Abstract
Valosin-containing protein (VCP/p97) is a member of the conserved type II AAA+ (ATPases associated with diverse cellular activities) family of proteins with multiple biological functions, especially in protein homeostasis. Mutations in VCP/p97 are reportedly related to unique autosomal dominant diseases, which may worsen cardiac function. Although the structure of VCP/p97 has been clearly characterized, with reports of high abundance in the heart, research focusing on the molecular mechanisms underpinning the roles of VCP/p97 in the cardiovascular system has been recently undertaken over the past decades. Recent studies have shown that VCP/p97 deficiency affects myocardial fibers and induces heart failure, while overexpression of VCP/p97 eliminates ischemia/reperfusion injury and relieves pathological cardiac hypertrophy caused by cardiac pressure overload, which is related to changes in the mitochondria and calcium overload. However, certain studies have drawn opposing conclusions, including the mitigation of ischemia/reperfusion injury via inhibition of VCP/p97 ATPase activity. Nevertheless, these emerging studies shed light on the role of VCP/p97 and its therapeutic potential in cardiovascular diseases. In other words, VCP/p97 may be involved in the development of cardiovascular disease, and is anticipated to be a new therapeutic target. This review summarizes current findings regarding VCP/p97 in the cardiovascular system for the first time, and discusses the role of VCP/p97 in cardiovascular disease.
Keywords: cardiovascular diseases, iNOS, mitochondria, VCP/p97
Introduction
Valosin-containing protein (VCP/p97) was firstly identified in 1982 by Moir et al. [1], when they genetically screened Saccharomyces cerevisiae. This protein is strongly associated with cell cycle arrest and was therefore called Cdc48 (cell division cycle 48). In Drosophila [2], VCP/p97 is localized to the endoplasmic reticulum (ER) and belongs to the type II AAA+ ATPase family; hence, it is also referred to as the transitional endoplasmic reticulum ATPase (TER ATPase). Mammalian homologs have been reported as a precursor of the small peptide valosin [3], known as valosin-containing protein (VCP/p97). The term VCP/p97 has been used to represent this protein in this review.
VCP/p97 is a molecular chaperone involved in the endoplasmic reticulum-associated degradation pathway (ERAD) [4]. More specifically, VCP/p97 is recruited to the ER membrane by binding with membrane adapters, including UBX domain-containing protein 1 (UBXD1), p47, and nuclear protein localization protein 4 (Npl4) [5–7]. VCP/p97 captures and extracts misfolded proteins through its putative channel by ATP hydrolysis, and subsequently targets misfolded proteins for proteasomal degradation. In addition to ERAD, VCP/p97 is involved in most organelle-mediated protein degradation processes, including the degradation of proteins in the outer mitochondrial membrane [8], nucleus [9] and co-translational degradation in ribosomes [10]. Additionally, VCP/p97 also plays a critical role in DNA damage [11]. In summary, VCP/p97 is a multifunctional protein that has considerable impact on protein metabolism and intracellular homeostasis.
Among various tissues, VCP/p97 is present in cardiac tissues in high abundance, only second to skeletal muscle. Dilated cardiomyopathy is observed in patients with IBMPFD (inclusion body myopathy (IBM), Paget disease (PDB), neurological components frontotemporal dementia (FTD)) who experience pain due to VCP/p97 mutations [12]. Silencing of TER94 (a homolog of VCP/p97 in Drosophila) also seriously damages heart function in Drosophila [13], which together highlights the importance of VCP/p97 in the heart. Current research has initially revealed the role of VCP/p97 in ischemia–reperfusion and hypertensive heart disease, but there is still controversy. This review summarizes the role of VCP/p97 in the cardiovascular system, particularly focusing on the underlying pathophysiological mechanisms, and sheds light on the remaining problems involving basic research surrounding cardiovascular diseases, thus aiming to raise awareness and interest concerning VCP/p97 in the cardiovascular system.
The VCP/p97 gene
The VCP/p97 gene is located on chromosome 9p13.3, and contains 17 exons. More than 50 missense mutations involving the VCP/p97 gene are related to unique autosomal dominant diseases [14]. These diseases are collectively referred to as ‘multisystem protein diseases', including IBMPFD [15], which denotes a combination of inclusion body myopathy (IBM), Paget disease (PDB), and neurological components frontotemporal dementia (FTD). These patients eventually die from muscle weakness and heart failure.
Single missense mutations of VCP/p97 can lead to IBMPFD [16]. These pathogenic single missense mutations are mainly located at the N-terminus of VCP/p97 (N domain or D1 domain). Among the mutations, the R155H substitution is the most common mutation in IBMPFD patients [17], while the A232E mutation results in the most serious clinical manifestations. VCP/p97 mutations also account for 1%–2% of the familial amyotrophic lateral sclerosis (ALS) cases [18]. Pathogenic VCP/p97 mutations lead to reductions in SUMOylation and the formation of VCP/p97 hexamers under stress. They also affect cofactor binding and degradation of endoplasmic reticulum-associated proteins, which ultimately renders cells vulnerable to stress [19]. Additionally, adenine nucleotide transferase is dysregulated due to pathogenic mutations involving VCP/p97, resulting in reduced ATP levels [20]. Utilization of the Cre-LoxP technology for excision of the VCP/p97 R155H missense mutation was anticipated as a correctional approach for this mutation [21], but currently there are no effective treatments for these genetic mutations.
The VCP/p97 protein
The VCP/p97 protein has 806 amino acids, and a predicted molecular weight of 97 kDa [22].
It is markedly abundant, accounting for ∼1% of the total cellular proteins. Generally, VCP/p97 is soluble in the cytosol, and is found to be associated with membrane structures, including the endoplasmic reticulum [23], golgi apparatus [24], mitochondria [25], and endosomes [26]. Additionally, VCP/p97 has been shown to play a role in the nucleus, mainly related to nuclear protein quality control [27].
VCP/p97 belongs to the type II AAA+ ATPase family [28]. It usually forms hexamers in cells, and its assembly is independent of ATP [29]. VCP/p97 has two ATPase domains, referred to as D1 and D2, which are connected by a short peptide linker [30]. The D1 domain is also connected by the N-D1 linker to the N-terminal domain, which mediates interactions between VCP/p97 and most of its cofactors, including Ufd1-Npl4, p37, p47, and so on [31–33]. Additionally, there are certain other proteins that bind to the C-terminal tail linked to the D2 domain. For example, UBXD1 and phospholipase A2-activating proteins occupy the C-terminus of VCP/p97 [25,34].
To date, at least 40 proteins that interact with VCP/p97 have been identified [35,36]. These proteins usually have a common binding motif and conserved binding modules, such as UBX (ubiquitin regulatory X) domain, UBXL (UBX like) domain, VIM (VCP/p97-interacting motif), and VBM (VCP/p97-binding motif) [37]. For example, the cofactor alveolar soft part sarcoma locus (ASPL), which has the highest affinity with VCP/p97, relys on its extended UBX domain (eUBX) [38]. These bind to different sites on VCP/p97, and then guide VCP/p97 to different membrane structures in order to perform a variety of biological functions. The VCP/p97 hexamer has many exposed domains, thereby allowing combinations of multiple cofactors at the same time; hence, the functions of VCP/p97 are diversified, and the regulation of VCP/p97 can be quite precise.
VCP/p97 and cardiovascular diseases
Imbalance in protein homeostasis is a hallmark of various cardiovascular diseases [39], including myocardial infarction, heart failure, and diabetic cardiomyopathy. Considering the core role of VCP/p97 in protein homeostasis, VCP/p97 is thought to be involved in the cardiovascular system. For example, VCP/p97 K524A transgenic mice exhibit cardiomyopathy with the accumulation of ubiquitinated proteins [10]. In addition to protein homeostasis, VCP/p97 also plays a vital role in the maintenance of mitochondrial function and the promotion of cardiomyocyte survival [40]. In the following sections, we have summarized certain basic findings concerning VCP/p97 in cardiovascular diseases.
VCP/p97 and ischemia/reperfusion injury
VCP/p97 and iNOS
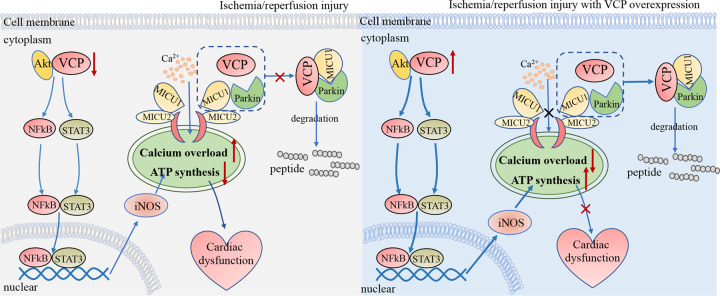
Ischemia–reperfusion is a process in which part of the myocardium receives insufficient blood supply due to clogged blood vessels in the ischemic phase, and then blood returns to the ischemic area in the reperfusion phase [41]. Both ischemia and reperfusion cause myocardial tissue damage [42,43]. Ischemic preconditioning for ischemic myocardium effectively relieves ischemia/reperfusion injury [44]. In the ischemic preconditioning process, endogenous NO produced within the myocardium regulates myocardial contraction and vessel vasodilation, thus reducing the area of myocardial infarction and improving endothelial function [45]. The synthesis of NO is mainly mediated by inducible NO synthase (iNOS) [46], which places iNOS in a crucial position in ischemic preconditioning [47]. VCP/p97 is a protective factor in ischemia/reperfusion injury, supported by a 50% reduction in the myocardial infarction area in VCP/p97 transgenic mice compared with that in wild-type mice after ischemia/reperfusion injury [48]. Moreover, overexpression of VCP/p97 in cardiomyocytes significantly reduces celandine-induced myocardial apoptosis [49]. Further mechanistic studies have revealed that VCP/p97 exerts its effects encompassing myocardial protection by elevating the production of NO via iNOS (Figure 1). VCP/p97 promotes iNOS expression in a dose-dependent manner, which largely relies on NF-kB (Figure 1), as the proteolytic effects of VCP overexpression are abolished by the addition of the NF-kB inhibitor, SN50 [49].
Figure 1. VCP's effects on cardiac ischemia/reperfusion injury.
The decreased VCP in ischemia reperfusion leads to a decrease in the physically interaction of VCP with Akt and the activation of NF-kB and STAT3 in cardiomyocytes, NF-kB and STAT3 are the transcriptional factors of iNOS, which has been proven to improve the respiratory function of mitochondria. In addition, the physically association of VCP, MICU1, and Parkin decreased, which reduced the degradation of MICU1, resulting in the continuous opening of mitochondrial permeability transition pore, calcium overload and decreased respiratory function in the mitochondria. Therefore, the decline of iNOS and the respiratory function of mitochondria jointly promote the cardiac dysfunction under ischemia–reperfusion. The overexpression of VCP enhances the interaction of VCP and Akt, which results in an increase in NF-kB and STAT3 into the nuclear, and the production of iNOS, thereby enhancing the mitochondrial respiratory function of cardiomyocytes. Meanwhile, the physically interaction of VCP, MICU1 and Parkin is enhanced under conditions of VCP/p97 overexpression, which accelerates the degradation of MICU1, reduces the opening frequency of mitochondrial permeability transition pore, and inhibits mitochondrial calcium overload. Thus the increase in iNOS production and the improvement of mitochondrial respiratory function together protect the cardiac dysfunction under ischemia–reperfusion.
VCP/p97 and mitochondria
Heart is a high-energy consumption organ, which relies on abundant mitochondria to provide energy; more than 90% of the ATP requirement is fulfilled by mitochondrial aerobic oxidation [50]. Patient-derived VCP/p97-mutant fibroblasts display low mitochondrial membrane potential and reduced ATP levels [51]. Mutation, or lack of VCP/p97 in neurons, aggravates mitochondrial dysfunction, resulting in reduced ATP synthesis, which renders neurons more vulnerable to ischemia [52], thereby indicating the roles of VCP/p97 in the regulation of mitochondrial function.
Under physiological conditions, VCP/p97, which is recruited by UBX1 to carbonyl cyanide 3-chlorophenylhydrazone (CCCP)-depolarized mitochondria, promotes mitochondrial autophagy for removal of damaged mitochondria and maintenance of mitochondrial fidelity [25]. VCP/p97 also mediates E3 ubiquitin ligase Parkin-induced Mfn1 degradation, and PINK1 degradation, to maintain mitochondrial proteostasis [53]. Under pathological conditions, the VCP/p97 mutant (VCP/p97 R155C or VCP/p97 R191Q) induces adenine nucleotide transferase dysregulation, resulting in a reduction in the rate of ADP or ATP translocation on the mitochondrial membrane [20]. Furthermore, VCP mutants migrate more slowly than endogenous VCP to the outer mitochondrial membrane, and also inhibit the localization of its cofactors Npl4 and p47 in damaged mitochondria, which collectively inhibits mitochondrial autophagy [54].
In the myocardial tissue, hypoxic-ischemic stimulation promotes the preferential accumulation of VCP/p97 in mitochondria [48]. Compared with wild-type mice, mitochondrial respiration capacity and ATP synthesis capacity in VCP/p97 transgenic mice are significantly enhanced, and the opening of mitochondrial permeability transition pore (mPTP) on mitochondria is consistently inhibited, thereby leading to considerable elimination of mitochondrial calcium overload caused by calcium influx [48]. A mechanistic study showed that VCP/p97 regulation of mitochondrial respiratory functions was dependent on iNOS. The effect of VCP/p97 overexpression on the enhancement of mitochondrial respiratory function is abolished with genetic deletion of iNOS in VCP/p97 transgenic mice, or by inhibition of iNOS activity with the iNOS inhibitor 1400W. The protective effects of VCP/p97 on mitochondrial calcium overload may partially rely on mitochondrial Ca2+ uptake protein 1 (MICU1) [55], which is an activator of mitochondrial Ca2+ unidirectional transporter (MCU), and is responsible for mitochondrial calcium uptake [56]. VCP/p97 physically associates with MICU1 through Parkin, and promotes post-translational protein degradation of MICU1, which may contribute to reduction in the frequency of mPTP opening and the damage caused by mitochondrial calcium overload (Figure 1).
Overexpression of VCP/p97 in transgenic mice reveals an important role in anti-ischemia/reperfusion injury by promoting iNOS production and by improving mitochondrial function. A VCP/p97 inhibitor (KUS121) was also shown to attenuate ischemia–reperfusion injury in various animal models, including murine and porcine ischemia and reperfusion injury models. Specifically, KUS121 treatment increases the level of ATP, reduces ER stress, and improves mitochondrial function, thereby reducing myocardial cell death caused by ischemia–reperfusion injury [57]. We hypothesize that this contradictory result may be associated with the method of activation and inhibition of VCP/p97 activity. First, VCP/p97 transgenic mice exhibit certain physiological processes other than VCP/p97 ATPase activity, including endoplasmic reticulum (ER)–associated degradation, lysosomal protein degradation, and so on [10], which may triggers systematic protective effects after ischemia/reperfusion injury, whereas KUS121 only inhibits VCP/p97ATPase activity. Second, VCP/p97 transgenic mice have undergone systemic changes since the embryonic stage compared with the short-term effects of KUS121, which may not provide sufficient time for adaptation. Therefore, both temporary inhibition of the ATPase activity of VCP/p97 after ischemia and generation of VCP/p97 transgenic mice before ischemia–reperfusion injury demonstrates strong anti-ischemia/reperfusion effects.
VCP/p97 and hypertensive heart disease
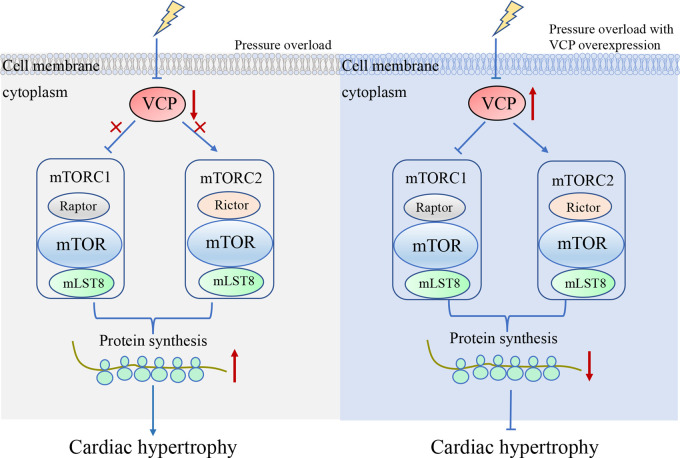
Hypertension usually leads to cardiac hypertrophy, a disease manifested by hypertrophy of single cardiomyocytes and enhanced rates of protein synthesis [58]. Myocardial hypertrophy is an independent pathological risk factor for many cardiovascular diseases, including myocardial infarction [59] and heart failure [60]. mTOR (mammalian target of rapamycin) is an evolutionarily conserved serine/tyrosine kinase which interacts with specific adaptor proteins to form two different macromolecular complexes, called mTORC1 (mTOR complex 1) and mTORC2 (mTOR complex 2) [61]. These two complexes are essential for the development of adaptive myocardial hypertrophy caused by pressure overload. The absence of mTORC2 damages compensatory myocardial hypertrophy [62], and partial inhibition of mTORC1 may eliminate pathological cardiac hypertrophy and improve left ventricular function [63]. Zhou N et al. [64] found that VCP/p97 expression is time- and dose-dependently reduced in pressure overload. And overexpression VCP/p97 protects heart from cardiac hypertrophy, supported by the facts that VCP/p97 transgenic mice shown relatively normal cardiac structure and cardiac function compared with wild-type mice in the presence of cardiac pressure overload [64] (Figure 2). Pressure overload suppresses the expression of VCP/p97 which activated mTORC2 and attenuates the inhibitive effect of VCP/p97 on mTORC1 signaling, subsequently promotes the protein synthesis pathway (Figure 2). While VCP/p97 overexpression restores the pressure overload-suppressed VCP/p97 and represses the pressure overload-induced activation of mTORC1, protects the heart against pressure overload-induced cardiac hypertrophy [65] (Figure 2).
Figure 2. VCP's effects on pressure overload induced cardiac hypertrophy.
Pressure overload inhibits VCP in cardiomyocytes, reduces VCP's activation of mTORC2 signaling pathway and its inhibitory effect on mTORC1 signaling pathway, and ultimately leads to increased protein synthesis and cell apoptosis; However, overexpression of VCP can effectively restored the activation of mTORC2 signaling pathway and inhibited mTORC1 signaling pathway, and resisting myocardial hypertrophy caused by pressure overload.
In the progression of cardiac hypertrophy, there are multiple molecular alterations [66]. One of these alterations involves changes to Akt activity, which plays a crucial role in the regulation of a variety of cellular processes ranging from cell survival to aging [67]. As described previously, Akt activates VCP/p97 in persistent hypoxic states [68]. Interestingly, in a study by Zhou et al.[64], VCP/p97 also promotes Akt phosphorylation under physiological conditions. Two-dimensional gel electrophoresis and mass spectrometry combined with immunoprecipitation experiments have confirmed that VCP/p97 and Akt bind to each other [69]. As a kinase protein, Akt can directly phosphorylate VCP/p97, whereas VCP/p97 is not a kinase protein, and does not possess phosphorylation ability. Given the fact that the activity of mTORC2 is up-regulated in VCP/p97 transgenic mice, and that Akt acts downstream of mTORC2, it is suggested that VCP/p97 may first activate mTORC2, and then promote Akt phosphorylation [64]. This finding indicates that interactions between Akt and VCP/p97 may involve both direct and indirect regulation, and these two proteins may exhibit different modes of action under different physiological conditions. Since Akt is a central protein involved in many cellular processes in cardiac hypertrophy, further studies focusing on the relationship between VCP/p97 and Akt will help to further reveal the roles of VCP/p97 in cardiac hypertrophy.
However, the protective effects of VCP/p97 in myocardial hypertrophy remain controversial. A recent study showed that overexpression of VCP/p97 did not alleviate the progression of myocardial hypertrophy caused by pressure overload. After 4 to 8 weeks of transverse aortic constriction (TAC) surgery, the left ventricular internal diameter and fractional shortening of VCP/p97 transgenic mice were not significantly different from those of control mice [10]. The authors assumed that VCP/p97 represented 1% of the total protein; hence, the effect of overexpressing VCP/p97 on the myocardium was minimal. It is worth noting that in this study, mice in the control group did not exhibit significant differences in the cardiac structure and function after receiving TAC surgery for 4 and 8 weeks, which prompted us to question the reliability of the cardiac pressure overload model. In general, long-term pressure overload inevitably leads to dramatic changes in the cardiac structure and function. Therefore, conclusions based on this model are also debatable. However, whether VCP/p97 exerts protective effects in myocardial hypertrophy requires further investigation.
Summary and outlook
Cardiovascular diseases, including myocardial infarction and heart failure, are important causes of high mortality rates in the elderly [70]. Traditional studies have attempted to identify risk factors related to the occurrence of cardiovascular diseases, and to reduce such risks by means of therapeutic drugs or gene knockout [71]. In recent years, it has been proven that endogenous protective factors within cardiomyocytes considerably help to reduce the risk of cardiovascular disease. VCP/p97 is a typical endogenous protective factor, which has long been considered to be associated with physiological processes that maintain protein homeostasis [72]. Recent studies have shown that VCP/p97 may be a potential therapeutic target in cardiovascular diseases. Reduction or abnormal function can cause a variety of diseases. The activation or overexpression of VCP/p97 can effectively delay the progression of diseases. In particular, overexpression of VCP/p97 can effectively increase the level of high-energy phosphate in the myocardium, resist mitochondrial calcium overload, and promote cell survival [48].
Existing research suggests that VCP/p97 has a strong potential to be a new therapeutic target for ischemic-reperfusion injury and pressure overload-induced cardiac hypertrophy, although there remain some opposing views. If VCP/p97 is to be seriously considered, then more research should be conducted to determine whether VCP/p97 has general myocardial protective effects, and the mechanisms by which it is involved in such processes. In particular, the role of VCP/p97 in protection against non-ischemic heart disease, including diabetic cardiomyopathy and idiopathic dilated cardiomyopathy should be elucidated. Additionally, many cardiovascular diseases caused by pressure overload, including pathological cardiac hypertrophy, result in protein homeostasis imbalance [73]. The mechanism by which VCP/p97 maintains myocardial protein homeostasis should be studied. Moreover, since the functions of mitochondria are diverse, it is possible that VCP/p97 may also regulate other aspects of mitochondria (including redox balance, glucose oxidation, and lipid oxidation), in spite of respiratory function and calcium hemostasis. Further research is needed to explore the complex relationships involving mitochondria and VCP/p97.
VCP/p97 mutations affect cardiac function. As mentioned before, mice that overexpress mutant VCP/p97 (VCP/p97 K524A) are prone to cardiomyopathy under cardiac pressure overload [10] due to loss of ATPase activity. The ATPase activity of VCP/p97 is a basic requirement for its normal function because it provides the energy required for extraction of ubiquitinated proteins from membrane structures (such as the endoplasmic reticulum) [74]. In line with this canonical view, the mutation of VCP/p97 involving Lys524 undermines the efficiency of protein clearance in the ERAD pathway, and affects nuclear integrity. However, this view has been challenged recently. A new substance (KUS121), which inhibits the ATPase activity of VCP/p97, has been shown to exert protective effects in in vivo myocardial infarction models, and in in vitro cardiomyocyte hypoxia models [57], which is contradictory to a study involving VCP/p97 K524A transgenic mice [10]. Although the structure of VCP/p97 has been clearly determined, more research is needed to clarify discrepancies in particular pathophysiological processes.
In summary, VCP/p97 plays important roles in the cardiovascular system. It may become a promising target for the treatment of cardiovascular diseases; however, the underlying molecular mechanisms await further investigation.
Perspectives
Importance of the field: VCP/p97 is a type II AAA+ ATPase that is conserved and abundantly expressed in a variety of tissues and cells, and is involved in a variety of physiological processes. This protein is increasingly associated with human diseases including cardiovascular system diseases, which has inspired people to further explore its important role in cardiovascular system.
Summary of current thinking: VCP/p97 deficiency damages myocardial fibers and induce heart failure, while VCP/p97 overexpression improves ischemia–reperfusion injury and pressure overload induced cardiac hypertrophy. Emerging evidence also indicates VCP/p97 plays an important role in maintaining normal mitochondrial respiratory function of cardiomyocytes and promoting its survival.
Future directions: Many unresolved problems remain about the role of VCP/p97 in the cardiovascular field. For example, the protective effect of VCP/p97 on myocardial hypertrophy caused by ischemia–reperfusion injury and pressure overload is still controversial, studies are particularly required to understand its underlying molecular mechanisms.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- CCCP
carbonyl cyanide 3-chlorophenylhydrazone
- Cdc48
cell division cycle 48
- ER
endoplasmic reticulum
- ERAD
endoplasmic reticulum-associated degradation pathway
- IBMPFD
inclusion body myopathy, Paget disease, neurological components frontotemporal dementia
- iNOS
inducible NO synthase
- MCU
mitochondrial Ca2+ unidirectional transporter
- MICU1
mitochondrial Ca2+ uptake protein 1
- mPTP
mitochondrial permeability transition pore
- mTOR
mammalian target of rapamycin
- mTORC1
mTOR complex 1
- mTORC2
mTOR complex 2
- Npl4
nuclear protein localization protein 4
- TAC
transverse aortic constriction
- TER
ATPase transitional endoplasmic reticulum ATPase
- UBX
ubiquitin regulatory X
- UBXD1
UBX domain-containing protein 1
- VBM
VCP/p97-binding motif
- VCP/p97
valosin-containing protein
- VIM
VCP/p97-interacting motif
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [81570261, 82070316] and Chinese Cardiovascular Association-Access Fund [2020-CCA-ACCESS-059].
Author Contribution
H.S. designed and wrote the manuscript draft; Y.P., W.H., N.Z., and D.W.W. assisted in critical revising of the manuscript; N.Z. gave economical support for this paper.
References
- 1.Moir, D., Stewart, S.E., Osmond, B.C. and Botstein, D. (1982) Cold-sensitive cell-division-cycle mutants of yeast: isolation, properties, and pseudoreversion studies. Genetics 100, 547–563 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magyar, A. and Váradi, A. (1990) Molecular cloning and chromosomal localization of a sarco/endoplasmic reticulum-type Ca2+-ATPase of Drosophila melanogaster. Biochem. Biophys. Res. Commun. 173, 872–877 10.1016/S0006-291X(05)80867-8 [DOI] [PubMed] [Google Scholar]
- 3.Koller, K.J. and Brownstein, M.J. (1987) Use of a cDNA clone to identify a supposed precursor protein containing valosin. Nature 325, 542–545 10.1038/325542a0 [DOI] [PubMed] [Google Scholar]
- 4.Zhong, X., Shen, Y., Ballar, P., Apostolou, A., Agami, R. and Fang, S. (2004) AAA ATPase p97/valosin-containing protein interacts with gp78, a ubiquitin ligase for endoplasmic reticulum-associated degradation. J. Biol. Chem. 279, 45676–45684 10.1074/jbc.M409034200 [DOI] [PubMed] [Google Scholar]
- 5.Nagahama, M., Ohnishi, M., Kawate, Y., Matsui, T., Miyake, H., Yuasa, K.et al. (2009) UBXD1 is a VCP-interacting protein that is involved in ER-associated degradation. Biochem. Biophys. Res. Commun. 382, 303–308 10.1016/j.bbrc.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 6.Chia, W.S., Chia, D.X., Rao, F., Bar Nun, S. and Geifman Shochat, S. (2012) ATP binding to p97/VCP D1 domain regulates selective recruitment of adaptors to its proximal N-domain. PLoS ONE 7, e50490 10.1371/journal.pone.0050490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballar, P., Pabuccuoglu, A. and Kose, F.A. (2011) Different p97/VCP complexes function in retrotranslocation step of mammalian ER-associated degradation (ERAD). Int. J. Biochem. Cell Biol. 43, 613–621 10.1016/j.biocel.2010.12.021 [DOI] [PubMed] [Google Scholar]
- 8.Guo, X. and Qi, X. (2017) VCP cooperates with UBXD1 to degrade mitochondrial outer membrane protein MCL1 in model of Huntington's disease. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 552–559 10.1016/j.bbadis.2016.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu, Y., Nakagawa, T., Morohoshi, A., Nakagawa, M., Ishida, N., Suzuki, N.et al. (2019) Pathogenic mutations in the ALS gene CCNF cause cytoplasmic mislocalization of Cyclin F and elevated VCP ATPase activity. Hum. Mol. Genet. 28, 3486–3497 10.1093/hmg/ddz119 [DOI] [PubMed] [Google Scholar]
- 10.Brody, M.J., Vanhoutte, D., Bakshi C, V., Liu, R., Correll, R.N., Sargent, M.A.et al. (2019) Disruption of valosin-containing protein activity causes cardiomyopathy and reveals pleiotropic functions in cardiac homeostasis. J. Biol. Chem. 294, 8918–8929 10.1074/jbc.RA119.007585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dantuma, N.P., Acs, K. and Luijsterburg, M.S. (2014) Should I stay or should I go: VCP/p97-mediated chromatin extraction in the DNA damage response. Exp. Cell Res. 329, 9–17 10.1016/j.yexcr.2014.08.025 [DOI] [PubMed] [Google Scholar]
- 12.Kimonis, V. (1993) Inclusion body myopathy with paget disease of bone and/or frontotemporal dementia. In GeneReviews (Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K.et al., eds), University of Washington, Seattle [Google Scholar]
- 13.Viswanathan, M.C., Blice-Baum, A.C., Sang, T.-K. and Cammarato, A. (2016) Cardiac-restricted expression of VCP/TER94 rnai or disease alleles perturbs Drosophila heart structure and impairs function. J. Cardiovasc. Dev. Dis. 3, 19 10.3390/jcdd3020019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Tahan, S., Al-Obeidi, E., Yoshioka, H., Lakatos, A., Weiss, L., Grafe, M.et al. (2018) Novel valosin-containing protein mutations associated with multisystem proteinopathy. Neuromuscul. Disord. 28, 491–501 10.1016/j.nmd.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 15.Kimonis, V.E., Fulchiero, E., Vesa, J. and Watts, G. (2008) VCP disease associated with myopathy, Paget disease of bone and frontotemporal dementia: review of a unique disorder. Biochim. Biophys. Acta 1782, 744–748 10.1016/j.bbadis.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 16.Watts, G.D.J., Wymer, J., Kovach, M.J., Mehta, S.G., Mumm, S., Darvish, D.et al. (2004) Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat. Genet. 36, 377–381 10.1038/ng1332 [DOI] [PubMed] [Google Scholar]
- 17.Weihl, C.C., Dalal, S., Pestronk, A. and Hanson, P.I. (2006) Inclusion body myopathy-associated mutations in p97/VCP impair endoplasmic reticulum-associated degradation. Hum. Mol. Genet. 15, 189–199 10.1093/hmg/ddi426 [DOI] [PubMed] [Google Scholar]
- 18.Ritson, G.P., Custer, S.K., Freibaum, B.D., Guinto, J.B., Geffel, D., Moore, J.et al. (2010) TDP-43 mediates degeneration in a novel Drosophila model of disease caused by mutations in VCP/p97. J. Neurosci. 30, 7729–7739 10.1523/JNEUROSCI.5894-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, T., Xu, W., Qin, M., Yang, Y., Bao, P., Shen, F.et al. (2016) Pathogenic mutations in the Valosin-containing Protein/p97(VCP) N-domain inhibit the SUMOylation of VCP and lead to impaired stress response. J. Biol. Chem. 291, 14373–14384 10.1074/jbc.M116.729343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludtmann, M.H.R., Arber, C., Bartolome, F., de Vicente, M., Preza, E., Carro, E.et al. (2017) Mutations in valosin-containing protein (VCP) decrease ADP/ATP translocation across the mitochondrial membrane and impair energy metabolism in human neurons. J. Biol. Chem. 292, 8907–8917 10.1074/jbc.M116.762898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nalbandian, A., Llewellyn, K.J., Nguyen, C., Monuki, E.S. and Kimonis, V.E. (2015) Targeted excision of VCP R155H mutation by Cre-LoxP technology as a promising therapeutic strategy for valosin-containing protein disease. Hum. Gene Ther. Methods 26, 13–24 10.1089/hgtb.2014.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song, C., Xiao, Z., Nagashima, K., Li, C.-C.H., Lockett, S.J., Dai, R.-M.et al. (2008) The heavy metal cadmium induces valosin-containing protein (VCP)-mediated aggresome formation. Toxicol. Appl. Pharmacol. 228, 351–363 10.1016/j.taap.2007.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu, X. and Rapoport, T.A. (2018) Mechanistic insights into ER-associated protein degradation. Curr. Opin. Cell Biol. 53, 22–28 10.1016/j.ceb.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Totsukawa, G., Matsuo, A., Kubota, A., Taguchi, Y. and Kondo, H. (2013) Mitotic phosphorylation of VCIP135 blocks p97ATPase-mediated Golgi membrane fusion. Biochem. Biophys. Res. Commun. 433, 237–242 10.1016/j.bbrc.2013.02.090 [DOI] [PubMed] [Google Scholar]
- 25.Bento, A.C., Bippes, C.C., Kohler, C., Hemion, C., Frank, S. and Neutzner, A. (2018) UBXD1 is a mitochondrial recruitment factor for p97/VCP and promotes mitophagy. Sci. Rep. 8, 12415 10.1038/s41598-018-30963-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramanathan, H.N. and Ye, Y. (2012) The p97 ATPase associates with EEA1 to regulate the size of early endosomes. Cell Res. 22, 346–359 10.1038/cr.2011.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallagher, P.S., Clowes Candadai, S.V. and Gardner, R.G. (2014) The requirement for Cdc48/p97 in nuclear protein quality control degradation depends on the substrate and correlates with substrate insolubility. J. Cell Sci. 127, 1980–1991 10.1242/jcs.141838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saffert, P., Enenkel, C. and Wendler, P. (2017) Structure and function of p97 and Pex1/6 type II AAA+ complexes. Front. Mol. Biosci. 4, 33 10.3389/fmolb.2017.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rijal, R., Arhzaouy, K., Strucksberg, K.-H., Cross, M., Hofmann, A., Schröder, R.et al. (2016) Mutant p97 exhibits species-specific changes of its ATPase activity and compromises the UBXD9-mediated monomerisation of p97 hexamers. Eur. J. Cell Biol. 95, 195–207 10.1016/j.ejcb.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 30.Tang, W.K. and Xia, D. (2016) Role of the D1-D2 linker of human VCP/p97 in the asymmetry and ATPase activity of the D1-domain. Sci. Rep. 6, 20037 10.1038/srep20037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niwa, H., Ewens, C.A., Tsang, C., Yeung, H.O., Zhang, X. and Freemont, P.S. (2012) The role of the N-domain in the ATPase activity of the mammalian AAA ATPase p97/VCP. J. Biol. Chem. 287, 8561–8570 10.1074/jbc.M111.302778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodnar, N.O., Kim, K.H., Ji, Z., Wales, T.E., Svetlov, V., Nudler, E.et al. (2018) Structure of the Cdc48 ATPase with its ubiquitin-binding cofactor Ufd1-Npl4. Nat. Struct. Mol. Biol. 25, 616–622 10.1038/s41594-018-0085-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang, X., Gui, L., Zhang, X., Bulfer, S.L., Sanghez, V., Wong, D.E.et al. (2015) Altered cofactor regulation with disease-associated p97/VCP mutations. Proc. Natl Acad. Sci. U.S.A. 112, E1705–E1714 10.1073/pnas.1418820112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papadopoulos, C., Kirchner, P., Bug, M., Grum, D., Koerver, L., Schulze, N.et al. (2017) VCP/p97 cooperates with YOD1, UBXD1 and PLAA to drive clearance of ruptured lysosomes by autophagy. EMBO J. 36, 135–150 10.15252/embj.201695148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bodnar, N. and Rapoport, T. (2017) Toward an understanding of the Cdc48/p97 ATPase. F1000Res. 6, 1318 10.12688/f1000research.11683.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue, L., Blythe, E.E., Freiberger, E.C., Mamrosh, J.L., Hebert, A.S., Reitsma, J.M.et al. (2016) Valosin-containing protein (VCP)-adaptor interactions are exceptionally dynamic and subject to differential modulation by a vcp inhibitor. Mol. Cell. Proteomics 15, 2970–2986 10.1074/mcp.M116.061036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hänzelmann, P. and Schindelin, H. (2017) The interplay of cofactor interactions and post-translational modifications in the regulation of the AAA+ ATPase p97. Front. Mol. Biosci. 4, 21 10.3389/fmolb.2017.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arumughan, A., Roske, Y., Barth, C., Forero, L.L., Bravo-Rodriguez, K., Redel, A.et al. (2016) Quantitative interaction mapping reveals an extended UBX domain in ASPL that disrupts functional p97 hexamers. Nat. Commun. 7, 13047 10.1038/ncomms13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gouveia, M., Xia, K., Colón, W., Vieira, S.I. and Ribeiro, F. (2017) Protein aggregation, cardiovascular diseases, and exercise training: Where do we stand? Ageing Res. Rev. 40, 1–10 10.1016/j.arr.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 40.Sun, X. and Qiu, H. (2020) Valosin-containing protein, a calcium-associated atpase protein, in endoplasmic reticulum and mitochondrial function and its implications for diseases. Int. J. Mol. Sci. 21, 3842 10.3390/ijms21113842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lejay, A., Fang, F., John, R., Van, J.A.D., Barr, M., Thaveau, F.et al. (2016) Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J. Mol. Cell. Cardiol. 91, 11–22 10.1016/j.yjmcc.2015.12.020 [DOI] [PubMed] [Google Scholar]
- 42.Hausenloy, D.J. and Yellon, D.M. (2013) Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 123, 92–100 10.1172/JCI62874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eltzschig, H.K. and Eckle, T. (2011) Ischemia and reperfusion–from mechanism to translation. Nat. Med. 17, 1391–1401 10.1038/nm.2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donato, M., Evelson, P. and Gelpi, R.J. (2017) Protecting the heart from ischemia/reperfusion injury: an update on remote ischemic preconditioning and postconditioning. Curr. Opin. Cardiol. 32, 784–790 10.1097/HCO.0000000000000447 [DOI] [PubMed] [Google Scholar]
- 45.Bolli, R. (2001) Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J. Mol. Cell. Cardiol. 33, 1897–1918 10.1006/jmcc.2001.1462 [DOI] [PubMed] [Google Scholar]
- 46.Förstermann, U. and Sessa, W.C. (2012) Nitric oxide synthases: regulation and function. Eur. Heart J. 33, 829–837. 837a–837d 10.1093/eurheartj/ehr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu, X., Ge, L., Niu, L., Lian, X., Ma, H. and Pang, L. (2018) The dual role of inducible nitric oxide synthase in myocardial ischemia/reperfusion injury: friend or foe? Oxid. Med. Cell. Longev. 2018, 8364848 10.1155/2018/8364848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lizano, P., Rashed, E., Stoll, S., Zhou, N., Wen, H., Hays, T.T.et al. (2017) The valosin-containing protein is a novel mediator of mitochondrial respiration and cell survival in the heart in vivo. Sci. Rep. 7, 46324 10.1038/srep46324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lizano, P., Rashed, E., Kang, H., Dai, H., Sui, X., Yan, L.et al. (2013) The valosin-containing protein promotes cardiac survival through the inducible isoform of nitric oxide synthase. Cardiovasc. Res. 99, 685–693 10.1093/cvr/cvt136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pohjoismäki, J.L. and Goffart, S. (2017) The role of mitochondria in cardiac development and protection. Free Radic. Biol. Med. 106, 345–354 10.1016/j.freeradbiomed.2017.02.032 [DOI] [PubMed] [Google Scholar]
- 51.Nalbandian, A., Llewellyn, K.J., Gomez, A., Walker, N., Su, H., Dunnigan, A.et al. (2015) In vitro studies in VCP-associated multisystem proteinopathy suggest altered mitochondrial bioenergetics. Mitochondrion 22, 1–8 10.1016/j.mito.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim, N.C., Tresse, E., Kolaitis, R.-M., Molliex, A., Thomas, R.E., Alami, N.H.et al. (2013) VCP is essential for mitochondrial quality control by PINK1/Parkin and this function is impaired by VCP mutations. Neuron 78, 65–80 10.1016/j.neuron.2013.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guardia-Laguarta, C., Liu, Y., Lauritzen, K.H., Erdjument-Bromage, H., Martin, B., Swayne, T.C.et al. (2019) PINK1 content in mitochondria is regulated by ER-associated degradation. J. Neurosci. 39, 7074–7085 10.1523/JNEUROSCI.1691-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimura, Y., Fukushi, J., Hori, S., Matsuda, N., Okatsu, K., Kakiyama, Y.et al. (2013) Different dynamic movements of wild-type and pathogenic VCPs and their cofactors to damaged mitochondria in a parkin-mediated mitochondrial quality control system. Genes Cells 18, 1131–1143 10.1111/gtc.12103 [DOI] [PubMed] [Google Scholar]
- 55.Stoll, S., Xi, J., Ma, B., Leimena, C., Behringer, E.J., Qin, G.et al. (2019) The valosin-containing protein protects the heart against pathological Ca2+ overload by modulating Ca2+ uptake proteins. Toxicol. Sci. 171, 473–484 10.1093/toxsci/kfz164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antony, A.N., Paillard, M., Moffat, C., Juskeviciute, E., Correnti, J., Bolon, B.et al. (2016) MICU1 regulation of mitochondrial Ca2+ uptake dictates survival and tissue regeneration. Nat. Commun. 7, 10955 10.1038/ncomms10955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ide, Y., Horie, T., Saito, N., Watanabe, S., Otani, C., Miyasaka, Y.et al. (2019) Cardioprotective effects of VCP modulator KUS121 in murine and porcine models of myocardial infarction. JACC Basic Transl. Sci. 4, 701–714 10.1016/j.jacbts.2019.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samak, M., Fatullayev, J., Sabashnikov, A., Zeriouh, M., Schmack, B., Farag, M.et al. (2016) Cardiac hypertrophy: An introduction to molecular and cellular basis. Med. Sci. Monit. Basic Res. 22, 75–79 10.12659/MSMBR.900437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asanuma, H. and Kitakaze, M. (2011) Does the treatment of both hypertension and cardiac hypertrophy not only prevent but also treat acute myocardial infarction? Circ. J. 75, 1061–1062 10.1253/circj.CJ-11-0292 [DOI] [PubMed] [Google Scholar]
- 60.Tham, Y.K., Bernardo, B.C., Ooi, J.Y.Y., Weeks, K.L. and McMullen, J.R. (2015) Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch. Toxicol. 89, 1401–1438 10.1007/s00204-015-1477-x [DOI] [PubMed] [Google Scholar]
- 61.Sciarretta, S., Forte, M., Frati, G. and Sadoshima, J. (2018) New insights into the role of mTOR signaling in the cardiovascular system. Circ. Res. 122, 489–505 10.1161/CIRCRESAHA.117.311147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shende, P., Xu, L., Morandi, C., Pentassuglia, L., Heim, P., Lebboukh, S.et al. (2016) Cardiac mTOR complex 2 preserves ventricular function in pressure-overload hypertrophy. Cardiovasc. Res. 109, 103–114 10.1093/cvr/cvv252 [DOI] [PubMed] [Google Scholar]
- 63.Zhang, D., Contu, R., Latronico, M.V.G., Zhang, J., Rizzi, R., Catalucci, D.et al. (2010) MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J. Clin. Invest. 120, 2805–2816 10.1172/JCI43008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou, N., Ma, B., Stoll, S., Hays, T.T. and Qiu, H. (2017) The valosin-containing protein is a novel repressor of cardiomyocyte hypertrophy induced by pressure overload. Aging Cell 16, 1168–1179 10.1111/acel.12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou, N., Stoll, S. and Qiu, H. (2017) VCP represses pathological cardiac hypertrophy. Aging (Albany NY) 9, 2469–2470 10.18632/aging.101357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shimizu, I. and Minamino, T. (2016) Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 97, 245–262 10.1016/j.yjmcc.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 67.Pillai, V.B., Sundaresan, N.R. and Gupta, M.P. (2014) Regulation of Akt signaling by sirtuins: its implication in cardiac hypertrophy and aging. Circ. Res. 114, 368–378 10.1161/CIRCRESAHA.113.300536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein, J.B., Barati, M.T., Wu, R., Gozal, D., Sachleben, L.R.J., Kausar, H.et al. (2005) Akt-mediated valosin-containing protein 97 phosphorylation regulates its association with ubiquitinated proteins. J. Biol. Chem. 280, 31870–31881 10.1074/jbc.M501802200 [DOI] [PubMed] [Google Scholar]
- 69.Vandermoere, F., El Yazidi-Belkoura, I., Slomianny, C., Demont, Y., Bidaux, G., Adriaenssens, E.et al. (2006) The valosin-containing protein (VCP) is a target of Akt signaling required for cell survival. J. Biol. Chem. 281, 14307–14313 10.1074/jbc.M510003200 [DOI] [PubMed] [Google Scholar]
- 70.Lloyd-Jones, D.M. (2016) Slowing progress in cardiovascular mortality rates: you reap what you sow. JAMA Cardiol. 1, 599–600 10.1001/jamacardio.2016.1348 [DOI] [PubMed] [Google Scholar]
- 71.MacRae, C.A., Roden, D.M. and Loscalzo, J. (2016) The future of cardiovascular therapeutics. Circulation 133, 2610–2617 10.1161/CIRCULATIONAHA.116.023555 [DOI] [PubMed] [Google Scholar]
- 72.van den Boom, J. and Meyer, H. (2018) VCP/p97-mediated unfolding as a principle in protein homeostasis and signaling. Mol. Cell 69, 182–194 10.1016/j.molcel.2017.10.028 [DOI] [PubMed] [Google Scholar]
- 73.Mailleux, F., Beauloye, C., Balligand, J.-L., Horman, S. and Bertrand, L. (2018) Studying the role of AMPK in cardiac hypertrophy and protein synthesis. Methods Mol. Biol. 1732, 321–342 10.1007/978-1-4939-7598-3_21 [DOI] [PubMed] [Google Scholar]
- 74.Lan, B., Chai, S., Wang, P. and Wang, K. (2017) VCP/p97/Cdc48, a linking of protein homeostasis and cancer therapy. Curr. Mol. Med. 17, 608–618 10.2174/1566524018666180308111238 [DOI] [PubMed] [Google Scholar]