Pseudomonas aeruginosa and Staphylococcus aureus are frequently coisolated from multiple infection sites, including the lungs of individuals with cystic fibrosis (CF) and nonhealing diabetic foot ulcers. Coinfection with P. aeruginosa and S. aureus has been shown to produce worse outcomes compared to infection with either organism alone. Furthermore, the ability of these pathogens to form biofilms enables them to cause persistent infection and withstand antimicrobial therapy. In this study, we found that P. aeruginosa-secreted products dramatically increase the ability of the antibiotic norfloxacin to kill S. aureus biofilms. Understanding how interspecies interactions alter the antibiotic susceptibility of bacterial biofilms may inform treatment decisions and inspire the development of new therapeutic strategies.
KEYWORDS: Pseudomonas aeruginosa, Staphylococcus aureus, antibiotics, fluoroquinolone, biofilm
ABSTRACT
The thick mucus within the airways of individuals with cystic fibrosis (CF) promotes frequent respiratory infections that are often polymicrobial. Pseudomonas aeruginosa and Staphylococcus aureus are two of the most prevalent pathogens that cause CF pulmonary infections, and both are among the most common etiologic agents of chronic wound infections. Furthermore, the ability of P. aeruginosa and S. aureus to form biofilms promotes the establishment of chronic infections that are often difficult to eradicate using antimicrobial agents. In this study, we found that multiple LasR-regulated exoproducts of P. aeruginosa, including 2-heptyl-4-hydroxyquinoline N-oxide (HQNO), siderophores, phenazines, and rhamnolipids, likely contribute to the ability of P. aeruginosa PA14 to shift S. aureus Newman norfloxacin susceptibility profiles. Here, we observe that exposure to P. aeruginosa exoproducts leads to an increase in intracellular norfloxacin accumulation by S. aureus. We previously showed that P. aeruginosa supernatant dissipates the S. aureus membrane potential, and furthermore, depletion of the S. aureus proton motive force recapitulates the effect of the P. aeruginosa PA14 supernatant on shifting norfloxacin sensitivity profiles of biofilm-grown S. aureus Newman. From these results, we hypothesize that exposure to P. aeruginosa PA14 exoproducts leads to increased uptake of the drug and/or an impaired ability of S. aureus Newman to efflux norfloxacin. Surprisingly, the effect observed here of P. aeruginosa PA14 exoproducts on S. aureus Newman susceptibility to norfloxacin seemed to be specific to these strains and this antibiotic. Our results illustrate that microbially derived products can alter the ability of antimicrobial agents to kill bacterial biofilms.
IMPORTANCE Pseudomonas aeruginosa and Staphylococcus aureus are frequently coisolated from multiple infection sites, including the lungs of individuals with cystic fibrosis (CF) and nonhealing diabetic foot ulcers. Coinfection with P. aeruginosa and S. aureus has been shown to produce worse outcomes compared to infection with either organism alone. Furthermore, the ability of these pathogens to form biofilms enables them to cause persistent infection and withstand antimicrobial therapy. In this study, we found that P. aeruginosa-secreted products dramatically increase the ability of the antibiotic norfloxacin to kill S. aureus biofilms. Understanding how interspecies interactions alter the antibiotic susceptibility of bacterial biofilms may inform treatment decisions and inspire the development of new therapeutic strategies.
INTRODUCTION
Cystic fibrosis (CF) is a genetic multiorgan disease. Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene result in a defective CFTR channel that causes ionic imbalances leading to thick mucus buildup in the airway, which promotes chronic polymicrobial infection. Two organisms that are commonly coisolated from the airways of individuals with CF are Pseudomonas aeruginosa and Staphylococcus aureus (1). Individually, these pathogens are associated with poor clinical outcomes (2–5); however, it has become clear that coinfection with both P. aeruginosa and S. aureus can lead to worse prognoses compared to monospecies infections with either of these organisms individually (5–9). In addition, these pathogens frequently coexist in chronic infections of nonhealing wounds, such as diabetic foot ulcers (10, 11).
Both P. aeruginosa and S. aureus have the ability to form robust biofilms. The biofilm mode of growth enables microbes to survive in the face of extreme environmental challenges, including high doses of antimicrobial agents (12). Moreover, it is becoming increasingly evident that interspecies interactions can have profound consequences on antibiotic susceptibility profiles within polymicrobial biofilm communities (13–19). In particular, multiple studies have uncovered interactions between P. aeruginosa and S. aureus that influence drug tolerance in diverse ways (13, 17, 20–29).
In this study, we identified an interaction between P. aeruginosa PA14 and S. aureus Newman that potentiates the activity of the nucleic acid synthesis inhibitor norfloxacin against S. aureus biofilms. Alone, norfloxacin has a modest impact on S. aureus Newman biofilm cell viability; however, we observed a striking 3- to 4-log increase in the ability of norfloxacin to kill S. aureus Newman biofilms in the presence of secreted factors produced by P. aeruginosa PA14. Our data are consistent with a model whereby P. aeruginosa PA14 exoproducts increase intracellular levels of norfloxacin within S. aureus Newman cells, leading to increased efficacy of this antibiotic. Thus, our findings could have implications for the treatment of persistent multispecies infections in the context of chronic pulmonary diseases and nonhealing wounds.
RESULTS
P. aeruginosa PA14 supernatant increases S. aureus Newman biofilm sensitivity to norfloxacin.
Previously, we performed a screen using Biolog Phenotype MicroArray panels to assess changes in the susceptibility of S. aureus to antibiotics in response to P. aeruginosa-secreted products (24, 28). This approach allowed us to survey a large diversity of compounds for their antimicrobial activity alone versus in the presence of P. aeruginosa exoproducts. In this screen, we found that P. aeruginosa PA14 cell-free culture supernatant altered the ability of many compounds to kill S. aureus Newman, leading to either increased tolerance to some compounds or, alternatively, increased sensitivity to other compounds. Among the drugs that had increased efficacy against S. aureus Newman in the presence of P. aeruginosa PA14 supernatant were several DNA synthesis inhibitors, including the fluoroquinolone norfloxacin (28). Interestingly, we observed that P. aeruginosa PA14 exoproducts had the opposite effect on S. aureus Newman sensitivity to RNA synthesis inhibitors, whereby P. aeruginosa protected S. aureus from rifampin (a rifamycin class antibiotic) and novobiocin (an aminocoumarin class antibiotic) (28).
Our initial Biolog screen allowed us to assess whether combinations of different antimicrobial compounds and secreted products altered the sensitivity of planktonic S. aureus cells to these compounds and/or whether these combinations altered the ability of S. aureus to form a biofilm. To test whether preformed S. aureus biofilms exhibited altered sensitivity to norfloxacin in the presence of P. aeruginosa PA14 exoproducts, we allowed the establishment of S. aureus Newman biofilms for 6 h prior to the addition of P. aeruginosa supernatant and the antibiotic. Previously, we showed that sessile S. aureus Newman cells after 6 h of incubation behave as biofilms under our experimental conditions as judged by their high-level tolerance to multiple antibiotics (24). Additionally, we showed that exposure to P. aeruginosa supernatant alone does not alter the cell viability of S. aureus biofilms (24, 28).
Here, we found that treatment with P. aeruginosa PA14 supernatant enhanced norfloxacin’s efficacy against S. aureus Newman cells growing as early (6 h) biofilms. Specifically, P. aeruginosa supernatant increased the susceptibility of early S. aureus Newman biofilms to 12.5 μg/ml of norfloxacin by 2 to 3 logs (Fig. 1A) and to 25 μg/ml of norfloxacin by 4 logs (Fig. 1B). In contrast, P. aeruginosa PA14 exoproducts did not alter the susceptibility of preformed S. aureus Newman biofilms to the other DNA synthesis inhibitors tested (not shown), including other quinolones (28).
FIG 1.
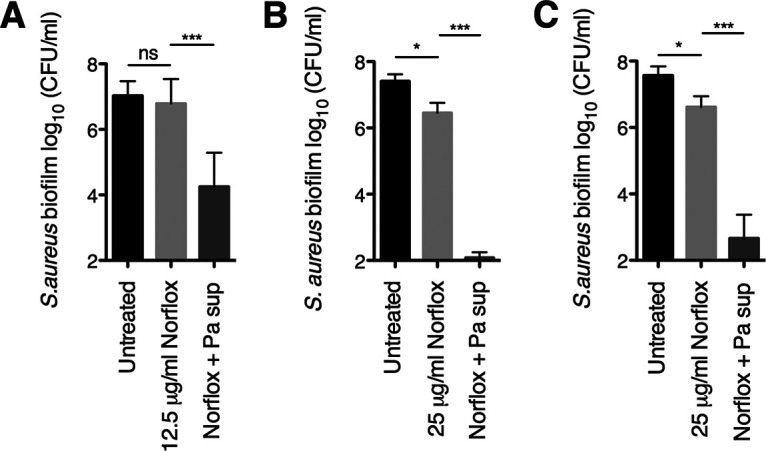
P. aeruginosa PA14 increases the susceptibility of early and mature S. aureus Newman biofilms to norfloxacin. (A and B) Biofilm disruption assays on plastic were performed with S. aureus Newman, P. aeruginosa PA14 supernatant (Pa sup), and norfloxacin (Norflox) at 12.5 μg/ml (A) or 25 μg/ml (B). Biofilms were grown for 6 h and exposed to the above treatments for 18 h, and then S. aureus biofilm CFU values were determined. (C) Biofilm disruption assays on plastic were performed with S. aureus Newman, P. aeruginosa PA14 supernatant (Pa sup), and norfloxacin (Norflox) at 25 μg/ml. Biofilms were grown for 24 h and exposed to the above treatments for 18 h, and then S. aureus biofilm CFU values were determined. Each column displays the average from at least six biological replicates, each with three technical replicates. Error bars indicate standard deviation (SD). ns, not significant; *, P < 0.05; ***, P < 0.001; values were determined by ordinary one-way analysis of variance (ANOVA) and Tukey’s multiple comparison posttest.
Additionally, we tested whether P. aeruginosa PA14 exoproducts can influence the ability of norfloxacin to kill more mature S. aureus Newman biofilms. In this experiment, we allowed S. aureus biofilms to establish for 24 h prior to treatment with supernatant and the antibiotic. Strikingly, we found that the combination of P. aeruginosa supernatant and 25 μg/ml of norfloxacin caused a 4-log reduction in the cell viability of 24-h-grown S. aureus biofilms compared to treatment with norfloxacin alone (Fig. 1C). Thus, these data indicate that P. aeruginosa PA14-produced factors alter the norfloxacin sensitivity profiles of early and more established S. aureus Newman biofilms to a similar degree.
Testing the contribution of P. aeruginosa exoproducts to the ability of P. aeruginosa PA14 to increase S. aureus Newman biofilm sensitivity to norfloxacin.
Next, we investigated which P. aeruginosa PA14-secreted products may be responsible for shifting S. aureus Newman biofilm sensitivity to norfloxacin. We first tested the contribution of P. aeruginosa-derived factors that were previously found to mediate other P. aeruginosa-S. aureus interactions that altered drug sensitivity profiles (13, 24, 25, 28, 30). In particular, we evaluated the contribution of 2-heptyl-4-hydroxyquinoline N-oxide (HQNO) and Pseudomonas quinolone signal (PQS), two components of the PQS quorum sensing system biosynthetic pathway, as well as the two siderophores produced by P. aeruginosa (pyoverdine and pyochelin) by examining P. aeruginosa PA14 strains with mutations in these factors (ΔpqsA, ΔpqsH, ΔpqsL, ΔpvdA, ΔpchE, ΔpvdA ΔpchE, and ΔpqsL ΔpvdA ΔpchE strains).
We tested the ability of supernatants prepared from the above P. aeruginosa PA14 strains to alter the susceptibility of S. aureus Newman biofilms to norfloxacin at a concentration of 25 μg/ml. We observed that the phenotypes of these individual mutant strains are highly variable (Fig. 2A). However, on average, we observed that supernatants from strains that are deficient in either the biosynthesis of the entire PQS pathway (ΔpqsA strain) or in the production of HQNO alone (ΔpqsL strain) each had a partial but statistically significant defect in increasing S. aureus Newman biofilm sensitivity to norfloxacin relative to the wild-type (WT) P. aeruginosa PA14 supernatant (Fig. 2A). Additionally, supernatant from strains lacking either PQS (ΔpqsH strain) or siderophores (ΔpvdA ΔpchE strain) had modest defects in shifting drug sensitivity that were not statistically significant. Furthermore, we observed that the supernatant from a P. aeruginosa strain lacking the production of HQNO and siderophores (ΔpqsL ΔpvdA ΔpchE triple mutant) had a similar phenotype as that of the ΔpqsA and ΔpqsL single deletion mutants (Fig. 2A).
FIG 2.
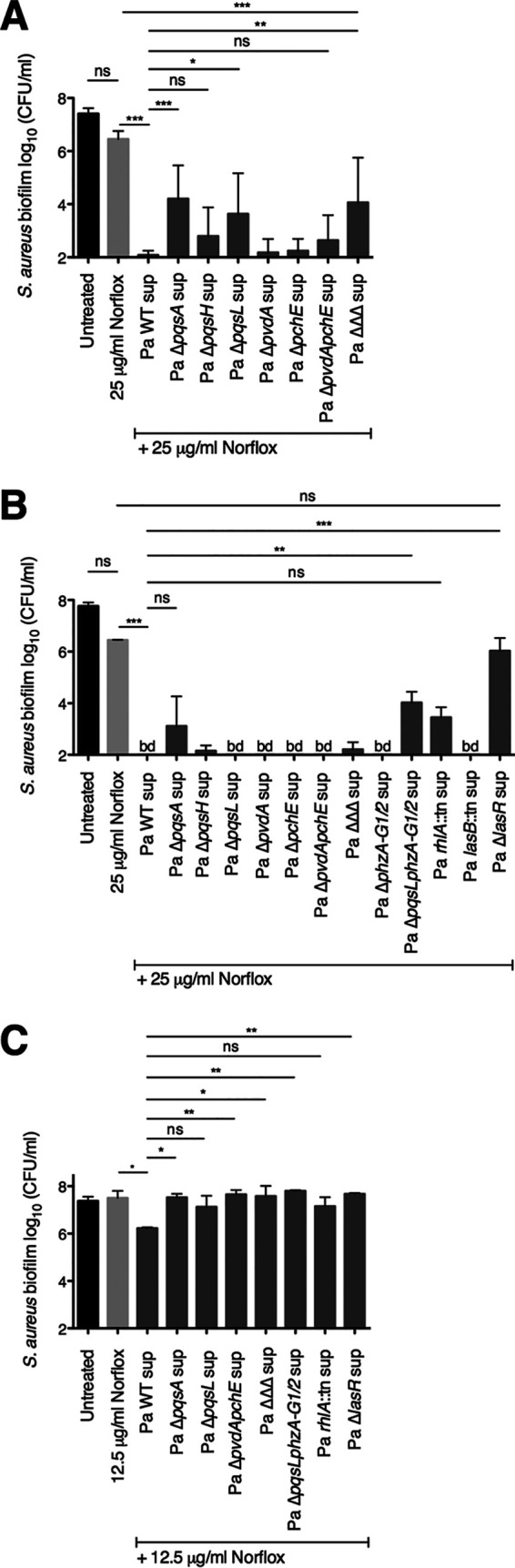
Testing the contribution of P. aeruginosa-secreted products to the ability of P. aeruginosa PA14 to shift S. aureus Newman biofilm sensitivity to norfloxacin. Biofilm disruption assays on plastic were performed with S. aureus Newman, wild-type P. aeruginosa PA14 and the specified deletion mutant supernatants (Pa sup), and norfloxacin (Norflox) at 25 μg/ml (A, B) or 12.5 μg/ml (C). Biofilms were grown for 6 h and exposed to the above treatments for 18 h, and S. aureus biofilm CFU values were determined. Each column displays the average from at least seven biological replicates (A) or the average from two biological replicates (B, C), each with three technical replicates. Error bars indicate SD. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; values were determined by ordinary one-way ANOVA and Tukey’s multiple comparison posttest.
Next, we assessed the potential contribution of other P. aeruginosa-secreted products to this interspecies interaction. We assayed the following P. aeruginosa PA14 strains harboring mutations in genes encoding regulatory proteins or genes required for the production of secreted products: ΔlasR strain (the master transcriptional regulator of the Las quorum sensing system), lasB::Tn strain (elastase), rhlA::Tn strain (rhamnolipids), and ΔphzA-G1/2 strain (phenazines) (Fig. 2B). Importantly, we previously showed that supernatants from each of the P. aeruginosa PA14 strains tested here do not alter the viability of S. aureus Newman biofilms in the absence of antibiotic (24, 28).
We found that supernatant from a strain that is unable to produce phenazines (ΔphzA-G1/2 strain) retained the ability to increase S. aureus Newman biofilm sensitivity to norfloxacin (Fig. 2B). However, supernatant from a strain that lacks both HQNO and phenazines (ΔpqsL ΔphzA-G1/2 strain) had a partial but significant defect in altering S. aureus antibiotic susceptibility relative to that of the wild-type P. aeruginosa PA14 supernatant. Additionally, supernatant from a strain that is deficient in the production of rhamnolipids (rhlA::Tn strain) led to a modest defect that was not statistically significant. Finally, supernatant from a strain lacking the quorum sensing system transcriptional regulator LasR (ΔlasR strain) completely lacked the ability to enhance the antistaphylococcal efficacy of norfloxacin (Fig. 2B). Based on the results from the genetic analyses described above, we hypothesize that multiple LasR-regulated factors (such as HQNO, siderophores, phenazines, and rhamnolipids) may contribute to the ability of P. aeruginosa to shift S. aureus norfloxacin susceptibility profiles.
The combination of P. aeruginosa PA14 supernatant with norfloxacin at a concentration of 25 μg/ml resulted in S. aureus Newman biofilm viability that approaches the limit of detection of our assay (Fig. 2A and B). Thus, we halved the concentration of norfloxacin used and tested the ability of a subset of P. aeruginosa strains to alter norfloxacin sensitivity profiles. At this lower dose of norfloxacin, P. aeruginosa PA14 supernatants from multiple strains (the ΔpqsA, ΔpvdA ΔpchE, ΔpqsL ΔpvdA ΔpchE, ΔpqsL ΔphzA-G1/2, and ΔlasR strains) had a statistically significant defect in shifting the drug susceptibility of S. aureus Newman biofilms (Fig. 2C). These results again suggest the possibility that multiple LasR-regulated factors play a role in this interspecies interaction.
Exogenous HQNO does not increase S. aureus Newman biofilm sensitivity to norfloxacin.
From the results above and our previous studies (24, 28), we hypothesized that the P. aeruginosa-produced small molecule HQNO may play a role in increasing the efficacy of norfloxacin against S. aureus biofilms. HQNO is a known inhibitor of the S. aureus electron transport chain (ETC) (20). Furthermore, this small molecule has been shown to impact S. aureus physiology in various ways, including driving a shift to fermentative growth (30) and increasing membrane fluidity (28). In previous studies performed by our laboratory, we discovered that the addition of pure HQNO alone was sufficient to alter S. aureus biofilm susceptibility to the cell wall-targeting antibiotic vancomycin (24) as well as the membrane-active compound chloroxylenol (28). Therefore, we next tested whether the addition of exogenous HQNO alone is sufficient to alter the efficacy of norfloxacin against S. aureus.
In a previous study, we determined that the concentration of HQNO in P. aeruginosa PA14 supernatants under our study conditions is ∼10 μg/ml (24). Here, we observed that the addition of HQNO at 100 μg/ml did not significantly alter the sensitivity of S. aureus Newman biofilms to norfloxacin at either concentration tested (12.5 μg/ml or 25 μg/ml norfloxacin) relative to that of treatment with the antibiotic alone (Fig. 3A and B, respectively). This result does not rule out the possibility that HQNO contributes to the ability of P. aeruginosa to alter norfloxacin efficacy; however, these data indicate that HQNO alone is not sufficient for this phenotype.
FIG 3.
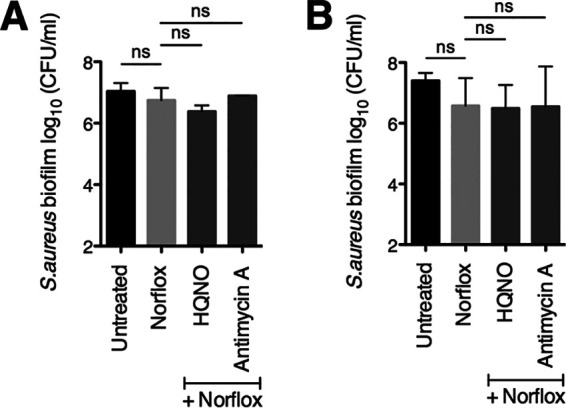
Addition of exogenous HQNO or antimycin A does not alter S. aureus Newman biofilm sensitivity to norfloxacin. Biofilm disruption assays on plastic were performed with S. aureus (Sa) Newman, HQNO at 100 μg/ml, antimycin A at 100 μg/ml, and norfloxacin (Norflox) at 12.5 μg/ml (A) or 25 μg/ml (B). Biofilms were grown for 6 h and exposed to the above treatments for 18 h, and S. aureus biofilm CFU values were determined. Each column displays the average from at least two biological replicates, each with three technical replicates. Error bars indicate SD. ns, not significant; values were determined by ordinary one-way ANOVA and Tukey’s multiple comparison posttest.
Furthermore, we tested whether treatment with antimycin A influences S. aureus susceptibility to norfloxacin, as this compound is known to inhibit complex III of the mammalian ETC, but it has been reported to be unable to inhibit the ETC of S. aureus (31, 32). Previously, we found that antimycin A can indeed deplete S. aureus membrane potential and that, similar to HQNO, antimycin A increases S. aureus biofilm sensitivity to chloroxylenol (28). Here, as in the case of HQNO, we observed that exposure of S. aureus Newman biofilms to antimycin A did not shift susceptibility to norfloxacin at either antibiotic concentration tested (Fig. 3).
Membrane-active compounds alter S. aureus Newman biofilm sensitivity to norfloxacin.
Norfloxacin is believed to enter S. aureus cells by diffusion; thus, it is possible that increasing membrane permeability or fluidity (which may influence membrane permeability) facilitates entry of the antibiotic. Our findings from a previous study suggest that HQNO has a fluidizing effect on the S. aureus cell membrane, which enhanced the efficacy of the membrane-active antiseptic chloroxylenol (28). Here, we tested whether altering membrane fluidity can impact S. aureus sensitivity to norfloxacin.
To test this hypothesis, we first treated S. aureus biofilms with the known fluidizing agent benzyl alcohol (33–35) in combination with norfloxacin. Previous studies have reported that treatment with 10 to 50 mM benzyl alcohol increases microbial cell membrane fluidity (33–37). We observed an approximate 1-log reduction in S. aureus Newman biofilm cell viability upon exposure to the combination of benzyl alcohol and norfloxacin relative to that of treatment with norfloxacin alone (Fig. 4A). In addition, we tested whether the opposite change—decreased fluidity—impacts S. aureus susceptibility to this antibiotic. Upon addition of dimethyl sulfoxide (DMSO), a compound that has been described to increase the rigidity of cell membranes (36, 37), we did not observe any changes in antibiotic sensitivity (Fig. 4B).
FIG 4.

Testing the effect of changing membrane fluidity on the efficacy of norfloxacin against S. aureus Newman biofilms. (A and B) Biofilm disruption assays on plastic were performed with S. aureus Newman, norfloxacin (Norflox) at 25 μg/ml and benzyl alcohol (BnOH) at 50 mM (A) or dimethyl sulfoxide (DMSO) at 1% and 6% (B). Biofilms were grown for 6 h and exposed to the above treatments for 18 h, and S. aureus biofilm CFU values were determined. Each column displays the average from at least three biological replicates, each with three technical replicates. (C) Biofilm disruption assays on plastic were performed with S. aureus Newman, norfloxacin (Norflox) at 12.5 μg/ml, and the specified fatty acids at 100 μg/ml. Biofilms were grown for 6 h and exposed to the above treatments for 18 h, and S. aureus biofilm CFU values were determined. Each column displays the average from two biological replicates, each with three technical replicates. Error bars indicate SD. ns, not significant; *, P < 0.05; values were determined by ordinary one-way ANOVA and Tukey’s multiple comparison posttest.
S. aureus is known to incorporate unsaturated fatty acids into the cell membrane (38, 39), which has been shown to increase membrane fluidity (39, 40). Here, we observe that the addition of unsaturated fatty acids increased S. aureus Newman biofilm sensitivity to norfloxacin by 2 to 3 logs relative to that of treatment with the drug alone (Fig. 4C). Therefore, these data suggest that modulating S. aureus membrane fluidity has the potential to influence the efficacy of norfloxacin.
It is possible that by increasing membrane fluidity, the above treatments also change the permeability of the membrane, which may allow for increased entry of norfloxacin into the cell and explain the enhancement of antibiotic-mediated killing that we observed. The findings that HQNO enhances membrane fluidity (28) but does not increase sensitivity to norfloxacin on its own (Fig. 3) indicate that HQNO-mediated changes in membrane fluidity are not sufficient to explain our findings in this study.
Exposure to P. aeruginosa PA14 exoproducts increases the intracellular accumulation of norfloxacin by S. aureus Newman.
As described above, a potential mechanism by which P. aeruginosa potentiates the antistaphylococcal efficacy of norfloxacin is via an increase in S. aureus intracellular antibiotic levels. Intracellular drug accumulation can be influenced by changes in uptake as well as efflux. In S. aureus, norfloxacin uptake is thought to occur via simple diffusion (41–43). In contrast, norfloxacin efflux is mediated by multidrug efflux pumps that are powered by the proton motive force (PMF) (44). Thus, norfloxacin efflux can be inhibited via the addition of the proton ionophore carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (44, 45).
Here, we tested whether P. aeruginosa-secreted factors alter the intracellular accumulation of norfloxacin by S. aureus. In this experiment, we assessed intracellular S. aureus norfloxacin levels by taking advantage of norfloxacin’s intrinsic fluorescence. In previous studies, fluoroquinolone uptake assays were conducted using drug concentrations ranging from 5 to 20 μg/ml (46–49). Based on this range, we selected a norfloxacin concentration of 10 μg/ml for a 30-min-long treatment of planktonic S. aureus cells in this experiment. As a positive control, we showed that exposure of the cells to CCCP at a concentration of 5 μM led to a significant increase in S. aureus Newman intracellular levels of norfloxacin relative to those associated with exposure to drug alone (Fig. 5A). As an additional control, we verified that exposure to P. aeruginosa PA14 supernatants did not contribute to the fluorescence signal in the absence of norfloxacin treatment (Fig. 5A).
FIG 5.
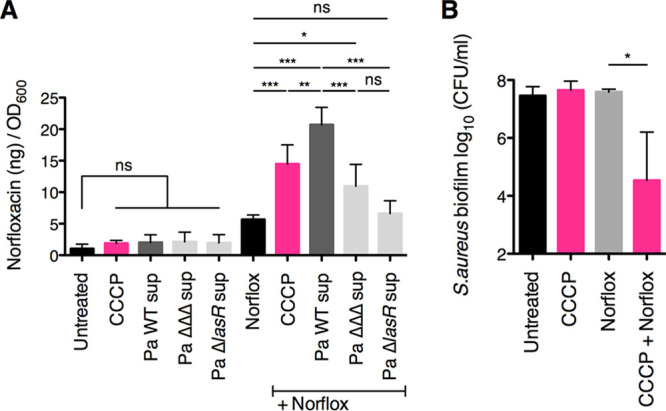
Exposure to P. aeruginosa PA14 exoproducts increases S. aureus Newman intracellular norfloxacin levels. Antibiotic internalization assays were performed with S. aureus Newman, wild-type P. aeruginosa PA14 and the specified deletion mutant supernatants (Pa sup), carbonyl cyanide 3-chlorophenylhydrazone (CCCP) at 5 μM, and norfloxacin (Norflox) at 10 μg/ml. Intracellular norfloxacin levels within planktonic S. aureus cells were quantified by measuring the intrinsic fluorescence at 440 nm following excitation at 281 nm, and a standard curve was used to relate fluorescence values to the amount of intracellular norfloxacin (ng) in each sample. Values are reported as the amount of intracellular norfloxacin per OD600 unit. Each column displays the average from at least two biological replicates, each with three technical replicates. (B) Biofilm disruption assays on plastic were performed with S. aureus Newman, CCCP at 5 μM, and norfloxacin (Norflox) at 25 μg/ml. Biofilms were grown for 6 h and exposed to the above treatments for 18 h, and S. aureus biofilm CFU values were determined. Each column displays the average from three biological replicates, each with three technical replicates. Error bars indicate SD. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; values were determined by ordinary one-way ANOVA and Tukey’s multiple comparison posttest.
Interestingly, we found that exposure to wild-type P. aeruginosa PA14 supernatant led to S. aureus Newman intracellular levels of norfloxacin that were significantly higher than those associated with exposure to norfloxacin alone and also significantly higher than those associated with treatment with CCCP (Fig. 5A). Additionally, we observed that exposure to supernatant from the P. aeruginosa PA14 ΔpqsL ΔpvdA ΔpchE deletion mutant (the triple mutant) resulted in significantly lower S. aureus Newman intracellular norfloxacin levels relative to those associated with treatment with wild-type P. aeruginosa PA14 supernatant (Fig. 5A).
Furthermore, treatment with supernatant from the P. aeruginosa PA14 ΔlasR deletion mutant entirely abrogated the ability of the P. aeruginosa supernatant to increase S. aureus norfloxacin accumulation, leading to intracellular drug levels that were not significantly different from those associated with exposure to the drug alone (Fig. 5A). Together, these data suggest that treatment with P. aeruginosa PA14 exoproducts increases S. aureus Newman intracellular accumulation of norfloxacin, which may occur via modulation of influx and/or efflux of this antibiotic.
Moreover, the addition of CCCP at 5 μM significantly enhanced the antistaphylococcal efficacy of norfloxacin under our study conditions (Fig. 5B), which suggests that a decrease in PMF can potentiate the activity of norfloxacin against S. aureus biofilms perhaps via allowing increased intracellular accumulation of this antibiotic.
P. aeruginosa PA14 supernatant does not impact the expression of selected S. aureus Newman antibiotic transporters.
Previously, our laboratory performed a transcriptome sequencing (RNA-seq) experiment to determine whether S. aureus gene expression changes during coculture with P. aeruginosa (30). We observed that multiple S. aureus drug transporters are differentially regulated in the presence of P. aeruginosa (these data are summarized in Table 1). However, the following caveats to this experiment should be considered: S. aureus was cocultured with P. aeruginosa PA14 instead of being exposed to P. aeruginosa cell-free culture supernatants, a different S. aureus strain was used (8325-4), and the RNA-seq experiment was performed on a single biological replicate.
TABLE 1.
Testing strains with transposon insertions in genes coding for S. aureus antibiotic transporters that were differentially regulated upon coculture with P. aeruginosa cellsa
| Gene IDb | Tn IDc | Gene | Gene annotation | Fold changed |
|---|---|---|---|---|
| 02418 | NE12 | lmrS | Drug resistance transporter, EmrB/QacA subfamily | −6.7 |
| 01876 | NE17 | Putative drug transporter | −3.7 | |
| 02700 | NE179 | mdeAe | Multidrug resistance protein | −14.9 |
| 02003 | NE370 | Toxin exporting ABC transporter, permease-ATP-binding protein, putative | −11.4 | |
| 00315 | NE405 | mepAe | MATE efflux family protein | −7.8 |
| 02420 | NE531 | sdrMe | Putative drug transporter | −2.9 |
| 00078 | NE749 | sbnD | Putative drug transporter | +1.3 |
| 02818 | NE773 | Drug transporter | −6.0 | |
| 02629 | NE781 | Multidrug resistance protein B, drug resistance transporter | −5.1 | |
| 00246 | NE901 | Putative drug transporter | −1.8 | |
| 00703 | NE1034 | norAe | Multidrug resistance protein | −1.6 |
| 02531 | NE1041 | Putative drug transporter | −9.8 | |
| 01448 | NE1184 | norBe | Putative drug transporter | +11.7 |
| 02740 | NE1400 | Putative drug transporter | −4.1 | |
| 00058 | NE1804 | norCe | Putative drug transporter | −2.0 |
| 02419 | NE1616 | sepA | Hypothetical protein | −11.8 |
Reported are data from an RNA-seq experiment from a previous study from our laboratory (30). Briefly, S. aureus 8325-4 was cocultured with P. aeruginosa PA14 cells or grown in monococulture in MEM + l-Gln on CF-derived epithelial cells for 6 h prior to RNA isolation and sequencing. The fold change shown indicates the expression in coculture versus the monococulture of S. aureus, with negative numbers indicating lower expression in coculture. Note that only a single biological replicate was performed for this experiment.
SAOUHSC gene number.
Transposon identification number.
Coculture/monococulture from a single replicate RNA-seq experiment.
Genes coding for antibiotic transporters that are reported to efflux norfloxacin.
Norfloxacin is a substrate of several multidrug efflux pumps belonging to the major facilitator superfamily (MFS) (NorA, NorB, NorC, MdeA, and SdrM) as well as the multidrug and toxic compound extrusion (MATE) family member MepA (44, 50). Among these genes, only norB expression increased during coculture (11.7-fold increase in coculture) (Table 1). In contrast, all of the other known norfloxacin transporters were downregulated in the presence of P. aeruginosa cells. The expression of norA, norC, and sdrM was minimally altered during coculture (1.6-fold decrease, 2-fold decrease, and 2.9-fold decrease, respectively), whereas mdeA and mepA were strongly downregulated in the presence of P. aeruginosa cells (14.9-fold decrease and 7.8-fold decrease, respectively) (Table 1).
Moreover, the expression of two genes encoding putative drug transporters also decreased in the presence of P. aeruginosa cells: SAOUHSC_02003 decreased 11.4-fold in coculture, and SAOUHSC_02531 decreased 9.8-fold in coculture (Table 1). Similar changes in the expression of these two genes were observed in other studies that examined the transcriptional response of S. aureus to either coculture with P. aeruginosa (51) or exposure to anoxic conditions (52).
From the results above, we hypothesized that P. aeruginosa exoproducts decrease the expression of multiple S. aureus drug efflux pumps. To test this hypothesis, we performed quantitative reverse transcription-PCR (qRT-PCR) to evaluate the effect of P. aeruginosa PA14 supernatant on the expression of S. aureus Newman drug transporters. Based on the RNA-seq results above and their known role in norfloxacin efflux, we selected the following subset of genes: mdeA, mepA, sdrM, and norA.
Exposure to P. aeruginosa PA14 supernatants (prepared either from the wild-type strain or from the ΔlasR mutant) did not lead to significant changes in expression of the four drug transporters above relative to exposure to medium alone (Fig. 6). These results suggest that P. aeruginosa PA14 exoproducts have minimal impact on the expression of at least a subset of S. aureus Newman antibiotic transporters under our study conditions. Therefore, our data do not support a model whereby downregulation of S. aureus efflux pumps contributes to the P. aeruginosa-mediated increase in S. aureus intracellular norfloxacin levels and reduced biofilm cell viability that we observe.
FIG 6.
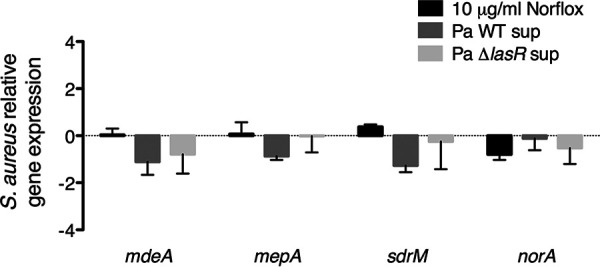
Exposure to P. aeruginosa PA14 exoproducts does not alter expression of a subset of S. aureus Newman antibiotic transporters. Quantitative reverse transcription-PCR (qRT-PCR) assays were performed to measure the expression of four S. aureus antibiotic transporters (mdeA, mepA, sdrM, and norA) in S. aureus Newman biofilm populations exposed to norfloxacin (Norflox) at 10 μg/ml, supernatants from wild-type P. aeruginosa PA14 supernatant (Pa WT sup) and the ΔlasR deletion mutant (Pa ΔlasR sup), or medium alone. Expression was normalized to S. aureus rpoB and is presented as relative to expression in medium alone. Each column displays the average from three biological replicates, each with three technical replicates. Error bars indicate SD. None of the above treatment conditions resulted in significant differences in expression for any of the genes tested relative to expression in medium alone by multiple Student t tests and the Holm-Šidák method for correcting multiple comparisons.
Testing the contribution of individual antibiotic transporters to the sensitivity of S. aureus JE2 biofilms to norfloxacin.
Multiple S. aureus efflux pumps are known to transport norfloxacin; however, we wanted to rule out the possibility that a single transporter was sufficient for conferring norfloxacin resistance under our experimental conditions. Thus, we tested whether S. aureus strains with transposon insertions in individual antibiotic transporter genes (listed in Table 1) were hypersensitive to the antibiotic. Treatment with norfloxacin at either concentration tested (12.5 μg/ml or 25 μg/ml) did not significantly reduce the biofilm cell viability of the parental strain (JE2 WT) or any of the transposon mutants (Fig. 7). Therefore, it is likely that multiple antibiotic transporters contribute to the resistance of S. aureus biofilms to norfloxacin under our conditions.
FIG 7.
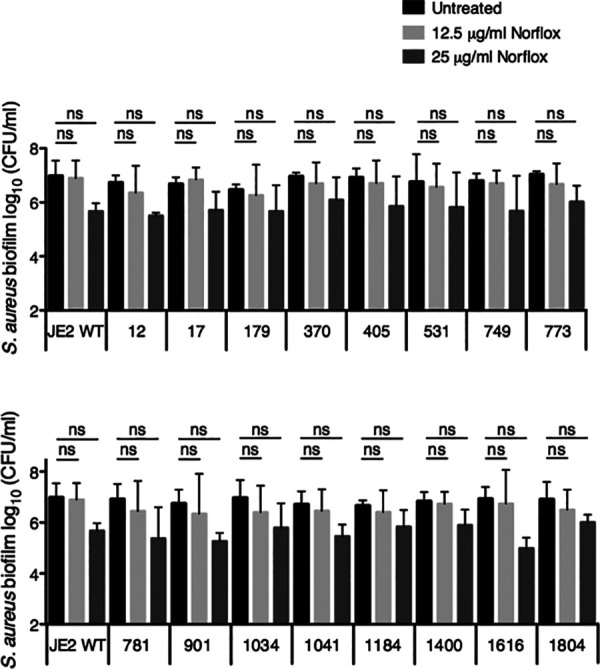
Testing the susceptibility of biofilm-grown S. aureus JE2 antibiotic transporter mutants to norfloxacin. Biofilm disruption assays on plastic were performed with the S. aureus JE2 parental strain or the specified transposon mutant (identified by Nebraska transposon mutant library number and described in Table 1) and norfloxacin (Norflox) at 12.5 μg/ml or 25 μg/ml. Biofilms were grown for 6 h and exposed to the above treatments for 18 h, and S. aureus biofilm CFU values were determined. Each column displays the average from two biological replicates, each with three technical replicates. Error bars indicate SD. ns, not significant; values were determined by ordinary one-way ANOVA and Bonferroni multiple comparison posttest.
The ability of P. aeruginosa to alter S. aureus sensitivity to fluoroquinolones is strain and antibiotic specific.
Finally, we investigated whether the P. aeruginosa-mediated shift in biofilm-grown S. aureus drug susceptibility is unique to the combination of strains and antibiotic used in this study (S. aureus Newman, P. aeruginosa PA14, and norfloxacin). Thus, we tested the following strain combinations: S. aureus JE2 paired with P. aeruginosa PA14 and S. aureus Newman paired with either P. aeruginosa PAO1 or each of the P. aeruginosa CF-derived clinical isolates SMC 1587 (mucoid strain) and SMC 1595 (nonmucoid strain). We have previously shown that exposure of S. aureus Newman biofilms to supernatant from these strains does not affect S. aureus viability (24).
Interestingly, P. aeruginosa supernatant-mediated changes in S. aureus susceptibility to norfloxacin appeared to be unique to the interaction between P. aeruginosa PA14 and S. aureus Newman; we did not observe a similar phenotype for the other S. aureus-P. aeruginosa strain combinations tested (Fig. 8). Furthermore, P. aeruginosa PA14 supernatant did not enhance the efficacy of other fluoroquinolones tested (ciprofloxacin, levofloxacin, or moxifloxacin) against S. aureus Newman biofilms (Fig. 9), suggesting that this phenotype may be unique to norfloxacin.
FIG 8.

Testing additional strains of S. aureus and P. aeruginosa. (A) Biofilm disruption assays on plastic were performed with S. aureus JE2, P. aeruginosa PA14 supernatant (PA14 sup), and norfloxacin (Norflox) at 12.5 μg/ml. Biofilms were grown for 6 h and exposed to the above treatments for 18 h, and S. aureus biofilm CFU values were determined. Each column displays the average from two biological replicates, each with three technical replicates. Error bars indicate SD. ns, not significant; values were determined by ordinary one-way ANOVA and Tukey’s multiple comparison posttest. (B) Biofilm disruption assays on plastic were performed with S. aureus Newman, supernatant (sup) from the specified strains of P. aeruginosa (PA14, PAO1, 1587, and 1595), and norfloxacin (Norflox) at 12.5 μg/ml. Biofilms were grown for 6 h and exposed to the above treatments for 18 h, and S. aureus biofilm CFU values were determined. Each column displays the average from two biological replicates, each with three technical replicates. Error bars indicate SD. ns, not significant; ***, P < 0.001; values were determined by ordinary one-way ANOVA and Dunnett’s multiple comparison posttest.
FIG 9.

P. aeruginosa PA14 supernatant does not alter the susceptibility of S. aureus Newman biofilms to other fluoroquinolones. Biofilm disruption assays on plastic were performed with S. aureus Newman, P. aeruginosa PA14 supernatant (Pa sup), and ciprofloxacin (Cipro) (A), levofloxacin (Levo) (B), or moxifloxacin (Moxi) (C), each at 10 μg/ml. Biofilms were grown for 6 h and exposed to the above treatments for 18 h, and S. aureus biofilm CFU values were determined. Each column displays the average from two biological replicates, each with three technical replicates. Error bars indicate SD. ns, not significant; *, P < 0.05; **, P < 0.01; values were determined by ordinary one-way ANOVA and Tukey’s multiple comparison posttest.
DISCUSSION
In this study, we discovered that P. aeruginosa PA14 influences the antistaphylococcal efficacy of the fluoroquinolone norfloxacin. Alone, norfloxacin at concentrations of 12.5 or 25 μg/ml has minimal efficacy against S. aureus Newman biofilms under our study conditions. Strikingly, we observed that P. aeruginosa PA14 cell-free culture supernatant increased the efficacy of this antibiotic against early (6 h) and more mature (24 h) S. aureus Newman biofilms by 3 to 4 logs. We found that multiple LasR-regulated exoproducts, including HQNO, siderophores, rhamnolipids, and phenazines, likely contribute to the ability of P. aeruginosa PA14 to shift the susceptibility of S. aureus Newman biofilms to norfloxacin.
A potential mechanism by which P. aeruginosa PA14 may be influencing the susceptibility of S. aureus Newman to norfloxacin is via an increase in intracellular drug levels within S. aureus cells. Indeed, we observed that exposure to P. aeruginosa PA14-secreted products led to an increase in S. aureus Newman norfloxacin accumulation. Increased drug accumulation may be a consequence of increased drug uptake, decreased efflux, or a combination of the two. The data presented here suggest that both mechanisms might be at work.
In support of a model of increased influx of this antibiotic upon exposure of S. aureus Newman to P. aeruginosa PA14 exoproducts are the following observations. Exposure to the known fluidizing agent benzyl alcohol led to a modest increase in drug efficacy, while the addition of exogenous unsaturated fatty acids (which increase membrane fluidity) (39, 40) enhanced norfloxacin’s ability to kill S. aureus Newman biofilms by multiple logs. Furthermore, P. aeruginosa-secreted factors HQNO and rhamnolipids have both been reported to alter multiple properties of the S. aureus cell membrane (28, 29). In particular, rhamnolipids have been shown to alter S. aureus membrane potential, fluidity, and permeability (29). Thus, it is possible that these exoproducts facilitate increased diffusion of norfloxacin across the S. aureus cell membrane.
Additionally, several of our findings support a model of decreased efflux. In particular, we previously observed that P. aeruginosa-secreted products can dissipate S. aureus membrane potential (28). Here, we observed that treatment with the PMF inhibitor CCCP, which is well known to inhibit the activity of S. aureus efflux pumps (45), increased S. aureus intracellular norfloxacin levels and enhanced the antistaphylococcal efficacy of norfloxacin under our study conditions, recapitulating the effect of P. aeruginosa PA14 supernatant on these phenotypes. Together, these data are consistent with a mechanism whereby P. aeruginosa PA14 exoproducts interfere with the ability of S. aureus Newman to effectively efflux the antibiotic.
One surprising finding is that the effect we observed for P. aeruginosa supernatants potentiating the action of norfloxacin appears to be specific for P. aeruginosa PA14, S. aureus Newman, and this antibiotic. Previous studies examining the impacts of vancomycin and chloroxylenol did not demonstrate such strain specificity (24, 28). Clearly, future work is needed to elucidate the precise mechanisms involved in this interspecies interaction.
Overall, our results underscore the impact of polymicrobial interactions on antimicrobial efficacy and highlight the wide-ranging effects of P. aeruginosa exoproducts on S. aureus physiology. As we search for new strategies to combat recalcitrant infections caused by biofilm-based polymicrobial infections, we must consider the impact of this particular environment on the antibiotic discovery pipeline. That is, most antibiotics are developed and tested in the context of single microbes, but efficacy in such systems does not always translate to polymicrobial settings. For the example presented here, norfloxacin, but not the other quinolone antibiotics tested, showed robust activity versus S. aureus biofilms in the presence of P. aeruginosa exoproducts; this is not an observation one would predict or expect based on the activity of these antibiotics versus pure cultures. Thus, in addition to using polymicrobial models to potentially identify new antimicrobial strategies, such communities could serve as “testing platforms” to identify efficacious antibiotics in vitro versus polymicrobial infections and thus guide therapeutic choices in vivo.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The S. aureus and P. aeruginosa strains used in this study are listed in Table 2. S. aureus was grown in tryptic soy broth (TSB), and P. aeruginosa was grown in lysogeny broth (LB). Overnight cultures were grown shaking at 37°C for 12 to 14 h.
TABLE 2.
Strains used in this study
| Species and strain | Strain no. | Phenotype | Source or reference |
|---|---|---|---|
| S. aureus | |||
| Newman | SMC 1007 | MSSAa | 54 |
| JE2 | S. aureus TMLc | MRSAb | 55 |
| JE2 NE12e (lmrS::Tn) | S. aureus TML | 55 | |
| JE2 NE17e | S. aureus TML | 55 | |
| JE2 NE179e (mdeA::Tn) | S. aureus TML | 55 | |
| JE2 NE370e | S. aureus TML | 55 | |
| JE2 NE405e (mepA::Tn) | S. aureus TML | 55 | |
| JE2 NE531e (sdrM::Tn) | S. aureus TML | 55 | |
| JE2 NE749e (sbnD::Tn) | S. aureus TML | 55 | |
| JE2 NE773e | S. aureus TML | 55 | |
| JE2 NE781e | S. aureus TML | 55 | |
| JE2 NE901e | S. aureus TML | 55 | |
| JE2 NE1034e (norA::Tn) | S. aureus TML | 55 | |
| JE2 NE1041e | S. aureus TML | 55 | |
| JE2 NE1184e (norB::Tn) | S. aureus TML | 55 | |
| JE2 NE1400e | S. aureus TML | 55 | |
| JE2 NE1616e (sepA::Tn) | S. aureus TML | 55 | |
| JE2 NE1804e (norC::Tn) | S. aureus TML | 55 | |
| P. aeruginosa | |||
| PA14 | SMC 232 | Nonmucoid | 56 |
| PAO1 | SMC 17 | Nonmucoid | 57 |
| PA14 ΔpqsA mutant | SMC 5013 | L. G. Rahme | |
| PA14 ΔpqsH mutant | SMC 5017 | 58 | |
| PA14 ΔpqsL mutant | SMC 6216 | 30 | |
| PA14 ΔpvdA mutant | SMC 6596 | 30 | |
| PA14 ΔpchE mutant | SMC 6597 | 30 | |
| PA14 ΔpvdA ΔpchE mutant | SMC 6215 | 59 | |
| PA14 ΔpqsL ΔpvdA ΔpchE mutant | SMC 6219 | 30 | |
| PA14 ΔphzA-G1/2 mutant | SMC 5020 | 60 | |
| PA14 ΔpqsL ΔphzA-G1/2 mutant | SMC 6220 | L. M. Filkins | |
| PA14 rhlA::Tn strain | P. aeruginosa TMLd | 61 | |
| PA14 lasB::Tn strain | P. aeruginosa TML | 61 | |
| PA14 ΔlasR mutant | SMC 5021 | 62 | |
| Clinical isolate | SMC 1587 | Mucoid | 63 |
| Clinical isolate | SMC 1595 | Nonmucoid | 63 |
MSSA, methicillin-sensitive S. aureus.
MRSA, methicillin-resistant S. aureus.
S. aureus TML, S. aureus JE2 Nebraska mariner transposon mutant library.
P. aeruginosa TML, P. aeruginosa PA14 NR mariner transposon mutant library.
S. aureus antibiotic transporter mutant.
Preparation of P. aeruginosa supernatants.
Overnight liquid cultures of P. aeruginosa were diluted to an optical density at 600 nm (OD600) of 0.05, washed in phosphate-buffered saline (PBS), and resuspended in minimum essential medium (MEM; Thermo Fisher Scientific) supplemented with 2 mM l-glutamine (MEM + l-Gln). Next, each well of a plastic 6-well plate was inoculated with 2 ml of the P. aeruginosa suspensions and incubated at 37°C and 5% CO2 for 1 h. Afterward, unattached cells were removed and 2 ml of MEM + l-Gln was added to each well. Following incubation for an additional 22 to 24 h at 37°C and 5% CO2, the culture supernatant was collected, centrifuged at 5,000 × g for 5 min, and passed through a 0.22-μm filter. In subsequent experiments, wells containing P. aeruginosa supernatant received half the well volume of supernatant and half the well volume of either MEM + l-Gln or antibiotic solutions in MEM + l-Gln.
Biofilm disruption assay on plastic.
Overnight liquid cultures of S. aureus were diluted to an OD600 of 0.05, washed in PBS, and resuspended in MEM + l-Gln. Triplicate wells of a plastic 96-well plate were inoculated with 100 μl of the S. aureus suspensions and incubated at 37°C with 5% CO2. Unattached cells were removed 6 h postinoculation (h p.i.) or 24 h p.i. (as indicated), at which point MEM + l-Gln, antibiotic dilutions in MEM + l-Gln (norfloxacin, ciprofloxacin, levofloxacin, or moxifloxacin), P. aeruginosa supernatant, HQNO, antimycin A, benzyl alcohol, DMSO, stearic acid, oleic acid, linoleic acid, linolenic acid, or CCCP was added (total well volume of 90 μl). The plate was incubated at 37°C and 5% CO2 for 18 additional hours. Planktonic cells were removed, and then 50 μl of PBS was added to each well of the plate. Next, the wells were scraped using a solid multipin replicator to disrupt the biofilms. Biofilm cells were serially diluted 10-fold in PBS and plated on mannitol salt agar. Viable cell counts for all relevant experiments are reported as log10 transformed CFU per milliliter.
Norfloxacin accumulation assay.
Antibiotic internalization assays were performed as previously described (46–49) with modifications. Overnight liquid cultures of S. aureus were diluted to an OD600 of 2.0, washed in PBS, and resuspended in MEM + l-Gln. Next, 1 ml of the S. aureus suspension was mixed with 1 ml of either MEM + l-Gln, P. aeruginosa supernatant, or CCCP (for a final S. aureus OD600 of 1.0) and then added to a 6-well plate (total well volume of 2 ml). The plate was incubated at 37°C and 5% CO2 statically for 3 h. Cells were collected, washed once with 1 ml of PBS, and adjusted to an OD600 of 1.0. Then, cells were resuspended in 1 ml of either MEM + l-Gln, P. aeruginosa supernatant, or CCCP and added to 1 ml of PBS containing a final concentration of norfloxacin of 10 μg/ml. Afterward, cells were incubated at 37°C under agitation for 30 min. Cells were collected and washed once with 1 ml of PBS. Next, cells were resuspended in 2 ml of 0.1 M glycine-HCl buffer (pH of 3), followed by measurement of OD600 and incubation at room temperature under agitation overnight (∼15 h). After centrifugation at 10,000 × g for 5 min, supernatants were transferred to a new tube. For each sample, 150 μl of supernatant was transferred to each well of flat-bottomed black 96-well plates. The intrinsic fluorescence of norfloxacin was measured at 440 nm following excitation at 281 nm. A standard curve was used to relate fluorescence values to the amount of intracellular norfloxacin (nanograms) in each sample. Values are reported as the amount of intracellular norfloxacin (nanograms) per OD600.
RNA extraction.
Overnight liquid cultures of S. aureus were diluted to an OD600 of 0.05, washed in PBS, and resuspended in MEM + l-Gln. Duplicate 6-well plates per condition were inoculated with the S. aureus suspensions (total well volume of 2 ml) and incubated at 37°C with 5% CO2. Unattached cells were removed at 24 h p.i., at which point antibiotic dilutions in MEM + l-Gln, P. aeruginosa supernatant, and MEM + l-Gln were added (total well volume of 2 ml). The plate was incubated at 37°C and 5% CO2 for 3 additional hours. Supernatants were discarded, and then 300 μl of RNAlater (Qiagen) was added to each well of the plate. Biofilm cells were removed from the bottom of each well using plastic cell scrapers and centrifuged at 6,000 × g for 5 min at 4°C. Following removal of supernatants, total RNA was isolated using PureZOL RNA isolation reagent (Bio-Rad) according to the manufacturer’s instructions, including homogenization by bead beating (5 cycles of 30 s). Afterward, Turbo DNA-free DNase treatment (Thermo Fisher Scientific) was performed according to the manufacturer’s instructions followed by PCR to test for DNA contamination.
Quantitative reverse transcription-PCR.
cDNA synthesis and quantitative reverse transcription-PCR (qRT-PCR) were performed using the iTaq Universal SYBR green one-step kit (Bio-Rad) according to the manufacturer's instructions. The qRT-PCR primers used were designed for each S. aureus gene of interest and are listed in Table 3. S. aureus rpoB was selected to normalize gene expression as determined previously (30). For each treatment condition, cycle threshold (CT) values for each gene of interest were normalized to rpoB CT values. Changes in gene expression were assessed by the ΔΔCT method (53). Expression data are presented as rpoB-normalized gene expression for each treatment condition relative to S. aureus exposed to medium alone.
TABLE 3.
Primers used in this study
| Primer name | Sequence | Source |
|---|---|---|
| Sa NWMN_2314_mdeA qPCR F | GTCCGCACTTTGCAACATTA | This study |
| Sa NWMN_2314_mdeA qPCR R | TGTCATCATCAATGGCATCA | This study |
| Sa NWMN_0327_mepA qPCR F | GGGCACCAATGGTTTCTATG | This study |
| Sa NWMN_0327_mepA qPCR R | GTACCCAAAGCTGCACCAAC | This study |
| Sa NWMN_2070_sdrM qPCR F | TGTGGCAATTGCAGGATTAC | This study |
| Sa NWMN_2070_sdrM qPCR R | TGATCCAAAACCTTGTATCACG | This study |
| Sa NWMN_0664_norA qPCR F | AATGCCTGGTGTGACAGGTT | This study |
| Sa NWMN_0664_norA qPCR R | TCGCTGACATGTAGCCAAAG | This study |
| Sa NWMN_0504_rpoB qPCR F | TTATGCTGCACCTCTTCGTG | This study |
| Sa NWMN_0504_rpoB qPCR R | TGGGAAATCACCCATAAAGAC | This study |
ACKNOWLEDGMENTS
We thank Ambrose Cheung, Laura Filkins, and Deborah Hogan for providing bacterial strains.
This work was supported by National Institutes of Health grant R37 AI83256-06 and the Cystic Fibrosis Foundation (OTOOLE16G0) to G.A.O. and the Microbiology and Molecular Pathogenesis Training Grant (T32-AI007519) to G.O.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Cystic Fibrosis Foundation. 2015. Cystic Fibrosis Foundation patient registry 2015 annual data report. Cystic Fibrosis Foundation, Bethesda, MD. [Google Scholar]
- 2.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. 2002. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 3.Wolter DJ, Emerson JC, McNamara S, Buccat AM, Qin X, Cochrane E, Houston LS, Rogers GB, Marsh P, Prehar K, Pope CE, Blackledge M, Deziel E, Bruce KD, Ramsey BW, Gibson RL, Burns JL, Hoffman LR. 2013. Staphylococcus aureus small-colony variants are independently associated with worse lung disease in children with cystic fibrosis. Clin Infect Dis 57:384–391. doi: 10.1093/cid/cit270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Com G, Carroll JL, Castro MM, Tang X, Jambhekar S, Berlinski A. 2014. Predictors and outcome of low initial forced expiratory volume in 1 second measurement in children with cystic fibrosis. J Pediatr 164:832–838. doi: 10.1016/j.jpeds.2013.11.064. [DOI] [PubMed] [Google Scholar]
- 5.Limoli DH, Yang J, Khansaheb MK, Helfman B, Peng L, Stecenko AA, Goldberg JB. 2016. Staphylococcus aureus and Pseudomonas aeruginosa co-infection is associated with cystic fibrosis-related diabetes and poor clinical outcomes. Eur J Clin Microbiol Infect Dis 35:947–953. doi: 10.1007/s10096-016-2621-0. [DOI] [PubMed] [Google Scholar]
- 6.Hudson VL, Wielinski CL, Regelmann WE. 1993. Prognostic implications of initial oropharyngeal bacterial flora in patients with cystic fibrosis diagnosed before the age of two years. J Pediatr 122:854–860. doi: 10.1016/S0022-3476(09)90007-5. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbluth DB, Wilson K, Ferkol T, Schuster DP. 2004. Lung function decline in cystic fibrosis patients and timing for lung transplantation referral. Chest 126:412–419. doi: 10.1378/chest.126.2.412. [DOI] [PubMed] [Google Scholar]
- 8.Maliniak ML, Stecenko AA, McCarty NA. 2016. A longitudinal analysis of chronic MRSA and Pseudomonas aeruginosa co-infection in cystic fibrosis: a single-center study. J Cyst Fibros 15:350–356. doi: 10.1016/j.jcf.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Limoli DH, Hoffman LR. 2019. Help, hinder, hide and harm: what can we learn from the interactions between Pseudomonas aeruginosa and Staphylococcus aureus during respiratory infections? Thorax 74:684–692. doi: 10.1136/thoraxjnl-2018-212616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gjødsbøl K, Christensen JJ, Karlsmark T, Jørgensen B, Klein BM, Krogfelt KA. 2006. Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 3:225–231. doi: 10.1111/j.1742-481X.2006.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Körber A, Schmid EN, Buer J, Klode J, Schadendorf D, Dissemond J. 2010. Bacterial colonization of chronic leg ulcers: current results compared with data 5 years ago in a specialized dermatology department. J Eur Acad Dermatol Venereol 24:1017–1025. doi: 10.1111/j.1468-3083.2010.03570.x. [DOI] [PubMed] [Google Scholar]
- 12.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. 2010. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman LR, Déziel E, D'Argenio DA, Lépine F, Emerson J, McNamara S, Gibson RL, Ramsey BW, Miller SI. 2006. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 103:19890–19895. doi: 10.1073/pnas.0606756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan RP, Fouhy Y, Garcia BF, Watt SA, Niehaus K, Yang L, Tolker-Nielsen T, Dow JM. 2008. Interspecies signalling via the Stenotrophomonas maltophilia diffusible signal factor influences biofilm formation and polymyxin tolerance in Pseudomonas aeruginosa. Mol Microbiol 68:75–86. doi: 10.1111/j.1365-2958.2008.06132.x. [DOI] [PubMed] [Google Scholar]
- 15.Harriott MM, Noverr MC. 2009. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob Agents Chemother 53:3914–3922. doi: 10.1128/AAC.00657-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armbruster CE, Hong W, Pang B, Weimer KED, Juneau RA, Turner J, Swords WE. 2010. Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio 1:e00102-10. doi: 10.1128/mBio.00102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalton T, Dowd SE, Wolcott RD, Sun Y, Watters C, Griswold JA, Rumbaugh KP. 2011. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS One 6:e27317. doi: 10.1371/journal.pone.0027317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vega NM, Allison KR, Samuels AN, Klempner MS, Collins JJ. 2013. Salmonella typhimurium intercepts Escherichia coli signaling to enhance antibiotic tolerance. Proc Natl Acad Sci U S A 110:14420–14425. doi: 10.1073/pnas.1308085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orazi G, O’Toole GA. 2019. “It takes a village”: mechanisms underlying antimicrobial recalcitrance of polymicrobial biofilms. J Bacteriol 202:e00530-19. doi: 10.1128/JB.00530-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lightbown JW. 1954. An antagonist of streptomycin and dihydrostreptomycin produced by Pseudomonas aeruginosa. J Gen Microbiol 11:477–492. doi: 10.1099/00221287-11-3-477. [DOI] [PubMed] [Google Scholar]
- 21.Lebrun M, de Repentigny J, Mathieu LG. 1978. Diminution of the antibacterial activity of antibiotics in cultures and in experimental mixed infections. Can J Microbiol 24:154–161. (In French.) doi: 10.1139/m78-028. [DOI] [PubMed] [Google Scholar]
- 22.DeLeon S, Clinton A, Fowler H, Everett J, Horswill AR, Rumbaugh KP. 2014. Synergistic interactions of Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro wound model. Infect Immun 82:4718–4728. doi: 10.1128/IAI.02198-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaudoin T, Yau YCW, Stapleton PJ, Gong Y, Wang PW, Guttman DS, Waters V. 2017. Staphylococcus aureus interaction with Pseudomonas aeruginosa biofilm enhances tobramycin resistance. NPJ Biofilms Microbiomes 3:25. doi: 10.1038/s41522-017-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orazi G, O’Toole GA. 2017. Pseudomonas aeruginosa alters Staphylococcus aureus sensitivity to vancomycin in a biofilm model of cystic fibrosis infection. mBio 8:e00873-17. doi: 10.1128/mBio.00873-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radlinski L, Rowe SE, Kartchner LB, Maile R, Cairns BA, Vitko NP, Gode CJ, Lachiewicz AM, Wolfgang MC, Conlon BP. 2017. Pseudomonas aeruginosa exoproducts determine antibiotic efficacy against Staphylococcus aureus. PLoS Biol 15:e2003981. doi: 10.1371/journal.pbio.2003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tavernier S, Crabbé A, Hacioglu M, Stuer L, Henry S, Rigole P, Dhondt I, Coenye T. 2017. Community composition determines activity of antibiotics against multispecies biofilms. Antimicrob Agents Chemother 61:e00301-17. doi: 10.1128/AAC.00302-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tognon M, Köhler T, Gdaniec BG, Hao Y, Lam JS, Beaume M, Luscher A, Buckling A, van Delden C. 2017. Co-evolution with Staphylococcus aureus leads to lipopolysaccharide alterations in Pseudomonas aeruginosa. ISME J 11:2233–2243. doi: 10.1038/ismej.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orazi G, Ruoff KL, O’Toole GA. 2019. Pseudomonas aeruginosa increases the sensitivity of biofilm-grown Staphylococcus aureus to membrane-targeting antiseptics and antibiotics. mBio 10:e01501-19. doi: 10.1128/mBio.01501-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radlinski LC, Rowe SE, Brzozowski R, Wilkinson AD, Huang R, Eswara P, Conlon BP. 2019. Chemical induction of aminoglycoside uptake overcomes antibiotic tolerance and resistance in Staphylococcus aureus. Cell Chem Biol 26:1355–1364. doi: 10.1016/j.chembiol.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Filkins LM, Graber JA, Olson DG, Dolben EL, Lynd LR, Bhuju S, O'Toole GA. 2015. Coculture of Staphylococcus aureus with Pseudomonas aeruginosa drives S. aureus towards fermentative metabolism and reduced viability in a cystic fibrosis model. J Bacteriol 197:2252–2264. doi: 10.1128/JB.00059-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith L. 1954. Bacterial cytochromes. Difference spectra. Arch Biochem Biophys 50:299–314. doi: 10.1016/0003-9861(54)90045-4. [DOI] [PubMed] [Google Scholar]
- 32.Lightbown JW, Jackson FL. 1956. Inhibition of cytochrome systems of heart muscle and certain bacteria by the antagonists of dihydrostreptomycin: 2-alkyl-4-hydroxyquinoline N-oxides. Biochem J 63:130–137. doi: 10.1042/bj0630130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedlander G, Le Grimellec C, Giocondi M-C, Amiel C. 1987. Benzyl alcohol increases membrane fluidity and modulates cyclic AMP synthesis in intact renal epithelial cells. Biochim Biophys Acta 903:341–348. doi: 10.1016/0005-2736(87)90224-0. [DOI] [PubMed] [Google Scholar]
- 34.Shigapova N, Török Z, Balogh G, Goloubinoff P, Vígh L, Horváth I. 2005. Membrane fluidization triggers membrane remodeling which affects the thermotolerance in Escherichia coli. Biochem Biophys Res Commun 328:1216–1223. doi: 10.1016/j.bbrc.2005.01.081. [DOI] [PubMed] [Google Scholar]
- 35.Cebrián G, Condón S, Mañas P. 2016. Influence of growth and treatment temperature on Staphylococcus aureus resistance to pulsed electric fields: relationship with membrane fluidity. Innovat Food Sci Emerg Technol 37:161–169. doi: 10.1016/j.ifset.2016.08.011. [DOI] [Google Scholar]
- 36.Veerman ECI, Valentijn-Benz M, Nazmi K, Ruissen ALA, Walgreen-Weterings E, van Marle J, Doust AB, van't Hof W, Bolscher JGM, Amerongen A. 2007. Energy depletion protects Candida albicans against antimicrobial peptides by rigidifying its cell membrane. J Biol Chem 282:18831–18841. doi: 10.1074/jbc.M610555200. [DOI] [PubMed] [Google Scholar]
- 37.Dyrda G, Boniewska-Bernacka E, Man D, Barchiewicz K, Słota R. 2019. The effect of organic solvents on selected microorganisms and model liposome membrane. Mol Biol Rep 46:3225–3232. doi: 10.1007/s11033-019-04782-y. [DOI] [PubMed] [Google Scholar]
- 38.White DC, Frerman FE. 1968. Fatty acid composition of the complex lipids of Staphylococcus aureus during the formation of the membrane-bound electron transport system. J Bacteriol 95:2198–2209. doi: 10.1128/JB.95.6.2198-2209.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sen S, Sirobhushanam S, Johnson SR, Song Y, Tefft R, Gatto C, Wilkinson BJ. 2016. Growth-environment dependent modulation of Staphylococcus aureus branched-chain to straight-chain fatty acid ratio and incorporation of unsaturated fatty acids. PLoS One 11:e0165300. doi: 10.1371/journal.pone.0165300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keweloh H, Diefenbach R, Rehm H-J. 1991. Increase of phenol tolerance of Escherichia coli by alterations of the fatty acid composition of the membrane lipids. Arch Microbiol 157:49–53. doi: 10.1007/BF00245334. [DOI] [PubMed] [Google Scholar]
- 41.Furet YX, Deshusses J, Pechère JC. 1992. Transport of pefloxacin across the bacterial cytoplasmic membrane in quinolone-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 36:2506–2511. doi: 10.1128/aac.36.11.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCaffrey C, Bertasso A, Pace J, Georgopapadakou NH. 1992. Quinolone accumulation in Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Antimicrob Agents Chemother 36:1601–1605. doi: 10.1128/aac.36.8.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piddock LJ, Jin YF, Ricci V, Asuquo AE. 1999. Quinolone accumulation by Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli. J Antimicrob Chemother 43:61–70. doi: 10.1093/jac/43.1.61. [DOI] [PubMed] [Google Scholar]
- 44.Kumar S, Mukherjee MM, Varela MF. 2013. Modulation of bacterial multidrug resistance efflux pumps of the major facilitator superfamily. Int J Bacteriol 2013:204141. doi: 10.1155/2013/204141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bentaboulet M, Kepes A. 1977. Counter-transport mediated by the lactose permease of Escherichia coli. Biochim Biophys Acta 471:125–134. doi: 10.1016/0005-2736(77)90400-x. [DOI] [PubMed] [Google Scholar]
- 46.Chapman JS, Georgopapadakou NH. 1989. Fluorometric assay for fleroxacin uptake by bacterial cells. Antimicrob Agents Chemother 33:27–29. doi: 10.1128/aac.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mortimer PG, Piddock LJ. 1991. A comparison of methods used for measuring the accumulation of quinolones by Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. J Antimicrob Chemother 28:639–653. doi: 10.1093/jac/28.5.639. [DOI] [PubMed] [Google Scholar]
- 48.Begic S, Worobec EA. 2007. Fluoroquinolone resistance of Serratia marcescens: sucrose, salicylate, temperature, and pH induction of phenotypic resistance. Can J Microbiol 53:1239–1245. doi: 10.1139/W07-097. [DOI] [PubMed] [Google Scholar]
- 49.Martins D, McKay G, Sampathkumar G, Khakimova M, English AM, Nguyen D. 2018. Superoxide dismutase activity confers (p)ppGpp-mediated antibiotic tolerance to stationary-phase Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 115:9797–9802. doi: 10.1073/pnas.1804525115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costa SS, Viveiros M, Amaral L, Couto I. 2013. Multidrug efflux pumps in Staphylococcus aureus: an update. Open Microbiol J 7:59–71. doi: 10.2174/1874285801307010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tognon M, Köhler T, Luscher A, van Delden C. 2019. Transcriptional profiling of Pseudomonas aeruginosa and Staphylococcus aureus during in vitro co-culture. BMC Genomics 20:30. doi: 10.1186/s12864-018-5398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuchs S, Pané-Farré J, Kohler C, Hecker M, Engelmann S. 2007. Anaerobic gene expression in Staphylococcus aureus. J Bacteriol 189:4275–4289. doi: 10.1128/JB.00081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 54.Duthie ES. 1952. Variation in the antigenic composition of staphylococcal coagulase. J Gen Microbiol 7:320–326. doi: 10.1099/00221287-7-3-4-320. [DOI] [PubMed] [Google Scholar]
- 55.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 57.Holloway BW, Morgan AF. 1986. Genome organization in Pseudomonas. Annu Rev Microbiol 40:79–105. doi: 10.1146/annurev.mi.40.100186.000455. [DOI] [PubMed] [Google Scholar]
- 58.Cugini C, Morales DK, Hogan DA. 2010. Candida albicans-produced farnesol stimulates Pseudomonas quinolone signal production in LasR-defective Pseudomonas aeruginosa strains. Microbiology 156:3096–3107. doi: 10.1099/mic.0.037911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Wilks JC, Danhorn T, Ramos I, Croal L, Newman DK. 2011. Phenazine-1-carboxylic acid promotes bacterial biofilm development via ferrous iron acquisition. J Bacteriol 193:3606–3617. doi: 10.1128/JB.00396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dietrich LEP, Price-Whelan A, Petersen A, Whiteley M, Newman DK. 2006. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol 61:1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 61.Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, Wu G, Villanueva J, Wei T, Ausubel FM. 2006. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci U S A 103:2833–2838. doi: 10.1073/pnas.0511100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hogan DA, Vik A, Kolter R. 2004. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol 54:1212–1223. doi: 10.1111/j.1365-2958.2004.04349.x. [DOI] [PubMed] [Google Scholar]
- 63.Yu Q, Griffin EF, Moreau-Marquis S, Schwartzman JD, Stanton BA, O'Toole GA. 2012. In vitro evaluation of tobramycin and aztreonam versus Pseudomonas aeruginosa biofilms on cystic fibrosis-derived human airway epithelial cells. J Antimicrob Chemother 67:2673–2681. doi: 10.1093/jac/dks296. [DOI] [PMC free article] [PubMed] [Google Scholar]


