 (1.68–2.43) |
| Not Tested and No STIs | 1358 (9.5%) | 1436 (8.6%) | 0.006 | 138 (9.6%) | 1.00 | 1.00 |
Unadjusted and adjusted odds ratios were generated from logistic regression models.
This excludes those that tested positive for HIV prior to their first attendance in the pre-PrEP period (n = 226) and PrEP period (n = 332).
Includes the day of first attendance in a period. STI only includes gonorrhoea and chlamydia. Patients have been assigned to only one category based on their highest risk factor.
P values come from a hypothesis test for equal proportions in the pre-PrEP and PrEP periods.
aOR, adjusted odds ratio; CI, confidence interval; IQR, interquartile range; NaSH, National Sexual Health Information System; OR, odds ratio; PEP, postexposure prophylaxis; PrEP, preexposure prophylaxis; STI, sexually transmitted infection.
One-fifth of MSM attending in the PrEP period were prescribed PrEP at least once (3256/16 273, 19.5%) (Table 1 and SDC7 for PrEP regimen). Compared with those who had attended services within the last 2 years, those who last attended over 2 years ago (aOR 0.53, 95% CI 0.47–0.59) and those who had never previously attended services (aOR 0.51, 95% CI 0.47–0.57) were less likely to be prescribed PrEP. MSM aged 25–39, 40–49 and 50+ years were more likely to have received PrEP than those aged 15–24 years (aOR 1.32, 95% CI 1.20–1.46; aOR 1.54, 95% CI 1.34–1.75; aOR 1.15, 95% CI 1.00–1.32, respectively). Those who reported previous and/or current injecting drug use were more likely to be prescribed PrEP (aOR 1.96, 95% CI 1.52–2.53). Compared with MSM who had never been tested for STIs or HIV within the previous 12 months, those more likely to be prescribed PrEP included those who had been tested for STIs and/or HIV (aOR 2.02, 95% CI 1.68–2.43), diagnosed with a nonrectal STI (aOR 2.63, 95% CI 2.09–3.31) and received PEP and/or had a rectal STI (aOR 4.82, 95% CI 3.94–5.81).
Nationwide trends in HIV diagnoses and recent infections
The number of new HIV diagnoses among MSM in both pre-PrEP periods (2013–2015 and 2015–2017) was similar (234 and 229) and reduced by 20% to 184 in the PrEP period (RRR 19.7%, 95% CI 2.5–33.8) (Table 2). The proportion of diagnoses made at sexual health clinics decreased from 61.1 to 50.7% and then down to 41.8% during the three periods. The estimated numbers of diagnoses involving recent infection increased from 61 to 73 between the two pre-PrEP periods but fell by 35% to 47 in the PrEP period (RRR 35.6%, 95% CI 7.1–55.4). The proportion of recent infections diagnosed in a sexual health clinic was similar in the two pre-PrEP periods (68.9 and 66.7%) but declined in the PrEP period to 53.2%.
Table 2.
Nationwide trends in HIV diagnoses and associated recent infections among MSM in Scotland for 24-month time periods before and after preexposure prophylaxis roll out.
| July 2013–June 2015(Early Pre-PrEP) | July 2015–June 2017(Immediately Pre-PrEP) | RRR (95% CI) from previous period | July 2017 to June 2019(PrEP period) | RRR (95% CI) from previous period | |
| Diagnoses, including those previously diagnosed outside Scotland | 342 | 339 | 0.9% (−15.1 to 14.7) | 298 | 12.1% (−2.7 to 24.7%) |
| Diagnosed in a Sexual Health Clinic | 191 | 166 | 13.1% (−7.0 to 29.4) | 142 | 14.5% (−7.0 to 31.6) |
| Previously diagnosed outside Scotland | 108 | 110 | 114 | ||
| New diagnoses, excluding those previously diagnosed outside Scotland | 234 | 229 | 2.1% (−17.4 to 18.4) | 184 | 19.7% (2.5–33.8) |
| Diagnosed in a Sexual Health Clinic | 143 | 116 | 18.9% (−3.6 to 36.5) | 77 | 33.6% (11.5–50.2) |
| Estimated recent infections among new diagnosesa | 61 | 73 | −19.7% (−68.1 to 14.8) | 47 | 35.6% (7.1–55.4) |
| Diagnosed in a Sexual Health Clinic | 42 | 48 | −14.3% (−72.9 to 24.5) | 25 | 47.9% (15.6–67.9) |
The source of these data is the Scottish national HIV diagnosis database.
CI, confidence interval; PrEP, preexposure prophylaxis; RRR, relative risk reduction.
HIV avidity data were missing for 19, 7 and 17% of samples in the early pre-PrEP, immediately pre-PrEP and PrEP periods, respectively; avidity status for those with missing information was estimated according to the avidity status of those with available data within each period.
HIV incidence among MSM attending sexual health clinics
Of 23 062 MSM who attended sexual health clinics in the study period, 12 276 (53.2%) had sufficient HIV testing data for inclusion in the incidence analysis (Tables, Supplemental Digital Content (SDC) S1 & S2). Of 3256 MSM prescribed PrEP, 579 (18%) did not contribute person-time while exposed to PrEP as they had no follow-up HIV test, with 316 (55%) of these first prescribed PrEP within the last 6 months of the study period.
HIV incidence in MSM reduced from 5.13 (95% CI 3.90–6.64) per 1000 person-years pre-PrEP to 3.25 (95% CI 2.30–4.47) in the PrEP period (Table, SDC S3). Adjusting for covariates, risk of HIV infection reduced by 43% in the PrEP period compared with pre-PrEP period (aIRR 0.57, 95% CI 0.37–0.87). Among those who had been prescribed PrEP at least once, the incidence was 1.74 (95% CI 0.58–4.14) per 1000 person-years; none of the four incident cases among those exposed had been taking PrEP at the time of likely HIV acquisition. Compared with the pre-PrEP period, risk of HIV in the PrEP period was reduced by 75% for those prescribed PrEP (aIRR 0.25, 95% CI 0.09–0.70) and 32% for those not prescribed PrEP (aIRR 0.68, 95% CI 0.43–1.05) (Table 3).
Table 3.
HIV incidence per 1000 person-years for the cohort of 12 276 MSM attending sexual health clinics in Scotland during the pre-PrEP and preexposure prophylaxis periods (i.e. from July 2015 to June 2019) and had multiple HIV tests sufficient to enable inclusion in the cohort for HIV incidence analysis.
| Number of MSM | Person-years (PY) | Incident HIV infections | Incidence per 1000 PY(95% CI) | Unadjusted incidence RR(95% CI) | Adjusted incidence RR(95% CI) | |
| Exposure perioda | ||||||
| Pre-PrEP period | 8831 | 10 524 | 54 | 5.13 (3.90–6.64) | 1.00 | 1.00 |
| PrEP period: never prescribed PrEP | 9600 | 8477 | 31 | 3.66 (2.53–5.12) | 0.71 (0.46–1.11) | 0.68 (0.43–1.05) |
| PrEP period: prescribed PrEP at least once | 2677 | 2295 | 4 | 1.74 (0.58–4.14) | 0.34 (0.12–0.94) | 0.25 (0.09–0.70) |
| Age (years) | ||||||
| 15--24 | 4180 | 6923 | 18 | 2.60 (1.60–4.02) | 1.00 | 1.00 |
| 25--39 | 5358 | 9261 | 50 | 5.40 (4.05–7.06) | 2.08 (1.21–3.56) | 2.15 (1.25–3.70) |
| 40+ | 2738 | 5112 | 21 | 4.11 (2.62–6.16) | 1.58 (0.84–2.96) | 1.81 (0.96–3.42) |
| Ethnicity | ||||||
| White | 9793 | 17 997 | 73 | 4.06 (3.20–5.07) | 1.00 | 1.00 |
| Non-White | 600 | 1021 | 6 | 5.88 (2.44–12.11) | 1.45 (0.63–3.33) | 1.30 (0.56–3.00) |
| Not known | 1883 | 2278 | 10 | 4.39 (2.25–7.79) | 1.08 (0.56–2.10) | 1.38 (0.70–2.73) |
| NHS Board of Residence | ||||||
| Greater Glasgow and Clyde | 4127 | 7251 | 28 | 3.86 (2.62–5.50) | 1.00 | 1.00 |
| Lothian | 3247 | 5664 | 32 | 5.65 (3.94–7.87) | 1.46 (0.88–2.43) | 1.56 (0.92–2.65) |
| Rest of Scotland | 4902 | 8381 | 29 | 3.46 (2.37–4.90) | 0.90 (0.53–1.51) | 1.07 (0.62–1.83) |
| Deprivation Quintile of Residence | ||||||
| 1–2 (Most deprived) | 4710 | 8316 | 34 | 4.09 (2.88–5.64) | 1.00 | 1.00 |
| 3–5 | 6244 | 10 709 | 47 | 4.39 (3.26–5.78) | 1.07 (0.69–1.67) | 1.06 (0.67–1.67) |
| Not known | 1322 | 2270 | 8 | 3.52 (1.66–6.65) | 0.86 (0.40–1.86) | 0.89 (0.41–1.92) |
| Ever injected drugs | ||||||
| No | 11 338 | 19 910 | 81 | 4.07 (3.25–5.03) | 1.00 | 1.00 |
| Yes | 224 | 398 | 6 | 15.06 (6.26–31.04) | 3.70 (1.62–8.48) | 3.14 (1.35–7.26) |
| Not known | 714 | 988 | 2 | 2.02 (0.40–6.49) | 0.50 (0.12–2.02) | 0.55 (0.13–2.30) |
| Risk Group | ||||||
| No high-risk behaviour | 9583 | 15 121 | 43 | 2.84 (2.09–3.79) | 1.00 | 1.00 |
| High-risk behaviourb | 3622 | 6175 | 46 | 7.45 (5.52–9.84) | 2.62 (1.73–3.97) | 2.89 (1.89–4.40) |
Incidence rate ratios were generated from Poisson regression models.
CI, confidence interval; PrEP, preexposure prophylaxis; RR, rate ratio.
Individuals can contribute to multiple exposure categories.
High-risk behaviour includes patients that had an STI (gonorrhoea or chlamydia) or were prescribed HIV PEP within 12 months prior to and including their first attendance over the follow-up period. Patients can move from no high-risk behaviour in the pre-PrEP period to high-risk behaviour in the PrEP-era.
Across the study period, risk of HIV infection was raised for those with a history of injecting drugs (aIRR 3.14, 95% CI 1.35–7.26) and those recently diagnosed with an STI or those who had been prescribed PEP (referred to as MSM with high-risk behaviour) (aIRR 2.89, 95% CI 1.89–4.40). However, the largest reduction in HIV incidence over time was observed among MSM with high-risk behaviour: from 10.87 (7.38–15.49) per 1000 person-years pre-PrEP to 2 (0.4–6.41) in the PrEP period (Fig. 1). Among MSM with high-risk behaviour, risk of HIV reduced by 83% for those prescribed PrEP compared with the pre-PrEP period (aIRR 0.17, 95% CI 0.04–0.71) (Table, SDC S4). Findings were unchanged in the sensitivity analysis applying the mid-point method for calculating incidence (Tables, SDC S5 & S6).
Fig. 1.
HIV incidence per 1000 person-years with 95% confidence intervals according to exposure category and defined risk group.
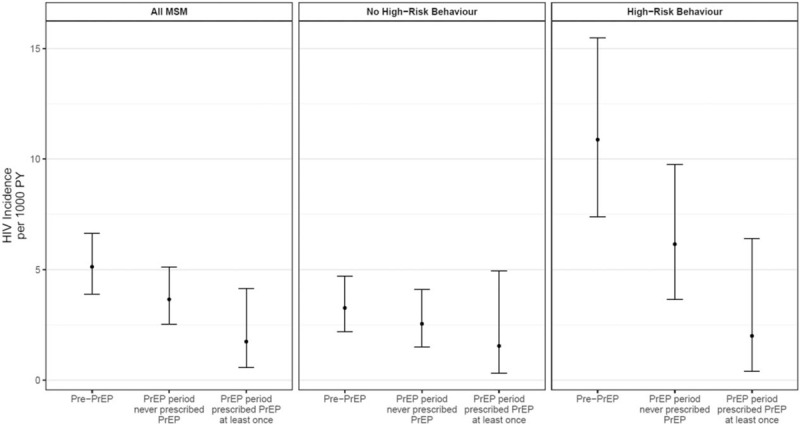
PrEP, preexposure prophylaxis; PY, person-years.
Discussion
In the first 2 years of Scotland's PrEP programme, national surveillance data showed that new HIV diagnoses in MSM fell by 20% overall and by 36% among those recently infected, compared with the 2 years before PrEP. These reductions occurred against 4 years of stable new HIV diagnoses and rising recent infections among MSM. Among MSM attending sexual health clinics, HIV incidence reduced from 5.13 to 3.25 per 1000 person-years in the pre-PrEP and PrEP periods, respectively. Compared with the pre-PrEP period, incidence of HIV in MSM was 75% lower among those prescribed PrEP at least once and 32% lower among those not prescribed PrEP, in the 2 years since PrEP was introduced.
MSM with high-risk behaviour (either PEP use or chlamydia/gonorrhoea in the last 12 months) benefitted most. Uptake of PrEP was higher in this group (31%) and incidence reduced from 11/1000 person-years in the pre-PrEP period to 2/1000 person-years among those exposed to PrEP, relating to 83% reduced risk.
Although the effectiveness of PrEP in MSM has been established in clinical trials [1–3], its impact on HIV incidence has not been demonstrated in a real-world national context until now. The retrospective cohort approach applied here, using existing datasets, enabled us to follow up a large national cohort of over 12 000 MSM contributing in excess of 20 000 person-years of follow-up, spanning time periods before and after the introduction of PrEP. Almost all (98%) MSM accessing state-funded PrEP in Scotland do so through sexual health clinics [10] so our dataset is likely to be comprehensive; the number of MSM in Scotland accessing PrEP privately appears to be low [8,20].
This study has several limitations. Incidence estimates are derived from those attending sexual health clinics so may not represent all MSM in Scotland. Our cohort of 12 276 MSM involved in incidence analysis relates to 20–25% of all MSM living in Scotland [17], but likely relates to a much larger proportion of those at high risk of HIV infection, given two-thirds (48/73) of MSM diagnosed with recently acquired HIV in the pre-PrEP period were diagnosed in sexual health clinics. Furthermore, our analysis relates directly to the setting for PrEP delivery.
To be included in the incidence analysis, individuals needed a previous negative HIV test and at least one follow-up test recorded on NaSH. Individuals who were diagnosed with HIV at their first ever clinic attendance were therefore excluded from the cohort, but this number also declined by 27% between pre-PrEP and PrEP periods (Table, SDC S2). There is uncertainty around the exact time of HIV acquisition for those we were able to follow up. To explore this, we adopted the mid-point approach to estimating incidence in sensitivity analysis; our findings were unchanged (Tables, SDC S5 & S6).
Frequency of HIV testing varied over time. MSM were tested on average 2.2 and 3.3 times in the pre-PrEP and PrEP periods, respectively; those prescribed PrEP were tested an average of 5.7 times in the PrEP period. Increased testing is likely to lead to earlier detection of HIV, and therefore, we may have underestimated the reduction in HIV incidence associated with PrEP exposure. However, this is likely to be minimal as only four new diagnoses were made in those exposed to PrEP.
We defined PrEP use as having been prescribed PrEP at least once. We were unable to determine the proportion of condomless anal intercourse episodes covered by PrEP as sexual behaviour and condom use are not recorded in a standardised way. As such, we do not know whether our findings represent ‘best case’ PrEP usage or a spectrum of use in which some take PrEP consistently and others more variably [21]. Furthermore, issues with NaSH reporting of STI test results precluded us from including previous infectious syphilis in our ‘with high risk-behaviour’ category.
The EPIC-NSW [5] implementation cohort study reported HIV incidence of 0.48 (95% CI 0.12–1.95) per 1000 person-years among MSM exposed to PrEP, which compared with 1.74 (95% CI 0.58–4.14) per 1000 person-years reported here. There were differences in study design that may have influenced the incidence rates observed. EPIC-NSW based analysis on 12 months’ follow-up and required participants to provide informed consent and attend follow up visits. The high median medication ratio (96.7%) suggests high participant adherence. In contrast, we report findings at 24 months after PrEP implementation, PrEP was provided as part of routine care, MSM who defaulted clinic appointments were not uniformly followed up, and we were unable to systematically measure adherence. Further, EPIC-NSW did not directly compare incidence in the PrEP exposed group to other key groups (i.e. MSM in the pre-PrEP period and MSM in the PrEP period who were not exposed to PrEP).
An American ecological analysis at the US jurisdiction level showed that areas with increases in PrEP coverage were associated with decreases in the estimated annual percentage change in HIV diagnosis rate, which were independent of levels of viral suppression among PLWHIV [22]. However, our analysis differs from this, as it follows up a cohort up over time (retrospectively) to provide national estimates of HIV incidence among MSM attending sexual health clinics before and after the PrEP programme rather than looking at associations between PrEP coverage and the annual percentage change in HIV diagnoses.
In Scotland, although reduction in HIV incidence was most pronounced in those prescribed PrEP, a modest reduction was also seen in other MSM attending sexual health services in that period. It is possible that PrEP may have reached the ‘core group’ of MSM (those engaging in frequent condomless anal intercourse with multiple partners) who without PrEP would contribute most to HIV transmission [23].
Although PrEP is likely to have contributed to the population level reduction in risk of HIV, other factors could have contributed. From 2015 onwards, earlier initiation of antiretroviral therapy in people living with HIV to prevent onward transmission [24] was believed to be reducing HIV incidence in some settings [25]. There was a modest increase in proportion of all PLWHIV in Scotland on antiretroviral therapy and with fully suppressed viral load over the study period. The UNAIDS 90 : 90:90 targets were met in 2018 [26]. However, the observed reduction in HIV diagnoses in MSM in the PrEP period was against a background of stable annual new HIV diagnoses in Scotland. Our finding that MSM with high-risk behaviour (previous PEP use and/or a bacterial STI) had greater uptake of PrEP and experienced a greater reduction in incidence (from 11/1000 in the pre-PrEP period to 2/1000 in the PrEP period among those exposed), provides further support for a direct impact of PrEP.
Shifts in condom use and changes in sexual behaviour could contribute to a reduction in HIV incidence. However, evidence from Scottish and international studies [27,28] suggests that sexual risk taking has increased in MSM (PrEP users and nonusers). Australian studies suggest that a rapid increase in PrEP use was associated with a rapid decrease in condom use and an increase in STIs [29].
PrEP was gradually scaled up in Scotland without a national mass media promotion campaign, to avoid demand exceeding clinic capacity. Mathematical modelling studies had suggested that rapidly recruited programmes with high coverage would be needed to quickly reduce HIV incidence in MSM [30]. Our findings show that reductions in incidence can be achieved through targeting PrEP at high risk populations attending routine sexual health services. However, Scotland's PrEP model includes free medication, comprehensive monitoring and adherence support in addition to blood borne virus and STI testing. The reductions in incidence reported here may not be possible in settings with less holistic PrEP care.
The Scottish PrEP programme has successfully attracted a group of MSM who are willing to attend specialist sexual health clinics for PrEP. The programme may have also benefitted MSM more broadly, shown by the reduction in HIV incidence in MSM who have not been prescribed PrEP. However, more work is needed to engage MSM who do not access sexual health services, including younger MSM.
Risk-based eligibility criteria need careful consideration. In our setting, expanding eligibility criteria to include MSM receiving PEP and those reporting any bacterial STI in the previous 12 months could potentially identify more MSM who could benefit.
We show that it is possible to achieve important reductions in HIV incidence in MSM when PrEP is implemented within routine care. Our findings suggest that PrEP can make a wider contribution, alongside other prevention interventions, in reducing population level risk of HIV for those not on PrEP. Although Scotland's PrEP programme provides PrEP for all who meet the eligibility criteria, only 2% of those prescribed PrEP were not MSM. Targeted community awareness-raising campaigns and PrEP provision in a wider range of settings will be needed to widen access to PrEP for all who may benefit.
Acknowledgements
We are grateful to the members of the Scottish National PrEP Coordination Group and the Monitoring and Research Subgroup (PrEP-MAR), Duncan McMaster, Stuart Wrigglesworth, David Henderson, Ross Cameron, the staff and patients of Scotland's sexual health services, and Health Protection Scotland (HPS) for funding this study.
C.E. led the study and was involved in all stages of the research process including co-designing the coding system for data collection, data analysis, data interpretation, leading manuscript writing and revisions. A.Y. assisted with study design, conducted the analyses under the supervision of S.H. and C.S.E., assisted with interpretation, manuscript writing and revisions. R.N. assisted with study design, analyses and was extensively involved in interpretation, manuscript writing and revision. D.G. assisted with study design, analyses, interpretation and manuscript revision. B.C. assisted with data collection, analyses, interpretation and manuscript revision. N.S. codesigned the coding system for data collection and was involved in data collection, interpretation and manuscript preparation and revision. L.W. assisted with data collection, analyses, interpretation and manuscript revision. S.H. conceived the study design, led methods for analysis and contributed substantially to interpretation, manuscript writing and revising. All authors approved the submitted manuscript.
Funding for this study was provided by Health Protection Scotland.
Conflicts of interest
C.E. reports research grants from National Institute of Health Research UK, Chief Scientist Office of Scotland, Engineering and Physical Sciences Research Council, UK Clinical Research Collaboration, Health Protection Scotland, European Centres for Disease Control.
S.H. reports research grants from Health Protection Scotland, National Institute of Health Research UK, Chief Scientist Office of Scotland, Medical Research Council, European Centre for Disease Prevention and Control, and European Monitoring Centre for Drugs and Drug Addiction; and honoraria from Gilead unrelated to submitted work.
R.N. reports research grants from National Institute of Health Research UK, Chief Scientist Office of Scotland and nonexecutive director membership of the Board of Public Health Scotland from April 2020.
A.Y., D.G., N.S., B.C. and L.W. report no competing interests.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373:2237–2246. [DOI] [PubMed] [Google Scholar]
- 3.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Preexposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molina JM, Charreau I, Spire B, Cotte L, Chas J, Capitant C, et al. Efficacy, safety, and effect on sexual behaviour of on-demand preexposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV 2017; 4:e402–e410. [DOI] [PubMed] [Google Scholar]
- 5.Grulich AE, Guy R, Amin J, Jin F, Selvey C, Holden J, et al. Population-level effectiveness of rapid, targeted, high-coverage roll-out of HIV preexposure prophylaxis in men who have sex with men: the EPIC-NSW prospective cohort study. Lancet HIV 2018; 5:e629–e637. [DOI] [PubMed] [Google Scholar]
- 6. Health Protection Scotland. HIV infection in Scotland: quarterly report to 30 September 2019, 2019. Available at: https://www.hps.scot.nhs.uk/web-resources-container/hiv-diagnoses-in-scotland-quarterly-report-to-30-september-2019 [Accessed 3 July 2020]. [Google Scholar]
- 7.Nandwani R. Preexposure prophylaxis is approved in Scotland. Lancet HIV 2017; 4:e238–e239. [DOI] [PubMed] [Google Scholar]
- 8. Health Protection Scotland and Information Services Division. Implementation of HIV PrEP in Scotland: first year report [Report], 2019. Available at: https://www.hps.scot.nhs.uk/web-resources-container/implementation-of-hiv-prep-in-scotland-first-year-report/ [Accessed 3 July 2020]. [Google Scholar]
- 9.Brady M, Rodger A, Asboe D, Cambiano V, Clutterbuck D, Desai M, et al. BHIVA/BASHH guidelines on the use of HIV preexposure prophylaxis (PrEP) 2018. HIV Med 2019; 20:S2–S80. [DOI] [PubMed] [Google Scholar]
- 10. Health Protection Scotland. Implementation of HIV PrEP in Scotland: second year report [Report], 2019. Available at: https://www.hps.scot.nhs.uk/web-resources-container/implementation-of-hiv-prep-in-scotland-second-year-report/ [Accessed 3 July 2020]. [Google Scholar]
- 11.McAuley A, Palmateer NE, Goldberg DJ, Trayner KM, Shepherd SJ, Gunson RN, et al. Re-emergence of HIV related to injecting drug use despite a comprehensive harm reduction environment: a cross-sectional analysis. Lancet HIV 2019; 6:e315–e324. [DOI] [PubMed] [Google Scholar]
- 12. Scottish Medicines Consortium. Emtricitabine/tenofovirdisoproxil 200 mg/245 mg film-coated tablets (Truvada) SMC No. (1225/17), 2017. Available at: https://www.scottishmedicines.org.uk/media/1620/emtricitabine_tenofovir_disoproxil_truvada_final_march_2017_for_website.pdf [Accessed 3 July 2020]. [Google Scholar]
- 13. McDaid L, Docherty S, Winter A. Review of the National Sexual Health System (NaSH) in Scotland: the potential for sexual health research, 2013. Available at: http://eprints.gla.ac.uk/90011/ [Accessed 3 July 2020]. [Google Scholar]
- 14.Goldberg D, Emslie J, Smyth W, Reid D. A system for surveillance of voluntary HIV testing: results of the first 2 years, 1989–1990. AIDS 1992; 6:495. [PubMed] [Google Scholar]
- 15.Shepherd SJ, McAllister G, Kean J, Wallace LA, Templeton KE, Goldberg DJ, et al. Development of an avidity assay for detection of recent HIV infections. J Virol Methods 2015; 217:42–49. [DOI] [PubMed] [Google Scholar]
- 16. Scottish Government. Scottish index of multiple deprivation 2020. Available at: https://www.gov.scot/collections/scottish-index-of-multiple-deprivation-2020/ [Accessed 3 July 2020]. [Google Scholar]
- 17. Office for National Statistics. Sexual orientation, UK: 2018, 2020. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/sexuality/bulletins/sexualidentityuk/2018 [Accessed 3 July 2020]. [Google Scholar]
- 18. Atkinson EJ, Crowson CS, Pedersen RA, Therneau TM. Poisson models for person-years and expected rates, 2008. Available at: https://www.mayo.edu/research/documents/biostat-81pdf/doc-10026981 [Accessed 3 July 2020]. [Google Scholar]
- 19.McDonald SA, Hutchinson SJ, Wallace LA, Cameron SO, Templeton K, McIntyre P, et al. Trends in the incidence of HIV in Scotland, 1988–2009. Sex Transm Infect 2012; 88:194–199. [DOI] [PubMed] [Google Scholar]
- 20.Gilson R, Clutterbuck D, Chen Z. Demand for preexposure prophylaxis for HIV and the impact on clinical services: Scottish men who have sex with men perspectives. Int J Std AIDS 2018; 29:273–277. [DOI] [PubMed] [Google Scholar]
- 21.Puppo C, Mabire X, Cotte L, Castro DR, Spire B, Cua E, et al. Community-based care in the ANRS-IPERGAY trial: the challenges of combination prevention. AIDS Educ Prev 2019; 31:259–272. [DOI] [PubMed] [Google Scholar]
- 22.Smith DK, Sullivan PS, Cadwell B, Waller LA, Siddiqi A, Mera-Giler R, et al. Evidence of an association of increases in preexposure prophylaxis coverage with decreases in human immunodeficiency virus diagnosis rates in the United States, 2012–2016. Clin Infect Dis 2020; doi:10.1093/cid/ciz1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watts C, Zimmerman C, Foss AM, Hossain M, Cox A, Vickerman P. Remodelling core group theory: the role of sustaining populations in HIV transmission. Sex Transm Infect 2010; 86:iii85–92. [DOI] [PubMed] [Google Scholar]
- 24. British HIV Association. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015 (2016 interim update), 2016. Available at: https://www.bhiva.org/file/RVYKzFwyxpgiI/treatment-guidelines-2016-interim-update.pdf [Accessed 3 July 2020]. [Google Scholar]
- 25. Public Health England. New HIV diagnoses fall by a third in the UK since 2015, 2019. Available at: https://www.gov.uk/government/news/new-hiv-diagnoses-fall-by-a-third-in-the-uk-since-2015 [Accessed 3 July 2020]. [Google Scholar]
- 26. Health Protection Scotland. HIV treatment and care in Scotland: summary report to 31 December 2018. Available at: https://www.hps.scot.nhs.uk/web-resources-container/hiv-treatment-and-care-in-scotland-summary-report-to-31-december-2018 [Accessed 18 November 2020]. [Google Scholar]
- 27.Holt M, Lea T, Mao L, Kolstee J, Zablotska I, Duck T, et al. Community-level changes in condom use and uptake of HIV preexposure prophylaxis by gay and bisexual men in Melbourne and Sydney, Australia: results of repeated behavioural surveillance in 2013-17. Lancet HIV 2018; 5:e448–e456. [DOI] [PubMed] [Google Scholar]
- 28.Werner RN, Gaskins M, Nast A, Dressler C. Incidence of sexually transmitted infections in men who have sex with men and who are at substantial risk of HIV infection: a meta-analysis of data from trials and observational studies of HIV preexposure prophylaxis. PLoS One 2018; 13:e0208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traeger MW, Cornelisse VJ, Asselin J, Price B, Roth NJ, Willcox J, et al. Association of HIV preexposure prophylaxis with incidence of sexually transmitted infections among individuals at high risk of HIV infection. JAMA 2019; 321:1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasaie P, Pennington J, Shah MS, Berry SA, German D, Flynn CP, et al. The impact of preexposure prophylaxis among men who have sex with men: an individual-based model. J Acq Immun Def Synd 2017; 75:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


